Variation of Electrical Resistivity and Charge Passed in High-Performance Concrete
Abstract
:1. Introduction
2. Materials and Methods
- Type II–V cement (TII-V) (moderate sulfate resistance cement of Type II and Type V blended) following ASTM C150 specification
- Ground granulated blast furnace slag of grade 100 (G100S)
- Ground granulated blast furnace slag of grade 120 (G120S)
- Class F fly Ash (F)
- Class C fly Ash (C)
- Silica fume (SF)
- Metakaolin (M)
3. Charge Passed Models
3.1. Charge Passed from Bulk Conductivity Model following RCPT Theory
3.2. Charge Passed Computation by Berke Model
4. Results and Discussion
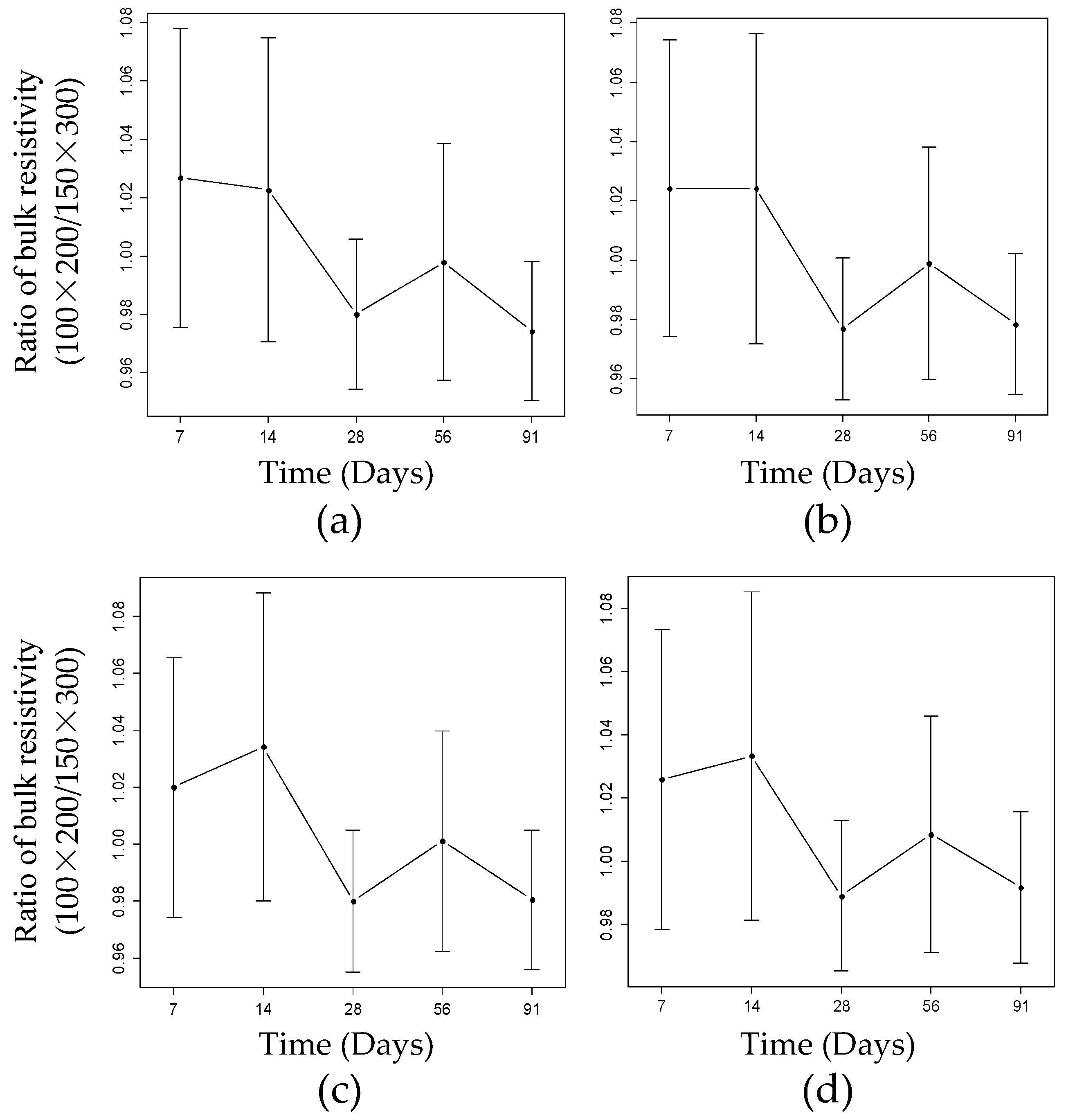
4.1. Influence of Geometric Size on the BR
4.2. Influence of Frequency on the BR
4.3. Charge Passed
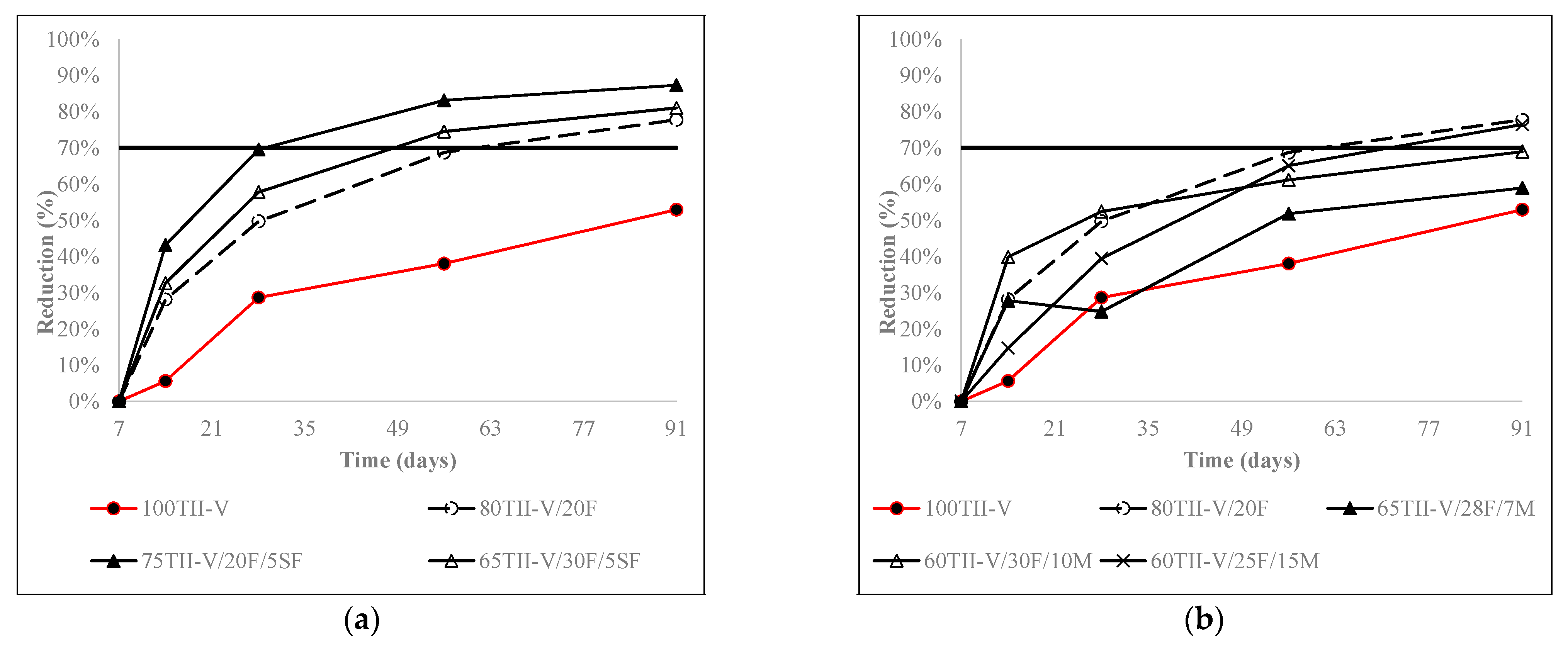
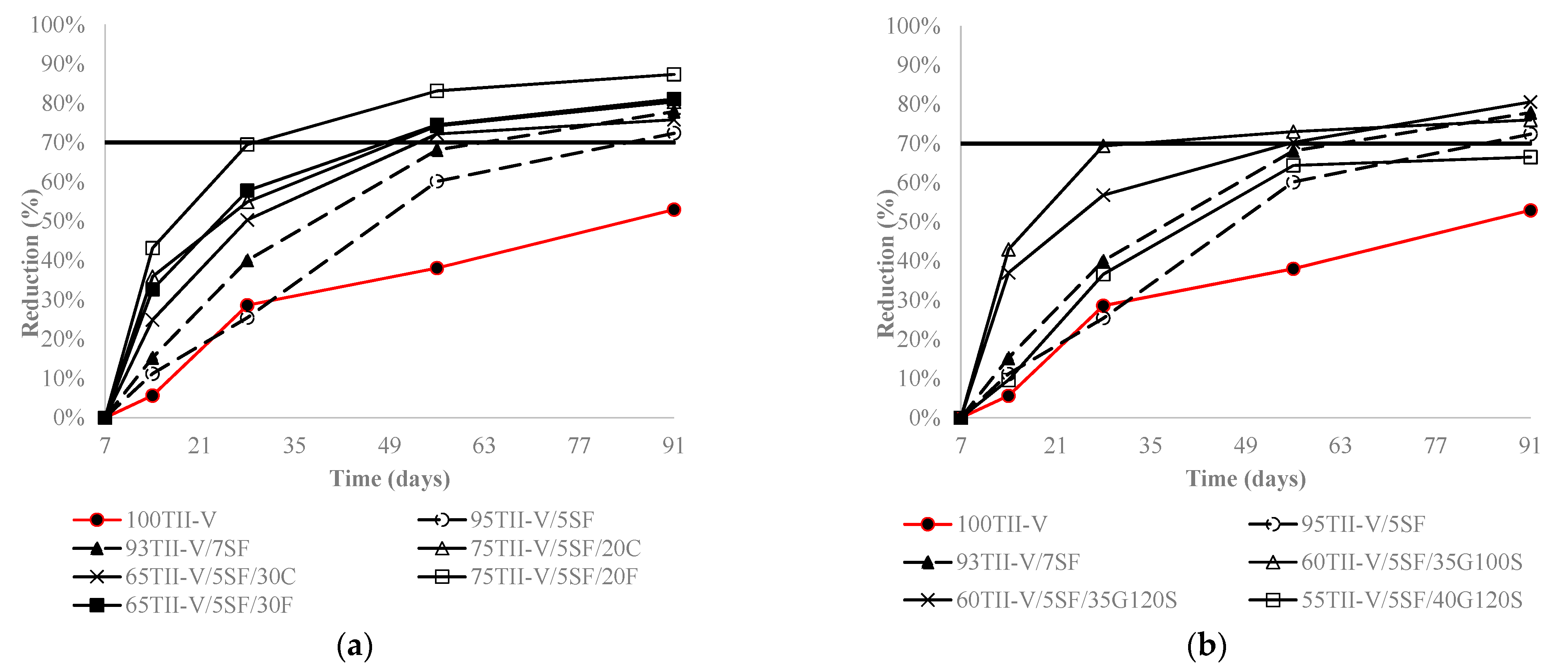
4.4. Charge Passed Reduction over an Extended Period
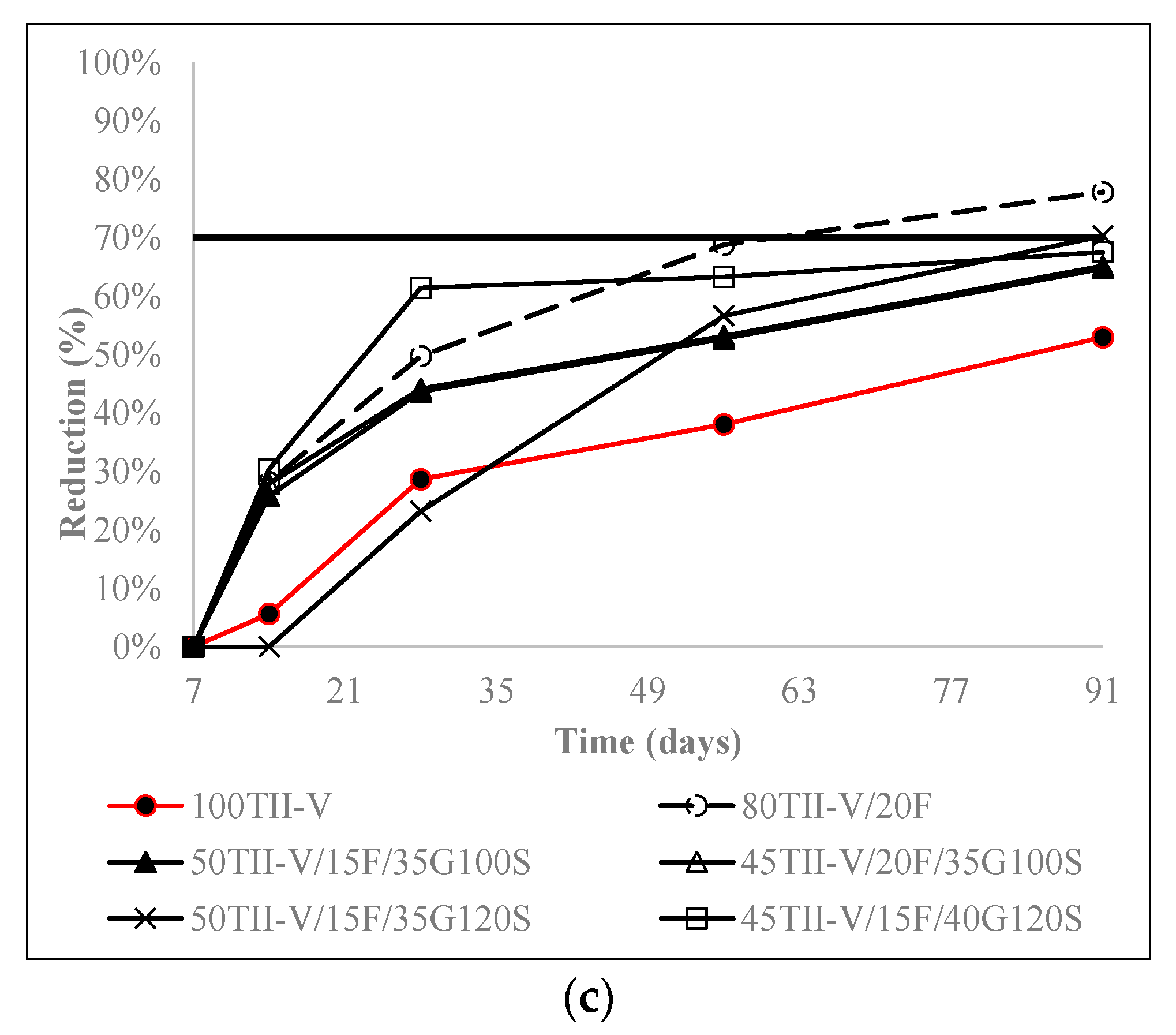
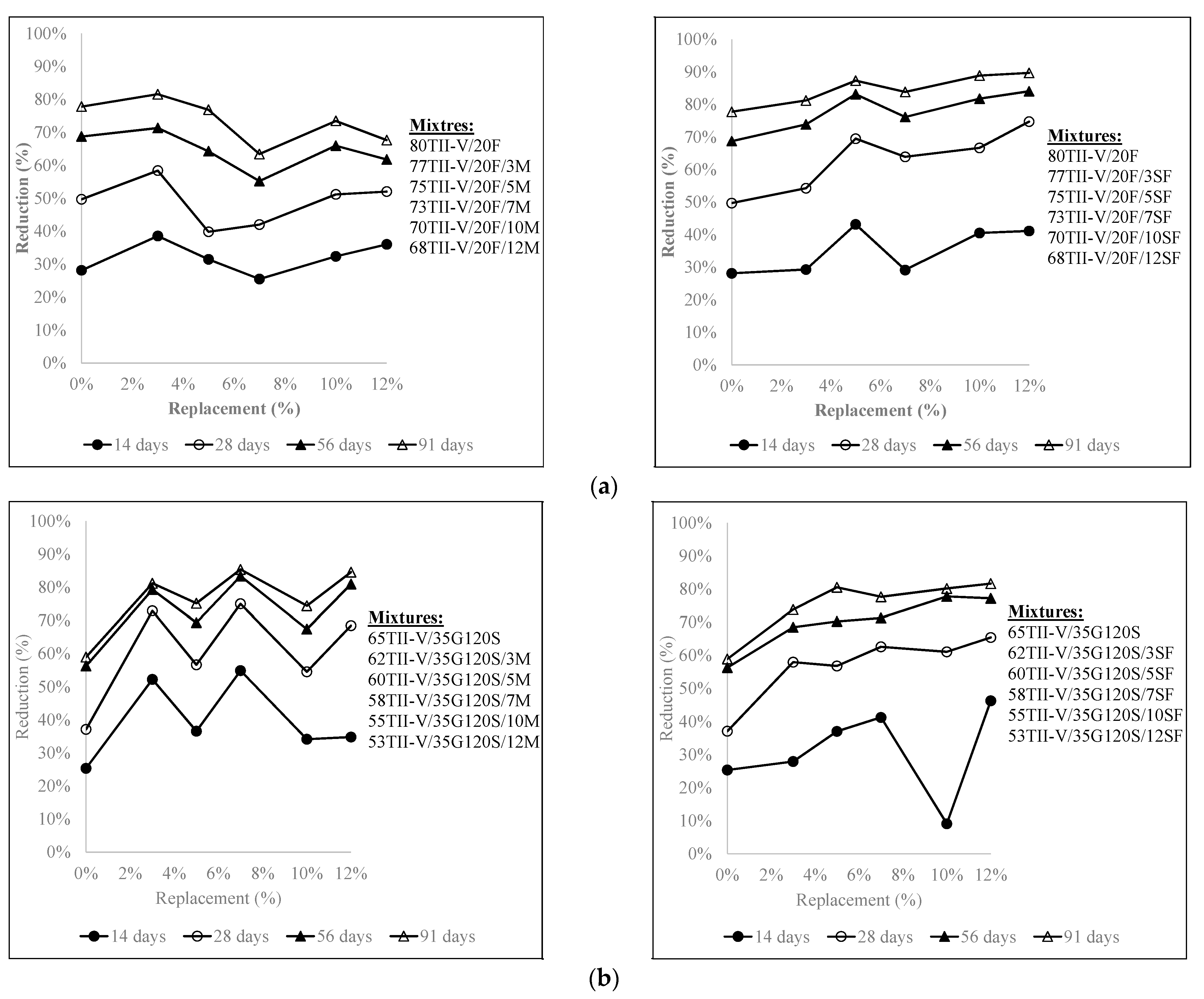
4.5. Influence of the Variation of Metakaolin and SF Replacement on Charge Passed Reduction
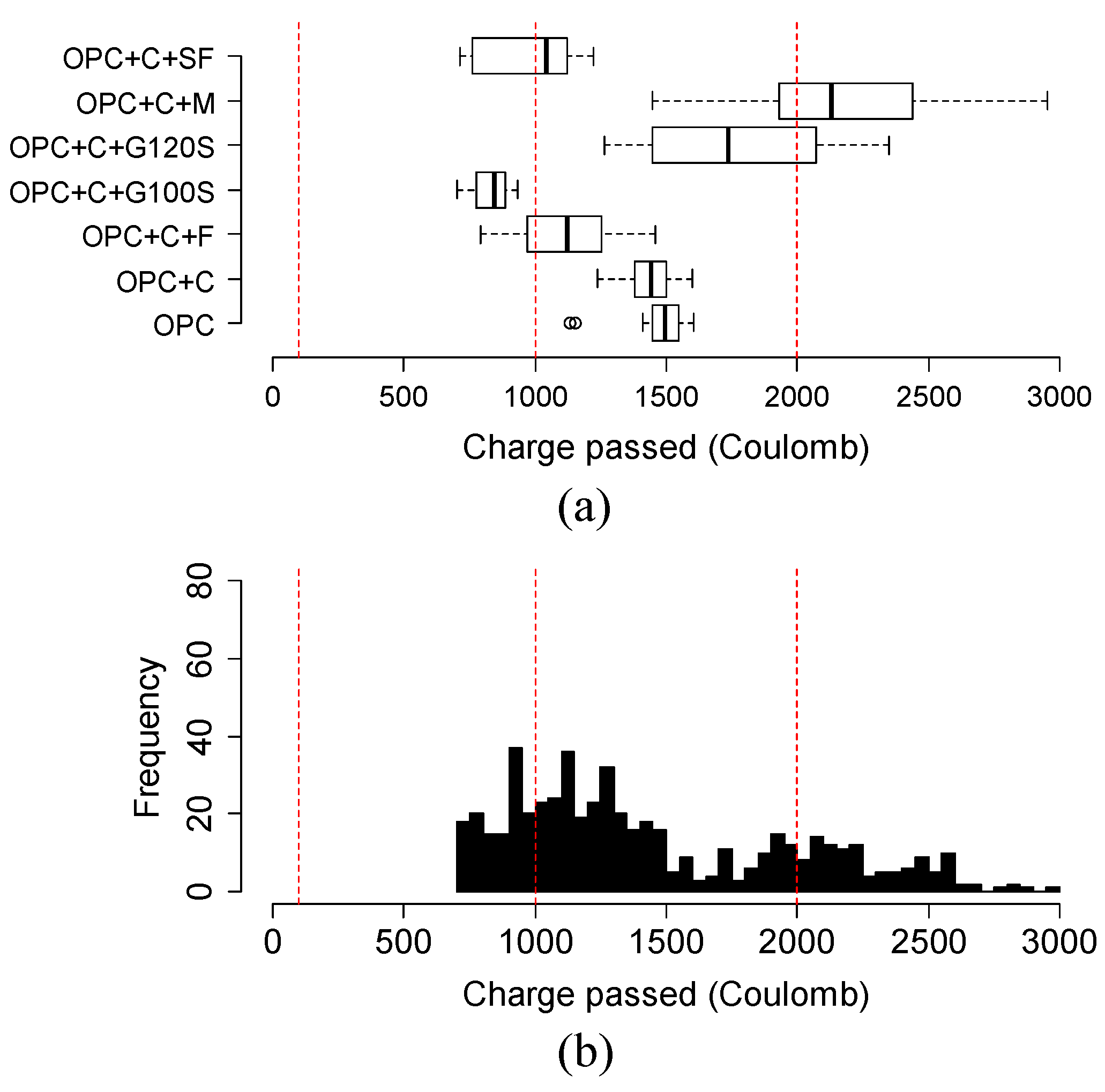
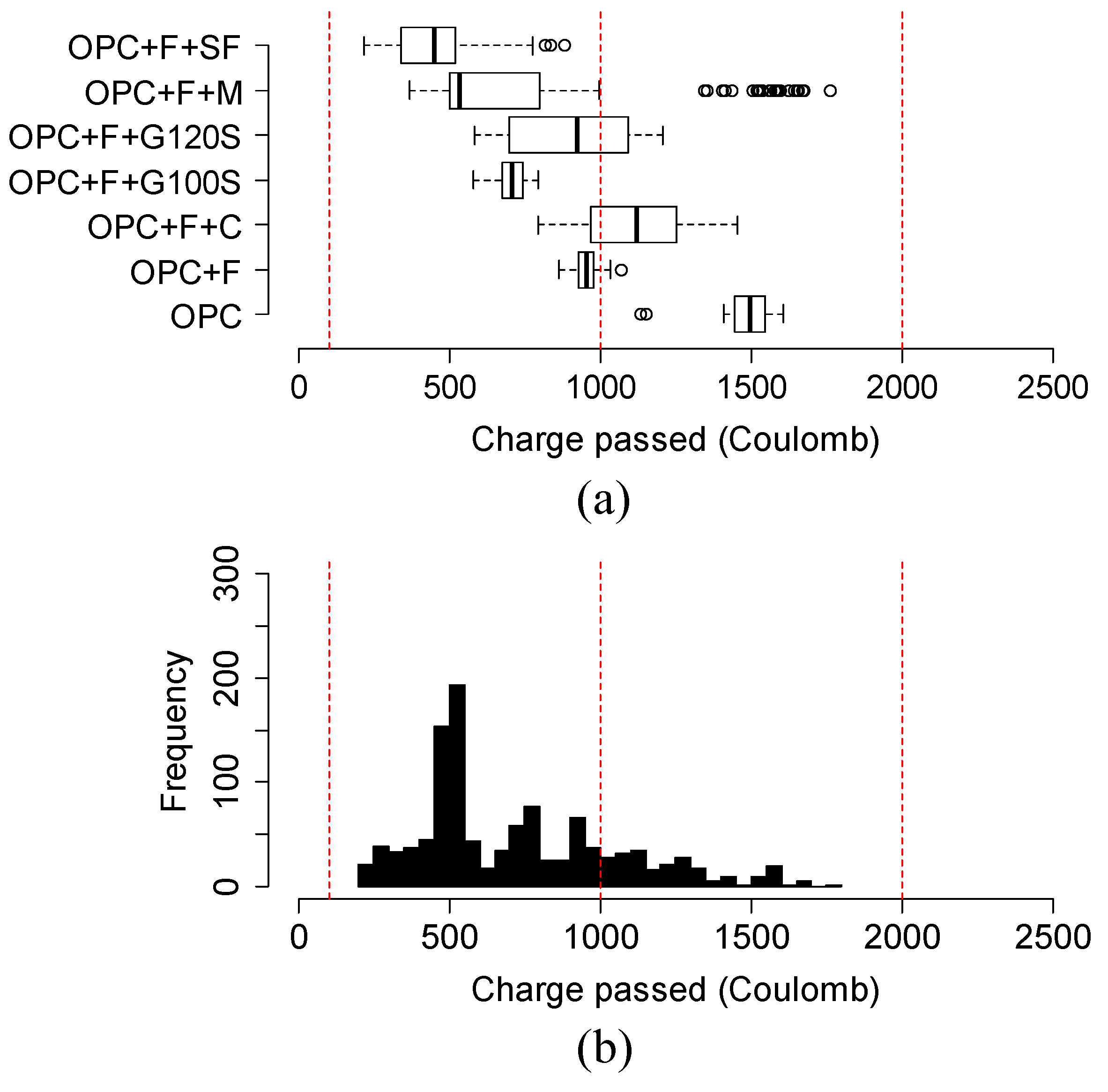
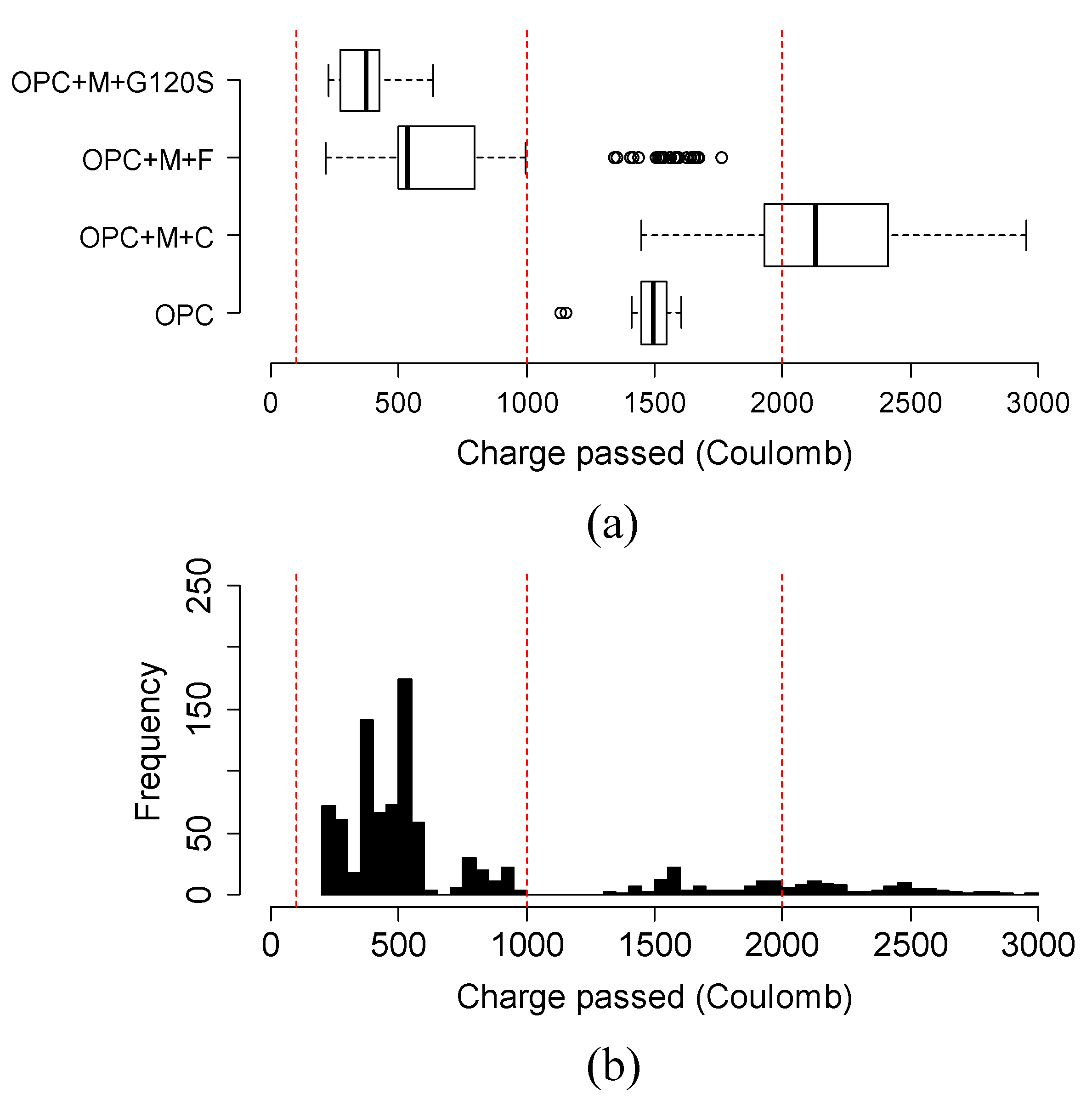
4.6. Variation and Distribution of Charge Passed of Different Groups of Ternary Mixtures
5. Conclusions
- The addition of SCMs in ternary mixtures, in general, helped to reduce the charge passed in a faster way compared to the binary mixtures. Specifically, in Class C fly ash-based mixtures, Class C fly ash combined with 35% slag G100S or with higher than 10% metakaolin obtained better results than the binary mixture. In metakaolin-based mixtures, the addition of 35% slag G120S are a better option for future bridge deck applications.
- In 20% fly ash-based ternary mixtures, the addition of 3% metakaolin or 12% silica fume resulted in the highest reduction of charge passed over time. 5% silica fume replacement in 20% fly ash in ternary mixtures can be considered an optimal solution. In addition, in 35% slag G120S-based ternary mixtures, the replacement of 12% silica fume provided the highest reduction at 91 days, and the addition of 3% to 12% silica fume provided a comparable reduction to each other. Furthermore, the addition of 3%, 7%, and 12% metakaolin provided a comparably higher amount of charge reduction over an extended period.
- The variation and distribution of charge passed data demonstrated that the addition of SCM provided a varying effect on the charge passed in the same group. Most ternary mixtures’ charge passed data fell within the range of low to very low chloride ion permeability class, except the ternary mixture of class C fly ash and metakaolin, which fell in moderate permeability.
- In practice, depending on the source and the availability of SCM, the first SCM in ternary mixtures design could be specified with confidence. Therefore, this study was significantly helpful in identifying the type and amount of second SCM in ternary mixtures to design low-charge-passed and high-durability concrete mixtures.
- The surface resistivity of concrete was determined by AASHTTO T 358, a newly developed standard, and the bulk resistivity of concrete was determined by AASHTTO TP 119 or ASTM C1876. All three instruments depicted consistency and accuracy in terms of computation of the charge passed for various concrete mixtures in an efficient way and within a very short period.
- The charge-passed distribution provided a useful tool for future research to compute the diffusion coefficients of HPC mixtures to assess chloride-induced corrosion.
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Snyder, K.A. The relationship between the formation factor and the diffusion coefficient of porous materials saturated with concentrated electrolytes: Theoretical and experimental considerations. J. Concr. Sci. Eng. 2001, 3, 216–222. [Google Scholar]
- Whittington, H.W.; McCarter, J.; Forde, M.C. The conduction of electricity through concrete. Mag. Concr. Res. 1981, 33, 48–60. [Google Scholar] [CrossRef]
- Weiss, W.J.; Shane, J.D.; Mieses, A.; Mason, T.O.; Shah, S.P. Aspects of monitoring moisture changes using electrical impedance spectroscopy. In Proceedings of the 2nd Symposium on Self-Desiccation and Its Importance in Concrete Technology, Lund, Sweden, 18 June 1999. [Google Scholar]
- Whiting, D. Rapid measurement of the chloride permeability of concrete. Public Roads 1981, 45, 101–112. [Google Scholar]
- Snyder, K.A.; Ferraris, C.; Martys, N.S.; Garboczi, E.J. Using Impedance Spectroscopy to Assess the Viability of the Rapid Chloride Test for Determining Concrete Conductivity. J. Res. Natl. Inst. Stand. Technol. 2000, 105, 497–509. [Google Scholar] [CrossRef]
- Nokken, M.R.; Hooton, R.D. Electrical conductivity as a pre-qualification and quality control tool. Concr. Int. 2006, 28, 61–66. [Google Scholar]
- Bentz, D.P. A virtual rapid chloride permeability test. Cem. Concr. Compos. 2007, 29, 723–731. [Google Scholar] [CrossRef]
- Riding, K.A.; Poole, J.L.; Schindler, A.K.; Juenger, M.C.G.; Folliard, K.J. Simplified Concrete Resistivity and Rapid Chloride Permeability Test Method. ACI Mater. J. 2008, 105, 390–394. [Google Scholar] [CrossRef]
- Kessler, R.J.; Powers, R.G.; Paredes, M.P.M. Resistivity Measurements of Water Saturated Concrete as an Indicator of Permeability. In Proceedings of the CORROSION 2005, Houston, TX, USA, 3–7 April 2005. [Google Scholar]
- Icenogle, P.J.; Rupnow, T.D. Development of Precision Statement for Concrete Surface Resistivity. Transp. Res. Rec. 2012, 2290, 38–43. [Google Scholar] [CrossRef]
- Piotr, S. Mechanical Properties of Ultra-High Performance Concrete with Partial Utilization of Waste Foundry Sand. Buildings 2020, 10, 11. [Google Scholar] [CrossRef]
- Qian, Y.; Dingyi, Y.; Yanghao, X.; Han, G.; Zhiming, M. Transport Properties Resistance Improvement of Ultra-High Performance Concrete after Exposure to Elevated Temperatures. Buildings 2021, 11, 416. [Google Scholar] [CrossRef]
- Mehta, P.K.; Monteiro, P.J.M. Concrete: Microstructure, Properties, and Materials, 4th ed.; McGraw-Hill Education: New York, NY, USA, 2014. (In English) [Google Scholar]
- Shi, C. Effect of mixing proportions of concrete on its electrical conductivity and the rapid chloride permeability test (ASTM C1202 or ASSHTO T277) results. Cem. Concr. Res. 2004, 34, 537–545. [Google Scholar] [CrossRef]
- Smith, K.M.; Schokker, A.J.; Tikalsky, P.J. Performance of Supplementary Cementitious Materials in Concrete Resistivity and Corrosion Monitoring Evaluations. ACI Mater. J. 2004, 101, 385–390. [Google Scholar] [CrossRef]
- Tikalsky, P.; Taylor, P.; Hanson, S.; Ghosh, P. Development of Performance Properties of Ternary Mixtures: Laboratory Study on Concrete; Technical Report; 2011. Available online: https://rosap.ntl.bts.gov/view/dot/24416 (accessed on 1 March 2011).
- Marriaga, J.L.; Claisse, P.; Ganjian, E. Application of traditional techniques on chloride resistance assessment of GGBS concrete. In Proceedings of the Second International Conference on Sustainable Construction Materials and Technologies, Ancona, Italy, 28–30 June 2010; pp. 1911–1921. [Google Scholar]
- Ghosh, P.; Tran, Q. Influence of parameters on surface resistivity of concrete. Cem. Concr. Compos. 2015, 62, 134–145. [Google Scholar] [CrossRef]
- Tran, Q.; Ghosh, P. Influence of Supplementary Cementitious Materials on Reduction and Variation of Charge Passed. In Proceedings of the Transportation Research Board 95th Annual Meeting, Washington, DC, USA, 10–14 January 2016; pp. 16–1195. [Google Scholar]
- Obla, K.H. Performance Criteria for Concrete Resistant to Chloride Ion Penetration. ACI Mater. J. 2019, 116, 139–148. [Google Scholar] [CrossRef]
- Moradllo, M.K.; Qiao, C.; Isgor, B.; Reese, S.; Weiss, W.J. Relating Formation Factor of Concrete to Water Absorption. ACI Mater. J. 2018, 115, 887–898. [Google Scholar] [CrossRef]
- Todak, H.; Lucero, C.; Weiss, W.J. Why is the air there? Thinking about freeze-thaw in terms of saturation. Concr. Focus 2015, 14, OC3–OC7. [Google Scholar]
- ASTM C192:2007; Standard Practice for Making and Curing Concrete Test Specimens in the Laboratory. ASTM International: West Conshohocken, PA, USA, 2007.
- AASHTO TP95:2011; Standard Method of Test for Surface Resistivity Indication of Concrete’s Ability to Resist Chloride Ion Penetration. American Association of State Highway and Transportation Officials: Washington, DC, USA, 2011.
- ASTM C1202-12:2012; Standard Test Method for Electrical Indication of Concrete’s Ability to Resist Chloride Ion Penetration. ASTM International: West Conshohocken, PA, USA, 2012.
- Ghosh, P.; Tran, Q. Correlation Between Bulk and Surface Resistivity of Concrete. Int. J. Concr. Struct. Mater. 2015, 9, 119–132. [Google Scholar] [CrossRef]
- Morris, W.; Moreno, E.I.; Sagüés, A.A. Practical evaluation of resistivity of concrete in test cylinders using a Wenner array probe. Cem. Concr. Res. 1996, 26, 1779–1787. [Google Scholar] [CrossRef]
- Berke, N.S.; Hicks, M.C. Estimating the life cycle of reinforced concrete decks and marine piles using laboratory diffusion and corrosion data. In Corrosion Forms and Control for Infrastructure; ASTM International: Philadelphia, PA, USA, 1992; pp. 207–231. [Google Scholar]
- Dinakar, P.; Babu, K.G.; Santhanam, M. Durability properties of high volume fly ash self compacting concretes. Cem. Concr. Compos. 2008, 30, 880–886. [Google Scholar] [CrossRef]
- Bleszynski, R.; Hooton, R.D.; Thomas, M.D.A.; Chris, A.R. Durability of Ternary Blend Concrete with Silica Fume and Blast-Furnace Slag: Laboratory and Outdoor Exposure Site Studies. ACI Mater. J. 2002, 99, 499–508. [Google Scholar] [CrossRef]
- Uysal, M.; Akyuncu, V. Durability performance of concrete incorporating Class F and Class C fly ashes. Constr. Build. Mater. 2012, 34, 170–178. [Google Scholar] [CrossRef]
- Wongkeo, W.; Thongsanitgarn, P.; Ngamjarurojana, A.; Chaipanich, A. Compressive strength and chloride resistance of self-compacting concrete containing high level fly ash and silica fume. Mater. Des. 2014, 64, 261–269. [Google Scholar] [CrossRef]
- Gesoğlu, M.; Güneyisi, E.; Özbay, E. Properties of self-compacting concretes made with binary, ternary, and quaternary cementitious blends of fly ash, blast furnace slag, and silica fume. Constr. Build. Mater. 2009, 23, 1847–1854. [Google Scholar] [CrossRef]




| No. | Mixture ID | 28-Day Compressive Strength (psi) | Replacement (%) | ||||||
|---|---|---|---|---|---|---|---|---|---|
| Cement | Class C Fly Ash | Class F Fly Ash | Slag G100 | Slag G120 | Silica Fume | Metakaolin | |||
| 1 | 100TII-V | 3927 | 100 | - | - | - | - | - | - |
| 2 | 80TII-V/20C | 3555 | 80 | 20 | - | - | - | - | - |
| 3 | 80TII-V/20F | 4077 | 80 | - | 20 | - | - | - | - |
| 4 | 95TII-V/5SF | 7841 | 95 | - | - | - | - | 5 | - |
| 5 | 93TII-V/7SF | 7539 | 93 | - | - | - | - | 7 | - |
| 6 | 65TII-V/35G100S | 5821 | 65 | - | - | 35 | - | - | - |
| 7 | 65TII-V/35G120S | 4481 | 65 | - | - | - | 35 | - | - |
| 8 | 60TII-V/20C/20F | 4230 | 60 | 20 | 20 | - | - | - | - |
| 9 | 75TII-V/20C/5SF | 6974 | 75 | 20 | - | - | - | 5 | - |
| 10 | 60TII-V/25C/15M | 5246 | 60 | 25 | - | - | - | - | 15 |
| 11 | 65TII-V/28C/7M | n/a | 65 | 28 | - | - | - | - | 7 |
| 12 | 65TII-V/30C/5SF | 7058 | 65 | 30 | - | - | - | 5 | - |
| 13 | 60TII-V/30C/10F | n/a | 60 | 30 | 10 | - | - | - | - |
| 14 | 60TII-V/30C/10M | n/a | 60 | 30 | - | - | - | - | 10 |
| 15 | 60TII-V/25F/15M | n/a | 60 | - | 25 | - | - | - | 15 |
| 16 | 65TII-V/28F/7M | n/a | 65 | - | 28 | - | - | - | 7 |
| 17 | 77TII-V/20F/3M | 4341 | 77 | - | 20 | - | - | - | 3 |
| 18 | 75TII-V/20F/5M | 4279 | 75 | - | 20 | - | - | - | 5 |
| 19 | 70TII-V/20F/10M | 4196 | 70 | - | 20 | - | - | - | 10 |
| 20 | 75TII-V/20F/5SF | 5264 | 75 | - | 20 | - | - | 5 | - |
| 21 | 70TII-V/20F/10SF | 2432 | 70 | - | 20 | - | - | 10 | - |
| 22 | 73TII-V/20F/7M | 6296 | 73 | - | 20 | - | - | - | 7 |
| 23 | 68TII-V/20F/12M | n/a | 68 | - | 20 | - | - | - | 12 |
| 24 | 77TII-V/20F/3SF | n/a | 77 | - | 20 | - | - | 3 | - |
| 25 | 73TII-V/20F/7SF | n/a | 73 | - | 20 | - | - | 7 | - |
| 26 | 68TII-V/20F/12SF | n/a | 68 | - | 20 | - | - | 12 | - |
| 27 | 60TII-V/30F/10C | 4345 | 60 | 10 | 30 | - | - | - | - |
| 28 | 65TII-V/30F/5SF | 5869 | 65 | - | 30 | - | - | 5 | - |
| 29 | 60TII-V/30F/10M | 5276 | 60 | - | 30 | - | - | - | 10 |
| 30 | 60TII-V/35G100S/5SF | 6318 | 60 | - | 35 | - | 5 | - | |
| 31 | 50TII-V/35G100S/15C | 6344 | 50 | 15 | - | 35 | - | - | - |
| 32 | 50TII-V/35G100S/15F | 6138 | 50 | - | 15 | 35 | - | - | - |
| 33 | 45TII-V/35G100S/20F | 4028 | 45 | - | 20 | 35 | - | - | - |
| 34 | 50TII-V/35G120S/15C | 5629 | 50 | 15 | - | - | 35 | - | - |
| 35 | 50TII-V/35G120S/15F | 4094 | 50 | - | 15 | - | 35 | - | - |
| 36 | 62TII-V/35G120S/3M | 5498 | 62 | - | - | - | 35 | - | 3 |
| 37 | 60TII-V/35G120S/5M | 6140 | 60 | - | - | - | 35 | - | 5 |
| 38 | 58TII-V/35G120S/7M | 5594 | 58 | - | - | - | 35 | - | 7 |
| 39 | 55TII-V/35G120S/10M | 5650 | 55 | - | - | - | 35 | - | 10 |
| 40 | 53TII-V/35G120S/12M | 7263 | 53 | - | - | - | 35 | - | 12 |
| 41 | 50TII-V/35G120S/15M | n/a | 50 | - | - | - | 35 | - | 15 |
| 42 | 62TII-V/35G120S/3SF | 6742 | 62 | - | - | - | 35 | 3 | - |
| 43 | 60TII-V/35G120S/5SF | n/a | 60 | - | - | - | 35 | 5 | - |
| 44 | 58TII-V/35G120S/7SF | 4000 | 58 | - | - | - | 35 | 7 | - |
| 45 | 55TII-V/35G120S/10SF | 4020 | 55 | - | - | - | 35 | 10 | - |
| 46 | 53TII-V/35G120S/12SF | 5900 | 53 | - | - | - | 35 | 12 | - |
| 47 | 45TII-V/40G120S/15C | 4600 | 45 | 15 | - | - | 40 | - | - |
| 48 | 45TII-V/40G120S/15F | 5178 | 45 | - | 15 | - | 40 | - | - |
| 49 | 55TII-V/40G120S/5SF | 5054 | 55 | - | - | - | 40 | 5 | - |
| 50 | 50TII-V/40G120S/10M | 4695 | 50 | - | - | - | 40 | - | 10 |
| No. | Mixture ID | Charge Passed following RCPT Theory (Coulombs) | Charge Passed by Berke Model (Coulombs) | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| From SR by Wenner Probe | From BR by Merlin | From BR (RCON) | From SR by Wenner Probe | From BR by Merlin | From BR (RCON) | ||||||||
| 10 KHz | 1 KHz | 0.1 KHz | 0.01 KHz | 10 KHz | 1 KHz | 0.1 KHz | 0.01 KHz | ||||||
| 1 | 100TII-V | 1493 | 1487 | - | - | - | - | 1152 | 1148 | - | - | - | - |
| 2 | 80TII-V/20C | 1454 | 1448 | - | - | - | - | 1116 | 1112 | - | - | - | - |
| 3 | 80TII-V/20F | 926 | 960 | - | - | - | - | 649 | 678 | - | - | - | - |
| 4 | 60TII-V/20C/20F | 1287 | 1287 | - | - | - | - | 964 | 965 | - | - | - | - |
| 5 | 60TII-V/30C/10F | 1236 | 1256 | - | - | - | - | 918 | 938 | - | - | - | - |
| 6 | 60TII-V/30F/10C | 1129 | 1132 | - | - | - | - | 823 | 828 | - | - | - | - |
| 7 | 75TII-V/20C/5SF | 737 | 747 | - | - | - | - | 493 | 502 | - | - | - | - |
| 8 | 50TII-V/35G120S/15C | 1472 | 1414 | - | - | - | - | 1133 | 1081 | - | - | - | - |
| 9 | 50TII-V/35G120S/15F | 990 | 1097 | - | - | - | - | 703 | 796 | - | - | - | - |
| 10 | 95TII-V/5SF | 948 | 991 | - | - | - | - | 667 | 705 | - | - | - | - |
| 11 | 93TII-V/7SF | 922 | 786 | - | - | - | - | 645 | 534 | - | - | - | - |
| 12 | 65TII-V/5SF/30C | 1258 | 1118 | - | - | - | - | 938 | 815 | - | - | - | - |
| 13 | 65TII-V/5SF/30F | 864 | 771 | - | - | - | - | 597 | 521 | - | - | - | - |
| 14 | 55TII-V/5SF/40G120S | 1241 | 1311 | - | - | - | - | 923 | 987 | - | - | - | - |
| 15 | 45TII-V/40G120S/15C | 2154 | 2032 | - | - | - | - | 1789 | 1838 | - | - | - | - |
| 16 | 45TII-V/40G120S/15F | 757 | 698 | - | - | - | - | 509 | 463 | - | - | - | - |
| 17 | 65TII-V/35G100S | 961 | 962 | - | - | - | - | 679 | 681 | - | - | - | - |
| 18 | 60TII-V/35G100S/5SF | 758 | 731 | - | - | - | - | 510 | 489 | - | - | - | - |
| 19 | 50TII-V/35G100S/15C | 943 | 809 | - | - | - | - | 664 | 553 | - | - | - | - |
| 20 | 50TII-V/35G100S/15F | 846 | 721 | - | - | - | - | 582 | 481 | - | - | - | - |
| 21 | 45TII-V/35G100S/20F | 752 | 693 | - | - | - | - | 505 | 459 | - | - | - | - |
| 22 | 60TII-V/30F/10M | 1611 | 1580 | 1442 | 1372 | 1288 | 1234 | 1262 | 1235 | 1239 | 1167 | 1082 | 1027 |
| 23 | 60TII-V/30C/10M | 2815 | 2568 | 2301 | 2179 | 2009 | 1929 | 2469 | 2215 | 2173 | 2035 | 1845 | 1758 |
| 24 | 50TII-V/40G120S/10M | 388 | 365 | 412 | 394 | 380 | 372 | 228 | 212 | 275 | 260 | 249 | 243 |
| 25 | 60TII-V/25F/15M | 804 | 791 | 733 | 704 | 687 | 678 | 548 | 537 | 549 | 524 | 508 | 500 |
| 26 | 60TII-V/25C/15M | 2429 | 2183 | 1940 | 1873 | 1836 | 1817 | 2067 | 1822 | 1770 | 1697 | 1657 | 1635 |
| 27 | 50TII-V/35G120S/15M | 371 | 386 | 393 | 378 | 366 | 358 | 216 | 227 | 260 | 248 | 238 | 232 |
| 28 | 65TII-V/28F/7M | 930 | 922 | 775 | 748 | 713 | 694 | 652 | 647 | 588 | 563 | 531 | 514 |
| 29 | 65TII-V/28C/7M | 1933 | 1933 | 1582 | 1512 | 1422 | 1333 | 1572 | 1574 | 1385 | 1311 | 1218 | 1127 |
| 30 | 77TII-V/20F/3M | 501 | 503 | 458 | 442 | 432 | 427 | 310 | 313 | 312 | 299 | 291 | 287 |
| 31 | 75TII-V/20F/5M | 552 | 543 | 490 | 479 | 469 | 463 | 348 | 344 | 339 | 330 | 321 | 316 |
| 32 | 70TII-V/20F/10M | 493 | 501 | 448 | 431 | 421 | 415 | 304 | 310 | 304 | 290 | 282 | 277 |
| 33 | 75TII-V/20F/5SF | 503 | 466 | 434 | 417 | 407 | 401 | 312 | 284 | 293 | 279 | 271 | 266 |
| 34 | 70TII-V/20F/10SF | 296 | 251 | 262 | 245 | 237 | 231 | 164 | 136 | 160 | 147 | 141 | 137 |
| 35 | 60TII-V/35G120S/5M | 435 | 435 | 385 | 372 | 363 | 356 | 262 | 261 | 254 | 243 | 236 | 230 |
| 36 | 55TII-V/35G120S/10M | 242 | 264 | 249 | 237 | 229 | 223 | 129 | 137 | 150 | 142 | 136 | 131 |
| 37 | 60TII-V/35G120S/5SF | 299 | 301 | 340 | 326 | 317 | 310 | 167 | 169 | 219 | 208 | 200 | 196 |
| 38 | 55TII-V/35G120S/10SF | 352 | 365 | 340 | 324 | 315 | 308 | 203 | 211 | 218 | 206 | 199 | 194 |
| 39 | 73TII-V/20F/7M | 498 | 515 | 509 | 493 | 477 | 464 | 308 | 322 | 355 | 341 | 327 | 317 |
| 40 | 68TII-V/20F/12M | 499 | 516 | 407 | 390 | 364 | 346 | 309 | 310 | 271 | 257 | 237 | 223 |
| 41 | 77TII-V/20F/3SF | 519 | 494 | 451 | 432 | 420 | 414 | 323 | 304 | 306 | 291 | 281 | 276 |
| 42 | 73TII-V/20F/7SF | 500 | 533 | 490 | 473 | 457 | 448 | 309 | 317 | 339 | 324 | 312 | 304 |
| 43 | 68TII-V/20F/12SF | 341 | 346 | 328 | 311 | 299 | 294 | 195 | 205 | 209 | 196 | 187 | 183 |
| 44 | 62TII-V/35G120S/3M | 547 | 570 | 519 | 502 | 486 | 474 | 344 | 363 | 363 | 348 | 335 | 326 |
| 45 | 58TII-V/35G120S/7M | 358 | 364 | 344 | 330 | 323 | 317 | 207 | 211 | 221 | 211 | 205 | 201 |
| 46 | 53TII-V/35G120S/12M | 254 | 248 | 236 | 225 | 218 | 211 | 137 | 134 | 141 | 133 | 128 | 123 |
| 47 | 62TII-V/35G120S/3SF | 586 | 625 | 582 | 567 | 550 | 537 | 375 | 401 | 416 | 404 | 389 | 378 |
| 48 | 58TII-V/35G120S/7SF | 422 | 379 | 356 | 344 | 333 | 324 | 252 | 190 | 230 | 221 | 213 | 206 |
| 49 | 53TII-V/35G120S/12SF | 344 | 328 | 301 | 290 | 284 | 279 | 197 | 186 | 189 | 180 | 176 | 172 |
| 50 | 65TII-V/35G120S | 1094 | 1110 | 1000 | 975 | 946 | 926 | 793 | 808 | 798 | 774 | 747 | 728 |
| Charge Passed (Coulombs) | Chloride Ion Penetrability |
|---|---|
| >4000 | High |
| 2000–4000 | Moderate |
| 1000–2000 | Low |
| 100–1000 | Very Low |
| <100 | Negligible |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Tran, Q.; Ghosh, P. Variation of Electrical Resistivity and Charge Passed in High-Performance Concrete. Materials 2022, 15, 6694. https://doi.org/10.3390/ma15196694
Tran Q, Ghosh P. Variation of Electrical Resistivity and Charge Passed in High-Performance Concrete. Materials. 2022; 15(19):6694. https://doi.org/10.3390/ma15196694
Chicago/Turabian StyleTran, Quang, and Pratanu Ghosh. 2022. "Variation of Electrical Resistivity and Charge Passed in High-Performance Concrete" Materials 15, no. 19: 6694. https://doi.org/10.3390/ma15196694





