Corrosion-Resistant Plug Materials for Geothermal Well Fluid Control
Abstract
:1. Introduction
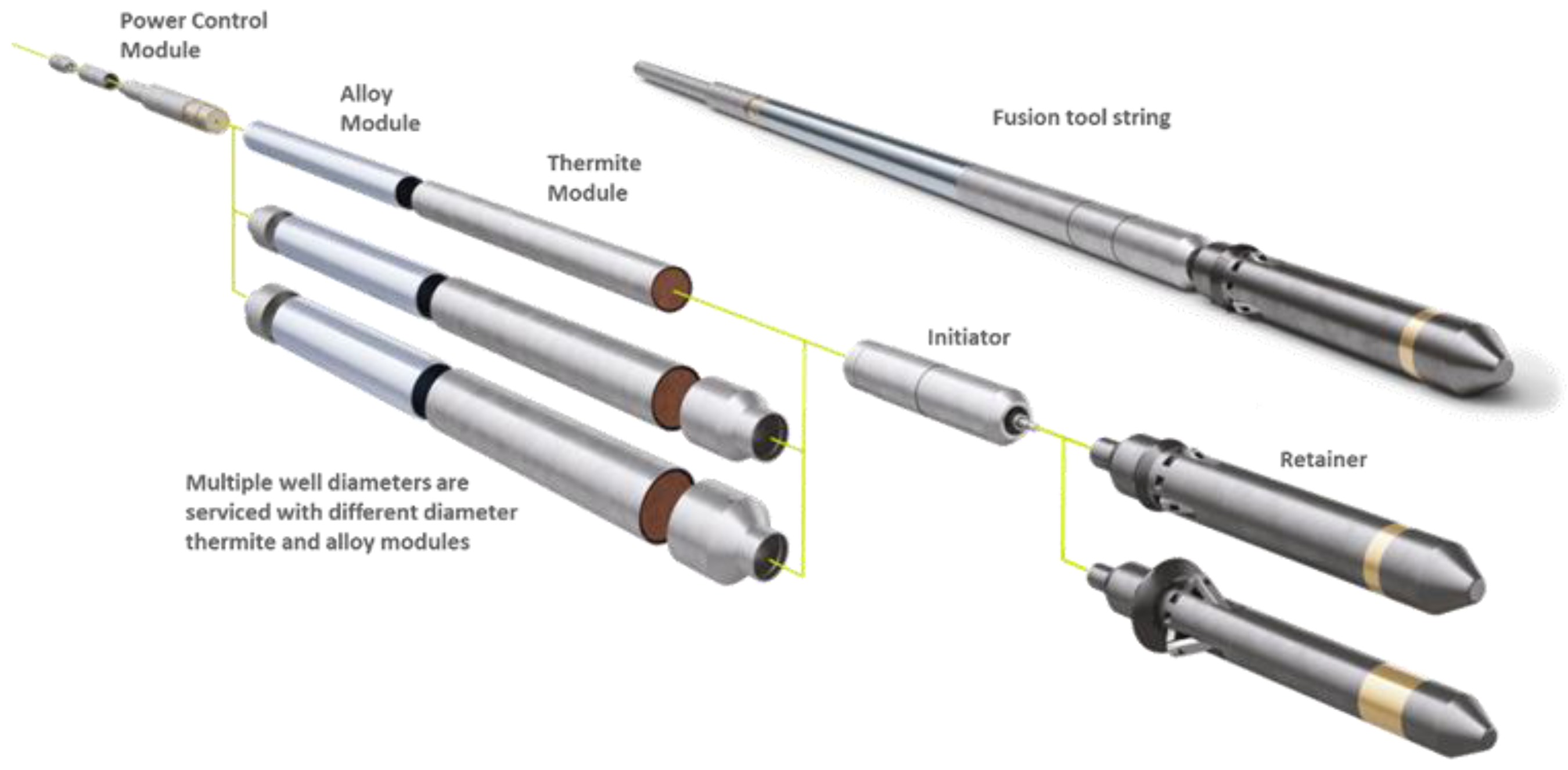
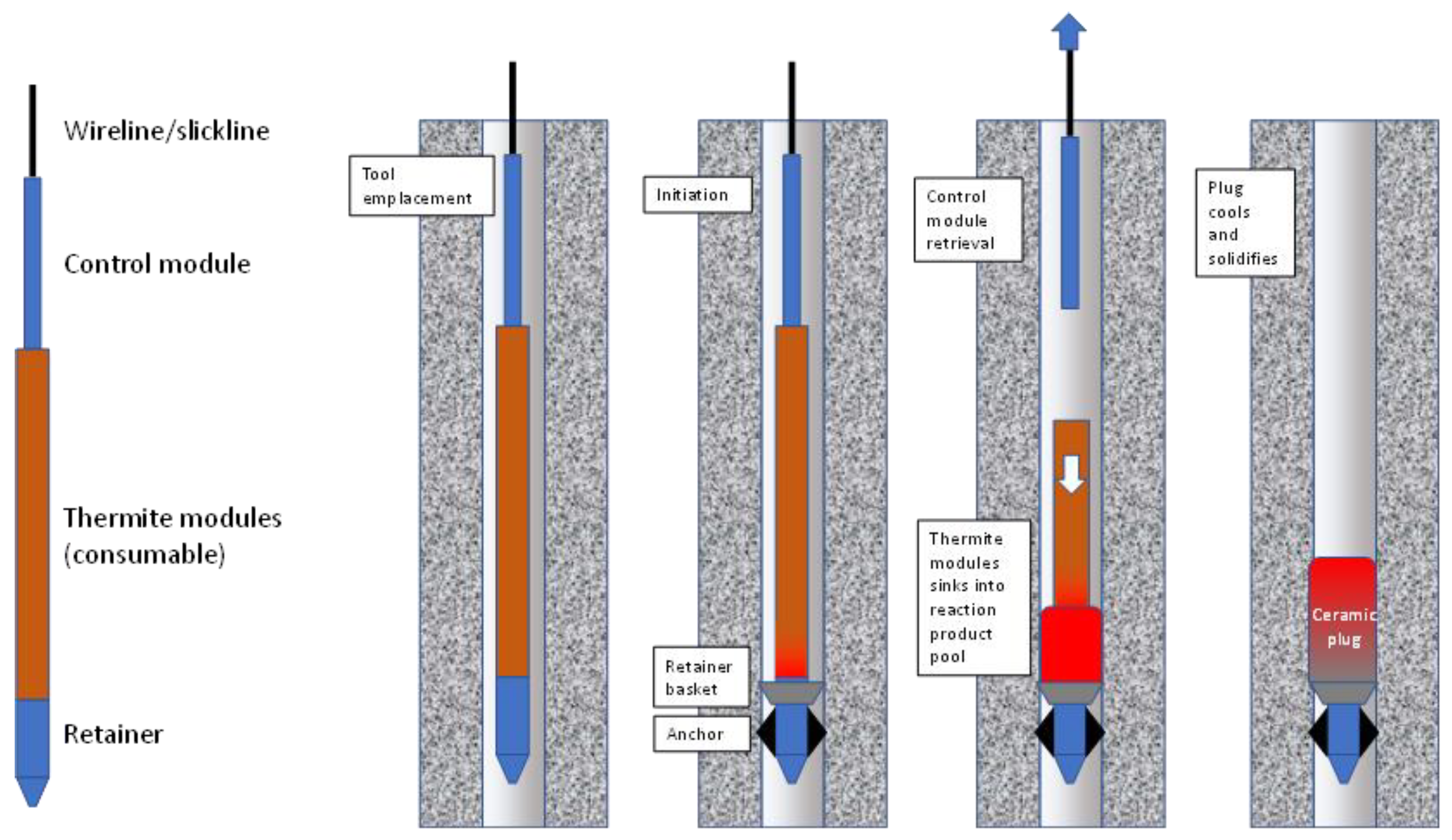
- Thermite-only ceramic plugs using consumable thermite housings
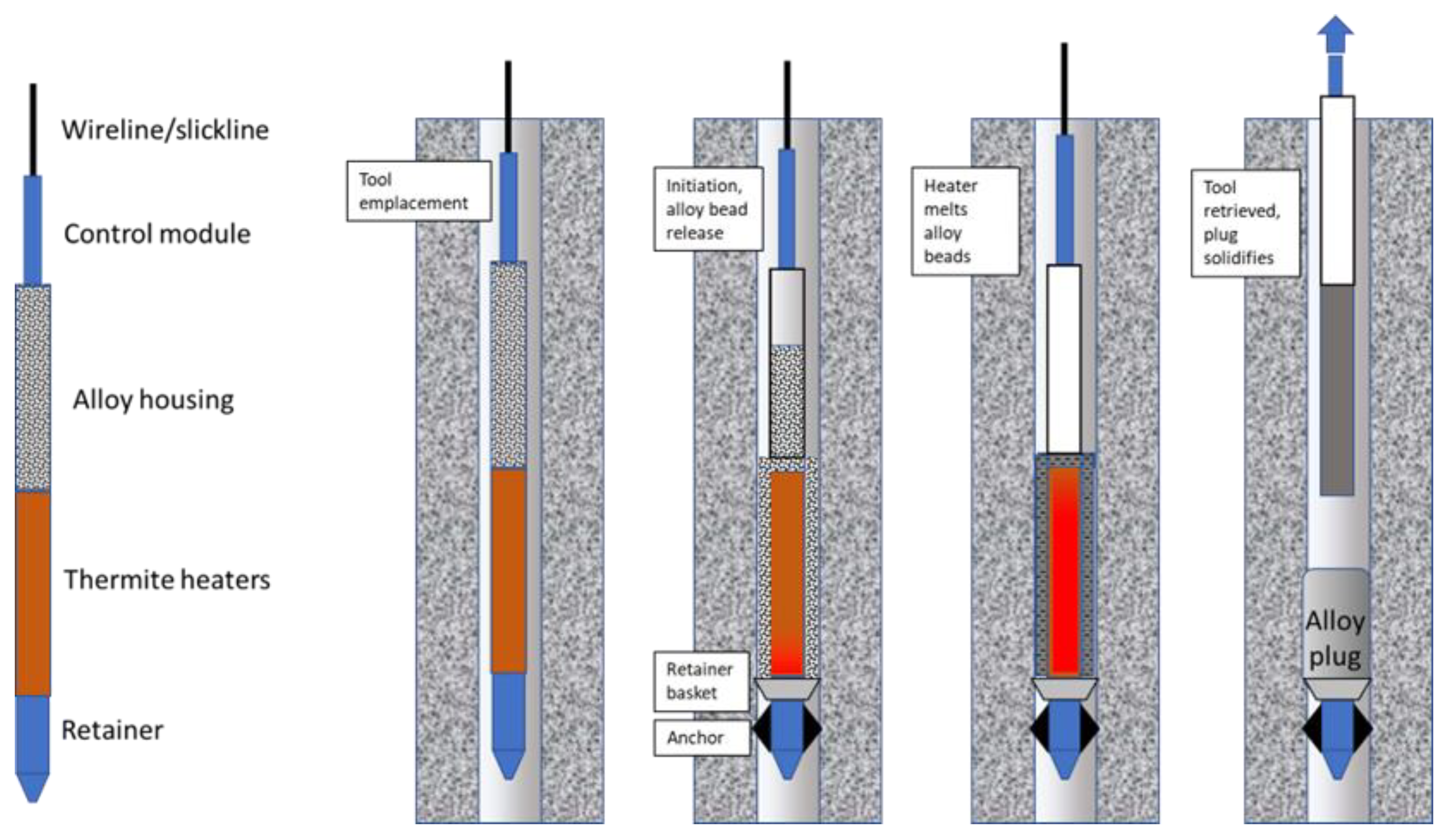
- Fusible metal plugs emplaced using retrievable thermite heaters
- Fusible metal seals in annuli between tubing and casing
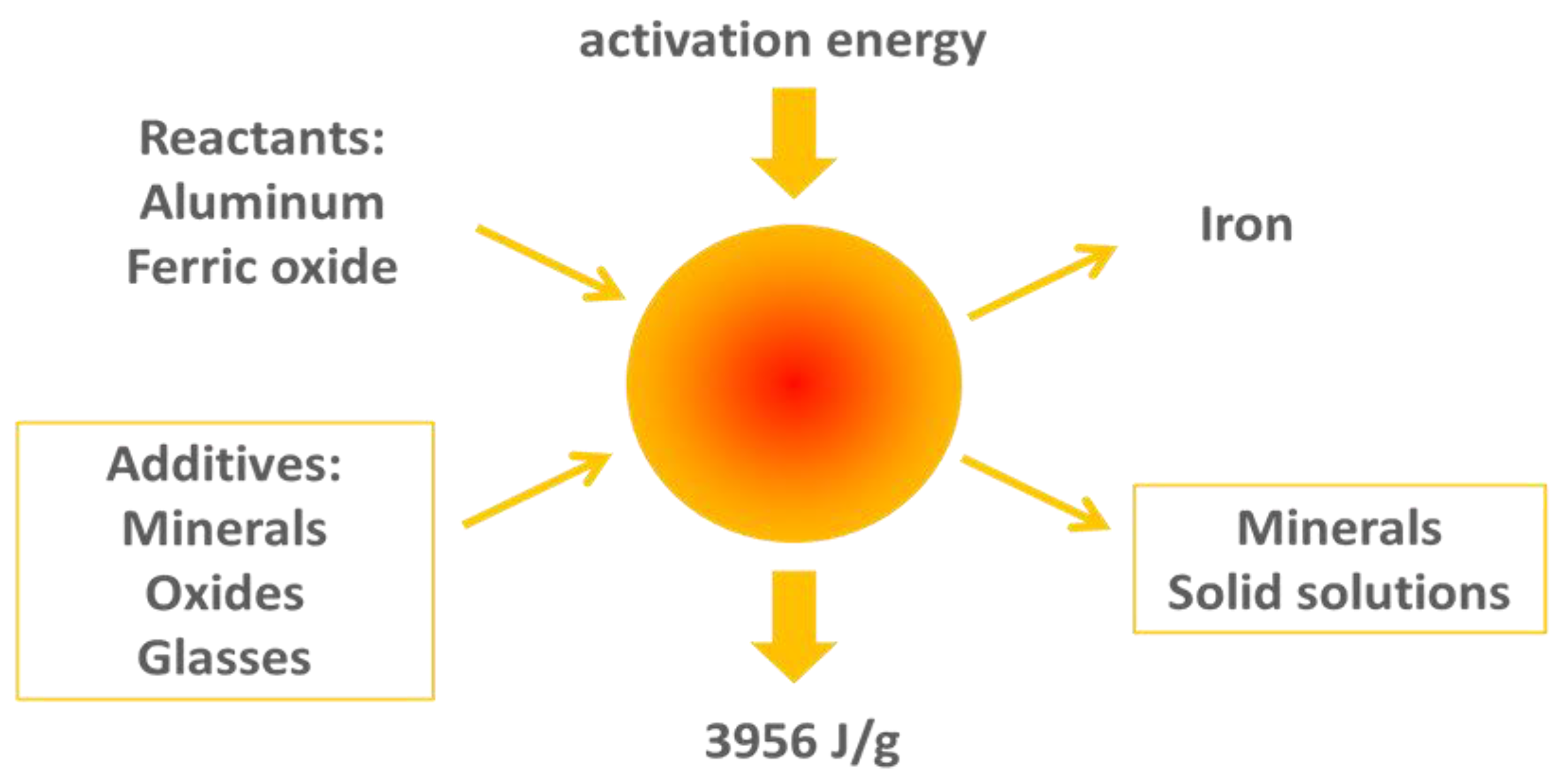
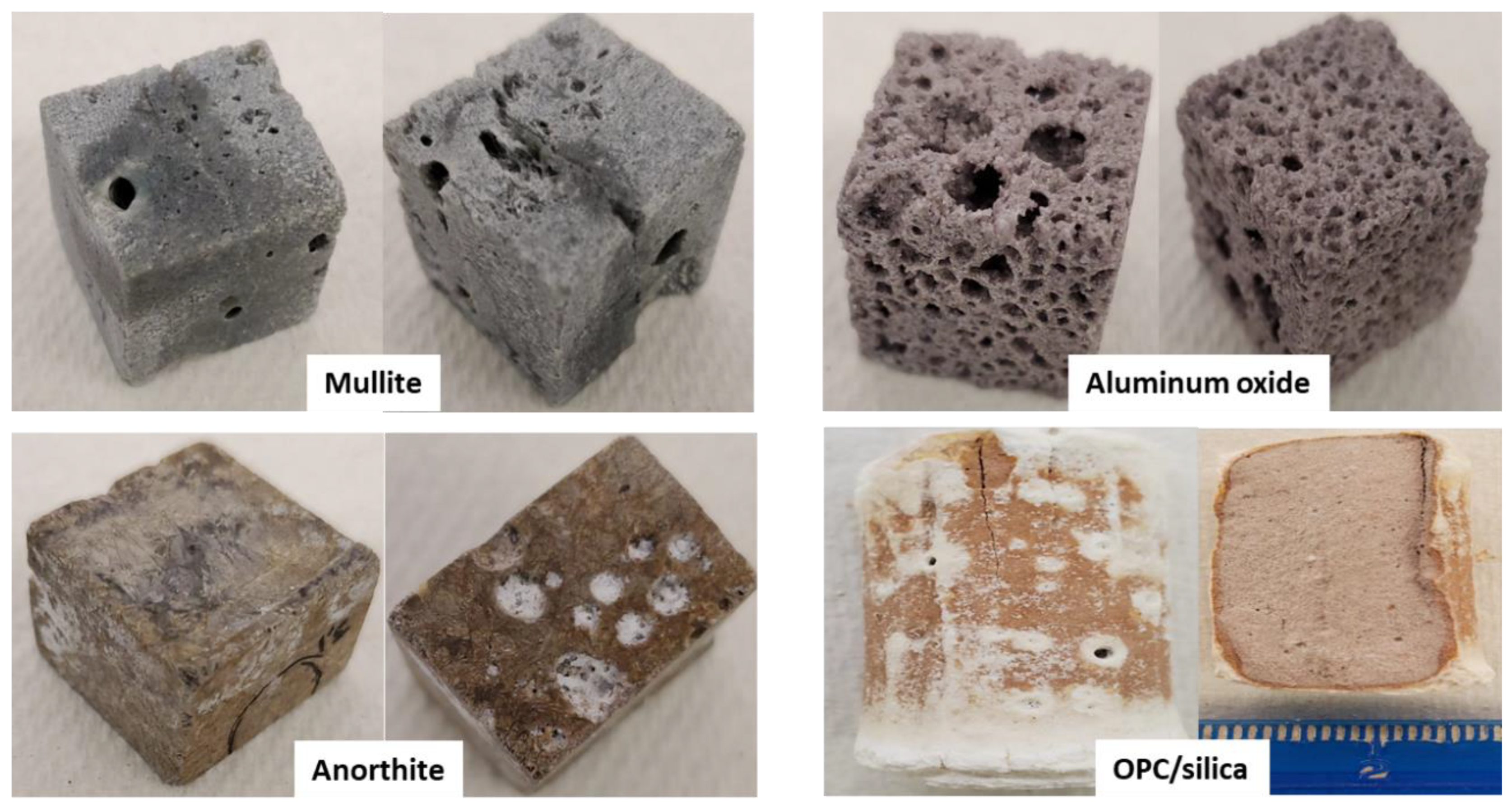
- Aluminum oxide—the product of the pure thermite reaction, or the aluminothermite formula diluted with aluminum oxide.
- Aluminosilicate—also called mullite, the product of the aluminothermite reactants diluted with SiO2.
- Anorthite—a feldspar material resulting from addition of specific oxides and minerals to the aluminothermite reactants.
2. Materials and Methods
3. Results
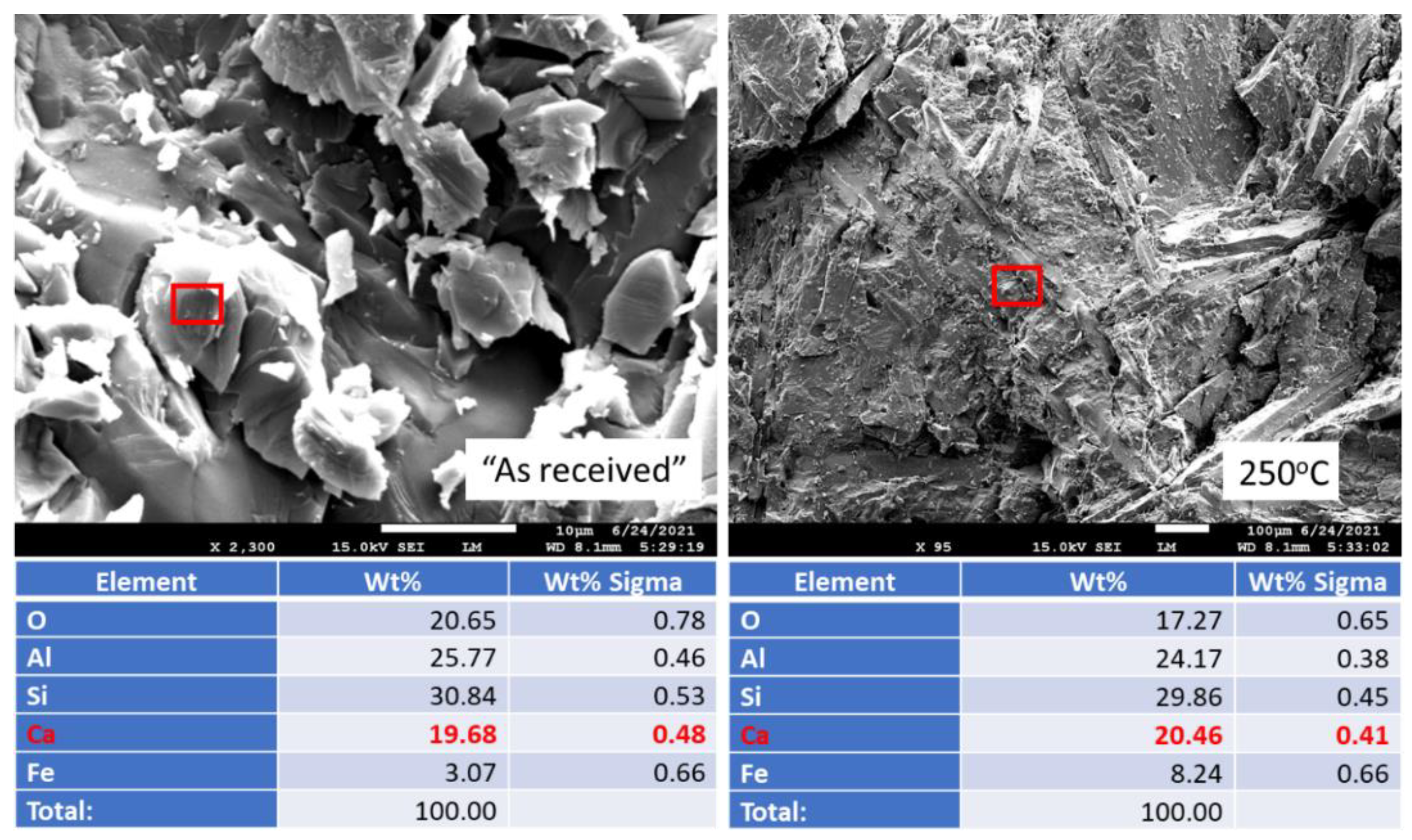
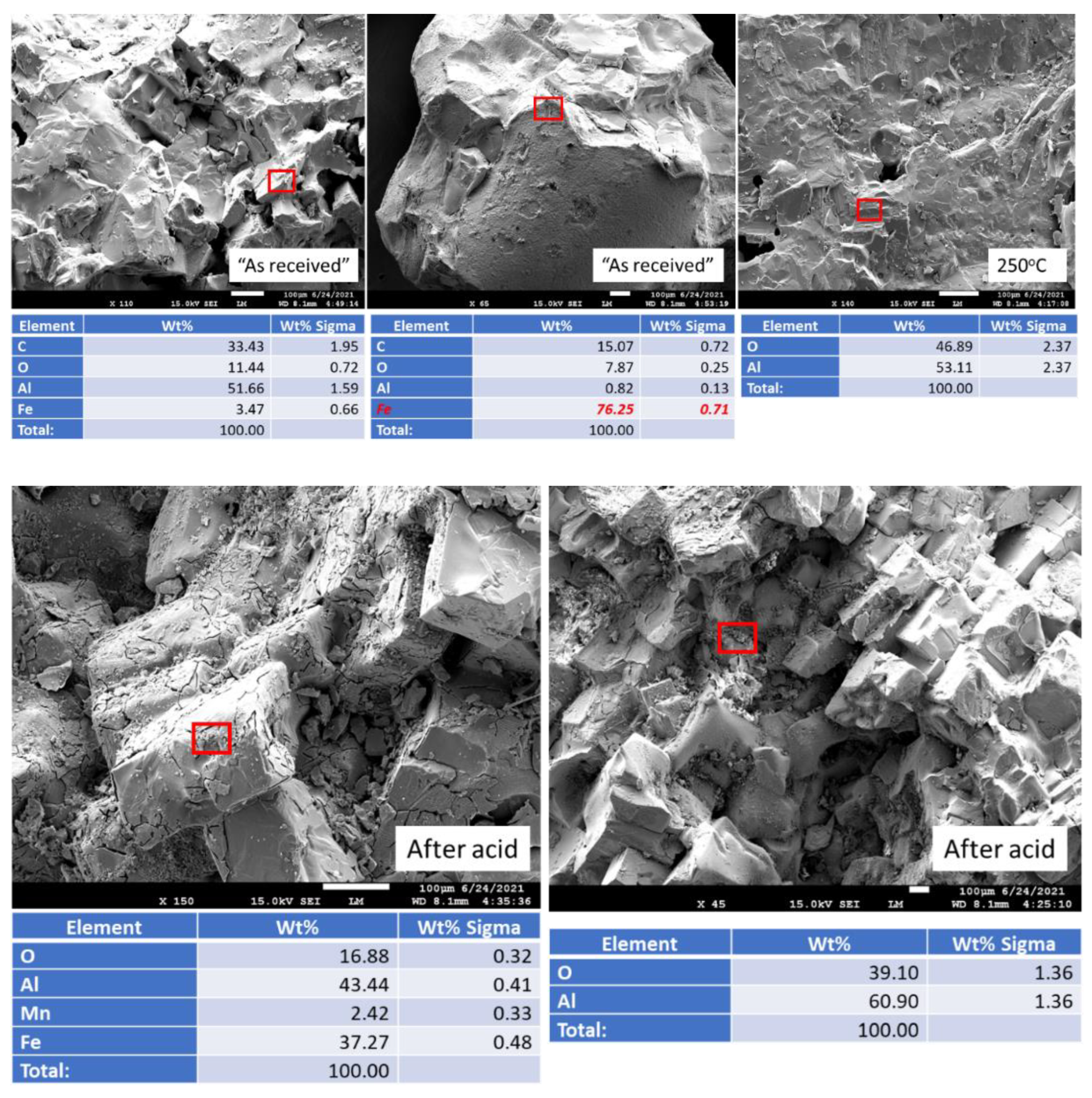
3.1. Composition of the Samples before Exposure Tests and 250 °C Autoclaved Samples
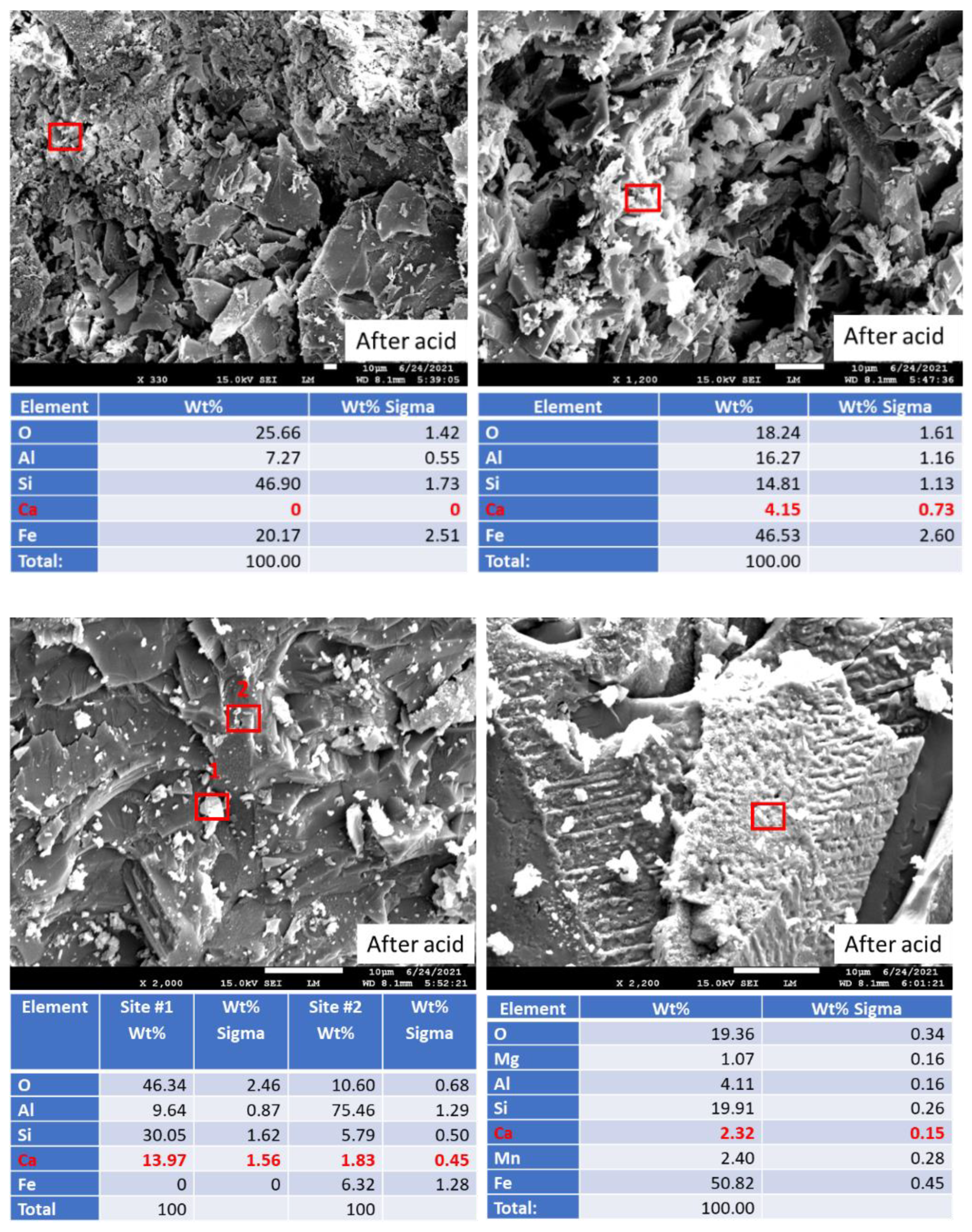
3.2. Acid Exposure Tests
3.2.1. Changes in Samples Appearances
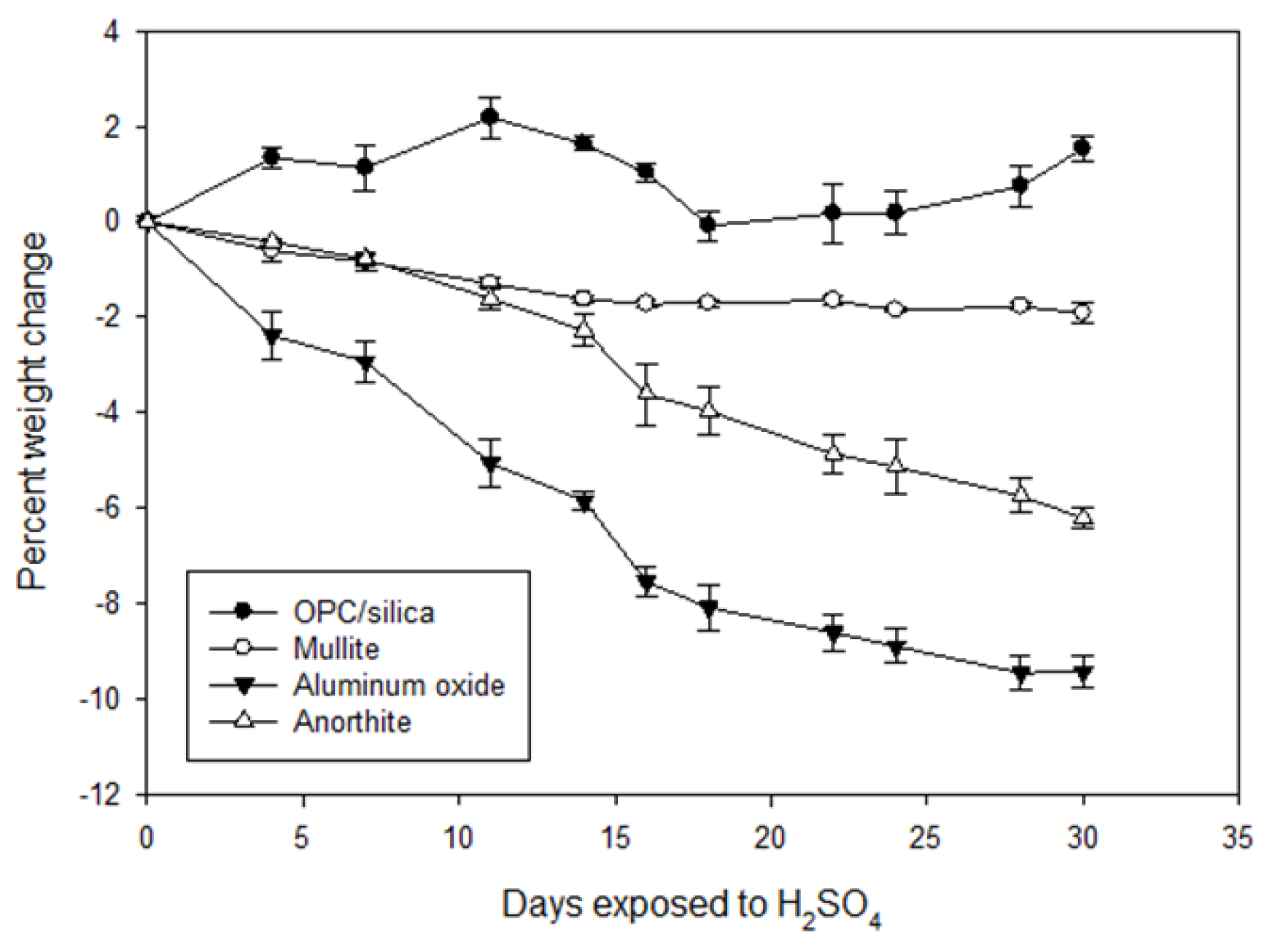
3.2.2. Changes in Weight and Mechanical Properties
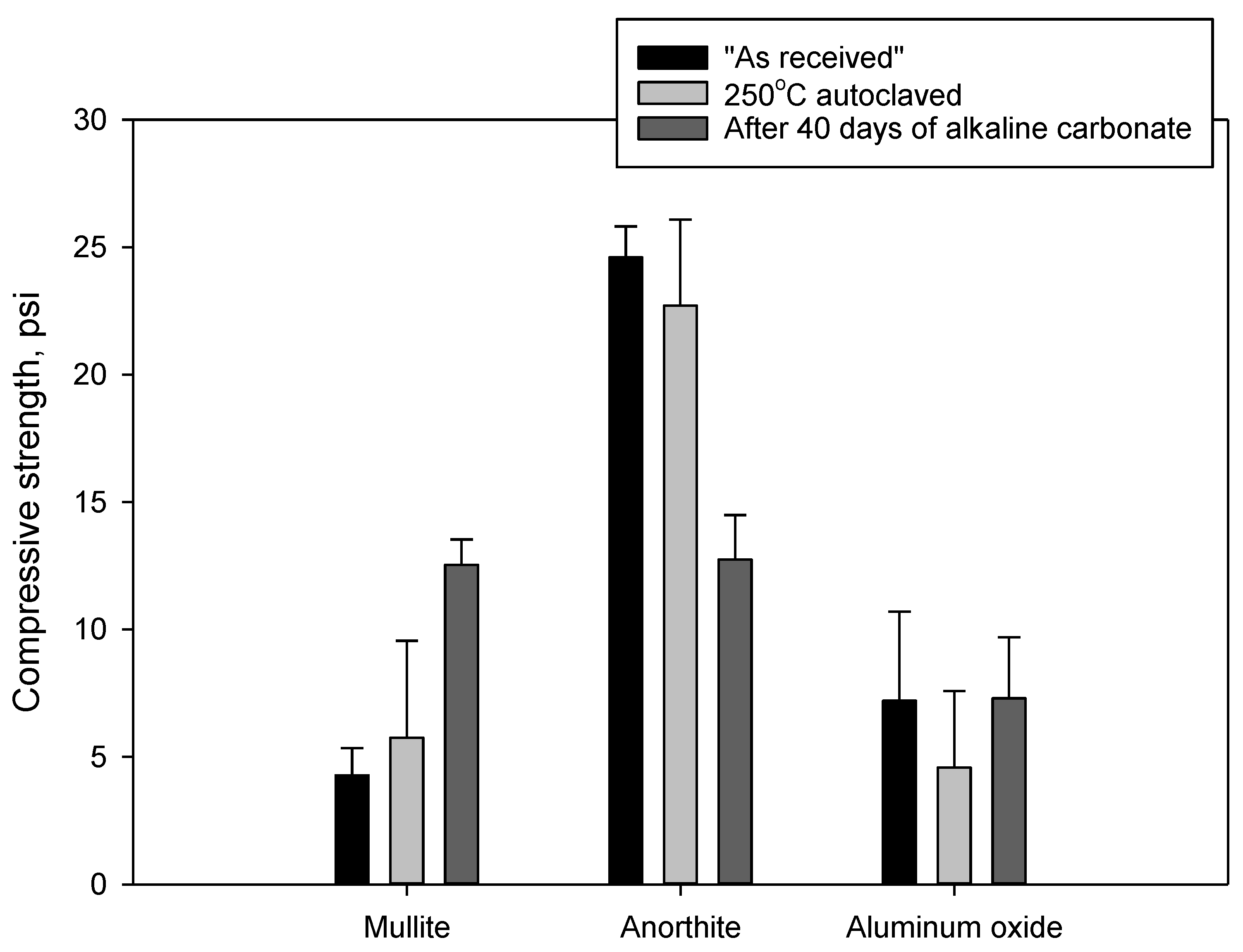
3.2.3. Compressive Strength and Porosity Changes
3.2.4. Elemental Composition of Sample Surfaces after the Acid Exposure
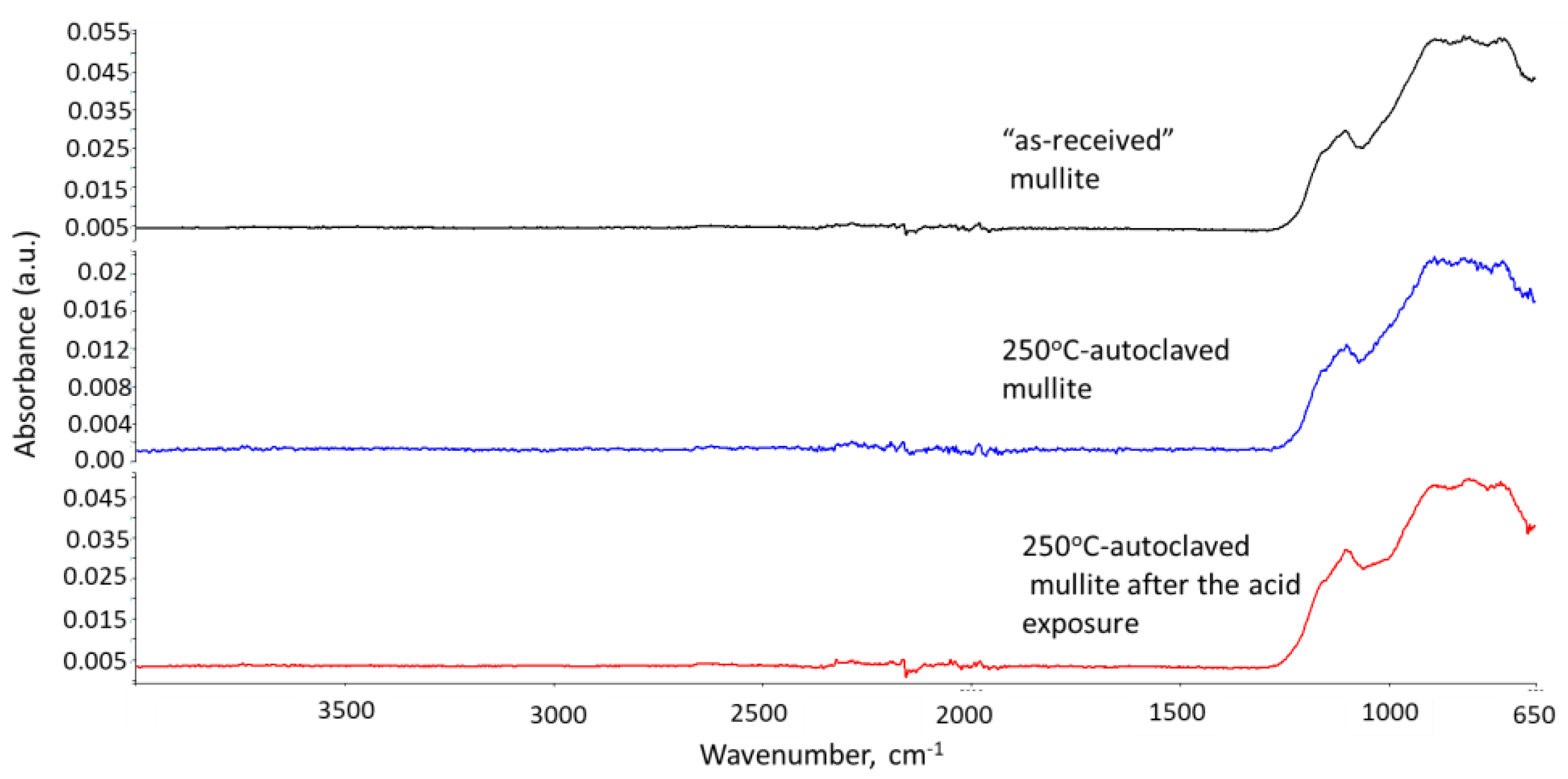
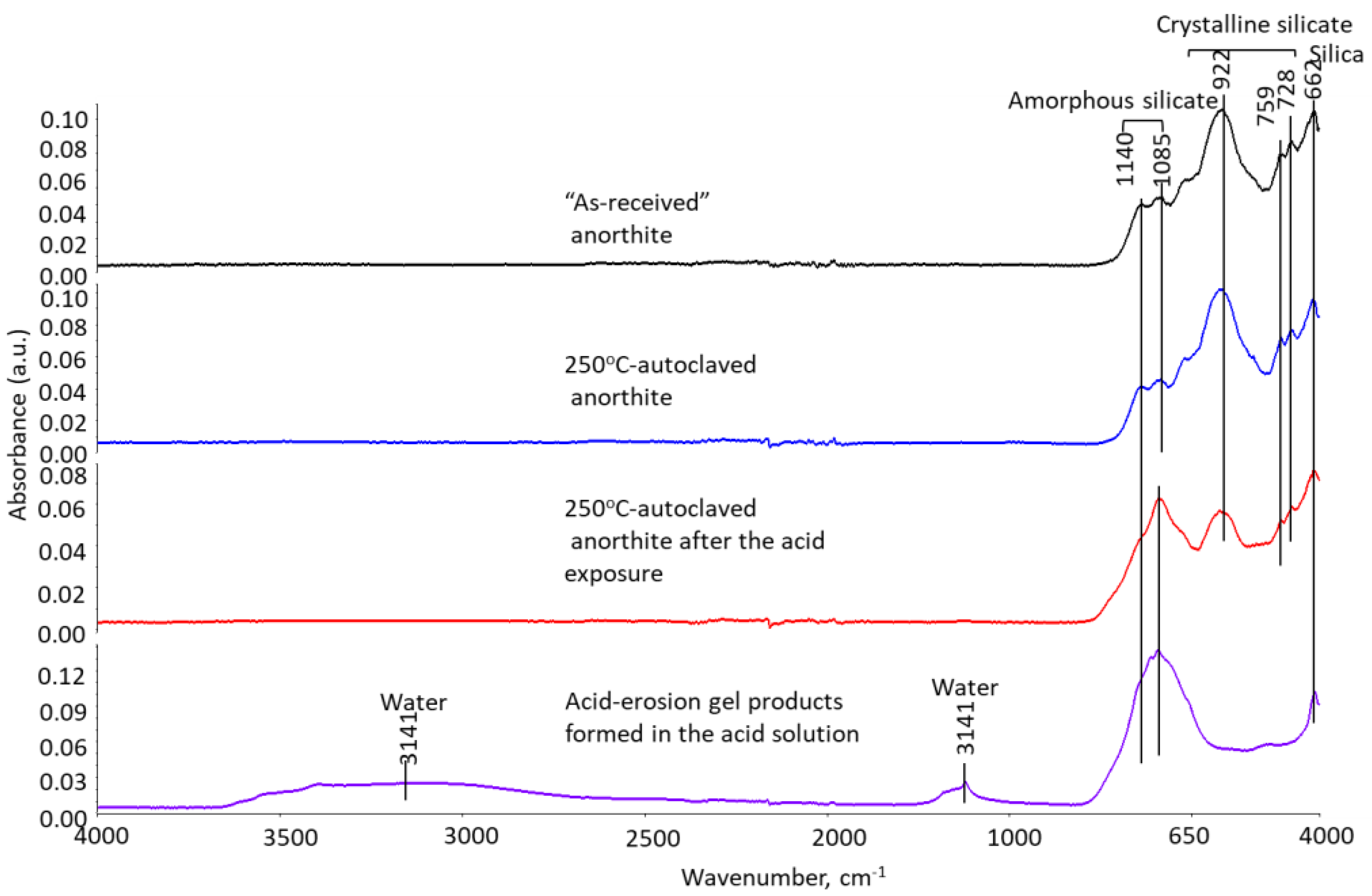
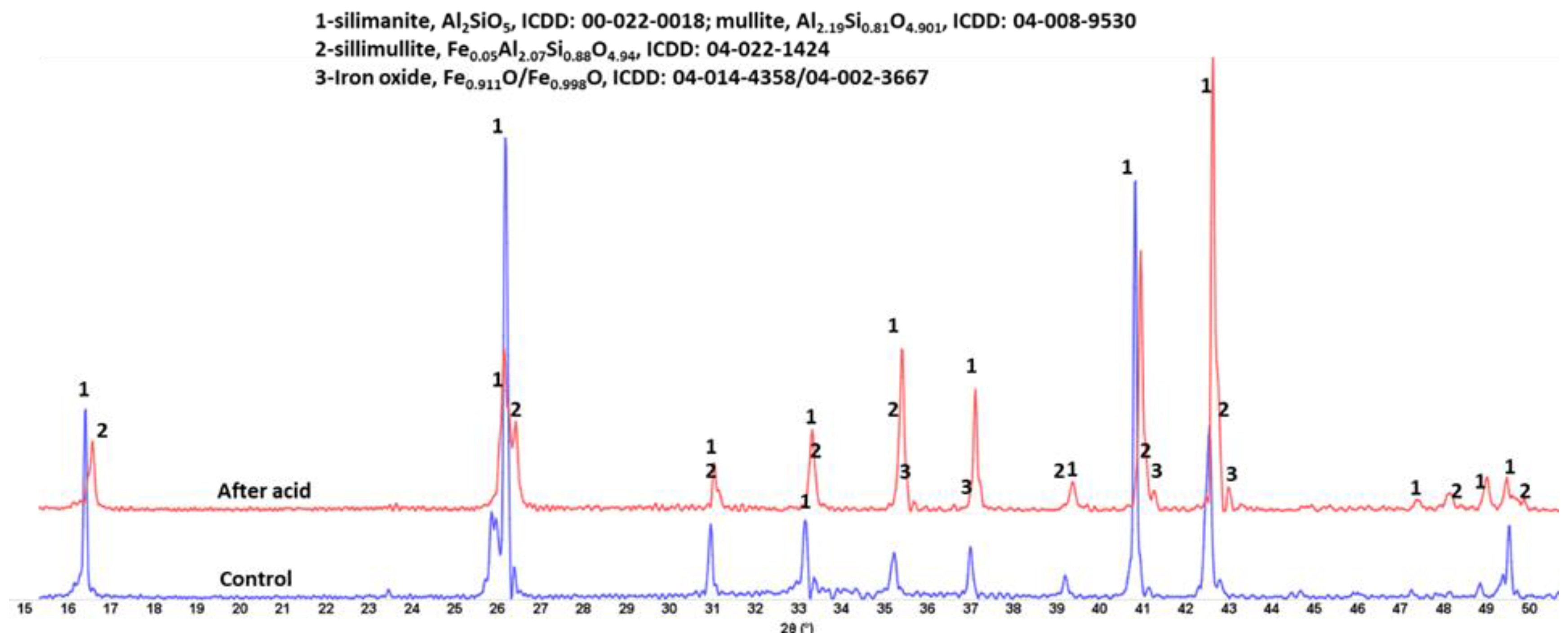
3.2.5. Phase Transitions during the Acid Exposure—FTIR and XRD Analyses
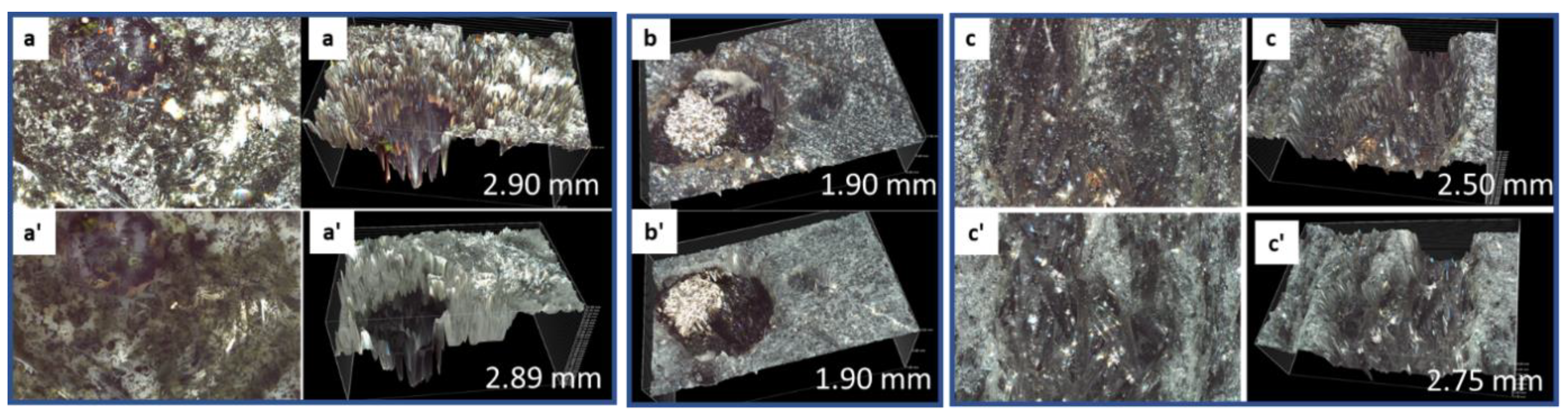
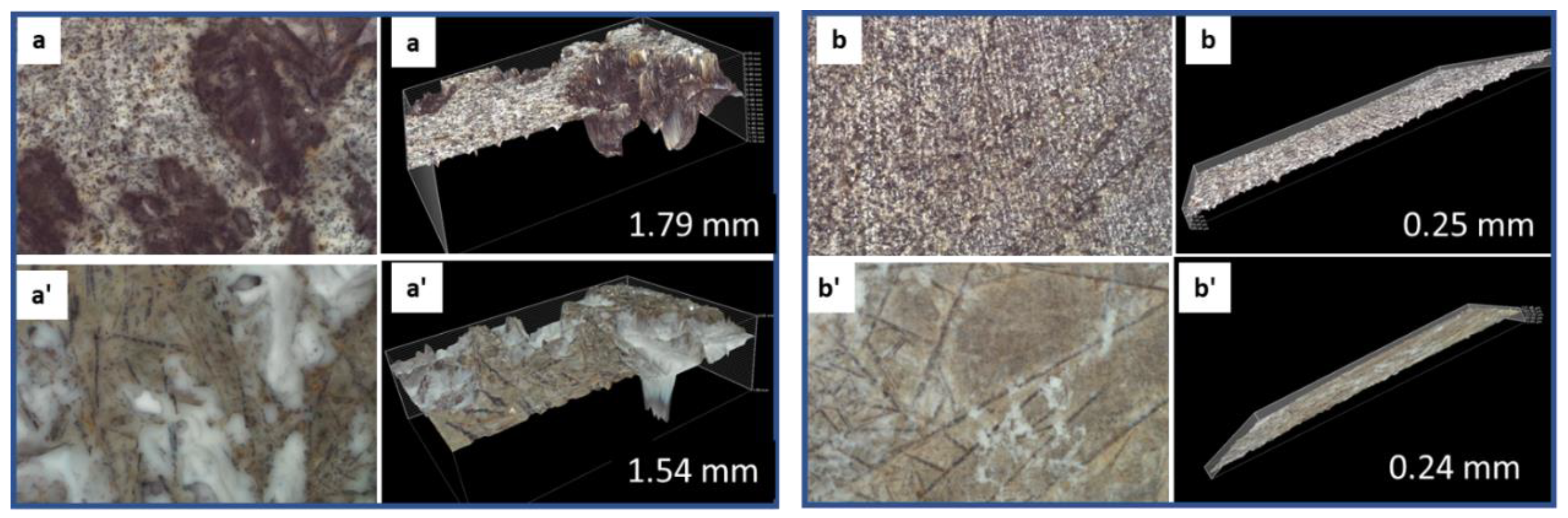
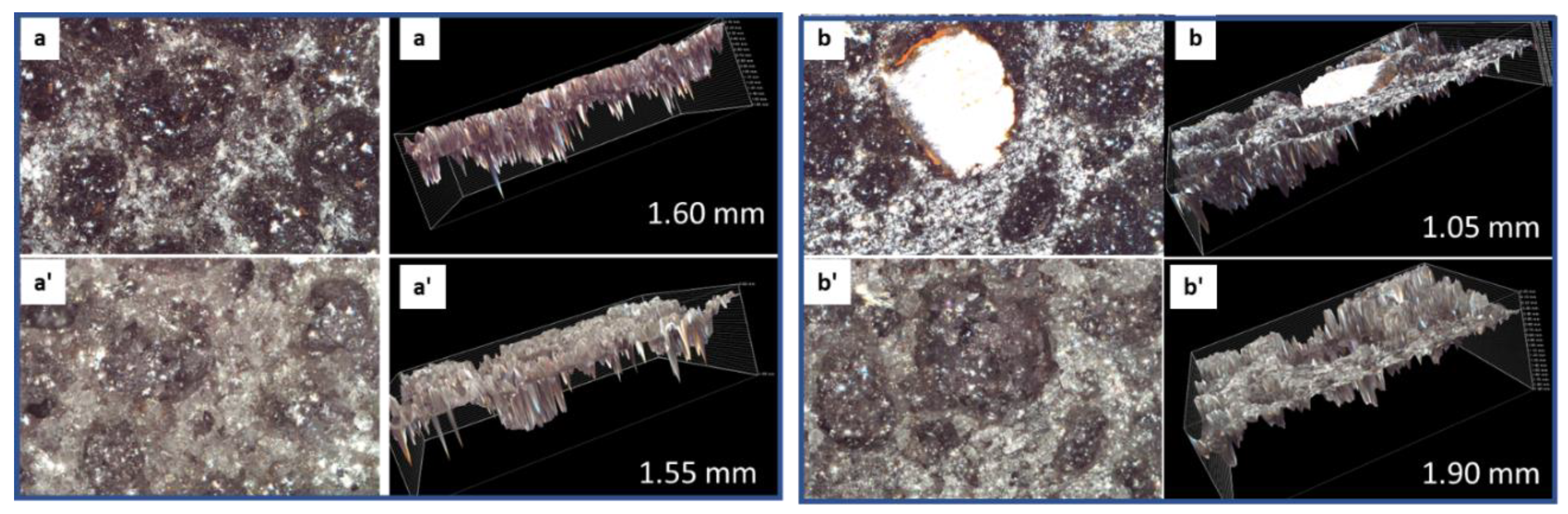
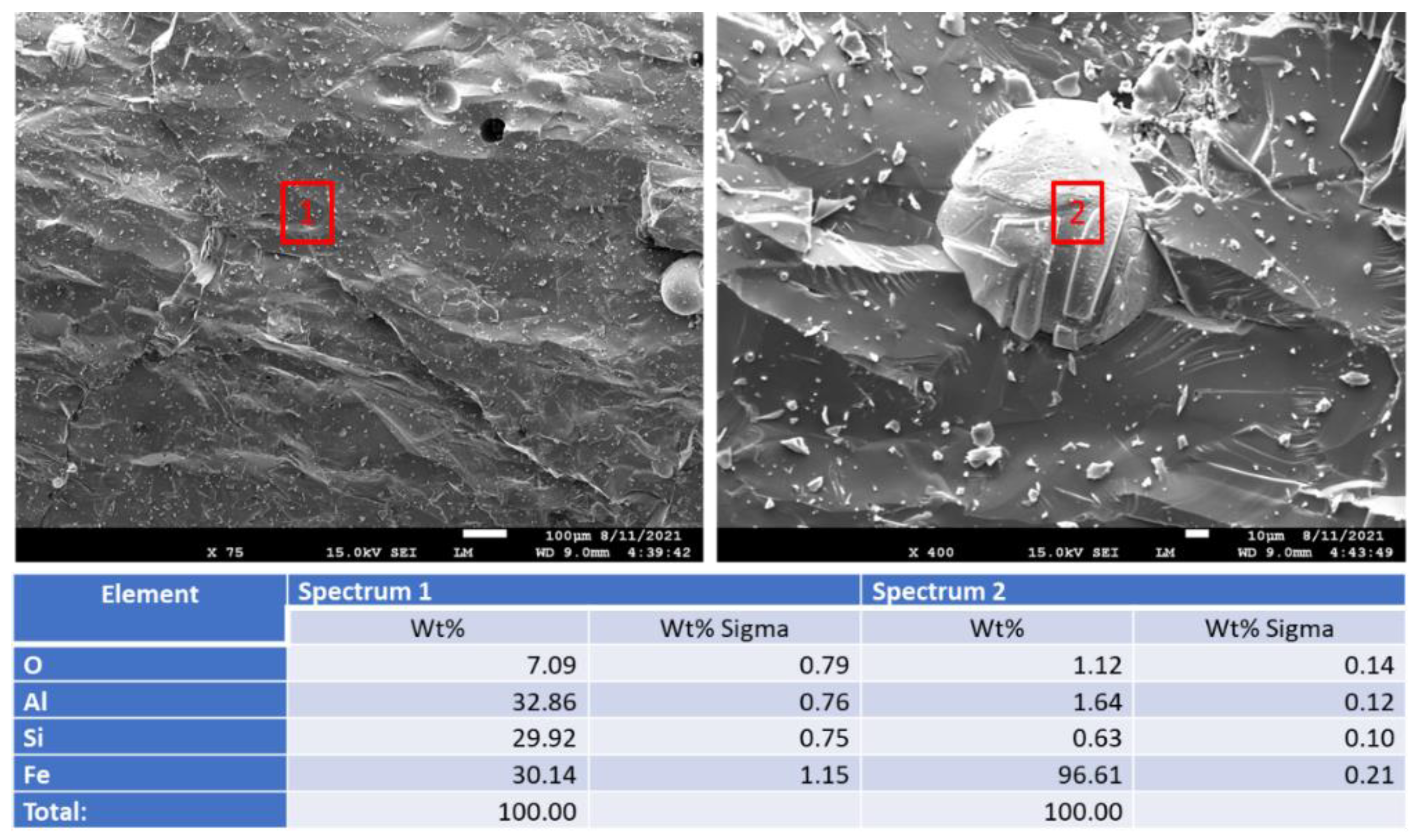
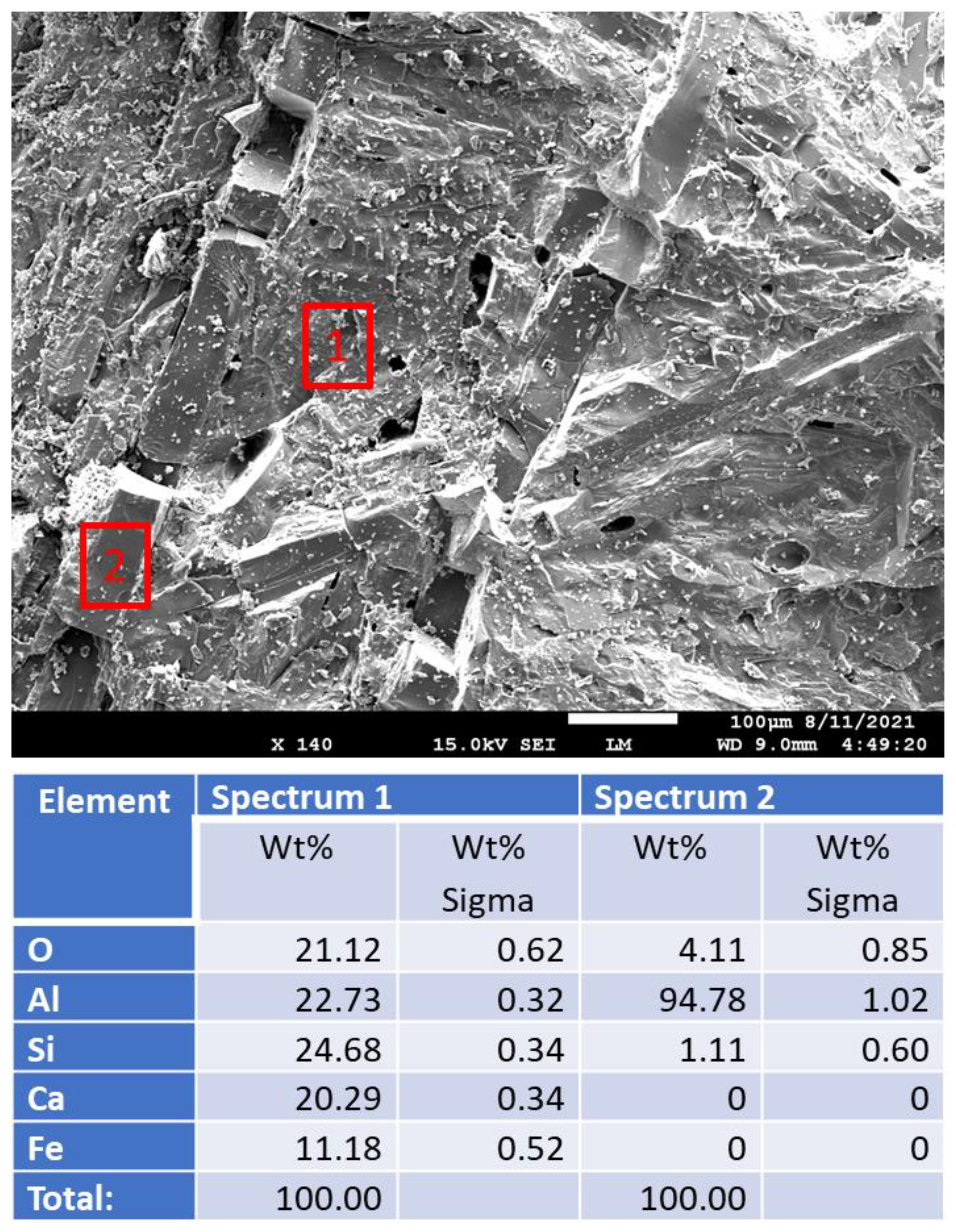
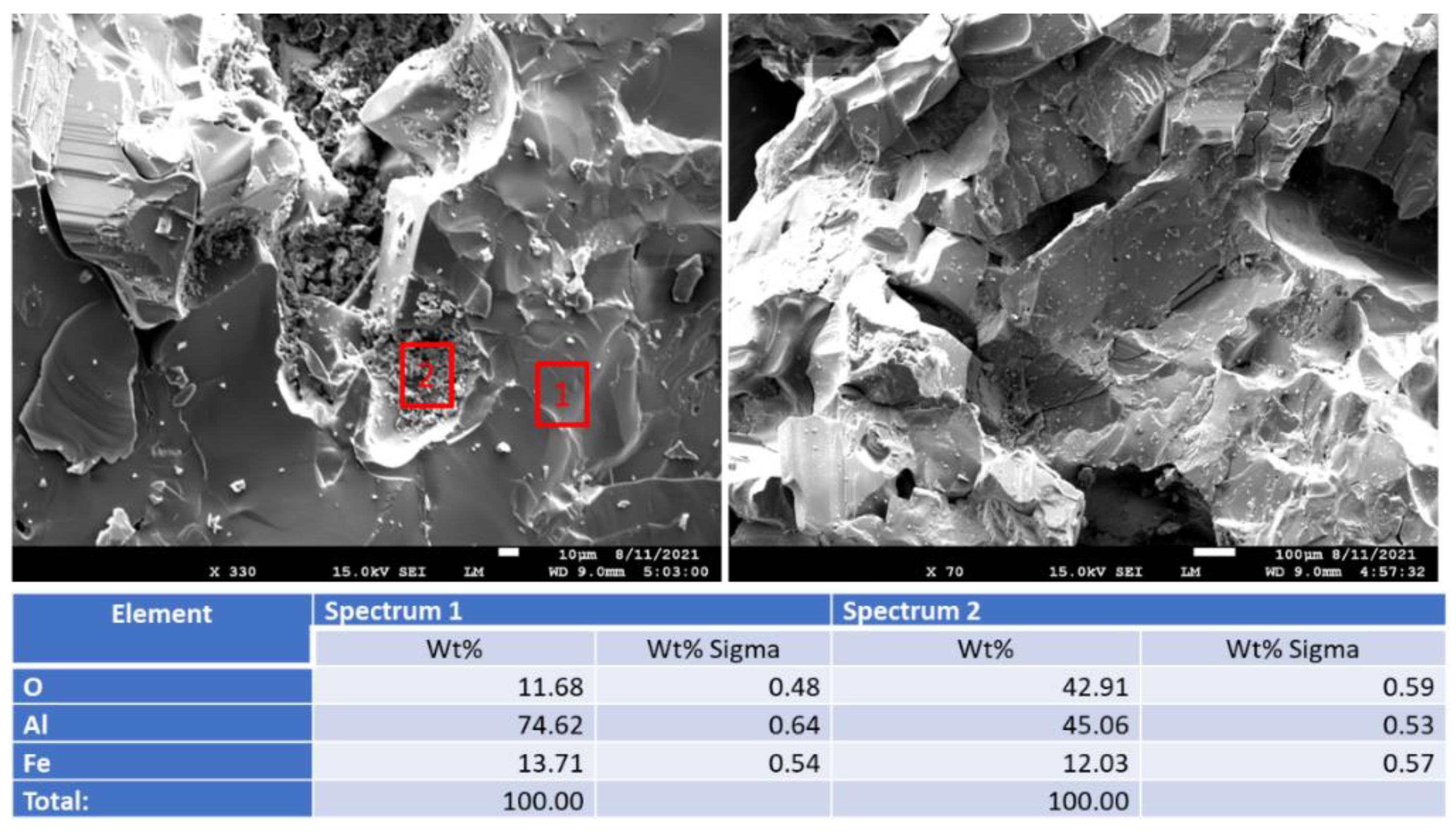
3.2.6. Morphological Changes—Scanning Electron Microscope and Energy Dispersive X-ray Analyses of Freshly Broken Surfaces
3.2.7. General Notes on Acid Exposure
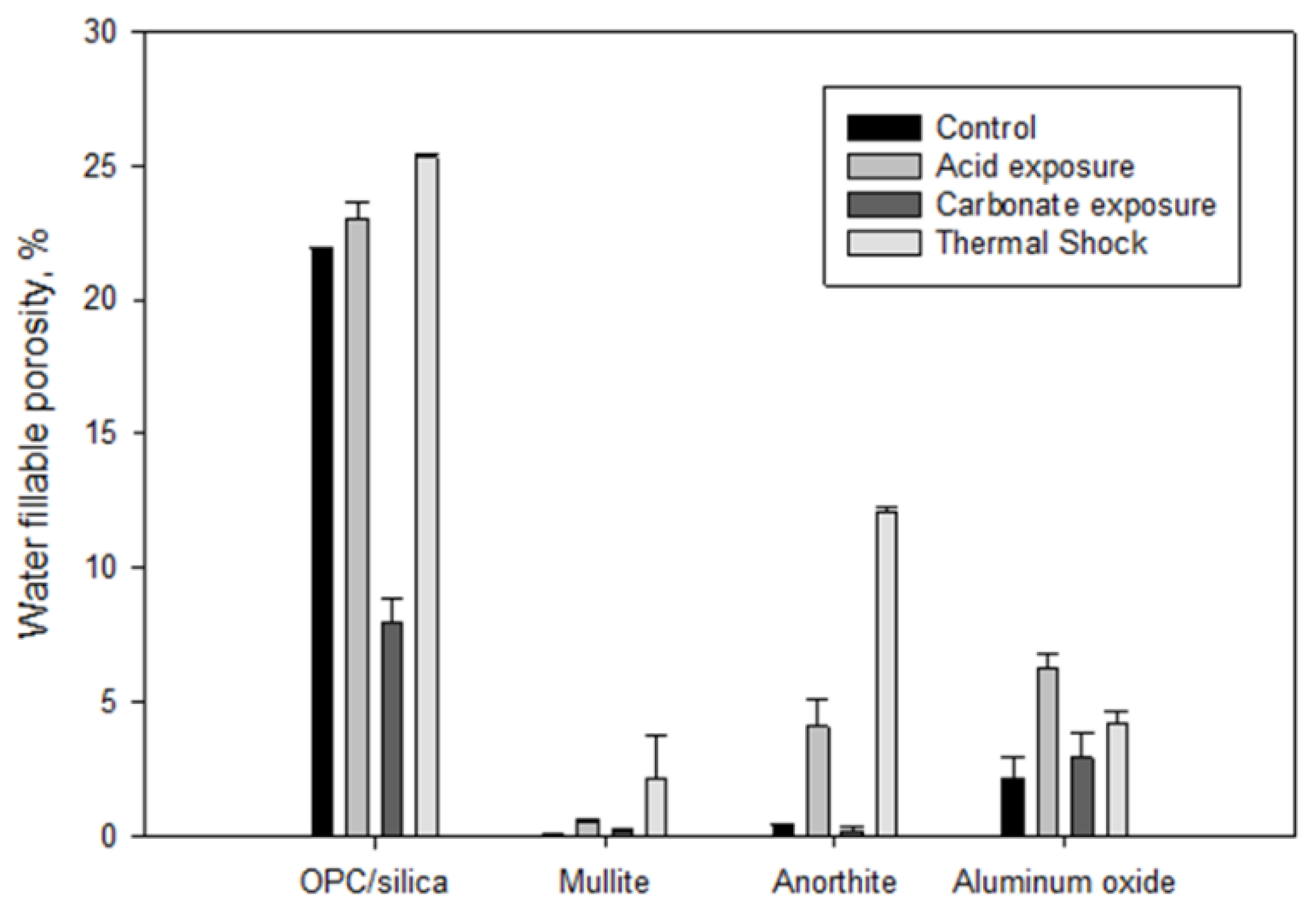
3.3. Alkaline Carbonation at 250 °C and Thermal Shock Tests (3 Cycles of 600 °C Heat → 10 min Cold Water Quenching)
3.3.1. Changes in Samples Appearances
3.3.2. Changes in Mechanical Properties
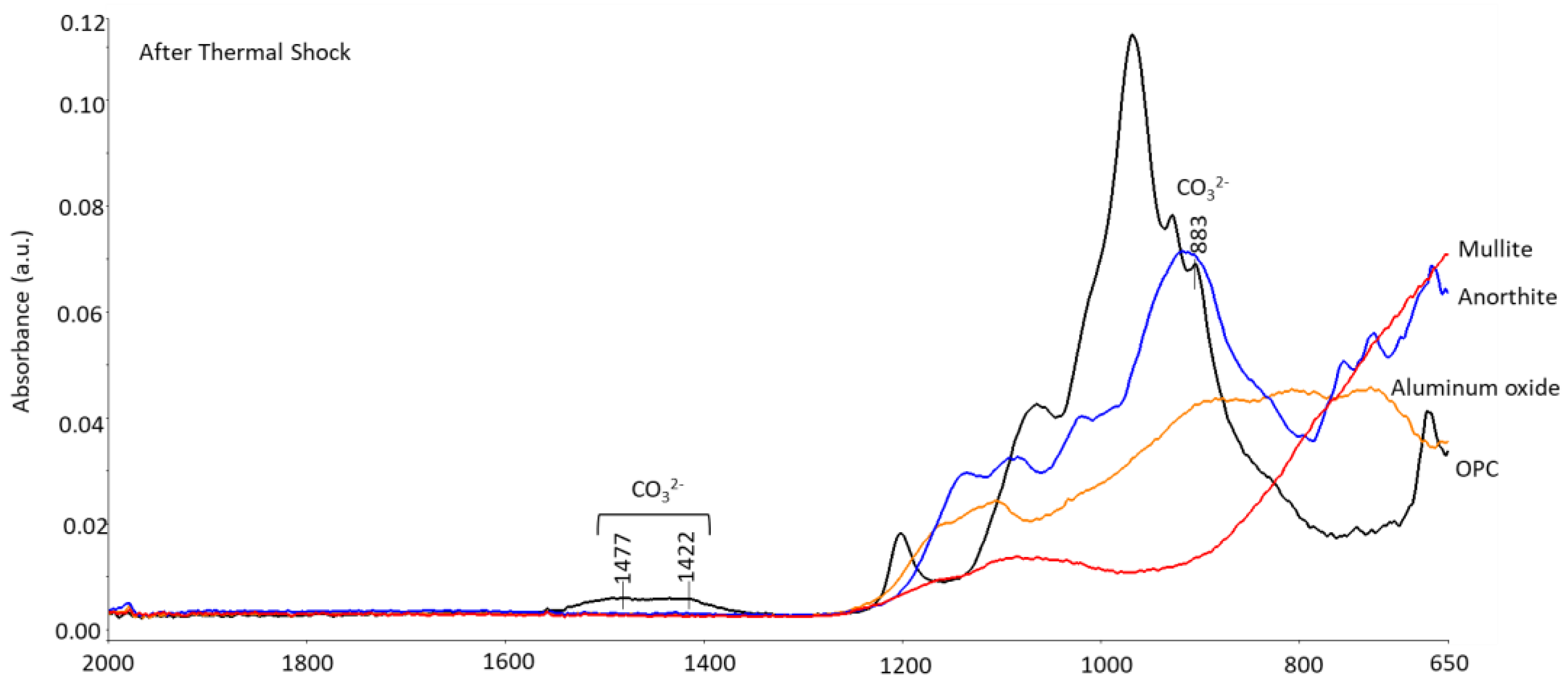
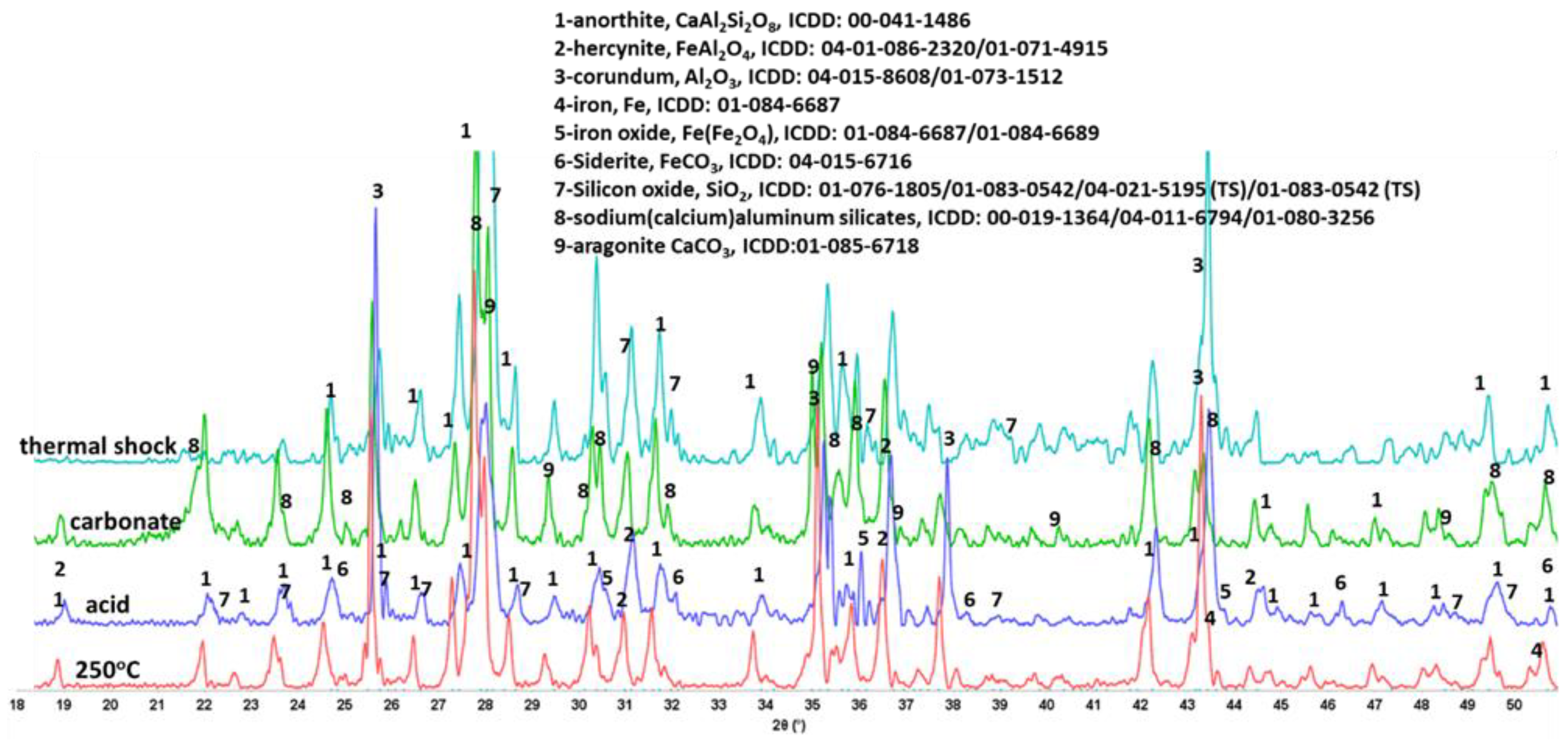
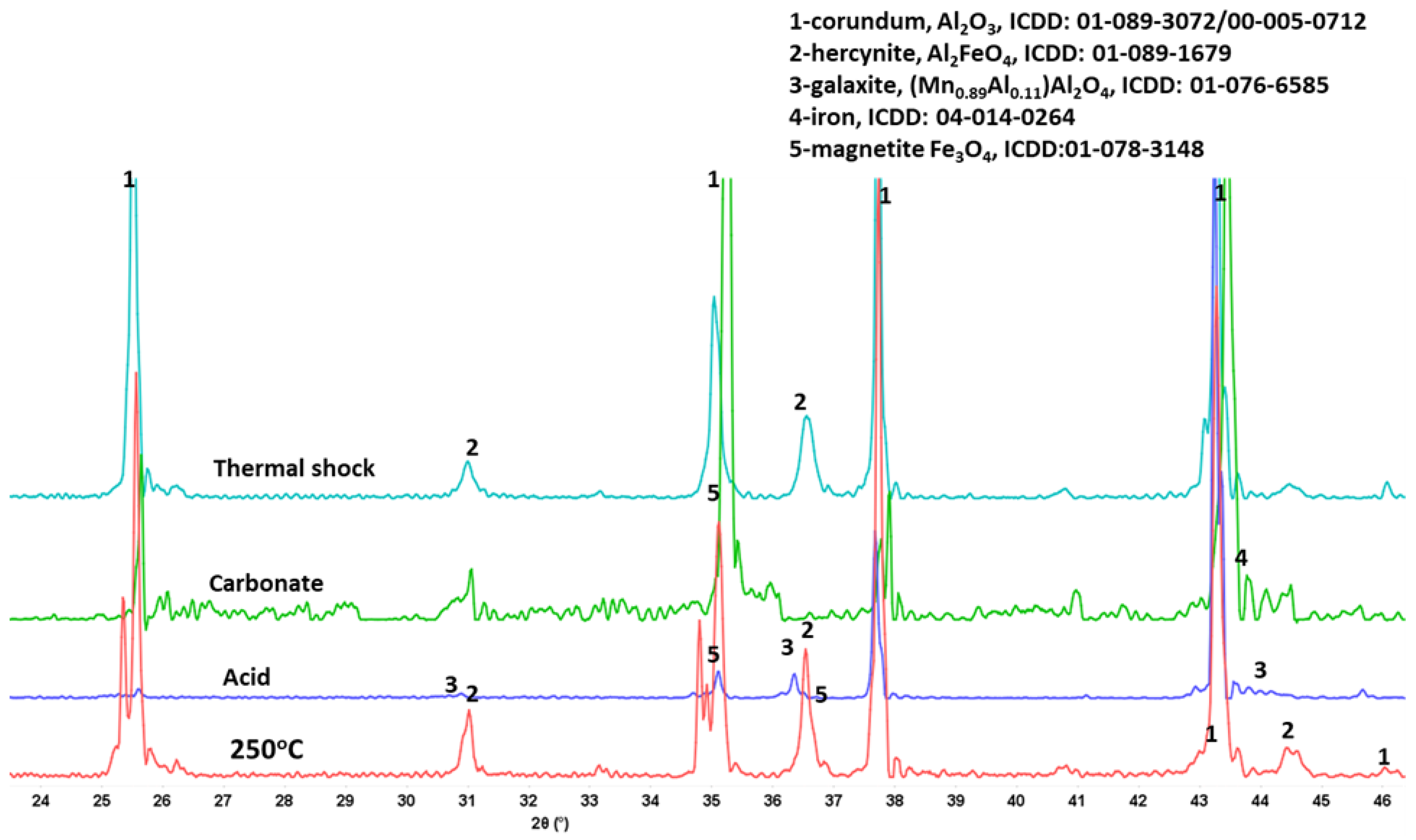
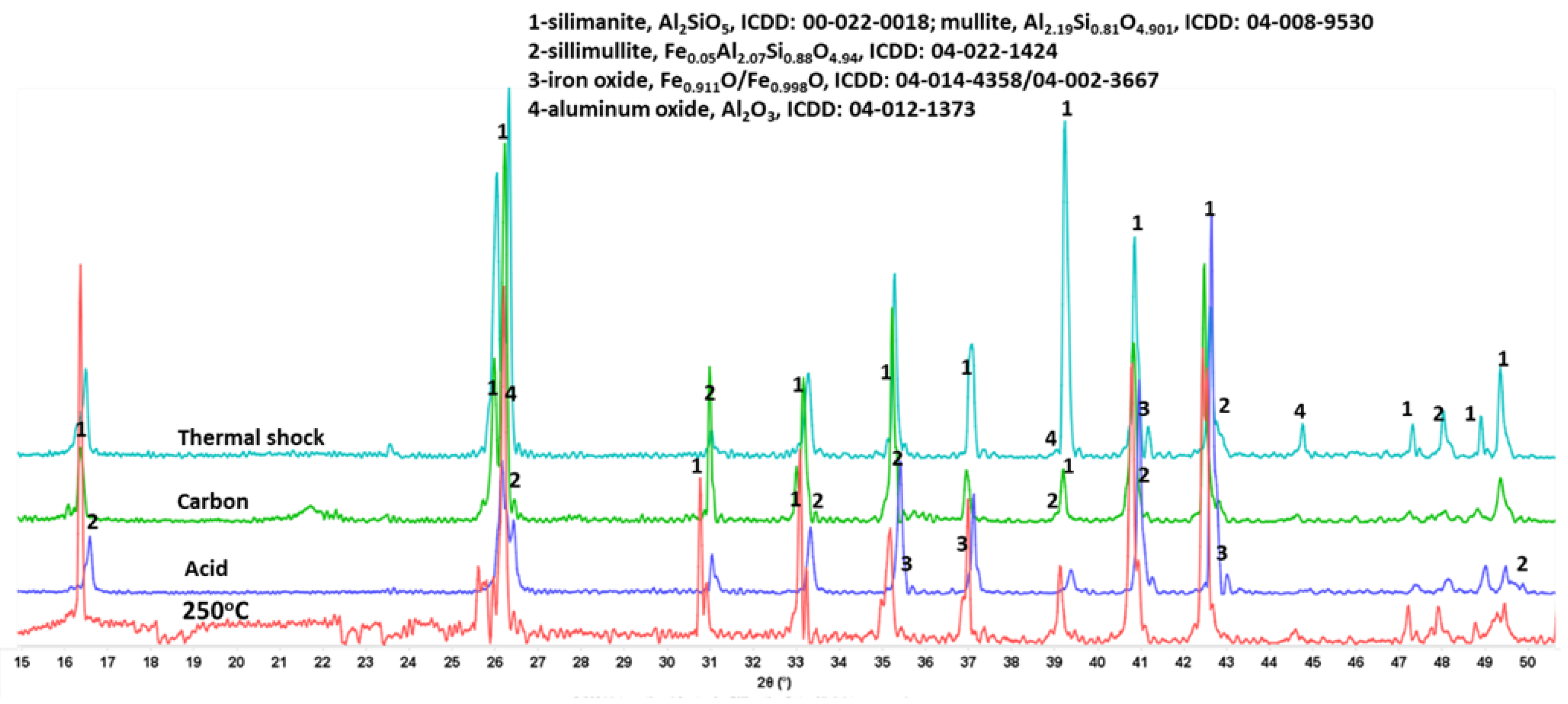
3.3.3. Phase Transitions during the Alkaline Carbonation and Thermal Shock—FTIR and XRD Analyses
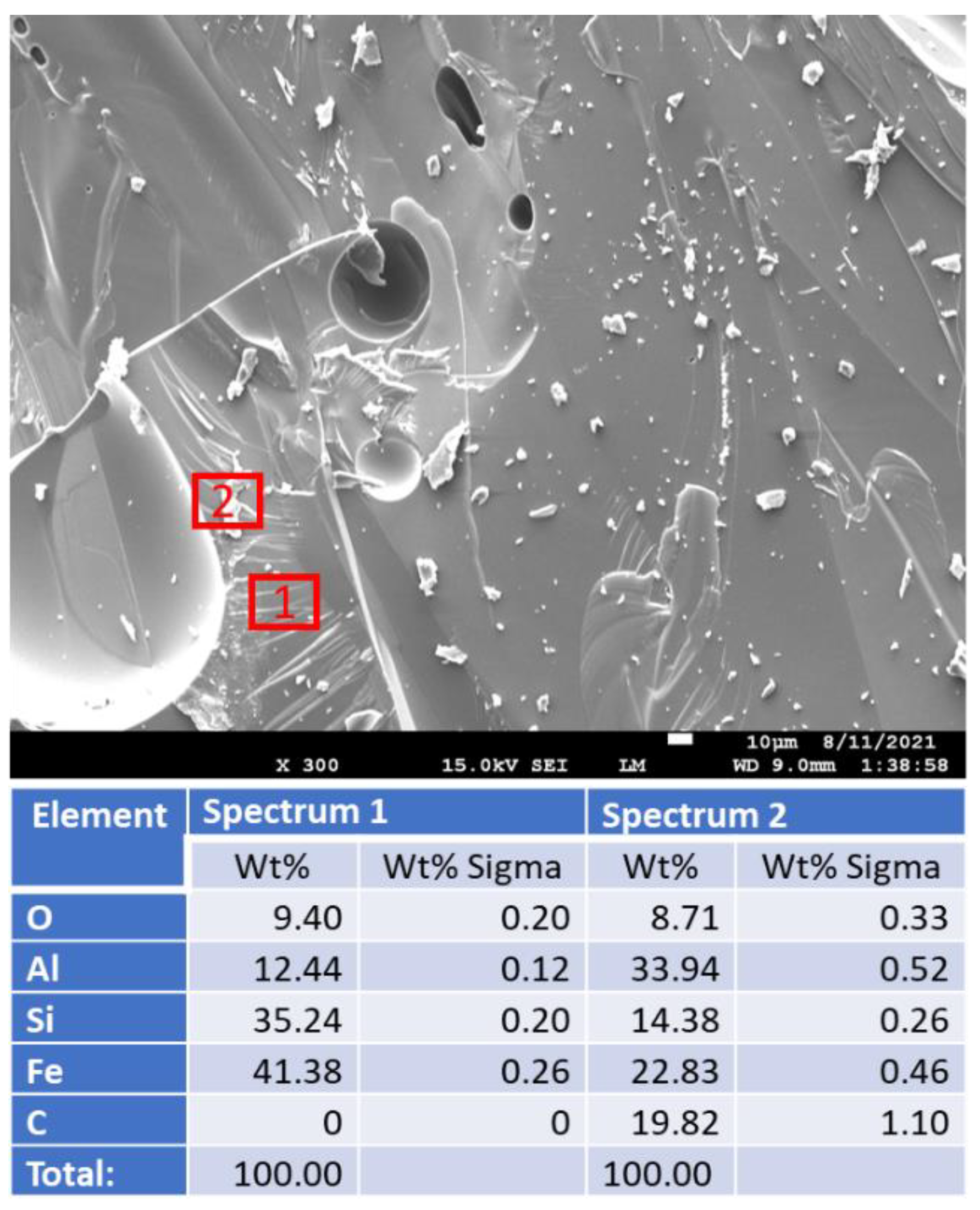
3.3.4. Morphological Changes—Scanning Electron Microscope and Energy Dispersive Elemental X-ray Analyses on Broken Sample Surfaces
3.3.5. General Notes on Alkaline Carbonation and Thermal Shock Test Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Achang, M.; Yanyao, L.; Radonjic, M. A Review of Past, Present, and Future Technologies for Permanent Plugging and Abandonment of Wellbores and Restoration of Subsurface Geologic Barriers. Environ. Eng. Sci. 2020, 37, 395–408. [Google Scholar] [CrossRef]
- Santra, A.; Sweatman, R. Understanding the long-term chemical and mechanical integrity of cement in a CCS environment. Energy Procedia 2011, 4, 5243–5250. [Google Scholar] [CrossRef]
- Olsen, R.; Leirvik, K.N.; Kvamme, B.; Kuznetsova, T. Effects of Sodium Chloride on Acidic Nanoscale Pores Between Steel and Cement. J. Phys. Chem. C 2016, 120, 29264–29271. [Google Scholar] [CrossRef]
- Recasens, M.; Garcia, S.; Mackay, E.; Delgado, J.; Maroto-Valer, M.M. Experimental Study of Wellbore Integrity for CO2 Geological Storage. Energy Procedia 2017, 114, 5249–5255. [Google Scholar] [CrossRef]
- Rahman, A.H.A.; Abdullah, B.; Vo, D.V.N. Modelling of Carbon Dioxide Leakage in Abandon Wells Using Computational Fluid Dynamics. Appl. Mech. Mater. 2014, 625, 780–783. [Google Scholar]
- Zhang, M.; Bachu, S. Review of integrity of existing wells in relation to CO2 geological storage: What do we know? Int. J. Greenh. Gas Control 2011, 5, 826–840. [Google Scholar] [CrossRef]
- Marangu, J.M.; Thiong’o, J.K.; Wachira, J.M. Review of Carbonation Resistance in Hydrated Cement Based Materials. J. Chem. 2019, 2019, 8489671. [Google Scholar] [CrossRef]
- Teodoriu, C.; Kosinowski, C.; Amani, M.; Schubert, J.; Shadravan, A. Wellbore integrity AND cement failure at hpht conditions. Int. J. Eng. Appl. Sci. 2013, 2, 2305–8269. [Google Scholar]
- Nelson, E.B.; Barlet-Gouedard, V. Thermal Cements. In Well Cementing; Nelson, E.B., Guillot, D., Eds.; Schlumberger: Sugar Land, TX, USA, 2006; pp. 319–341. [Google Scholar]
- Bergen, S.L.; Zemberekci, L.; Nair, S.D. A review of conventional and alternative cementitious materials for geothermal wells. Renew. Sustain. Energy Rev. 2022, 161, 112347. [Google Scholar] [CrossRef]
- Sugama, T.; Weber, L.; Brother, L. Sodium-polyphosphate-modified fly ash/calcium aluminate blend cement: Durability in wet, harsh geothermal environments. Mater. Lett. 2000, 44, 45–53. [Google Scholar] [CrossRef]
- Sugama, T.; Pyatina, T. Self-healing, re-adhering, and corrosion mitigating properties of fly ash-containing calcium aluminum phosphate cement composite at 300 °C hydrothermal temperature. Cem Concrete Comp. 2019, 99, 1–16. [Google Scholar] [CrossRef]
- Sugama, T. Advanced Cements for Geothermal Wells. BNL-77901-2007-IR; 2006. Available online: https://www.bnl.gov/isd/documents/35393.pdf (accessed on 21 September 2022).
- Lowry, B.; Nielson, D. Application of controlled-porosity ceramic material in geothermal drilling. In Proceedings of the 43d Workshop on Geothermal Reservoir Engineering, Stanford, CA, USA, 12–14 February 2018. [Google Scholar]
- Lowry, W.; Dunn, S.; Coates, K.; Duguid, A.; Wohletz, K. High Performance Ceramic Plugs for Borehole Sealing. In Proceedings of the High Level Radioactive Waste Management Conference, Charleston, SC, USA, 12–16 April 2015. [Google Scholar]
- Lowry, W.; Coates, K.; Wohletz, K.; Dunn, S.; Duguid, A.; Arnold, B.; Patera, E.; Groven, L. Ceramic Plugs for Deep Borehole Seals. In Proceedings of the Waste Management 2017 Conference, Phoenix, AZ, USA, 5–9 March 2017. [Google Scholar]
- Fischer, S.H.; Grubelich, M.C. Theoretical Energy Release of Thermites, Intermetallics, and Combustible Metals. In Proceedings of the Australian-American Joint Conference of Mines and Mine Countermeasures, Sydney, Australia, 12–16 July 1999. [Google Scholar]
- Sugama, T.; Pyatina, T. Alkali-Activated Cement Composites for High Temperature Geothermal Wells; Scientific Research Books: Wuhan, China, 2018. [Google Scholar]
- Pyatina, T.; Sugama, T. Acid resistance of calcium aluminate cement-fly ash F blends. Adv. Cem. Res. 2016, 28, 433–457. [Google Scholar] [CrossRef] [Green Version]
- Pyatina, T.; Sugama, T. Cements with supplementary cementitious materials for high-temperature geothermal wells. Geothermics 2020, 86, 101840. [Google Scholar] [CrossRef]
- Lamberet, S.; Guinot, D.; Lempereur, E.; Talley, J.; Alt, C. Field investigations of high performance calcium aluminate mortar for wastewater applications. In Calcium Aluminate Cements Proceedings of the Centenary Conference 2008; Fentiman, C.H., Mangabhai, R.J., Scrivener, K.L., Eds.; IHS BRE Press: Berkshire, UK, 2008; pp. 269–277. [Google Scholar]










| Sample Name | Averaged Elemental Composition, Major (Minor), (Expressed in the Form of Oxides, Mass %) | Crystalline Composition (ICDD Compound Number) |
|---|---|---|
| Aluminum oxide | Al2O3—60%, Fe2O3—5% (major) CaO—0.3%, MnO—0.2% (minor) | corundum, Al2O3, (01-089-3072/00-005-0712) (major) hercynite, Al2FeO4, (01-089-1679) galaxite, (Mn0.89Al0.11) Al2O4, (01-076-6585) iron, (04-014-0264) magnetite Fe3O4, (01-078-3148) |
| Anorthite | Al2O3—45%, SiO2—27%, CaO—15%, Fe2O3—10% (major) Cl—0.7% (minor) | anorthite, CaAl2Si2O8, (00-041-1486) (major) hercynite, FeAl2O4, (04-01-086-2320/01-071-4915) corundum, Al2O3, (04-015-8608/01-073-1512) iron, Fe, (01-084-6687) iron oxide, Fe(Fe2O4), (01-084-6687/01-084-6689) |
| Aluminum-silicate | Al2O3—60%, SiO2—30%, Fe2O3—10% (major) MnO—0.4%, CaO—0.4%, Cr2O3—0.1% (minor) | sillimanite, Al2SiO5, (00-022-0018); mullite, Al2.19Si0.81O4.901, (04-008-9530) sillimullite, Fe0.05Al2.07Si0.88O4.94, (04-022-1424) iron oxide, Fe0.911O/Fe0.998O, (04-014-4358/04-002-3667) |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lowry, B.; Louden, A.; Jerman, R.; Pyatina, T. Corrosion-Resistant Plug Materials for Geothermal Well Fluid Control. Materials 2022, 15, 6703. https://doi.org/10.3390/ma15196703
Lowry B, Louden A, Jerman R, Pyatina T. Corrosion-Resistant Plug Materials for Geothermal Well Fluid Control. Materials. 2022; 15(19):6703. https://doi.org/10.3390/ma15196703
Chicago/Turabian StyleLowry, Bill, Andrew Louden, Robin Jerman, and Tatiana Pyatina. 2022. "Corrosion-Resistant Plug Materials for Geothermal Well Fluid Control" Materials 15, no. 19: 6703. https://doi.org/10.3390/ma15196703




