Dispersion and Aggregation Fate of Individual and Co-Existing Metal Nanoparticles under Environmental Aqueous Suspension Conditions
Abstract
:1. Introduction
2. Materials and Methods
3. Results and Discussion
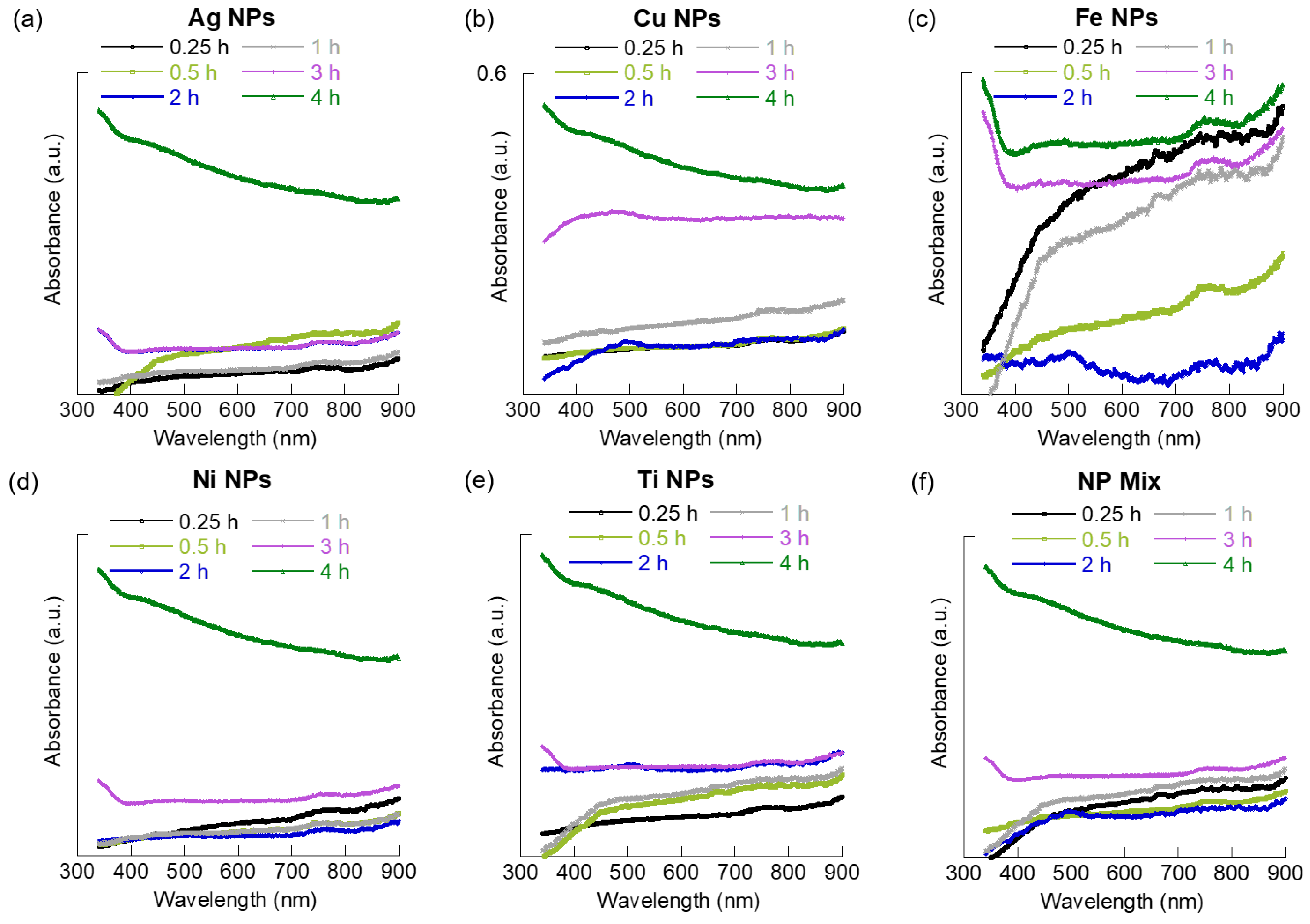
3.1. Suspension Time versus Dispersion Quality
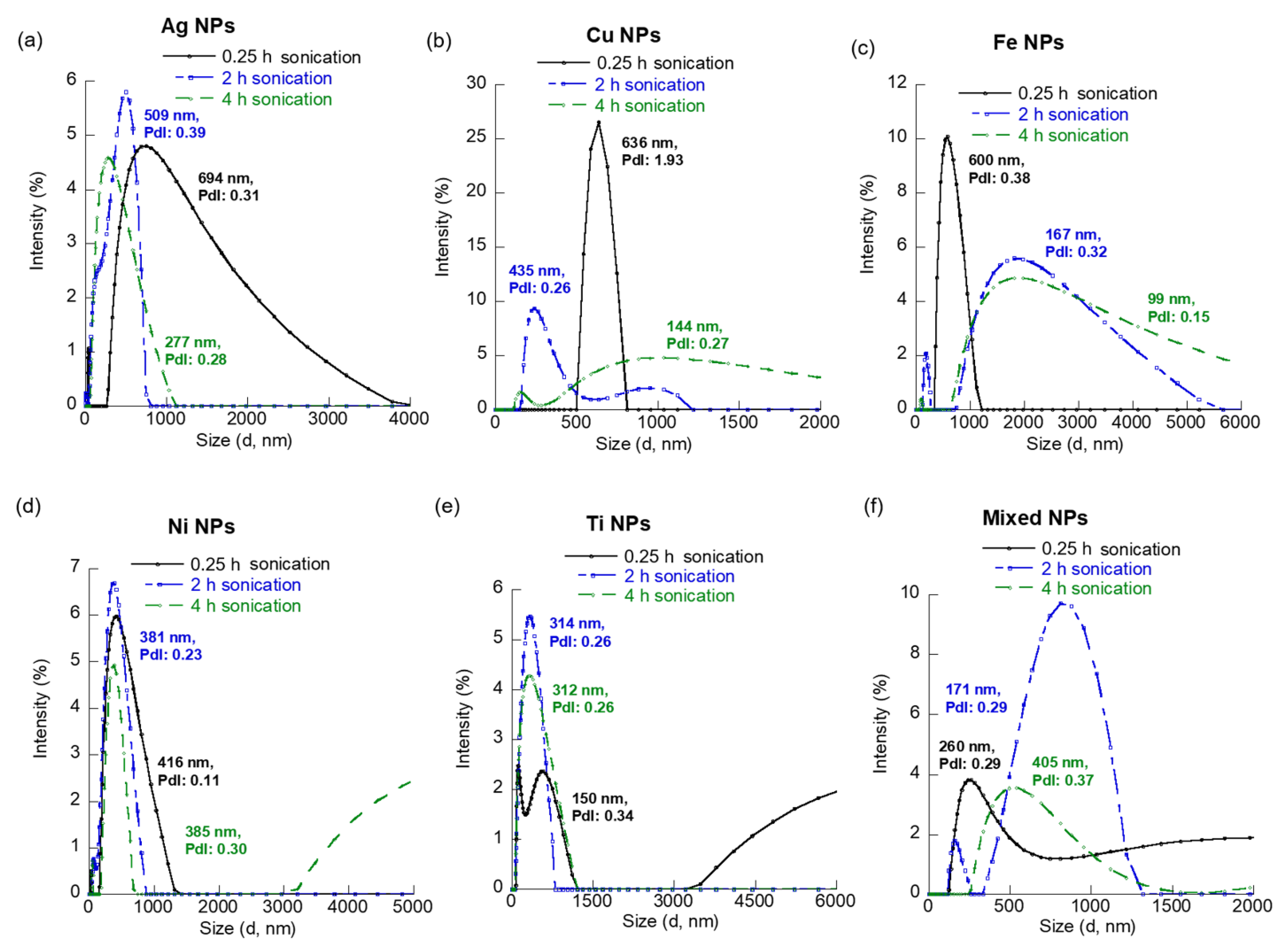
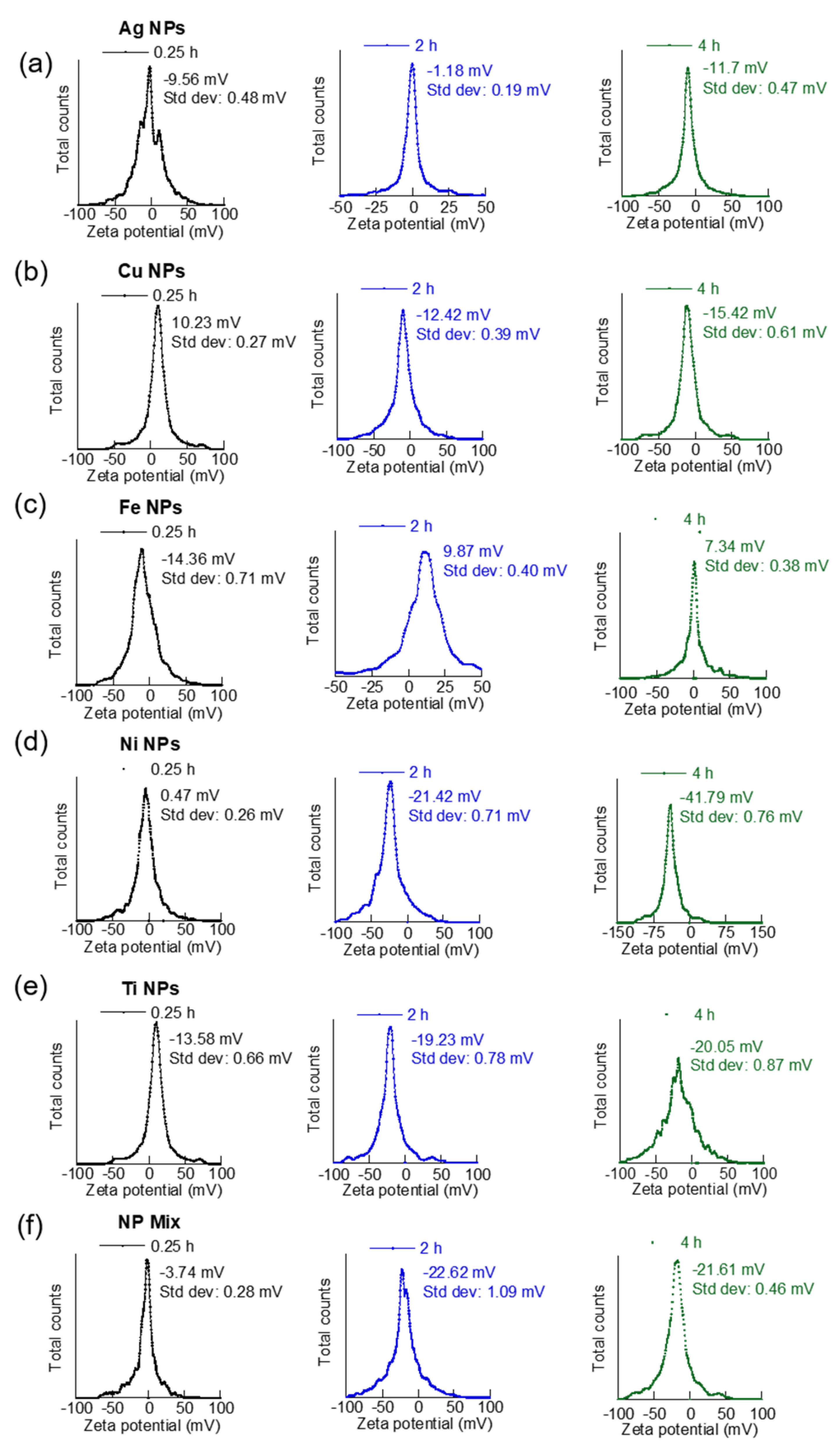
3.2. Suspension Time versus MNPs Aggregation and Dispersion
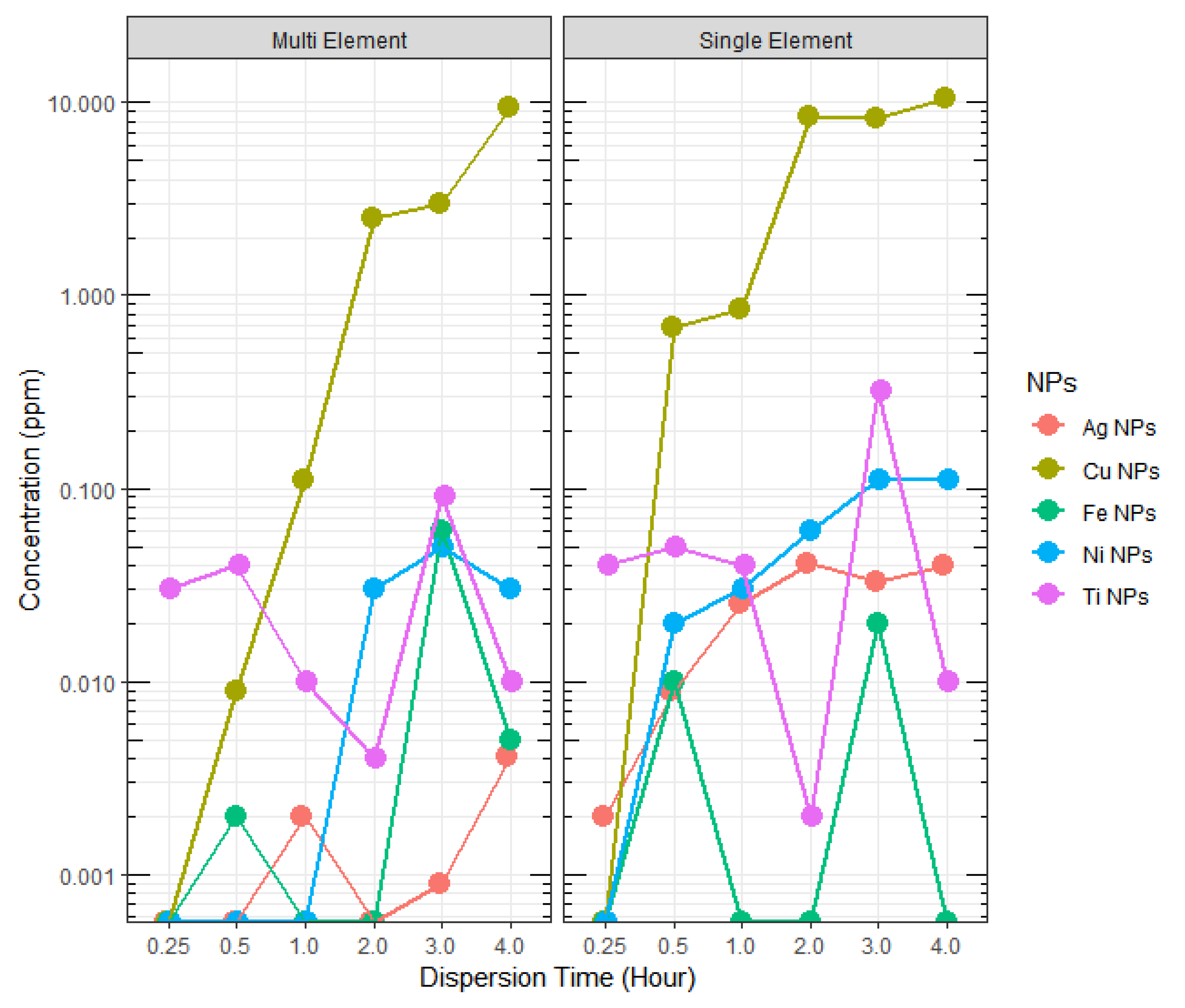
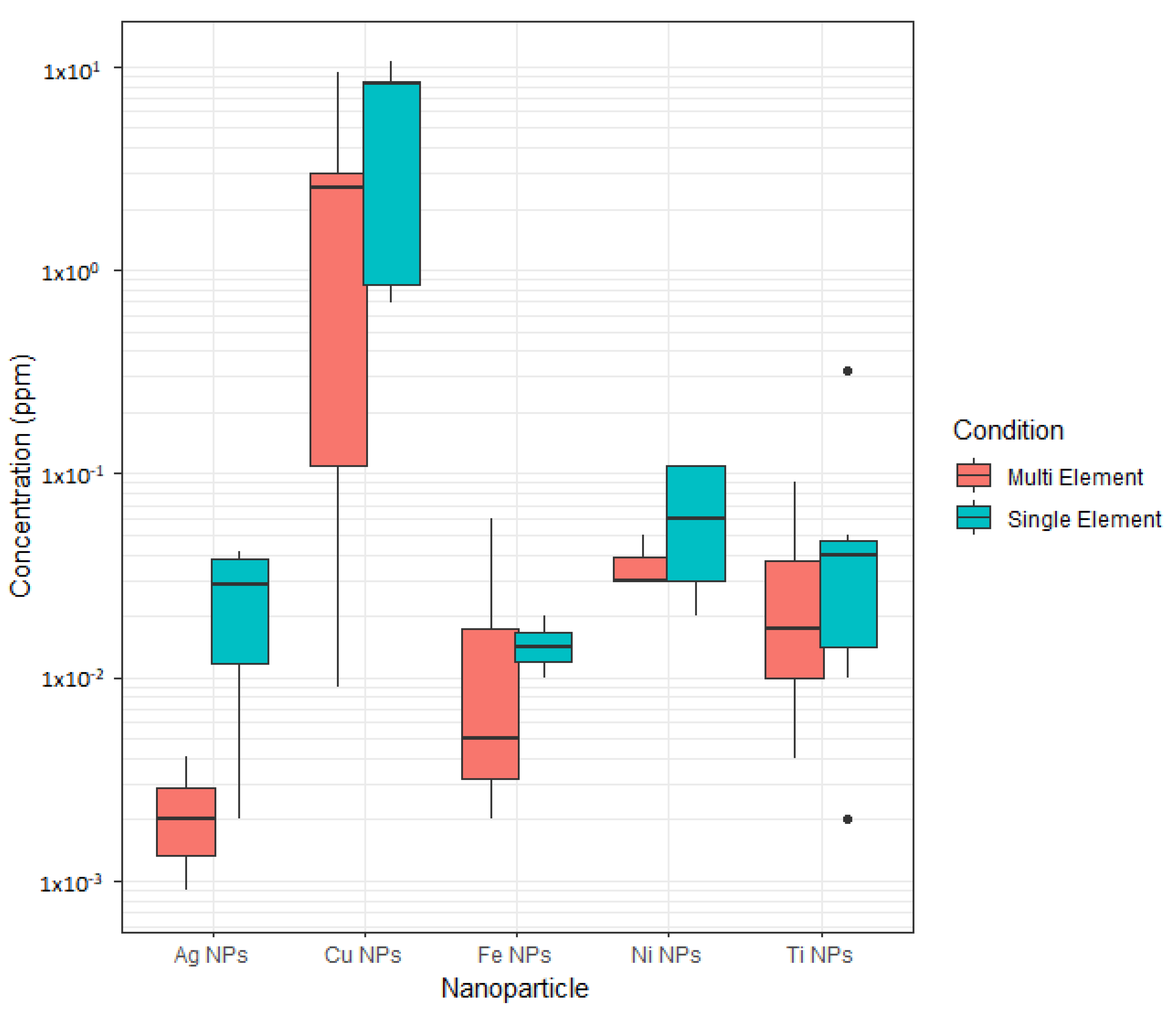
3.3. Sonication Time versus Dispersion Concentration
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- Markets, M. Metal Nanoparticles Market by metal (Platinum, Gold, Silver, Iron, Titanium, Copper, Nickel), End-use industry (Pharmaceutical & healthcare, Electrical & electronics, Catalyst, Personal care & cosmetics), and Region-Global Forecast to 2022. Mark. Res. Rep 2018, 4489142, 286. [Google Scholar]
- Loosli, F.; Wang, J.; Rothenberg, S.; Bizimis, M.; Winkler, C.; Borovinskaya, O.; Flamigni, L.; Baalousha, M. Sewage spills are a major source of titanium dioxide engineered (nano)-particle release into the environment. Environ. Sci. Nano 2019, 6, 763–777. [Google Scholar] [CrossRef] [PubMed]
- Wu, S.; Zhang, S.; Gong, Y.; Shi, L.; Zhou, B. Identification and quantification of titanium nanoparticles in surface water: A case study in Lake Taihu, China. J. Hazard. Mater. 2020, 382, 121045. [Google Scholar] [CrossRef] [PubMed]
- Quik, J.T.K.; Velzeboer, I.; Wouterse, M.; Koelmans, A.A.; van de Meent, D. Heteroaggregation and sedimentation rates for nanomaterials in natural waters. Water Res. 2014, 48, 269–279. [Google Scholar] [CrossRef]
- Bathi, J.R.; Moazeni, F.; Upadhyayula, V.K.K.; Chowdhury, I.; Palchoudhury, S.; Potts, G.E.; Gadhamshetty, V. Behavior of engineered nanoparticles in aquatic environmental samples: Current status and challenges. Sci. Total Environ. 2021, 793, 148560. [Google Scholar] [CrossRef]
- Tsai, W.-B.; Kao, J.-Y.; Wu, T.-M.; Cheng, W.-T. Dispersion of titanium oxide nanoparticles in aqueous solution with anionic stabilizer via ultrasonic wave. J. Nanopart. 2016, 2016, 6539581. [Google Scholar] [CrossRef]
- Peters, R.; van Bemmel, G.; Milani, N.; Hertog, G.; Undas, A.K.; Lee, M.; Bouwmeester, H. Detection of nanoparticles in Dutch surface waters. Sci. Total Environ. 2017, 621, 210–218. [Google Scholar] [CrossRef]
- Wang, J.; Nabi, M.M.; Mohanty, S.K.; Afrooz, A.R.M.N.; Cantando, E.; Aich, N.; Baalousha, M. Detection and quantification of engineered particles in urban runoff. Chemosphere 2020, 248, 126070. [Google Scholar] [CrossRef]
- Fang, J.; Shijirbaatar, A.; Lin, D.H.; Wang, D.J.; Shen, B.; Sun, P.D.; Zhou, Z.Q. Stability of co-existing ZnO and TiO2 nanomaterials in natural water: Aggregation and sedimentation mechanisms. Chemosphere 2017, 184, 1125–1133. [Google Scholar] [CrossRef]
- Tong, T.; Fang, K.; Thomas, S.A.; Kelly, J.J.; Gray, K.A.; Gaillard, J.-F. Chemical Interactions between Nano-ZnO and Nano-TiO2 in a Natural Aqueous Medium. Environ. Sci. Technol. 2014, 48, 7924–7932. [Google Scholar] [CrossRef]
- Lv, X.; Tao, J.; Chen, B.; Zhu, X. Roles of temperature and flow velocity on the mobility of nano-sized titanium dioxide in natural waters. Sci. Total Environ. 2016, 565, 849–856. [Google Scholar] [CrossRef] [PubMed]
- Heard, S.B.; Gienapp, C.B.; Lemire, J.F.; Heard, K.S. Transverse mixing of transported material in simple and complex stream reaches. Hydrobiologia 2001, 464, 207–218. [Google Scholar] [CrossRef]
- Saharia, A.M.; Zhu, Z.; Aich, N.; Baalousha, M.; Atkinson, J.F. Modeling the transport of titanium dioxide nanomaterials from combined sewer overflows in an urban river. Sci. Total Environ. 2019, 696, 133904. [Google Scholar] [CrossRef]
- Czernuszenko, W. Dispersion of pollutants in rivers. Hydrol. Sci. J. 1987, 32, 59–67. [Google Scholar] [CrossRef]
- Geertsen, V.; Tabarant, M.; Spalla, O. Behavior and Determination of Titanium Dioxide Nanoparticles in Nitric Acid and River Water by ICP Spectrometry. Anal. Chem. 2014, 86, 3453–3460. [Google Scholar] [CrossRef] [PubMed]
- Taurozzi, J.S.; Hackley, V.A.; Wiesner, M.R. Ultrasonic dispersion of nanoparticles for environmental, health and safety assessment—issues and recommendations. Nanotoxicology 2011, 5, 711–729. [Google Scholar] [CrossRef]
- Council, N.R. Challenges in Characterizing Small Particles: Exploring Particles from the Nano- to Microscale: A Workshop Summary; Masciangioli, J., Alper, J., Eds.; The National Academies Press: Washington, DC, USA, 2012; p. 100. [Google Scholar]
- Iqbal, J.; Abbasi, B.A.; Yaseen, T.; Zahra, S.A.; Shahbaz, A.; Shah, S.A.; Uddin, S.; Ma, X.; Raouf, B.; Kanwal, S.; et al. Green synthesis of zinc oxide nanoparticles using Elaeagnus angustifolia L. leaf extracts and their multiple in vitro biological applications. Sci. Rep. 2021, 11, 20988. [Google Scholar] [CrossRef]
- Shah, I.H.; Ashraf, M.; Sabir, I.A.; Manzoor, M.A.; Malik, M.S.; Gulzar, S.; Ashraf, F.; Iqbal, J.; Niu, Q.; Zhang, Y. Green synthesis and Characterization of Copper oxide nanoparticles using Calotropis procera leaf extract and their different biological potentials. J. Mol. Struct. 2022, 1259, 132696. [Google Scholar] [CrossRef]
- Park, C.; Park, H.; You, J.; Na, S.; Jang, K. Highly Sensitive and Real-Time Detection of Zinc Oxide Nanoparticles Using Quartz Crystal Microbalance via DNA Induced Conjugation. Materials 2022, 15, 6113. [Google Scholar] [CrossRef]
- Tareq, S.M.; Boutchuen, A.; Roy, S.; Zimmerman, D.; Jur, G.; Bathi, J.R.; Palchoudhury, S. A Dynamic Light Scattering Approach for Detection of Nanomaterials in Tennessee River. Water Resour. Res. 2021, 57, e2020WR028687. [Google Scholar] [CrossRef]
- Wilschefski, S.C.; Baxter, M.R. Inductively Coupled Plasma Mass Spectrometry: Introduction to Analytical Aspects. Clin. Biochem. Rev. 2019, 40, 115–133. [Google Scholar] [CrossRef] [PubMed]
- Choi, S.; Johnston, M.; Wang, G.-S.; Huang, C.P. A seasonal observation on the distribution of engineered nanoparticles in municipal wastewater treatment systems exemplified by TiO2 and ZnO. Sci. Total Environ. 2018, 625, 1321–1329. [Google Scholar] [CrossRef] [PubMed]
- Navarro, J.R.; Werts, M.H. Resonant light scattering spectroscopy of gold, silver and gold-silver alloy nanoparticles and optical detection in microfluidic channels. Analyst 2013, 138, 583–592. [Google Scholar] [CrossRef]
- Baalousha, M.; Lead, J.R. Nanoparticle dispersity in toxicology. Nat. Nanotechnol. 2013, 8, 308–309. [Google Scholar] [CrossRef] [PubMed]
- Hofmann, A.; Thierbach, S.; Semisch, A.; Hartwig, A.; Taupitz, M.; Rühl, E.; Graf, C. Highly monodisperse water-dispersable iron oxide nanoparticles for biomedical applications. J. Mater. Chem. 2010, 20, 7842–7853. [Google Scholar] [CrossRef]
- Badawy, A.M.E.; Luxton, T.P.; Silva, R.G.; Scheckel, K.G.; Suidan, M.T.; Tolaymat, T.M. Impact of Environmental Conditions (pH, Ionic Strength, and Electrolyte Type) on the Surface Charge and Aggregation of Silver Nanoparticles Suspensions. Environ. Sci. Technol. 2010, 44, 1260–1266. [Google Scholar] [CrossRef]
- Hotze, E.; Phenrat, E.; Lowry, G. Nanoparticle Aggregation: Challenges to Understanding Transport and Reactivity in the Environment. J. Environ. Qaulity 2010, 39, 1909–1924. [Google Scholar] [CrossRef] [PubMed] [Green Version]

| NP | Particle Diameter (nm) | Surface Area (m2/g) | True Density (g/cm3) | Purity (Trace Metal Basis) | MNPs Concentration in Single Element Solutions (mg/L) |
|---|---|---|---|---|---|
| Ag | 20–30 | 20 | 10.5 | 99.95% | 2 |
| Cu | 60–80 | 6–8 | 8.9 | 99.90% | 2 |
| Fe | 40–60 | 6–13 | 7.9 | 99.70% | 4 |
| Ni | 40–60 | 10–15 | 8.9 | 99.70% | 6 |
| Ti | 40–60 | 20 | 4.5 | 99.90% | 2 |
| NP | Sonication Time (Hours) | Average Diameter (nm) | PdI | ζ-Potential (mV) | U (μmcm/(Vs)) |
|---|---|---|---|---|---|
| Ag | 0.25 | 557.7 | 0.31 | −9.56 | −0.6775 |
| 2 | 4440.3 | 0.39 | −1.18 | −0.0833 | |
| 4 | 289.4 | 0.29 | −11.68 | −0.8279 | |
| Cu | 0.25 | 1317 | 1.93 | 10.22 | 0.7246 |
| 2 | 435.5 | 0.27 | −12.42 | −0.8798 | |
| 4 | 615.2 | 0.27 | −15.42 | −1.0929 | |
| Fe | 0.25 | 572.2 | 0.39 | −14.36 | −1.0178 |
| 2 | 1021.5 | 0.32 | 9.87 | 0.6994 | |
| 4 | 1618.1 | 0.15 | 7.34 | 0.5200 | |
| Ni | 0.25 | 338.5 | 0.11 | 0.47 | 0.0331 |
| 2 | 938.1 | 0.23 | −21.42 | −1.5177 | |
| 4 | 1618.1 | 0.15 | −41.79 | −2.8716 | |
| Ti | 0.25 | 458.2 | 0.34 | −13.85 | −0.9817 |
| 2 | 293.0 | 0.26 | −19.23 | −1.3624 | |
| 4 | 312.0 | 0.26 | −20.05 | −1.4208 | |
| Co-existing MNP | 0.25 | 466.0 | 0.29 | −3.74 | −0.2652 |
| 2 | 1064.6 | 0.29 | −22.62 | −1.6028 | |
| 4 | 1425.8 | 0.37 | −21.61 | −1.5312 |
| Dispersion Time (Hours) | Ag NPs (ppm) | Cu NPs (ppm) | Fe NPs (ppm) | Ni NPs (ppm) | Ti NPs (ppm) |
|---|---|---|---|---|---|
| 0.25 | 0.002 | ND * | ND * | ND * | 0.04 |
| 0.50 | 0.009 | 0.69 | 0.01 | 0.02 | 0.05 |
| 1.00 | 0.025 | 0.84 | ND * | 0.03 | 0.04 |
| 2.00 | 0.041 | 8.52 | ND * | 0.06 | 0.002 |
| 3.00 | 0.033 | 8.31 | 0.02 | 0.11 | 0.32 |
| 4.00 | 0.040 | 10.5 | ND * | 0.11 | 0.01 |
| Actual Concentration in Prepared Solution (ppm) | 2 | 2 | 4 | 6 | 2 |
| Dispersion Time | 0.25 h | 0.5 h | 1 h | 2 h | 3 h | 4 h |
|---|---|---|---|---|---|---|
| MNP | ||||||
| Fe | 0 | 0.250 | 0 | 0 | 0.500 | 0 |
| Ni | 0 | 0.333 | 0.500 | 1.00 | 1.83 | 1.83 |
| Ag | 0.100 | 0.450 | 1.25 | 2.05 | 1.65 | 2.00 |
| Ti | 2.00 | 2.50 | 2.015 | 0.100 | 16.0 | 0.500 |
| Cu * | 0 * | 34.5 * | 42.0 * | 426 * | 415 * | 525 * |
| Dispersion Time (h) | Ag NPs (ppm) | Cu NPs (ppm) | Fe NPs (ppm) | Ni NPs (ppm) | Ti NPs (ppm) |
|---|---|---|---|---|---|
| 0.25 | ND * | ND * | ND * | ND * | 0.03 |
| 0.50 | ND * | 0.009 | 0.002 | ND * | 0.04 |
| 1.00 | 0.002 | 0.11 | ND * | ND * | 0.01 |
| 2.00 | ND * | 2.53 | ND * | 0.03 | 0.004 |
| 3.00 | 0.0009 | 3.01 | 0.06 | 0.05 | 0.09 |
| 4.00 | 0.0041 | 9.41 | 0.005 | 0.03 | 0.01 |
| Actual Concentration in Prepared Solution (ppm) | 12 | 12 | 12 | 12 | 12 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bathi, J.R.; Roy, S.; Tareq, S.; Potts, G.E.; Palchoudhury, S.; Sweck, S.O.; Gadhamshetty, V. Dispersion and Aggregation Fate of Individual and Co-Existing Metal Nanoparticles under Environmental Aqueous Suspension Conditions. Materials 2022, 15, 6733. https://doi.org/10.3390/ma15196733
Bathi JR, Roy S, Tareq S, Potts GE, Palchoudhury S, Sweck SO, Gadhamshetty V. Dispersion and Aggregation Fate of Individual and Co-Existing Metal Nanoparticles under Environmental Aqueous Suspension Conditions. Materials. 2022; 15(19):6733. https://doi.org/10.3390/ma15196733
Chicago/Turabian StyleBathi, Jejal Reddy, Shuvashish Roy, Syed Tareq, Gretchen E. Potts, Soubantika Palchoudhury, Samantha O. Sweck, and Venkataramana Gadhamshetty. 2022. "Dispersion and Aggregation Fate of Individual and Co-Existing Metal Nanoparticles under Environmental Aqueous Suspension Conditions" Materials 15, no. 19: 6733. https://doi.org/10.3390/ma15196733





