Tuning the Hydration Acceleration Efficiency of Calcium Carbonate by Pre-Seeding with Calcium Silicate Hydrate
Abstract
:1. Introduction
2. Materials and Methods
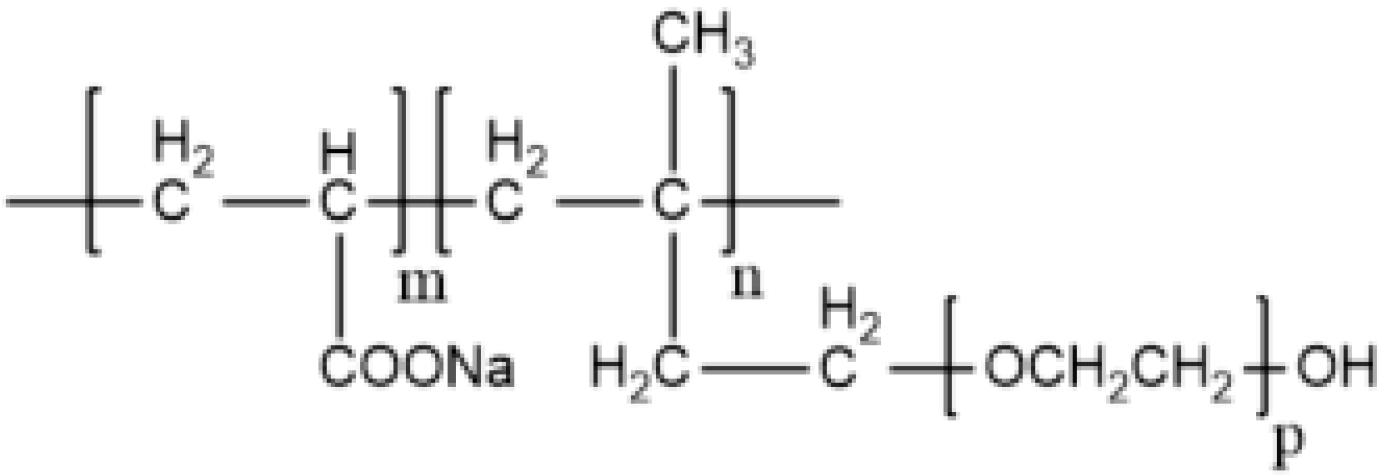
2.1. Materials
2.2. Preparation and Characterization of CC–CSH
2.2.1. Preparation of CC–CSH
2.2.2. Characterization of CC–CSH
2.3. Preparation of Mortar and Paste Samples
2.3.1. Mortar Preparation
2.3.2. Paste Preparation
2.4. Testing and Characterization of the Mortar and Paste Samples
2.4.1. Mortar Strength Tests
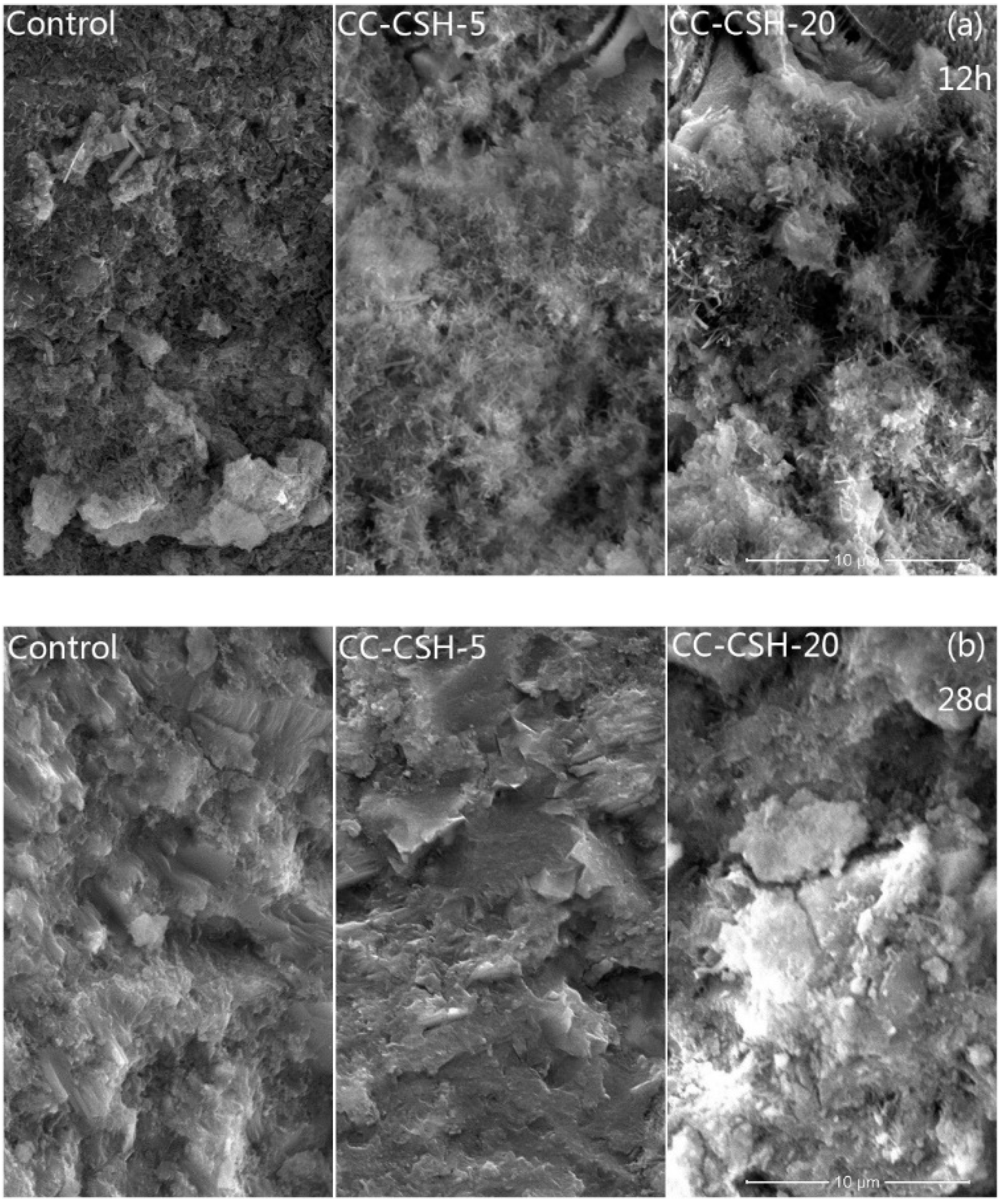
2.4.2. Microscopic Observations
2.4.3. Isothermal Calorimetry (IC) Tests
3. Results
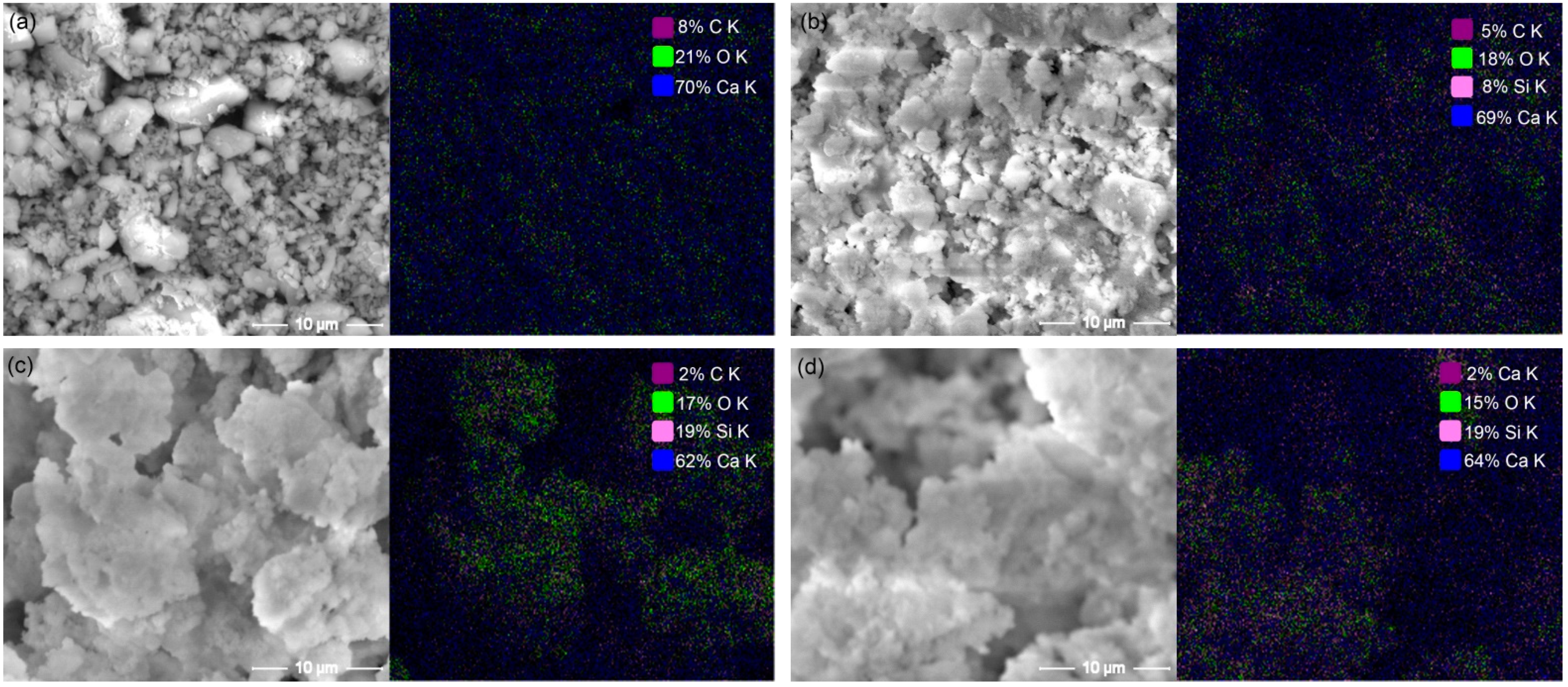
3.1. Characteristics of CC–CSH Particles
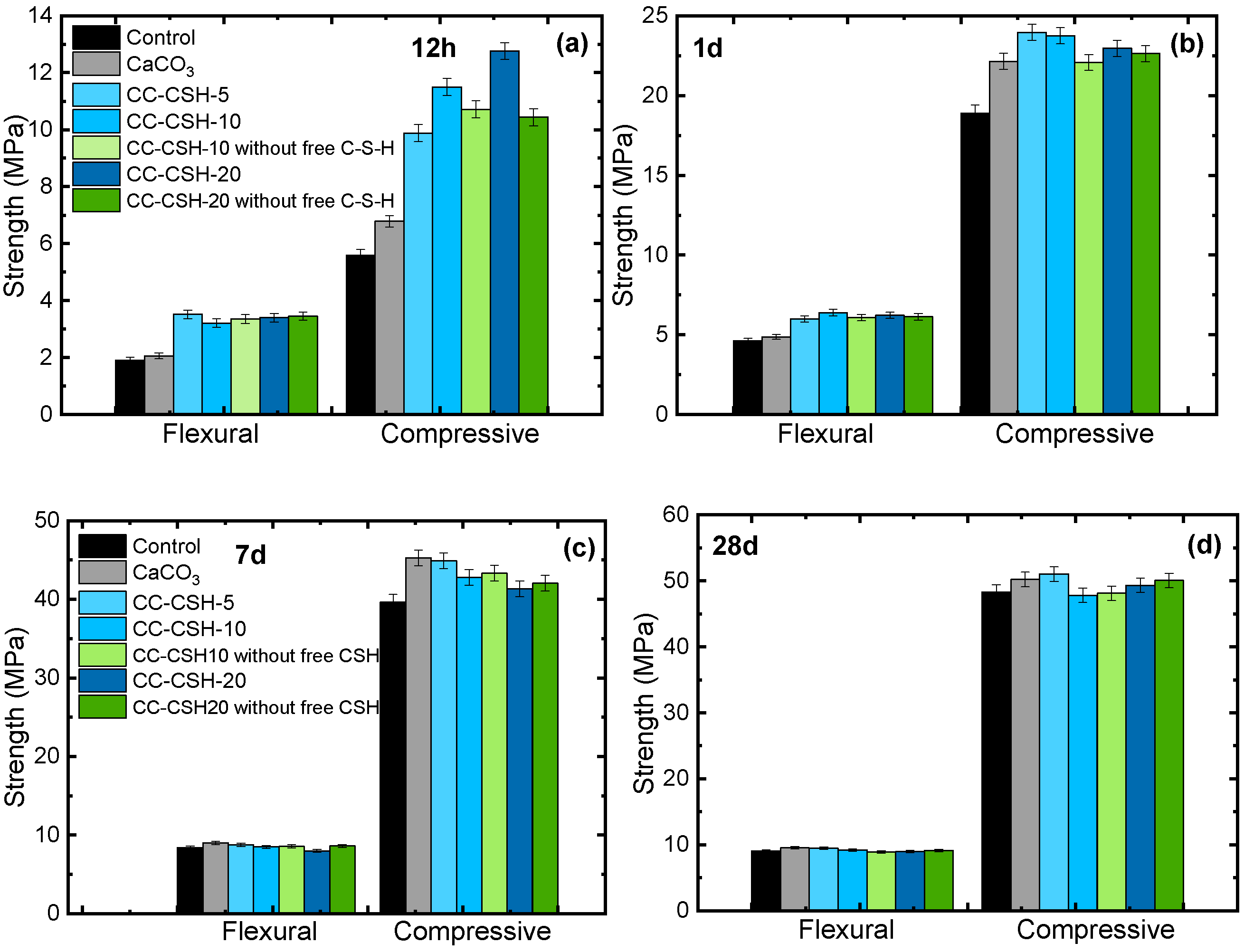
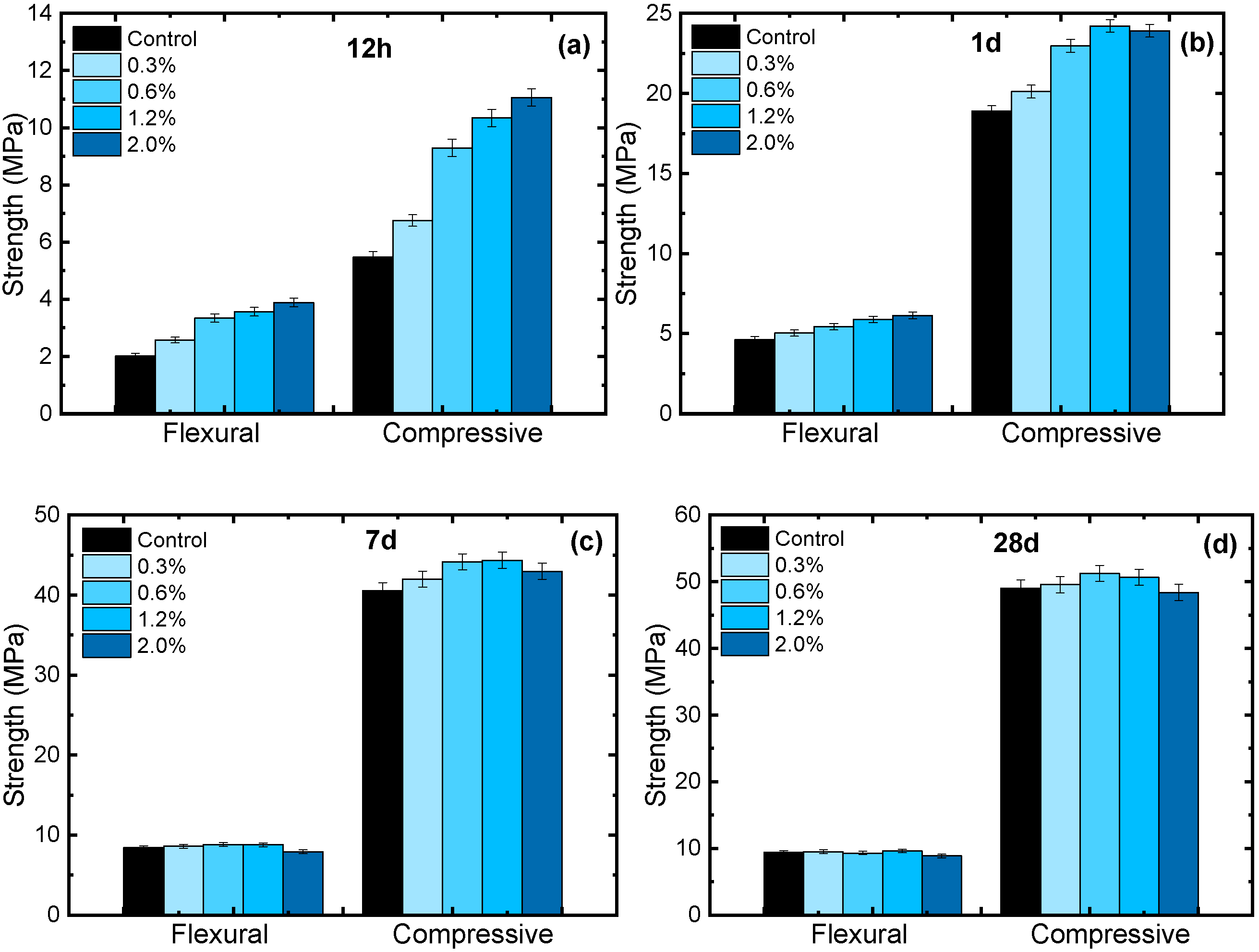
3.2. Effect of CC–CSH on Mortar Strength
3.3. Effect of CC–CSH on Cement Hydration
4. Conclusions
- (1)
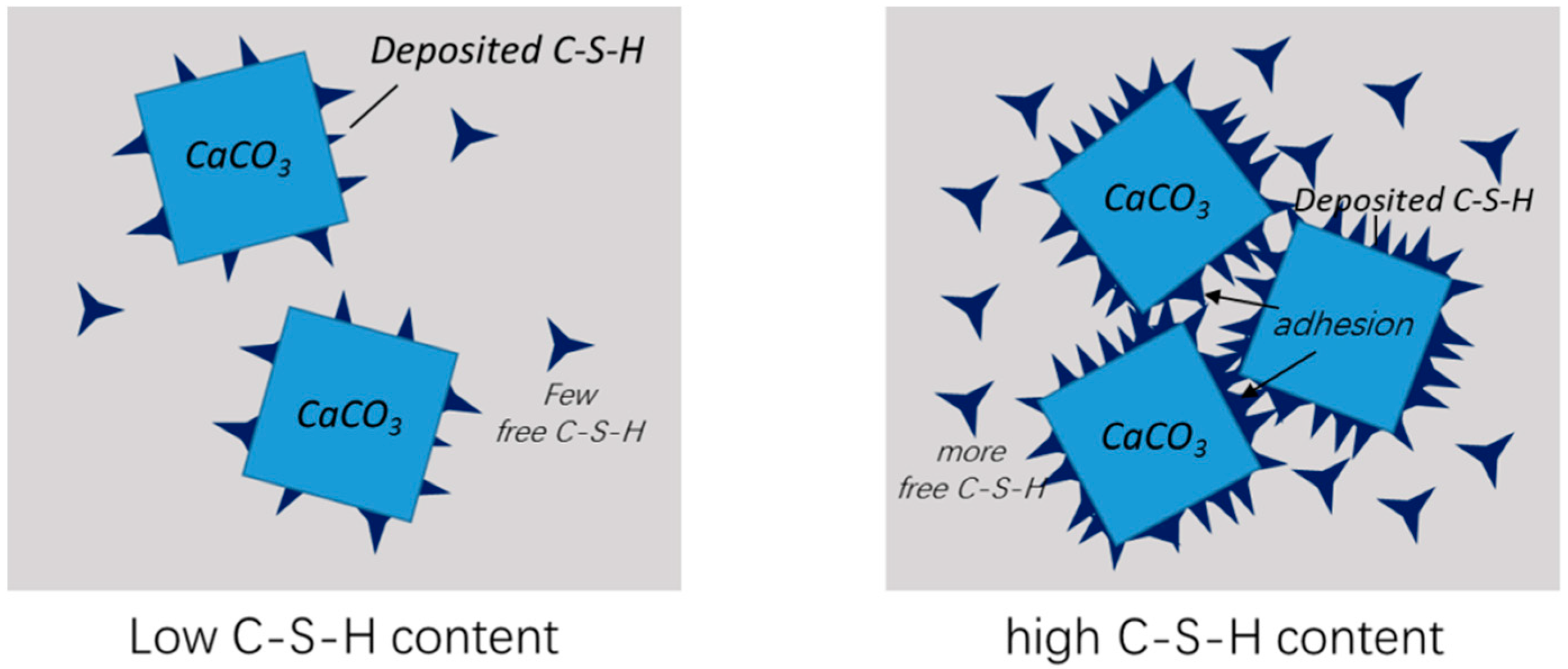
- C–S–H nanogels can be artificially grown on CaCO3 particles, achieved through the seeding effect of CaCO3. The amount of nanogels on the CaCO3 surface is restricted by the available surface. Excessive nanogels would exist in free form in solution. The maximum content of C–S–H on the surface is reached at the modification degree of 5–10% m (CaCO3).
- (2)
- The C–S–H nanogels on CaCO3 surface alters its interfacial properties and exhibit a tuning effect on the cement hydration. The effect strengthens with higher surface C–S–H coverage, and it was halted once surface coverage reached the maximum.
- (3)
- Pre-seeding CaCO3 with C–S–H can considerably enhance its strengthening effect on cement in early ages (<1 d). The enhancement is due to the better seeding effect on C–S–H. However, excessive C–S–H modification would weaken CC–CSH’s effect on mechanical properties at later ages (>1 d), which is caused by excessively fast hydration product growth and particle agglomeration. Considering strength at all ages, CC–CSH-5 showed the most balanced performance among all the CC–CSH samples. It can be inferred that CaCO3 and C–S–H exhibited synergistic effects at the modification degree of CC–CSH-5. According to the strength tests on dosage dependence, the optimal dosage of CC–CSH-5 is 0.6–1.2%.
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Mehta, P.K.; Monteiro, P.J.M. Concrete: Microstructure, Properties, and Materials; McGraw-Hill: New York, NY, USA, 2006. [Google Scholar]
- Chuah, S.; Pan, Z.; Sanjayan, J.G.; Wang, C.M.; Duan, W.H. Nano reinforced cement and concrete composites and new perspective from graphene oxide. Constr. Build. Mater. 2014, 73, 113–124. [Google Scholar]
- Scrivener, K.L.; John, V.M.; Gartner, E.M.; Environment, U.N. Eco-efficient cements: Potential economically viable solutions for a low-CO2 cement-based materials industry. Cem. Concr. Res. 2018, 114, 2–26. [Google Scholar]
- Silvestre, J.; Silvestre, N.; de Brito, J. Review on concrete nanotechnology. Eur. J. Environ. Civ. Eng. 2015, 20, 455–485. [Google Scholar]
- Sanchez, F.; Sobolev, K. Nanotechnology in concrete—A review. Constr. Build. Mater. 2010, 24, 2060–2071. [Google Scholar]
- Wang, J.; Qian, C.; Qu, J.; Guo, J. Effect of lithium salt and nano nucleating agent on early hydration of cement based materials. Constr. Build. Mater. 2018, 174, 24–29. [Google Scholar]
- Yang, H.; Monasterio, M.; Zheng, D.; Cui, H.; Tang, W.; Bao, X.; Chen, X. Effects of nano silica on the properties of cement-based materials: A comprehensive review. Constr. Build. Mater. 2021, 282, 122715. [Google Scholar]
- Singh, L.P.; Bhattacharyya, S.K.; Shah, S.P.; Mishra, G.; Sharma, U. Studies on early stage hydration of tricalcium silicate incorporating silica nanoparticles: Part II. Constr. Build. Mater. 2016, 102, 943–949. [Google Scholar]
- Land, G.; Stephan, D. Preparation and Application of Nanoscaled C-S-H as an Accelerator for Cement Hydration. In Nanotechnology in Construction; Sobolev, K., Shah, S.P., Eds.; Spinger: Cham, Switzerland, 2015; pp. 117–122. [Google Scholar]
- Camiletti, J.; Soliman, A.M.; Nehdi, M.L. Effect of nano-calcium carbonate on early-age properties of ultra-high-performance concrete. Magaz. Concr. Res. 2013, 65, 297–307. [Google Scholar]
- Liu, H.; Li, Q.; Su, D.; Yue, G.; Wang, L. Study on the Influence of Nanosilica Sol on the Hydration Process of Different Kinds of Cement and Mortar Properties. Materials 2021, 14, 3653. [Google Scholar]
- Kong, D.; He, G.; Pan, H.; Weng, Y.; Du, N.; Sheng, J. Influences and Mechanisms of Nano-C-S-H Gel Addition on Fresh Properties of the Cement-Based Materials with Sucrose as Retarder. Materials 2020, 13, 2345. [Google Scholar]
- Cao, M.; Ming, X.; He, K.; Li, L.; Shen, S. Effect of Macro-, Micro- and Nano-Calcium Carbonate on Properties of Cementitious Composites, A Review. Materials 2019, 12, 781. [Google Scholar]
- Lv, S.; Liu, J.; Sun, T.; Ma, Y.; Zhou, Q. Effect of GO nanosheets on shapes of cement hydration crystals and their formation process. Constr. Build. Mater. 2014, 64, 231–239. [Google Scholar]
- Rong, Z.; Zhao, M.; Wang, Y. Effects of Modified Nano-SiO2 Particles on Properties of High-Performance Cement-Based Composites. Materials 2020, 13, 646. [Google Scholar]
- Fan, Y.F.; Zhang, S.Y.; Wang, Q.; Shah, S.P. The effects of nano-calcined kaolinite clay on cement mortar exposed to acid deposits. Constr. Build. Mater. 2016, 102, 486–495. [Google Scholar]
- Zhang, M.H.; Li, H. Pore structure and chloride permeability of concrete containing nano-particles for pavement. Constr. Build. Mater. 2011, 25, 608–616. [Google Scholar]
- Mohseni, E.; Naseri, F.; Amjadi, R.; Khotbehsara, M.M.; Ranjbar, M.M. Microstructure and durability properties of cement mortars containing nano-TiO2 and rice husk ash. Constr. Build. Mater. 2016, 114, 656–664. [Google Scholar]
- Jee, H.; Park, J.; Zalnezhad, E.; Jeong, K.; Woo, S.M.; Seok, S.; Bae, S. Characterization of Titanium Nanotube Reinforced Cementitious Composites: Mechanical Properties, Microstructure, and Hydration. Materials 2019, 12, 1617. [Google Scholar]
- Li, W.-W.; Ji, W.-M.; Wang, Y.-C.; Liu, Y.; Shen, R.-X.; Xing, F. Investigation on the Mechanical Properties of a Cement-Based Material Containing Carbon Nanotube under Drying and Freeze-Thaw Conditions. Materials 2015, 8, 8780–8792. [Google Scholar]
- Han, B.; Sun, S.; Ding, S.; Zhang, L.; Yu, X.; Ou, J. Review of nanocarbon-engineered multifunctional cementitious composites. Compos. Part A Appl. Sci. Manuf. 2015, 70, 69–81. [Google Scholar]
- Paul, A.; Rashidi, M.; Kim, J.Y.; Jacobs, L.J.; Kurtis, K.E. The impact of sulfate-and sulfide-bearing sand on delayed ettringite formation. Cem. Concr. Compos. 2022, 125, 104323. [Google Scholar]
- Kabir, H.; Hooton, R.D.; Popoff, N.J. Evaluation of cement soundness using the ASTM C151 autoclave expansion test. Cem. Concr. Res. 2020, 136, 106159. [Google Scholar] [CrossRef]
- Li, Z.; Thomas, R.J.; Peethamparan, S. Alkali-silica reactivity of alkali-activated concrete subjected to ASTM C 1293 and 1567 alkali-silica reactivity tests. Cem. Concr. Res. 2019, 123, 105796. [Google Scholar] [CrossRef]
- Ghafari, E.; Costa, H.; Julio, E.; Portugal, A.; Duraes, L. The effect of nanosilica addition on flowability, strength and transport properties of ultra high performance concrete. Mater. Design 2014, 59, 1–9. [Google Scholar] [CrossRef]
- Lin, K.; Wu, C.; Chang, J. Advances in synthesis of calcium phosphate crystals with controlled size and shape. Acta Biomater. 2014, 10, 4071–4102. [Google Scholar] [PubMed]
- Li, X.; Ma, Y.; Shen, X.; Zhong, Y.; Li, Y. Study of Hydration and Microstructure of Mortar Containing Coral Sand Powder Blended with SCMs. Materials 2020, 13, 4248. [Google Scholar] [CrossRef]
- Bentz, D.P.; Ardani, A.; Barrett, T.; Jones, S.Z.; Lootens, D.; Peltz, M.A.; Sato, T.; Stutzman, P.E.; Tanesi, J.; Weiss, W.J. Multi-scale investigation of the performance of limestone in concrete. Constr. Build. Mater. 2015, 75, 1–10. [Google Scholar] [CrossRef]
- GB/T 175-2007Common Portland Cement; Administration of Quality Supervision: Beijing, China, 2017.
- GB/T 17671-1999Methods of Testing Cements–Determination of Strength; Administration of Quality Supervision: Beijing, China, 1999.
- Lesko, S.; Lesniewska, E.; Nonat, A.; Mutin, J.C.; Goudonnet, J.P. Investigation by atomic force microscopy of forces at the origin of cement cohesion. Ultramicroscopy 2001, 86, 11–21. [Google Scholar] [CrossRef]
- Aitcin, P.C.; Flatt, R.J. Science and Techlology of Concrete Admixtures; WoodHead Publishing: Amsterdam, The Netherlands, 2016. [Google Scholar]
- Aydin, S.; Baradan, B. Mechanical and microstructural properties of heat cured alkali-activated slag mortars. Mater. Design 2012, 35, 374–383. [Google Scholar] [CrossRef]
- Singh, L.P.; Karade, S.R.; Bhattacharyya, S.K.; Yousuf, M.M.; Ahalawat, S. Beneficial role of nanosilica in cement based materials—A review. Constr. Build. Mater. 2013, 47, 1069–1077. [Google Scholar]
- Nicoleau, L. Accelerated growth of calcium silicate hydrates: Experiments and simulations. Cem. Concr. Res. 2011, 41, 1339–1348. [Google Scholar] [CrossRef]
- Nicoleau, L.; Gädt, T.; Chitu, L.; Maier, G.; Paris, O. Oriented aggregation of calcium silicate hydrate platelets by the use of comb-like copolymers. Soft Matter 2013, 9, 4864. [Google Scholar] [CrossRef] [Green Version]

| Compound | Content (%) |
|---|---|
| SiO2 | 21.02 |
| Al2O3 | 4.50 |
| CaO | 63.66 |
| MgO | 1.65 |
| Fe2O3 | 2.90 |
| SO3 | 3.64 |
| K2O | 0.71 |
| Na2O | 0.18 |
| Free lime | 1.22 |
| Total | 99.48 |
| Loss on Ignition | 1.3% |
| Surface area | 355 m2/kg |
| Sample Name | CaCO3 (g) | PCE (Solid, g) | Ca(NO3)2 4H2O (g) | Na2SiO3 9H2O (g) | C–S–H(%) * | Ca/Si | Solid Content (%) |
|---|---|---|---|---|---|---|---|
| CaCO3 | 100.0 | 5.00 | -- | -- | -- | -- | 7.19 |
| CC–CSH-5 | 10.17 | 12.24 | 5.0 | 1.0 | 7.46 | ||
| CC–CSH-10 | 20.34 | 24.48 | 10.0 | 7.92 | |||
| CC–CSH-20 | 40.69 | 48.97 | 20.0 | 8.71 |
| Test | Sample | Cement (g) | Sample Dosage * | |
|---|---|---|---|---|
| % | (g) | |||
| Samples on fixed dosage | Control | 600 ± 0.1 | None | None |
| CaCO3 | 592.8 ± 0.1 | 1.2 | 7.20 ± 0.01 | |
| CC–CSH-5 | 592.8 ± 0.1 | 1.2 | 7.20 ± 0.01 | |
| CC–CSH-10 | 592.8 ± 0.1 | 1.2 | 7.20 ± 0.01 | |
| CC–CSH-20 | 592.8 ± 0.1 | 1.2 | 7.20 ± 0.01 | |
| Dosage dependence of the favored sample | Control | 600 ± 0.1 | None | None |
| CC–CSH-5 | 598.2 ± 0.1 | 0.3 | 1.80 ± 0.01 | |
| CC–CSH-5 | 596.4 ± 0.1 | 0.6 | 3.60 ± 0.01 | |
| CC–CSH-5 | 592.8 ± 0.1 | 1.2 | 7.20 ± 0.01 | |
| CC–CSH-5 | 588.0 ± 0.1 | 2.0 | 12.00 ± 0.01 | |
| Sample Name | Size * (103 nm) | Free Nano C–S–H | |
|---|---|---|---|
| Content ** (%) | Size (nm) | ||
| CaCO3 | 1.59 | -- | -- |
| CC–CSH-5 | 1.62 | 4.3 | 72.1 |
| CC–CSH-10 | 2.19 | 22.7 | 69.8 |
| CC–CSH-20 | 3.11 | 53.1 | 87.3 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yan, H.; Zhou, D.; Yang, Y.; Shu, X.; Yu, C.; Ran, Q. Tuning the Hydration Acceleration Efficiency of Calcium Carbonate by Pre-Seeding with Calcium Silicate Hydrate. Materials 2022, 15, 6726. https://doi.org/10.3390/ma15196726
Yan H, Zhou D, Yang Y, Shu X, Yu C, Ran Q. Tuning the Hydration Acceleration Efficiency of Calcium Carbonate by Pre-Seeding with Calcium Silicate Hydrate. Materials. 2022; 15(19):6726. https://doi.org/10.3390/ma15196726
Chicago/Turabian StyleYan, Han, Dongliang Zhou, Yong Yang, Xin Shu, Cheng Yu, and Qianping Ran. 2022. "Tuning the Hydration Acceleration Efficiency of Calcium Carbonate by Pre-Seeding with Calcium Silicate Hydrate" Materials 15, no. 19: 6726. https://doi.org/10.3390/ma15196726







