Influence of Heat Treatment on Microstructure, Mechanical Property, and Corrosion Behavior of Cold-Sprayed Zn Coating on Mg Alloy Substrate
Abstract
1. Introduction
2. Experimental Procedure
2.1. Coatings Preparation and Heat Treatment
2.2. Phase and Microstructure Characterization
2.3. Microhardness and Bond Adhesion Tensile Strength Test
2.4. Corrosion Testing
3. Results and Discussion
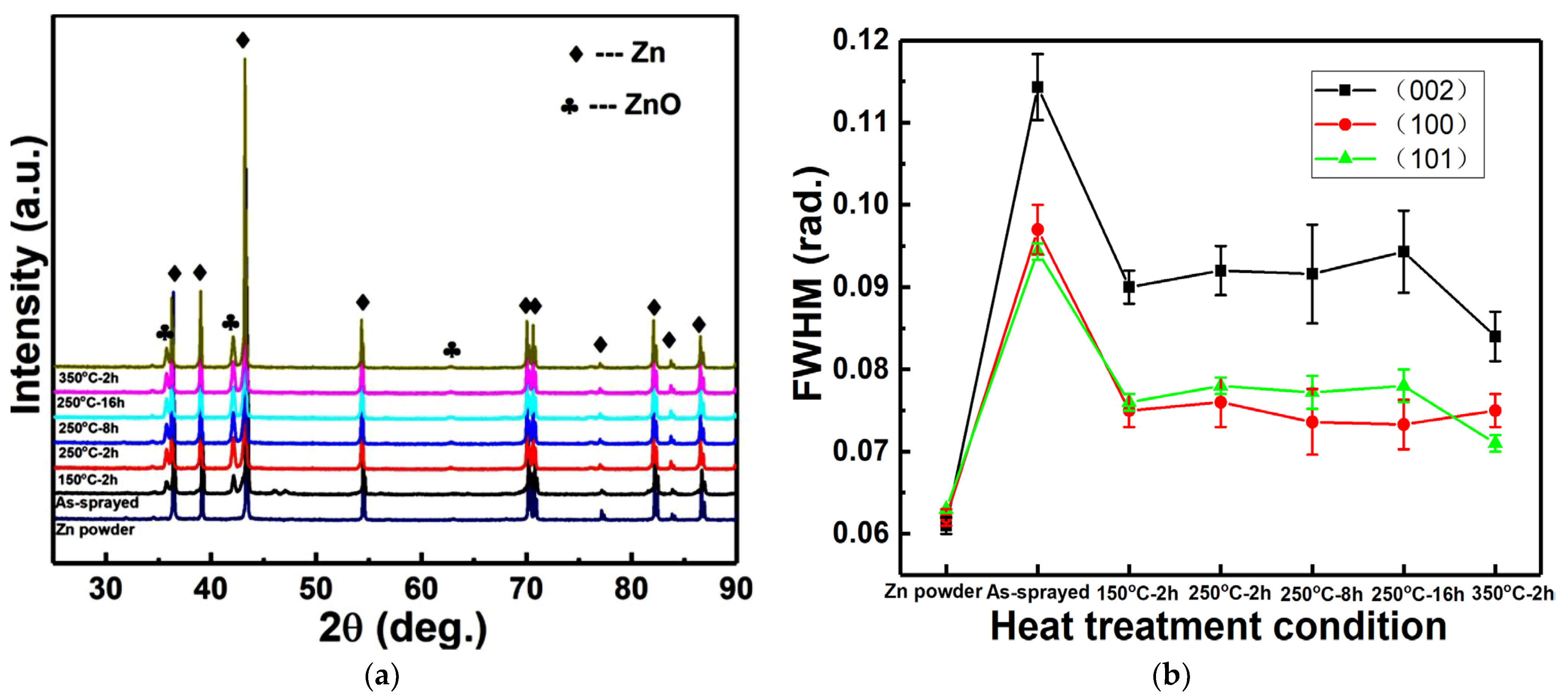
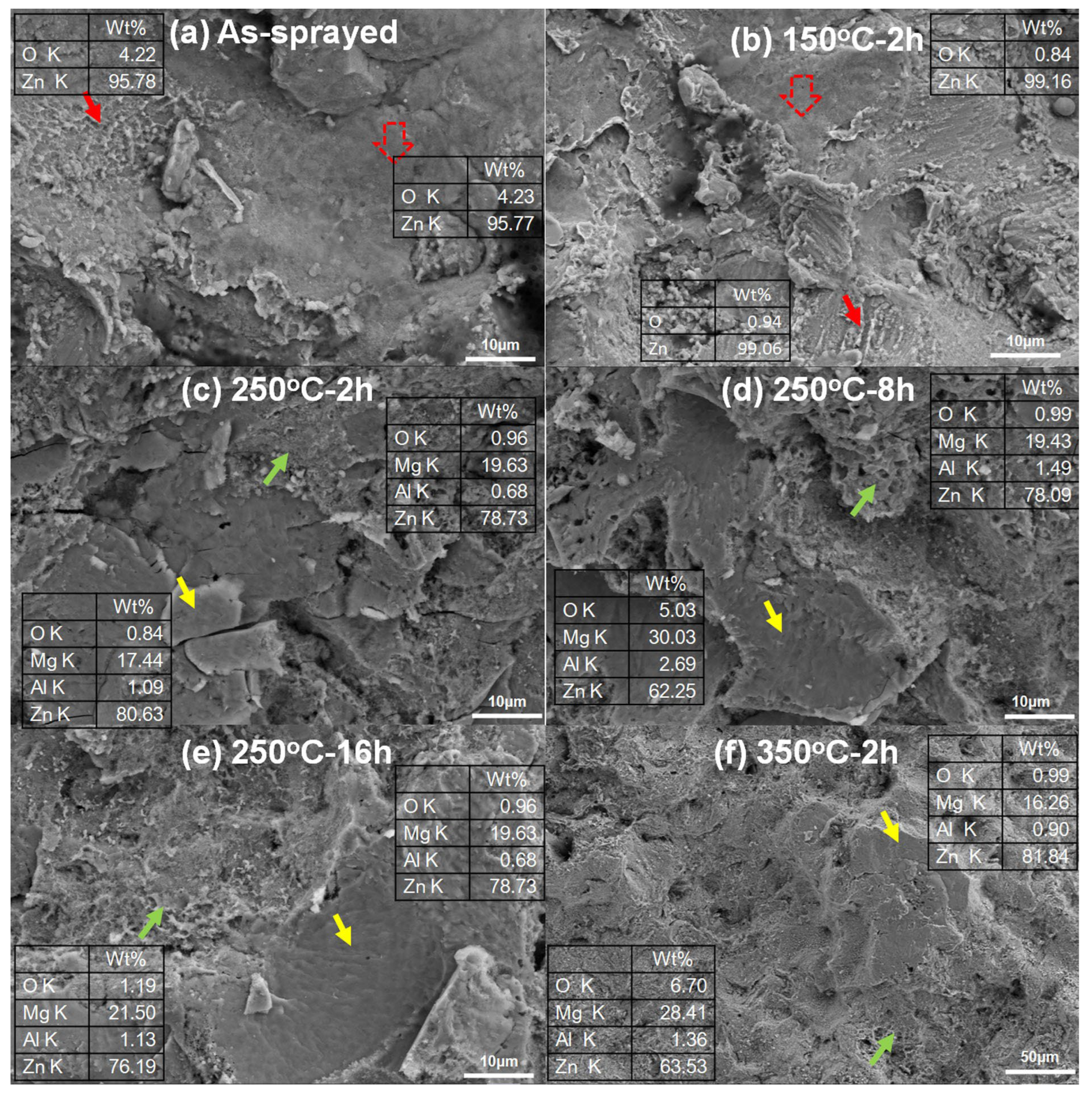
3.1. Phase Composition
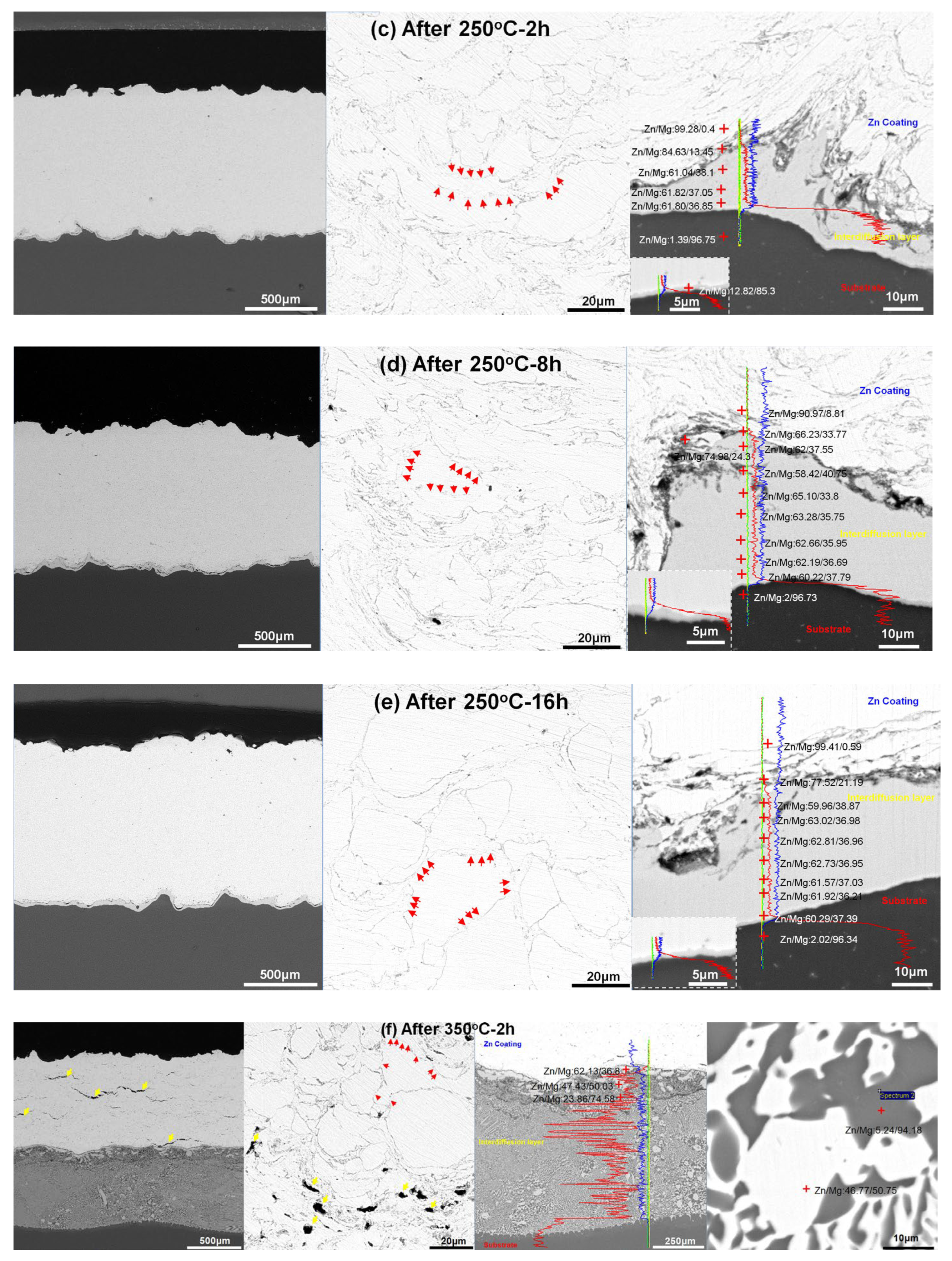
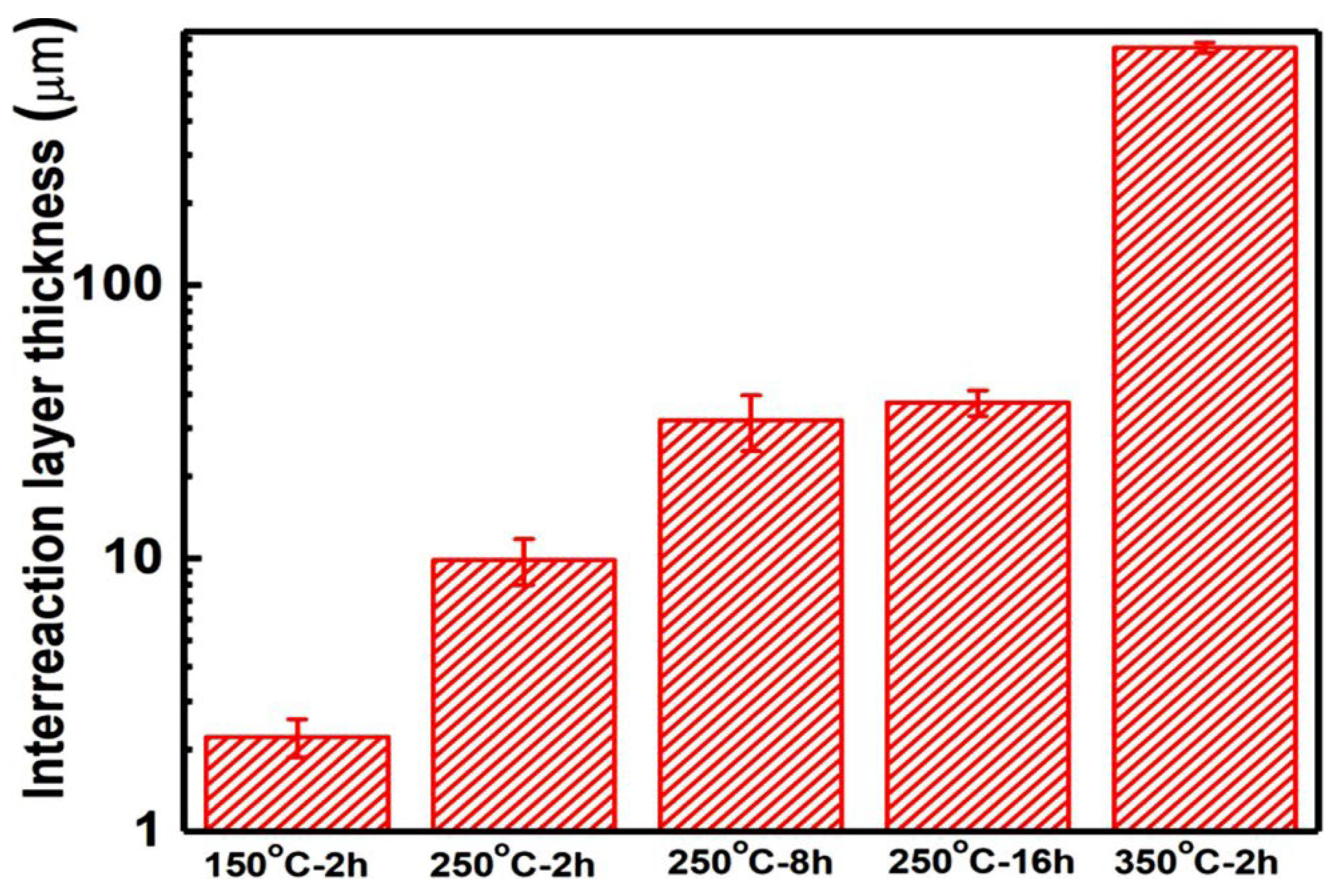
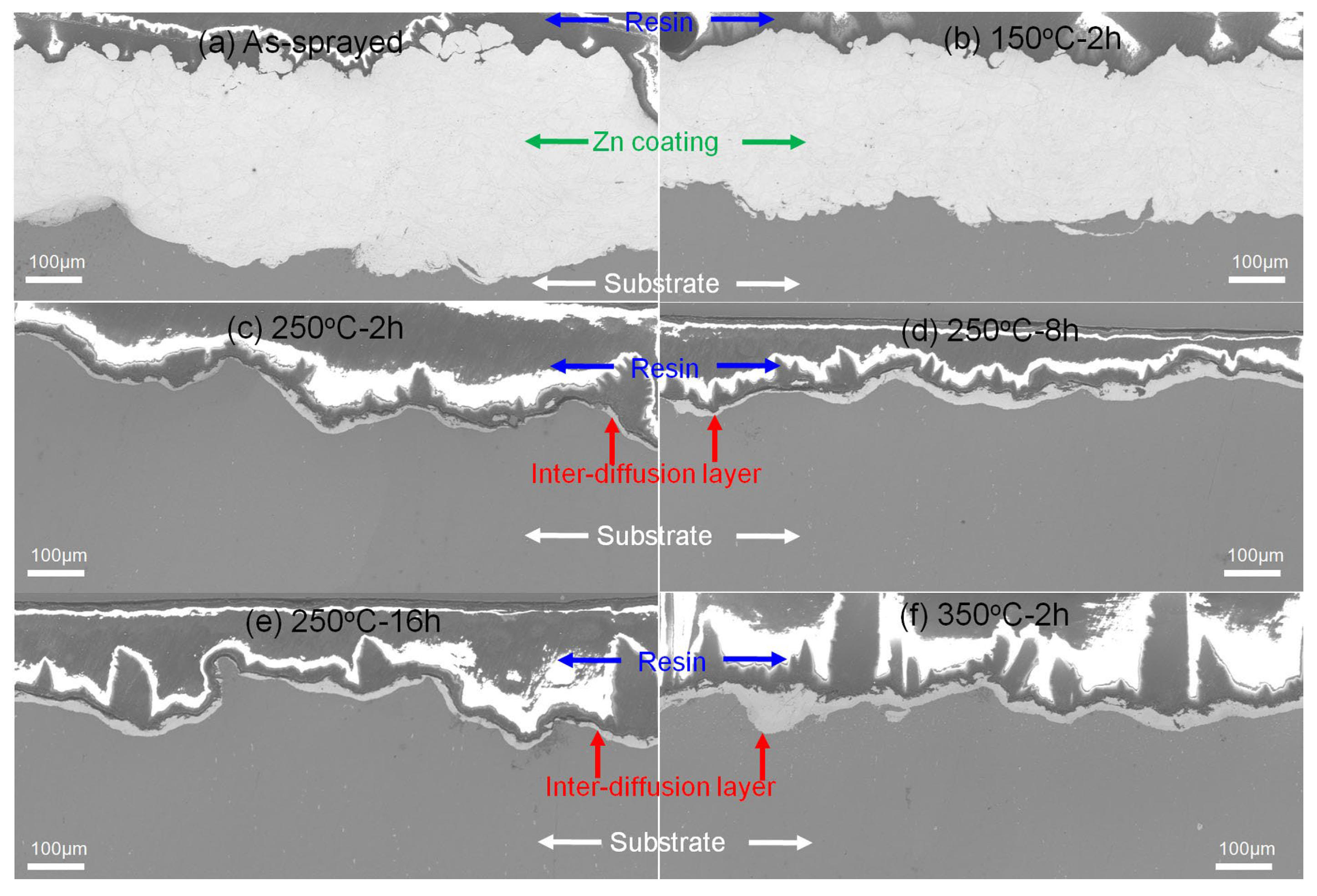
3.2. Cross-Sectional Microstructures after Heat Treatment
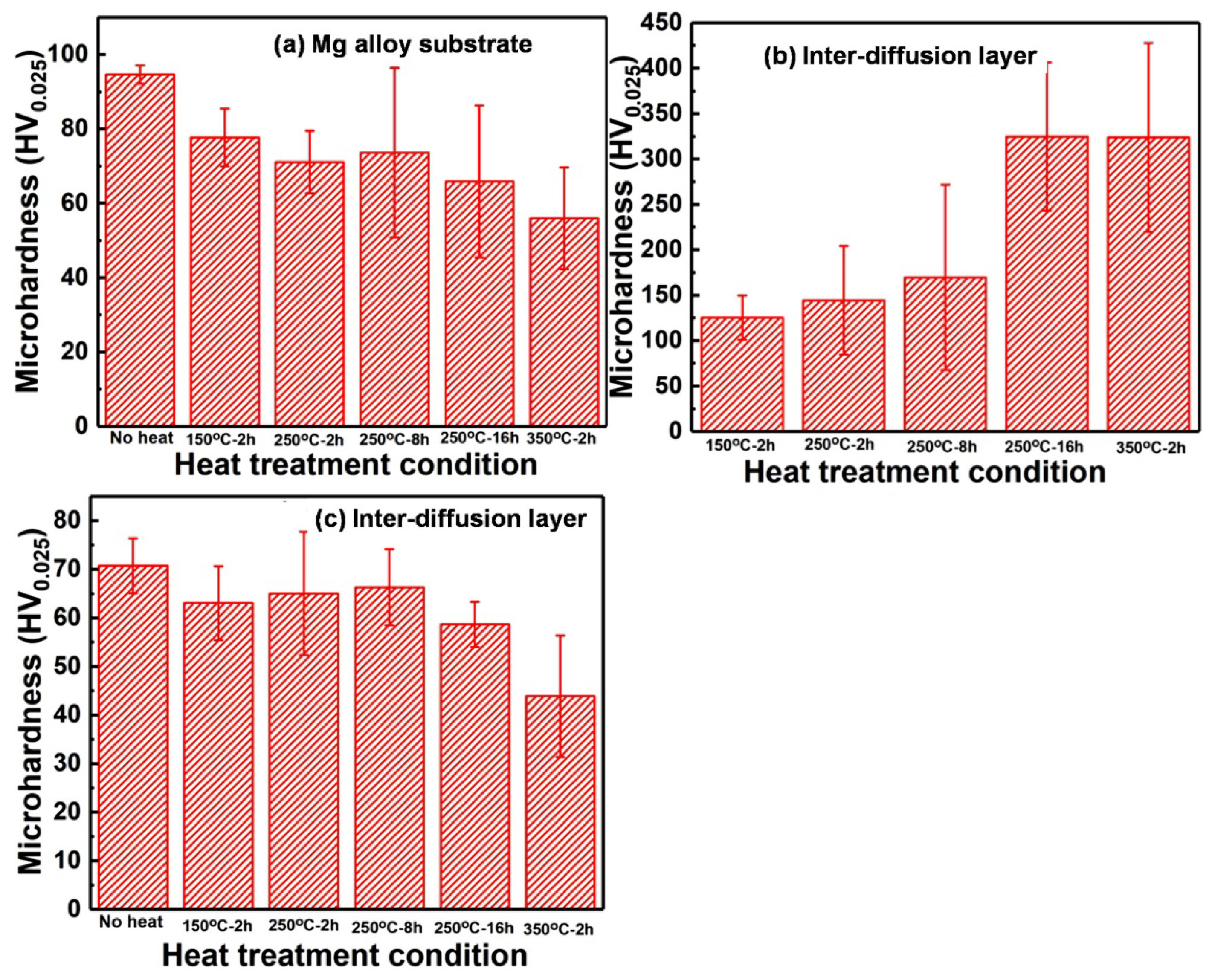
3.3. Microhardness after Heat Treatment
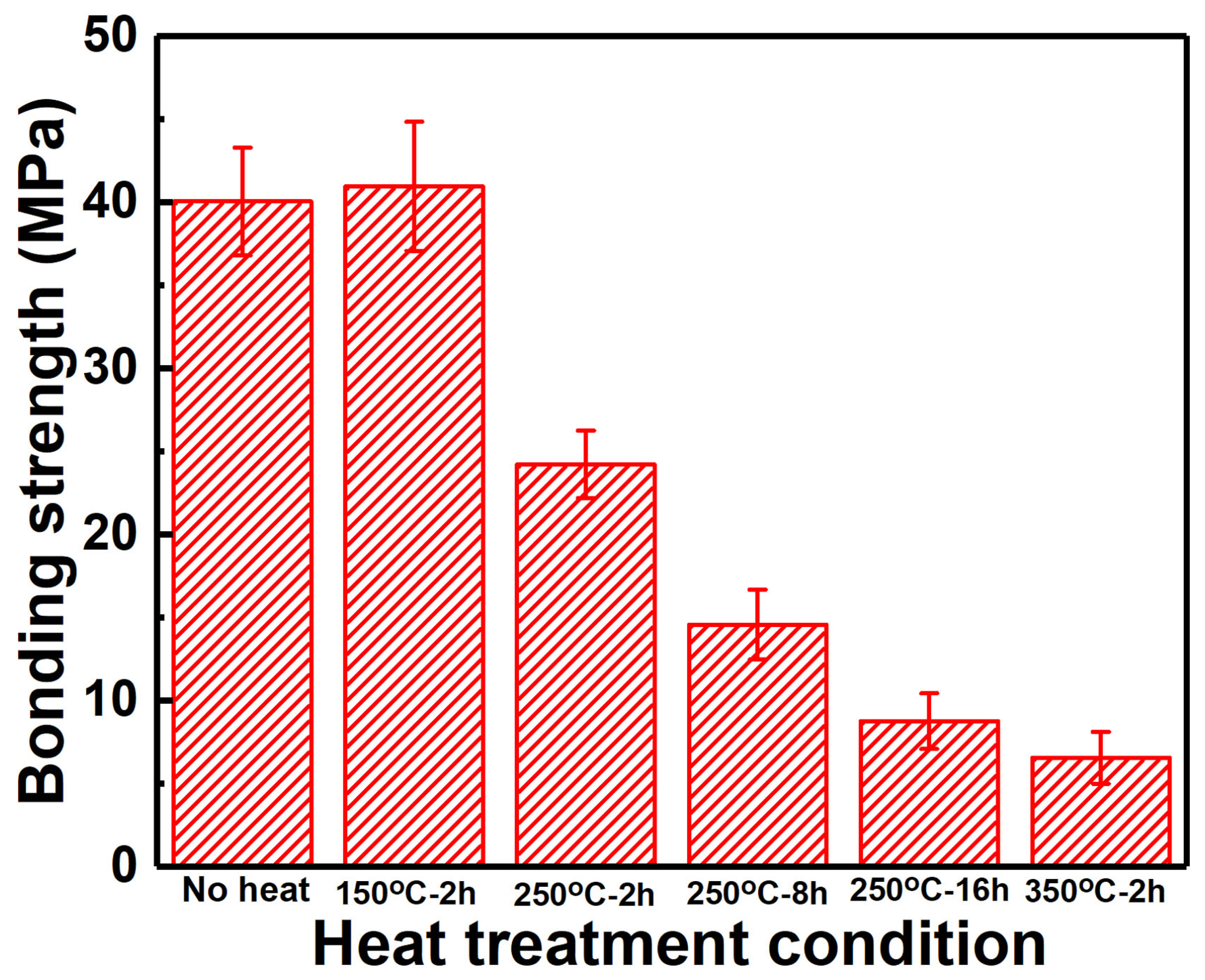
3.4. Tensile Strength after Heat Treatment
3.5. Polarization Curves
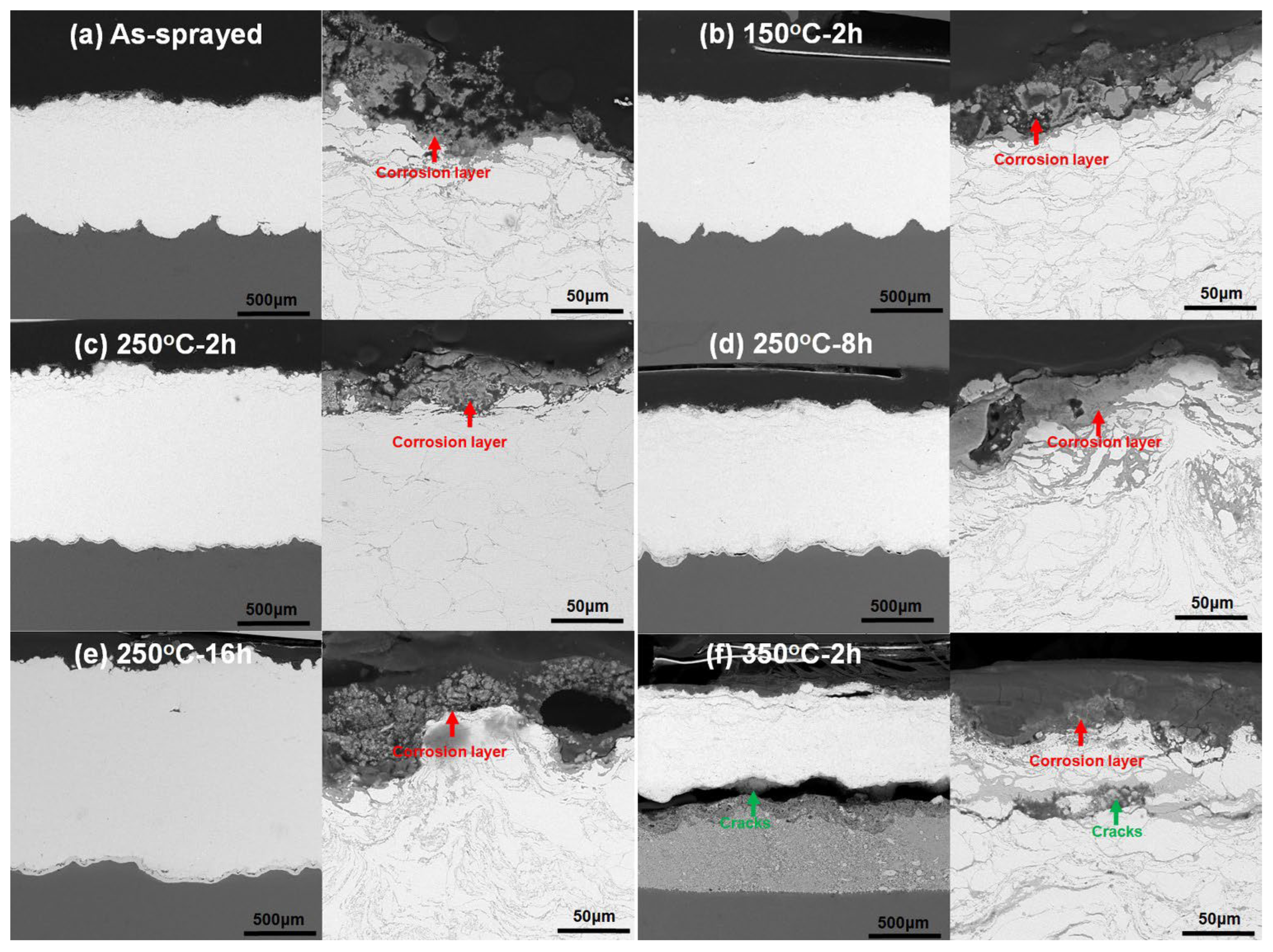
3.6. Corrosion Behavior
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Tsakiris, V.; Tardei, C.; Clicinschi, F.M. Biodegradable Mg alloys for orthopedic implants–A review. J. Magnes. Alloy. 2021, 9, 1884–1905. [Google Scholar] [CrossRef]
- Li, H.; Wen, J.; Liu, Y.; He, J.; Shi, H.; Tian, P. Progress in Research on Biodegradable Magnesium Alloys: A Review. Adv. Eng. Mater. 2020, 22, 2000213. [Google Scholar] [CrossRef]
- Dikici, B.; Esen, Z.; Duygulu, O.; Gungor, S. Corrosion of Metallic Biomaterials. In Advances in Metallic Biomaterials; Springer: Berlin/Heidelberg, Germany, 2015; Volume 3, pp. 275–303. [Google Scholar] [CrossRef]
- Zhao, D.; Witte, F.; Lu, F.; Wang, J.; Li, J.; Qin, L. Current status on clinical applications of magnesium-based orthopaedic implants: A review from clinical translational perspective. Biomaterials 2017, 112, 287–302. [Google Scholar] [CrossRef] [PubMed]
- Wu, G.; Ibrahim, J.M.; Chu, P.K. Surface design of biodegradable magnesium alloys—A review. Surf. Coat. Technol. 2012, 233, 2–12. [Google Scholar] [CrossRef]
- Zhuo, X.; Wu, Y.; Ju, J.; Liu, H.; Jiang, J.; Hu, Z.; Bai, J.; Xue, F. Recent progress of novel biodegradable zinc alloys: From the perspective of strengthening and toughening. J. Mater. Res. Technol. 2022, 17, 244–269. [Google Scholar] [CrossRef]
- Yuan, W.; Xia, D.; Wu, S.; Zheng, Y.; Guan, Z.; Rau, J.V. A review on current research status of the surface modification of Zn-based biodegradable metals. Bioact. Mater. 2021, 7, 192–216. [Google Scholar] [CrossRef] [PubMed]
- Li, H.F.; Shi, Z.Z.; Wang, L.N. Opportunities and challenges of biodegradable Zn-based alloys. J. Mater. Sci. Technol. 2020, 46, 136–138. [Google Scholar] [CrossRef]
- Prashar, G.; Vasudev, H. A comprehensive review on sustainable cold spray additive manufacturing: State of the art, challenges and future challenges. J. Clean. Prod. 2021, 310, 127606. [Google Scholar] [CrossRef]
- Li, W.; Cao, C.; Wang, G.; Wang, F.; Xu, Y.; Yang, X. ‘Cold spray +’ as a new hybrid additive manufacturing technology: A literature review. Sci. Technol. Weld. Join. 2019, 24, 420–445. [Google Scholar] [CrossRef]
- Chavan, N.M.; Kiran, B.; Jyothirmayi, A.; Phani, P.S.; Sundararajan, G. The Corrosion Behavior of Cold Sprayed Zinc Coatings on Mild Steel Substrate. J. Therm. Spray Technol. 2013, 22, 463–470. [Google Scholar] [CrossRef]
- Maledi, N.; Oladijo, O.; Botef, I.; Ntsoane, T.; Madiseng, A.; Moloisane, L. Influence of cold spray parameters on the microstructures and residual stress of Zn coatings sprayed on mild steel. Surf. Coat. Technol. 2017, 318, 106–113. [Google Scholar] [CrossRef]
- Li, W.-Y.; Li, C.-J.; Yang, G.-J. Effect of impact-induced melting on interface microstructure and bonding of cold-sprayed zinc coating. Appl. Surf. Sci. 2010, 257, 1516–1523. [Google Scholar] [CrossRef]
- Yao, H.-L.; Yi, Z.-H.; Yao, C.; Zhang, M.-X.; Wang, H.-T.; Li, S.-B.; Bai, X.-B.; Chen, Q.-Y.; Ji, G.-C. Improved corrosion resistance of AZ91D magnesium alloy coated by novel cold-sprayed Zn-HA/Zn double-layer coatings. Ceram. Int. 2020, 46, 7687–7693. [Google Scholar] [CrossRef]
- Yao, H.-L.; Hu, X.-Z.; Yi, Z.-H.; Xia, J.; Tu, X.-Y.; Li, S.-B.; Yu, B.; Zhang, M.-X.; Bai, X.-B.; Chen, Q.-Y.; et al. Microstructure and improved anti-corrosion properties of cold-sprayed Zn coatings fabricated by post shot-peening process. Surf. Coat. Technol. 2021, 422, 127557. [Google Scholar] [CrossRef]
- Frattolin, J.; Roy, R.; Rajagopalan, S.; Walsh, M.; Yue, S.; Bertrand, O.F.; Mongrain, R. A manufacturing and annealing protocol to develop a cold-sprayed Fe-316L stainless steel biodegradable stenting material. Acta Biomater. 2019, 99, 479–494. [Google Scholar] [CrossRef] [PubMed]
- Hussain, T.; McCartney, G.; Shipway, P.H.; Zhang, D. Bonding Mechanisms in Cold Spraying: The Contributions of Metallurgical and Mechanical Components. J. Therm. Spray Technol. 2009, 18, 364–379. [Google Scholar] [CrossRef]
- Bu, H.; Yandouzi, M.; Lu, C.; Jodoin, B. Post-heat Treatment Effects on Cold-Sprayed Aluminum Coatings on AZ91D Magnesium Substrates. J. Therm. Spray Technol. 2012, 21, 731–739. [Google Scholar] [CrossRef]
- Bu, H.; Yandouzi, M.; Lu, C.; Jodoin, B. Effect of heat treatment on the intermetallic layer of cold sprayed aluminum coatings on magnesium alloy. Surf. Coat. Technol. 2011, 205, 4665–4671. [Google Scholar] [CrossRef]
- Peng, H.; Chen, D.; Bai, X.; She, X.; Li, D.; Jiang, X. Ultrasonic spot welding of magnesium-to-aluminum alloys with a copper interlayer: Microstructural evolution and tensile properties. J. Manuf. Process. 2018, 37, 91–100. [Google Scholar] [CrossRef]
- Zhong, Q.; Pan, D.; Zuo, S.; Li, X.; Luo, H.; Lin, Y. Fabrication of Mg Zn intermetallic layer with high hardness and corrosion resistance on AZ31 alloy. Mater. Charact. 2021, 179, 111365. [Google Scholar] [CrossRef]
- Ma, L.; Long, W.; Qiao, P.; He, D.; Li, X. Development of a Binary Zn-Based Solder Alloy for Joining Wrought Magnesium Alloy AZ31B. J. Mater. Eng. Perform. 2012, 22, 118–122. [Google Scholar] [CrossRef]
- Das, S.K.; Kim, Y.-M.; Ha, T.K.; Jung, I.-H. Investigation of anisotropic diffusion behavior of Zn in hcp Mg and interdiffusion coefficients of intermediate phases in the Mg–Zn system. Calphad 2013, 42, 51–58. [Google Scholar] [CrossRef]
- Kammerer, C.C.; Behdad, S.; Zhou, L.; Betancor, F.; Gonzalez, M.; Boesl, B.; Sohn, Y.H. Diffusion Kinetics, Mechanical Properties, and Crystallographic Characterization of Intermetallic Compounds in the Mg-Zn Binary System. Intermetallics 2015, 67, 145–155. [Google Scholar] [CrossRef]
- Ouyang, Y.; Liu, K.; Peng, C.; Chen, H.; Tao, X.; Du, Y. Investigation of diffusion behavior and mechanical properties of Mg-Zn system. Calphad 2019, 65, 204–211. [Google Scholar] [CrossRef]
- Roy, V.; Nahak, B.; Yusufzai, M.; Vashista, M. Assessment of Plastic Deformation Upon Grinding Using X-Ray Diffraction Profiles. Int. J. Eng. Res. 2013, 2, 119–124. [Google Scholar]
- Wei, Y.K.; Luo, X.T.; Li, C.X.; Li, C.J. Optimization of In-situ Shot-peening Assisted Cold Spraying Parameters for Full Corrosion Protection of Mg Alloy by Fully Dense Al-based Alloy Coating. J. Therm. Spray Technol. 2017, 26, 173–183. [Google Scholar] [CrossRef]
- Luo, X.-T.; Wei, Y.-K.; Wang, Y.; Li, C.-J. Microstructure and mechanical property of Ti and Ti6Al4V prepared by an in-situ shot peening assisted cold spraying. Mater. Des. 2015, 85, 527–533. [Google Scholar] [CrossRef]
- Wei, Y.-K.; Luo, X.-T.; Ge, Y.; Chu, X.; Huang, G.-S.; Li, C.-J. Deposition of fully dense Al-based coatings via in-situ micro-forging assisted cold spray for excellent corrosion protection of AZ31B magnesium alloy. J. Alloy. Compd. 2019, 806, 1116–1126. [Google Scholar] [CrossRef]
- Min, Y.; Li, W.Y.; Chao, Z.; Liao, H. Effect of Vacuum Heat Treatment on Tensile Strength and Fracture Performance of Cold-sprayed Cu-4Cr-2Nb Coatings. Appl. Surf. Sci. 2011, 257, 5972–5976. [Google Scholar]
- Ma, L.; He, D.; Li, X.; Jiang, J. Microstructure and Mechanical Properties of Magnesium Alloy AZ31B Brazed Joint Using a Zn-Mg-Al Filler Metal. J. Mater. Sci. Technol. 2010, 26, 743–746. [Google Scholar] [CrossRef]
- Liu, L.; Tan, J.; Zhao, L.; Liu, X. The relationship between microstructure and properties of Mg/Al brazed joints using Zn filler metal. Mater. Charact. 2007, 59, 479–483. [Google Scholar] [CrossRef]
- Liu, L.; Tan, J.; Liu, X. Reactive brazing of Al alloy to Mg alloy using zinc-based brazing alloy. Mater. Lett. 2007, 61, 2373–2377. [Google Scholar] [CrossRef]
- Sample, C.M.; Champagne, V.K.; Nardi, A.T.; Lados, D.A. Factors governing static properties and fatigue, fatigue crack growth, and fracture mechanisms in cold spray alloys and coatings/repairs: A review. Addit. Manuf. 2020, 36, 101371. [Google Scholar] [CrossRef]
- Cizek, J.; Kovarik, O.; Cupera, J.; Kondas, J.; Bajer, T.; Siska, F.; Janovska, M.; Seiner, H. Measurement of mechanical and fatigue properties using unified, simple-geometry specimens: Cold spray additively manufactured pure metals. Surf. Coat. Technol. 2021, 412, 126929. [Google Scholar] [CrossRef]
- Huang, C.; Arseenko, M.; Zhao, L.; Xie, Y.; Elsenberg, A.; Li, W.; Gärtner, F.; Simar, A.; Klassen, T. Property prediction and crack growth behavior in cold sprayed Cu deposits. Mater. Des. 2021, 206, 109826. [Google Scholar] [CrossRef]
- Dean, S.W. Electrochemical methods of corrosion testing. In Electrochemical Techniques for Corrosion Engineering; Baboian, R., Ed.; U.S. Department of Energy Office of Scientific and Technical Information: Oak Ridge, TN, USA, 1977. [Google Scholar]
- Zhou, H.; Li, C.; Ji, G.; Fu, S.; Yang, H.; Luo, X.; Yang, G.; Li, C.-J. Local microstructure inhomogeneity and gas temperature effect in in-situ shot-peening assisted cold-sprayed Ti-6Al-4V coating. J. Alloy. Compd. 2018, 766, 694–704. [Google Scholar] [CrossRef]
- Vojtěch, D.; Kubásek, J.; Šerák, J.; Novák, P. Mechanical and Corrosion Properties of Newly Developed Biodegradable Zn-based Alloys for Bone Fixation. Acta Biomater. 2011, 7, 3515–3522. [Google Scholar] [CrossRef]
- Levy, G.K.; Goldman, J.; Aghion, E. The Prospects of Zinc as a Structural Material for Biodegradable Implants—A Review Paper. Metals 2017, 7, 402. [Google Scholar] [CrossRef]




| Samples | Immersion Time | βa (mV) | βc (mV) | Icorr (mA/cm2) | Ecorr (V) | Corrosion Rate (mm/year) |
|---|---|---|---|---|---|---|
| Substrate | 1 day | 690.6 ± 59.9 | 417.6 ± 144.3 | 3.12 ± 0.75 | −1.54 ± 0.01 | 29.9 ± 0.9 |
| As-sprayed | 1 day | 503.4 ± 122.2 | 331.5 ± 53.1 | 1.31 ± 0.71 | −1.05 ± 0.04 | 18.9 ± 7.7 |
| 7 days | 507.3 ± 331.4 | 312.2 ± 60.1 | 0.74 ± 0.25 | −1.06 ± 0.12 | 8.7 ± 3.7 | |
| 14 days | 540.6 ± 197.1 | 369.7 ± 66.5 | 1.07 ± 0.38 | −1.11 ± 0.18 | 9.9 ± 4.6 | |
| 150 °C-2 h | 1 day | 330.1 ± 204.3 | 254.6 ± 128.2 | 1.57 ± 0.72 | −1.04 ± 0.04 | 18.5 ± 7.6 |
| 7 days | 1123.1 ± 363.9 | 243.2 ± 115.4 | 1.17 ± 0.88 | −1.03 ± 0.07 | 12.5 ± 7.5 | |
| 14 days | 522.5 ± 119.8 | 433.2 ± 146.5 | 0.62 ± 0.23 | −1.02 ± 0.32 | 7.3 ± 3.5 | |
| 250 °C-2 h | 1 day | 619.6 ± 236.7 | 374.6 ± 116.9 | 1.92 ± 0.49 | −1.04 ± 0.05 | 19.9 ± 5.7 |
| 7 days | 725.9 ± 106.5 | 283.2 ± 73.9 | 1.14 ± 0.26 | −0.99 ± 0.16 | 13.5 ± 3.1 | |
| 14 days | 590.1 ± 193.4 | 386.4 ± 113.1 | 0.82 ± 0.36 | −1.12 ± 0.17 | 9.7 ± 4.2 | |
| 250 °C-8h | 1 day | 505.5 ± 232.9 | 309.1 ± 17.7 | 1.79 ± 0.29 | −1.02 ± 0.02 | 21.1 ± 3.5 |
| 7 days | 516.5 ± 233.1 | 307.9 ± 22.5 | 1.58 ± 0.61 | −0.99 ± 0.01 | 18.6 ± 7.2 | |
| 14 days | 482.5 ± 61.1 | 433.7 ± 201.7 | 0.73 ± 0.25 | −1.14 ± 0.24 | 8.6 ± 2.9 | |
| 250 °C-16h | 1 day | 455.9 ± 23.9 | 468.8 ± 285.8 | 1.98 ± 1.06 | −1.06 ± 0.05 | 21.3 ± 2.5 |
| 7 days | 726.1 ± 54.6 | 257.5 ± 123.5 | 0.78 ± 0.15 | −0.98 ± 0.02 | 9.2 ± 2.6 | |
| 14 days | 567.4 ± 69.3 | 277.1 ± 59.8 | 0.67 ± 0.16 | −0.97 ± 0.04 | 7.9 ± 1.9 | |
| 350 °C-2 h | 1 day | 383.5 ± 133.1 | 334.1 ± 157.7 | 1.47 ± 0.61 | −1.17 ± 0.26 | 17.3 ± 7.1 |
| 7 days | 592.2 ± 157.5 | 282.2 ± 67.9 | 1.14 ± 0.24 | −0.98 ± 0.02 | 13.4 ± 4.7 | |
| 14 days | 352.7 ± 59.2 | 357.6 ± 78.6 | 0.67 ± 0.22 | −1.29 ± 0.15 | 7.9 ± 2.3 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhou, Z.; Chen, X.; Hu, X.; Li, S.; Lv, M.; Xie, Y.; Yao, H.; Wang, H.; Bai, X. Influence of Heat Treatment on Microstructure, Mechanical Property, and Corrosion Behavior of Cold-Sprayed Zn Coating on Mg Alloy Substrate. Materials 2022, 15, 6721. https://doi.org/10.3390/ma15196721
Zhou Z, Chen X, Hu X, Li S, Lv M, Xie Y, Yao H, Wang H, Bai X. Influence of Heat Treatment on Microstructure, Mechanical Property, and Corrosion Behavior of Cold-Sprayed Zn Coating on Mg Alloy Substrate. Materials. 2022; 15(19):6721. https://doi.org/10.3390/ma15196721
Chicago/Turabian StyleZhou, Zhenpeng, Xiao Chen, Xiaozhen Hu, Sheng Li, Menglong Lv, Yiting Xie, Hailong Yao, Hongtao Wang, and Xiaobo Bai. 2022. "Influence of Heat Treatment on Microstructure, Mechanical Property, and Corrosion Behavior of Cold-Sprayed Zn Coating on Mg Alloy Substrate" Materials 15, no. 19: 6721. https://doi.org/10.3390/ma15196721
APA StyleZhou, Z., Chen, X., Hu, X., Li, S., Lv, M., Xie, Y., Yao, H., Wang, H., & Bai, X. (2022). Influence of Heat Treatment on Microstructure, Mechanical Property, and Corrosion Behavior of Cold-Sprayed Zn Coating on Mg Alloy Substrate. Materials, 15(19), 6721. https://doi.org/10.3390/ma15196721








