Effect of Thermal Treatment on Corrosion Behavior of AISI 316L Stainless Steel Manufactured by Laser Powder Bed Fusion
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials and Post-Processing Thermal Treatment
2.2. Microstructure Characterization
2.3. Electrochemical Tests
3. Results
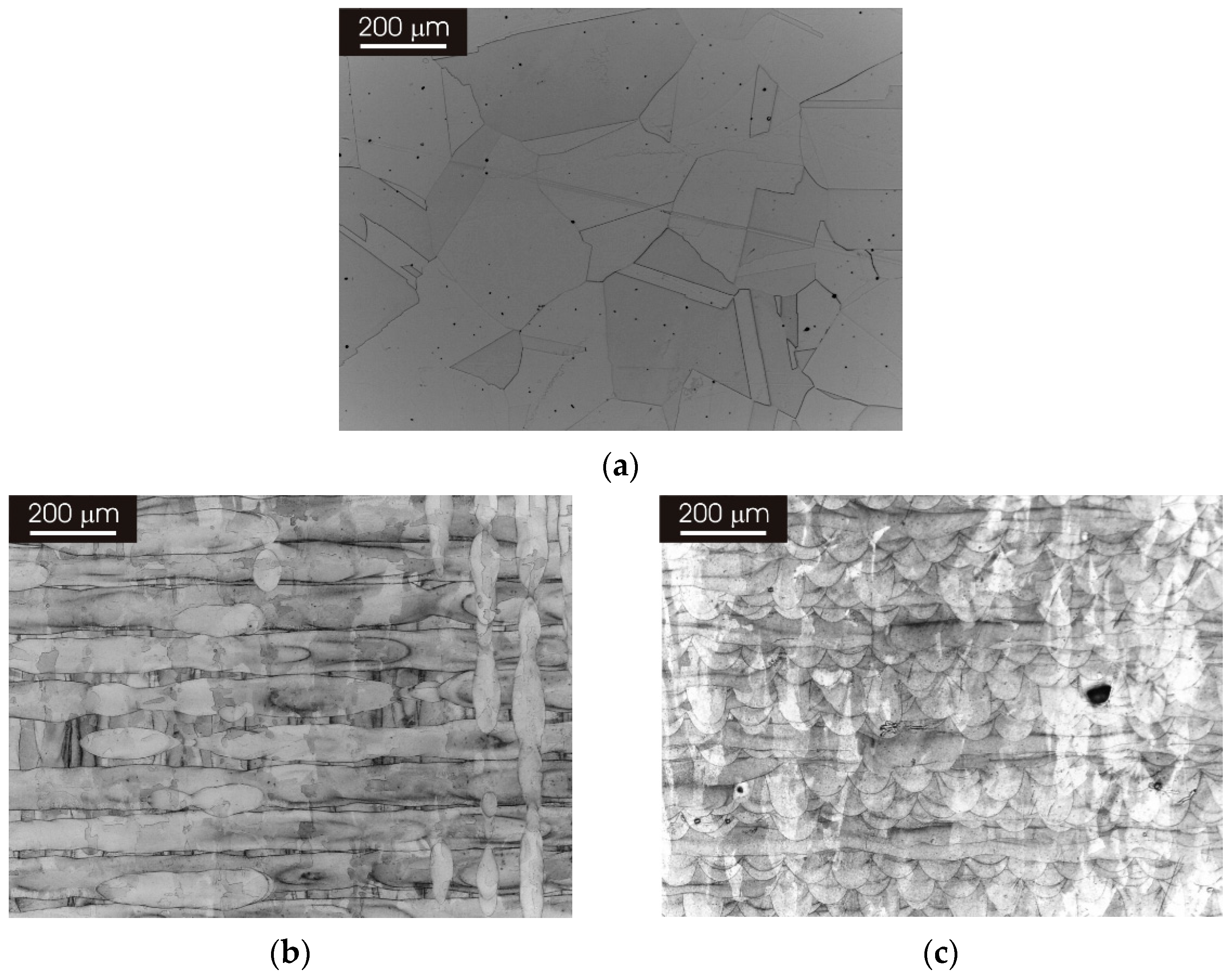
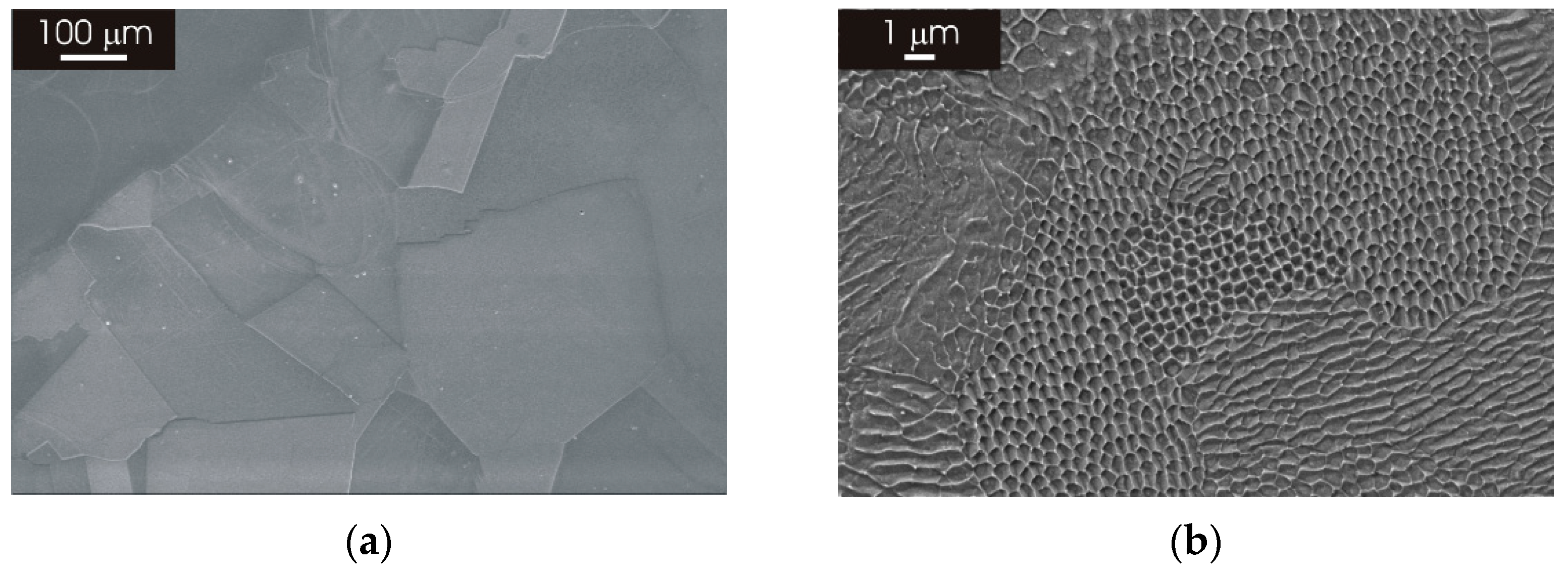
3.1. Microstructure of as Produced L-PBF AISI 316L Stainless Steel
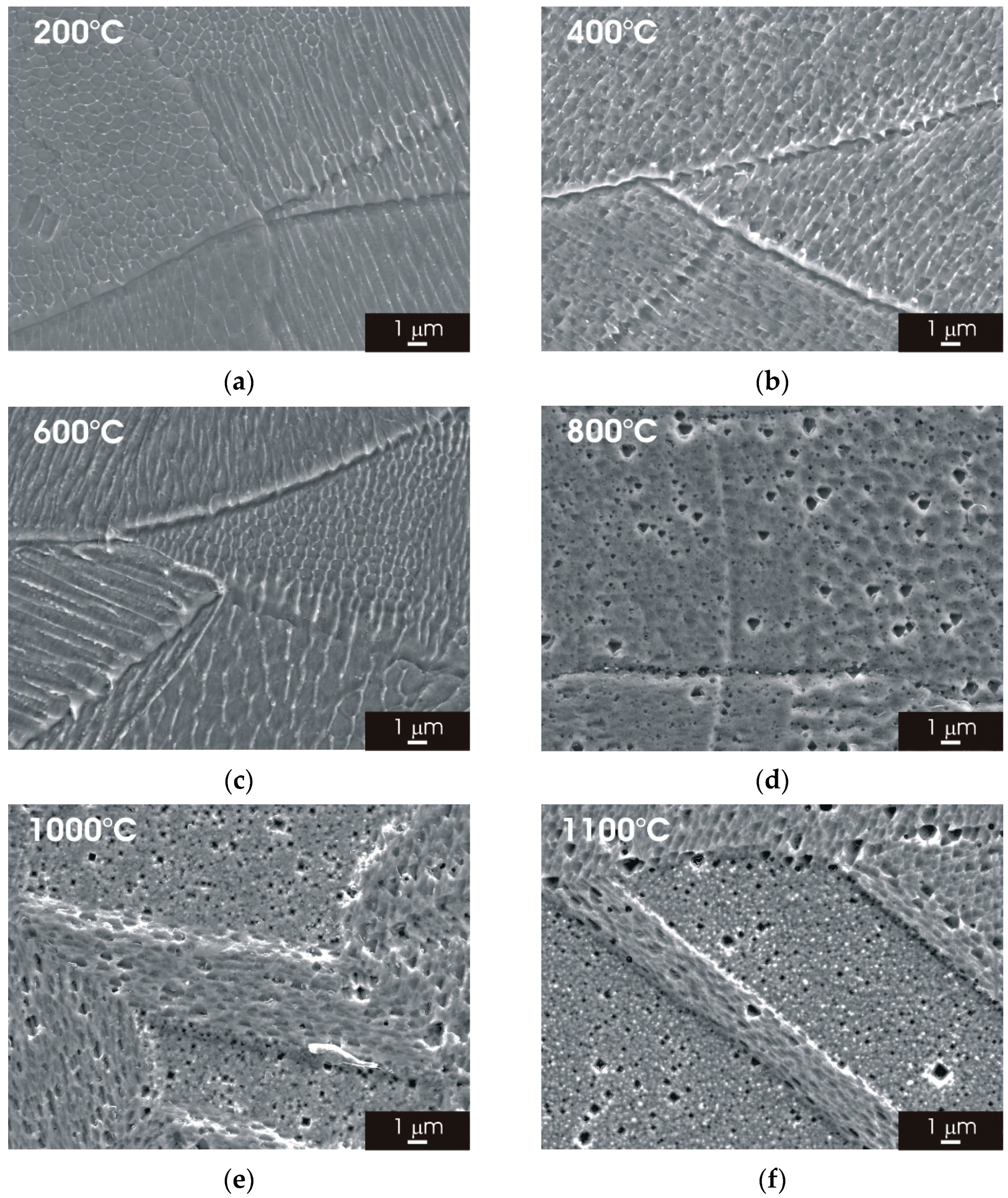
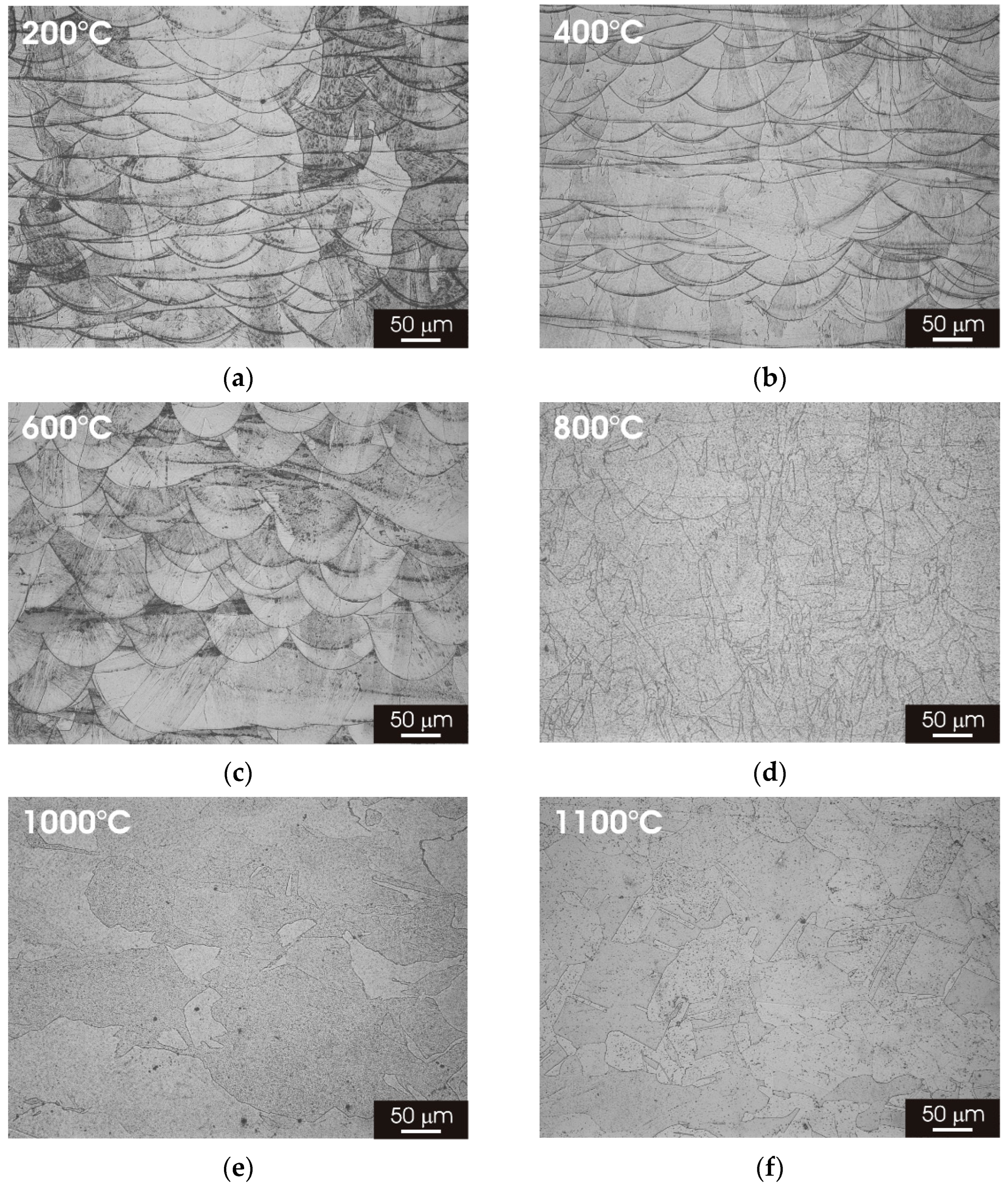
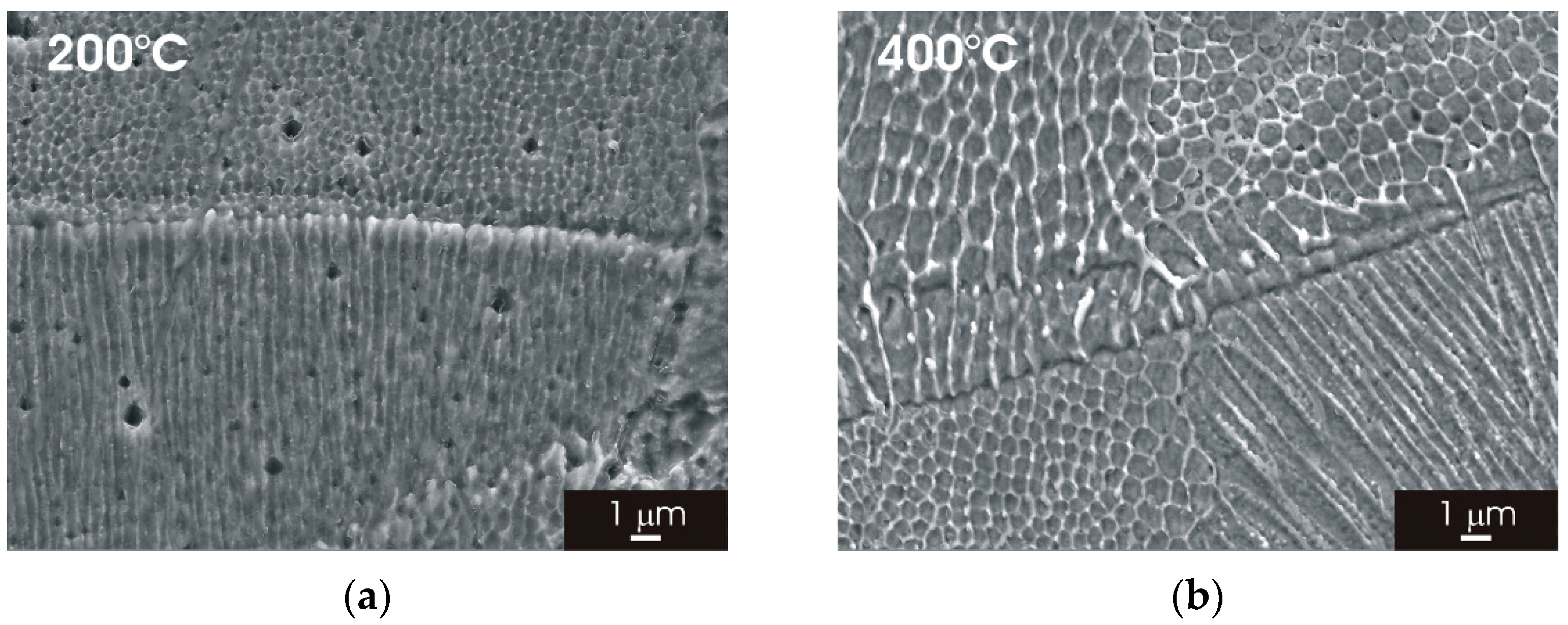
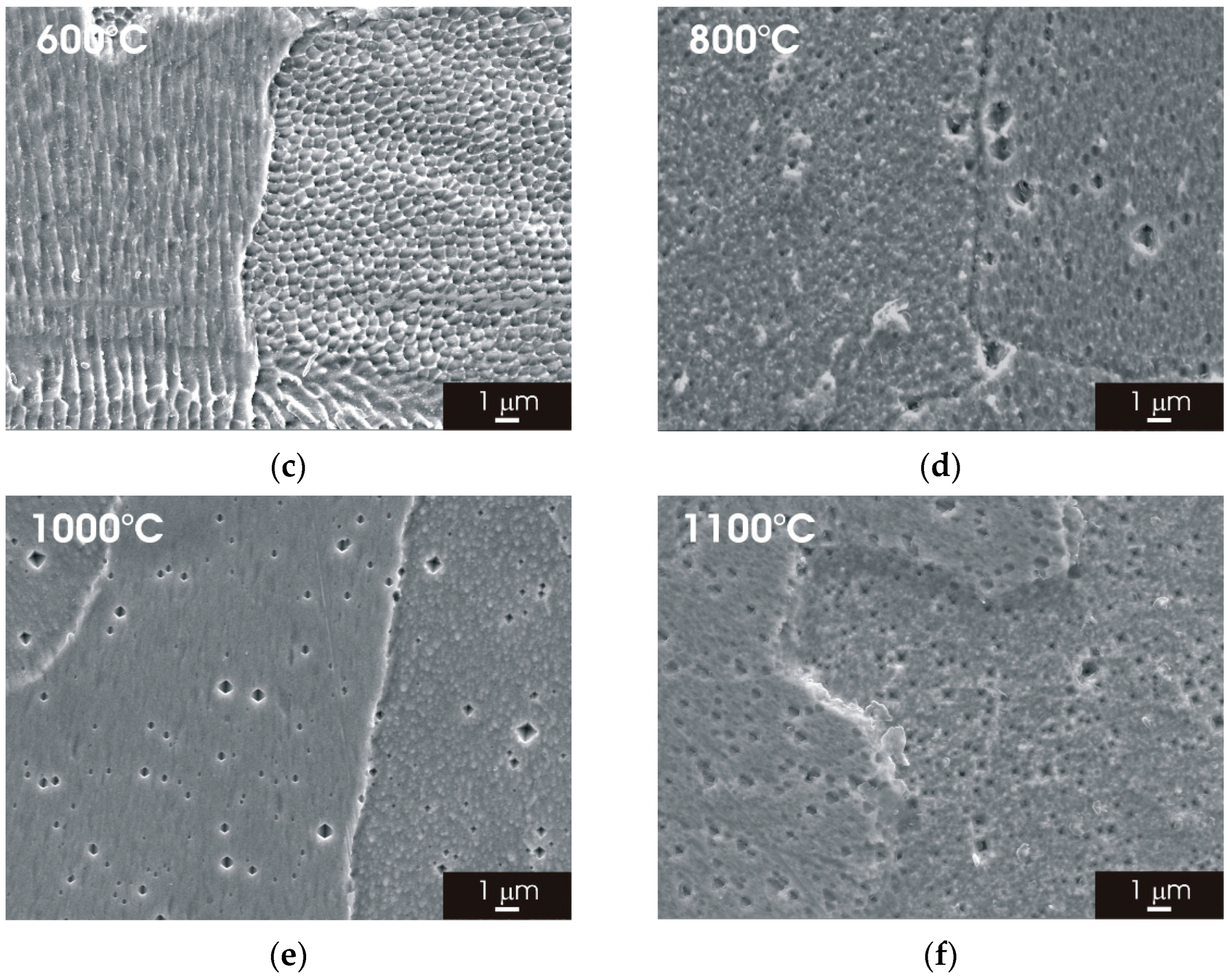
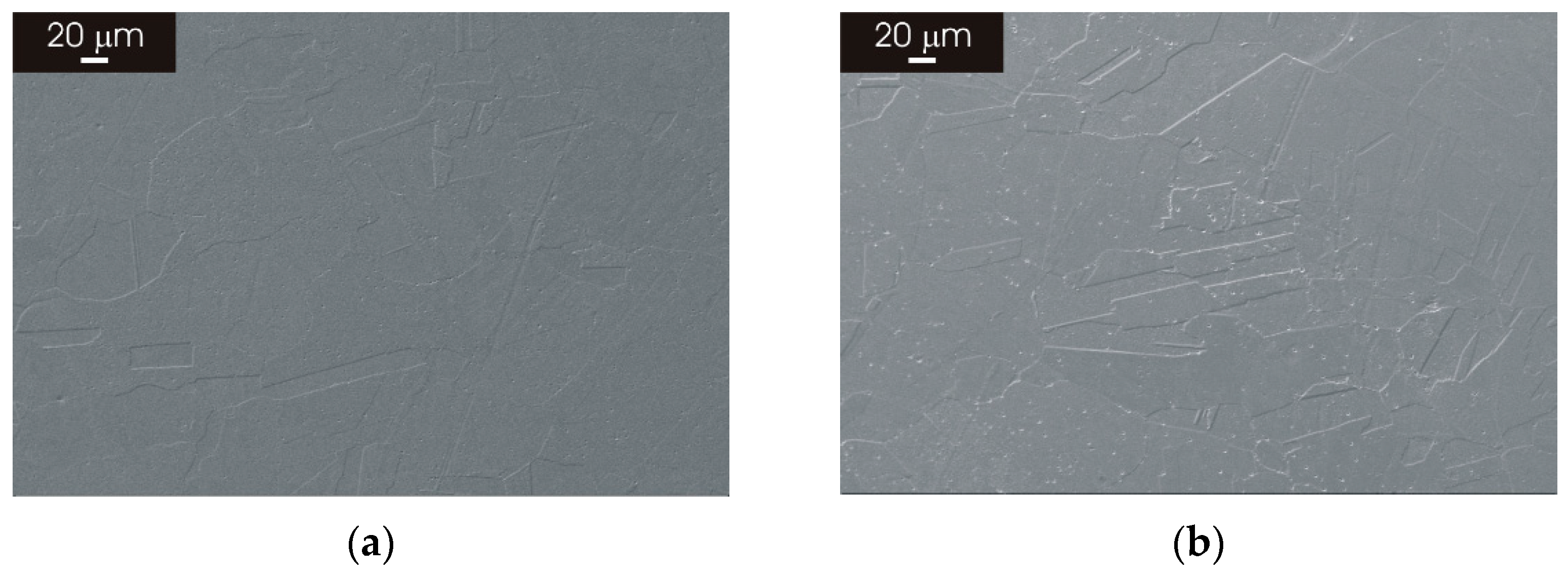
3.2. Microstructure of L-PBF AISI 316L Stainless Steel after Thermal Treatment
3.2.1. XY Surface
3.2.2. XZ Surface
3.3. Electrochemical Behaviour of L-PBF AISI 316L Stainless Steel after Thermal Treatment
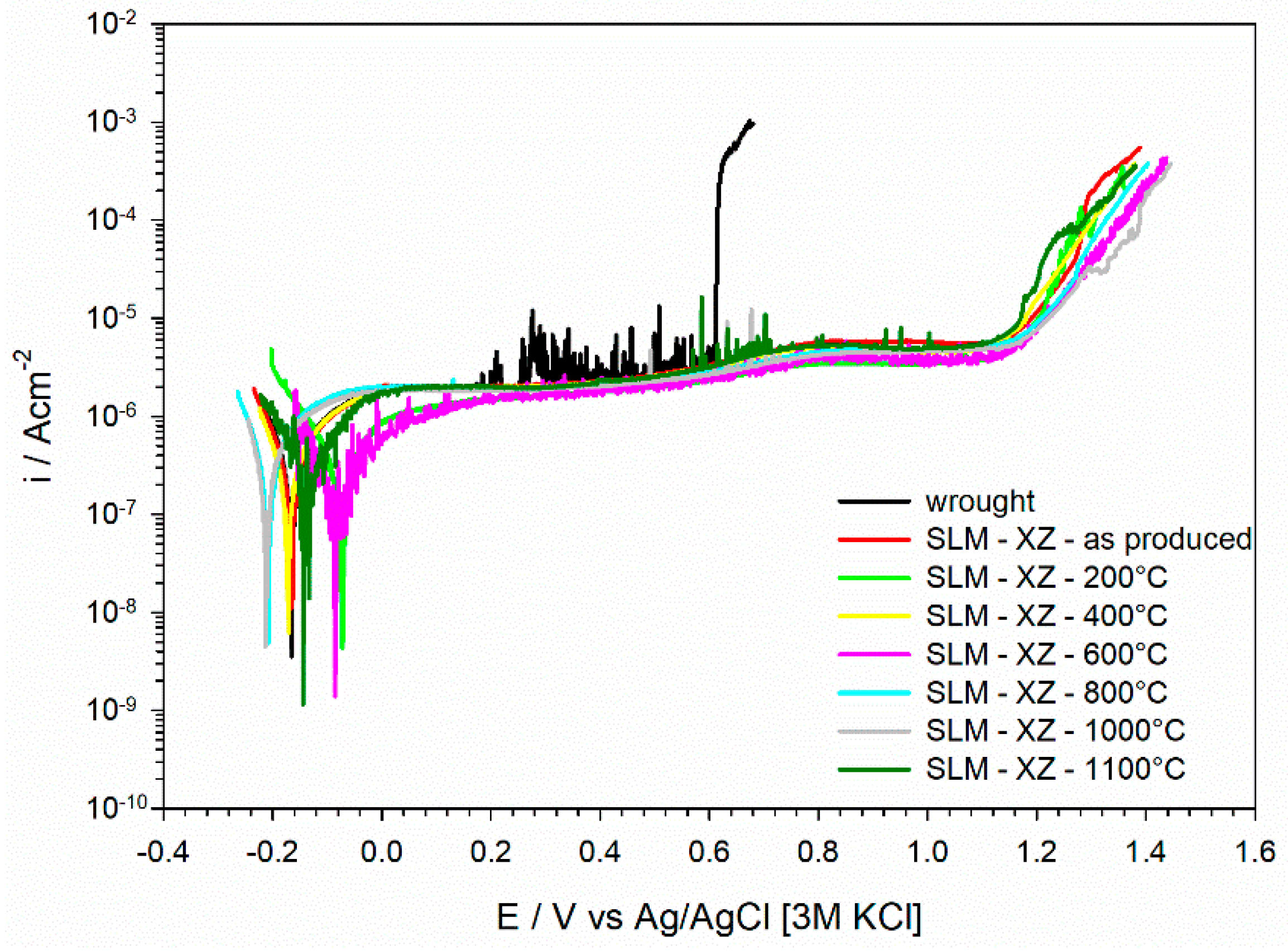
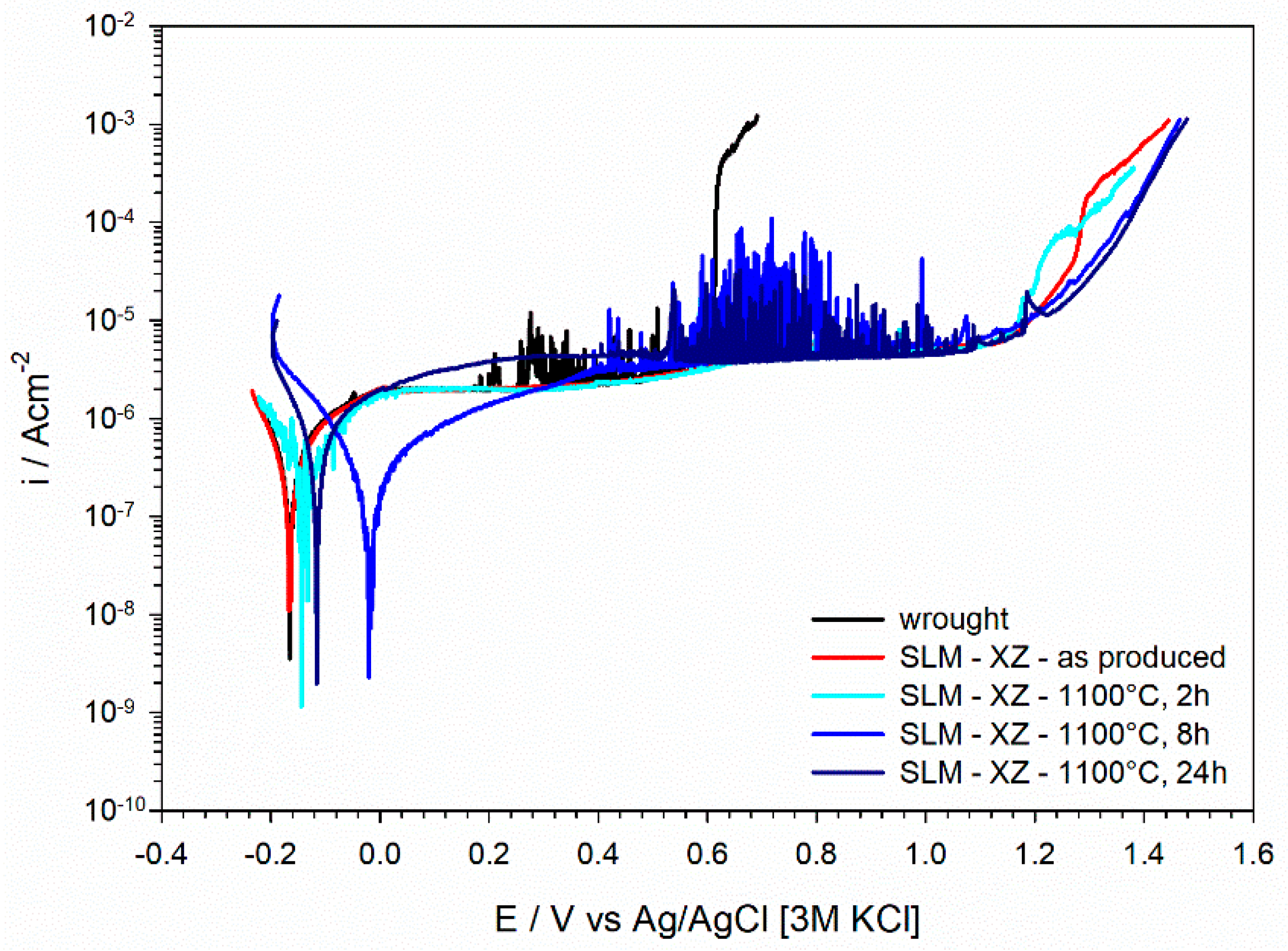
3.3.1. Potentiodynamic Polarization Curves
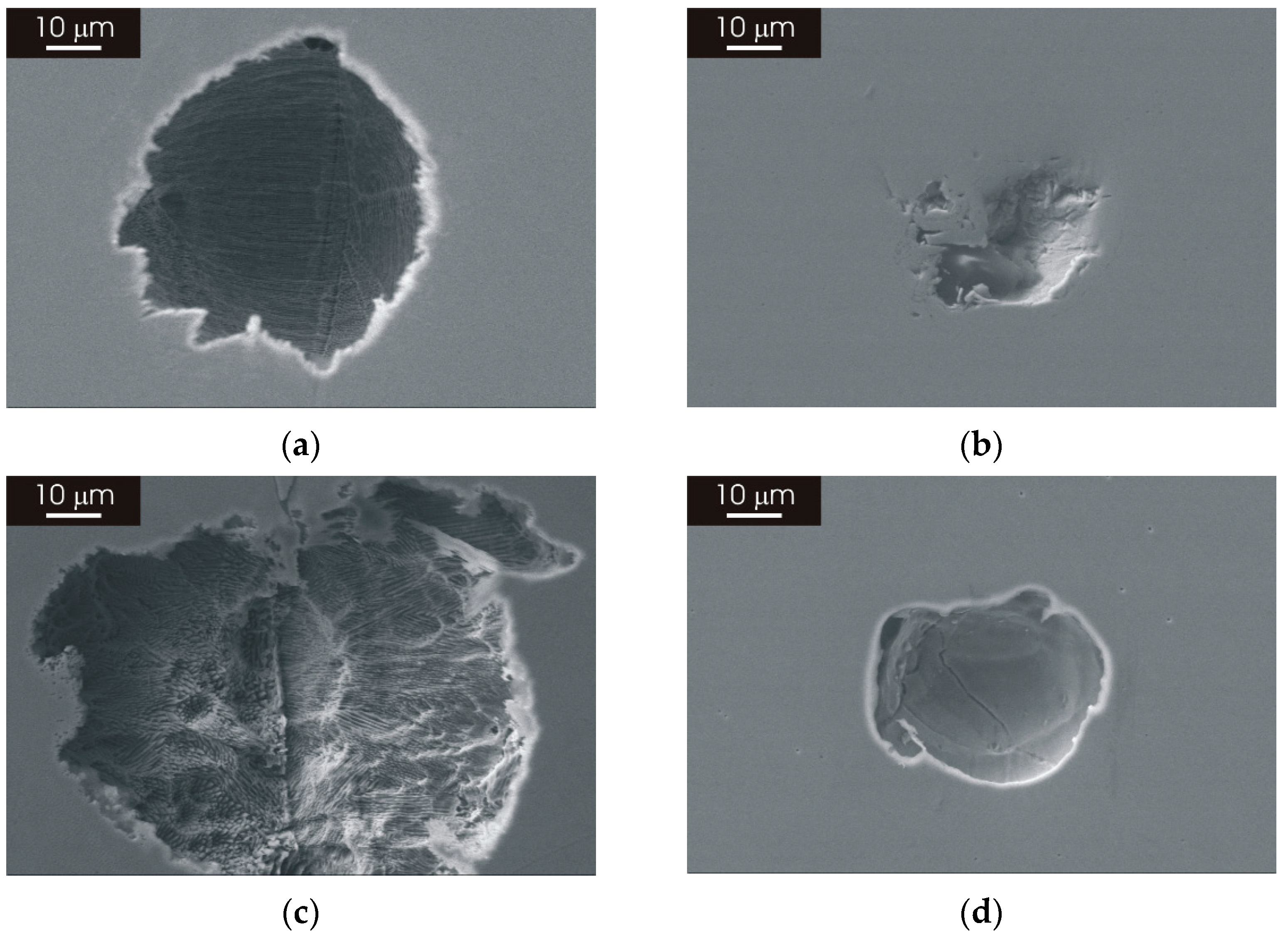
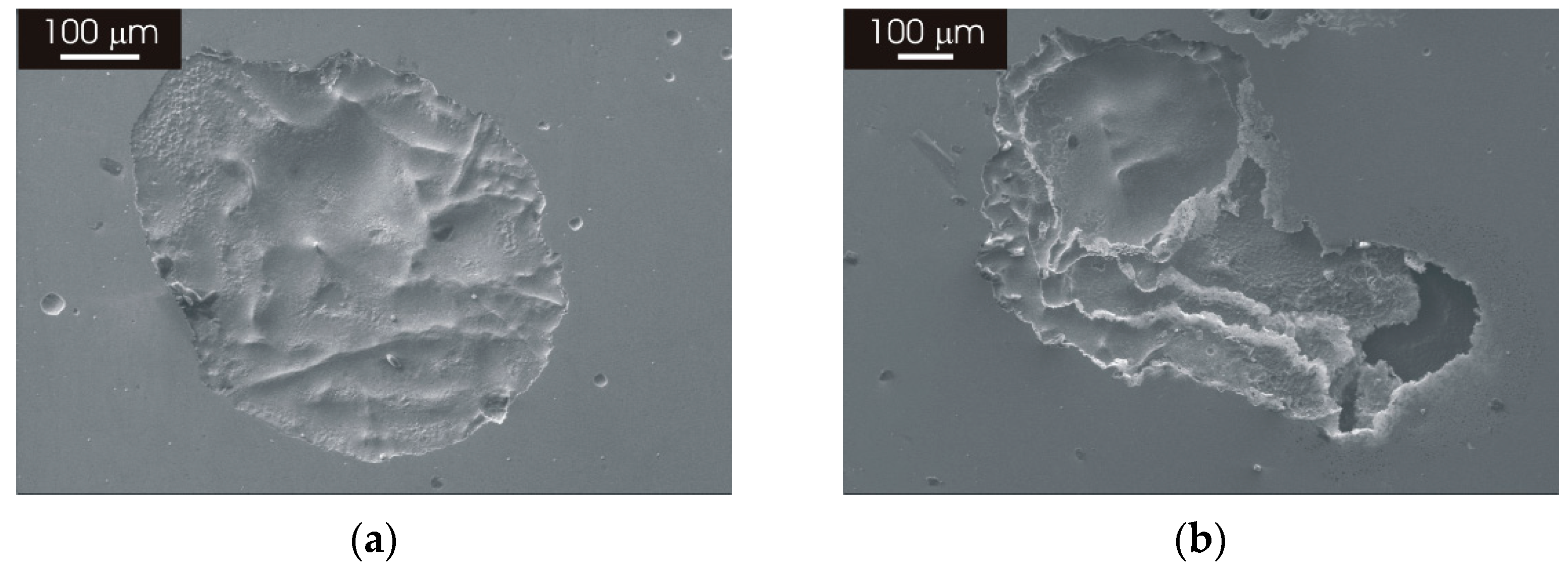
3.3.2. Morphology of Attack after Galvanostatic Polarization
3.4. Effect of Prolonged Thermal Treatment of L-PBF AISI 316L Stainless Steel
3.4.1. Microstructure
3.4.2. Potentiodynamic Polarization Curves and Morphology of Attack
4. Discussion
5. Conclusions
- The laser-scanning pattern typically observed for the XY surface of AISI 316L stainless steel manufactured by L-PBF progressively evolves into an austenitic structure with fine grains increasing the heat-treatment temperature. A similar modification was observed for the complex overlapping melt pools visible in the XZ surface.
- The modification of the microstructure due to thermal treatment does not affect the electrochemical behavior of the L-PBF stainless steel, which displays the same passive behavior of the material in the as-printed condition for heat treatments up to 1100 °C.
- Post-processing heat treatments (stress-relief thermal treatment or recrystallization annealing) can be performed without significantly affecting the high-corrosion resistance of the as-produced L-PBF stainless steel in the temperature range studied in this work. This expands the applicability of 3D-printed stainless-steel components.
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Gor, M.; Soni, H.; Wankhede, V.; Sahlot, P.; Grzelak, K.; Szachgluchowicz, I.; Kluczyński, J. A Critical Review on Effect of Process Parameters on Mechanical and Microstructural Properties of Powder-Bed Fusion Additive Manufacturing of SS316L. Materials 2021, 14, 6527. [Google Scholar] [CrossRef] [PubMed]
- Gorsse, S.; Hutchinson, C.; Gouné, M.; Banerjee, R. Additive Manufacturing of Metals: A Brief Review of the Characteristic Microstructures and Properties of Steels, Ti-6Al-4V and High-Entropy Alloys. Sci. Technol. Adv. Mater. 2017, 18, 584–610. [Google Scholar] [CrossRef] [PubMed]
- Gu, D.D.; Meiners, W.; Wissenbach, K.; Poprawe, R. Laser Additive Manufacturing of Metallic Components: Materials, Processes and Mechanisms. Int. Mater. Rev. 2012, 57, 133–164. [Google Scholar] [CrossRef]
- Hemmasian Ettefagh, A.; Guo, S.; Raush, J. Corrosion Performance of Additively Manufactured Stainless Steel Parts: A Review. Addit. Manuf. 2021, 37, 101689. [Google Scholar] [CrossRef]
- Murr, L.E.; Gaytan, S.M.; Ramirez, D.A.; Martinez, E.; Hernandez, J.; Amato, K.N.; Shindo, P.W.; Medina, F.R.; Wicker, R.B. Metal Fabrication by Additive Manufacturing Using Laser and Electron Beam Melting Technologies. J. Mater. Sci. Technol. 2012, 28, 1–14. [Google Scholar] [CrossRef]
- Murr, L.E.; Martinez, E.; Amato, K.N.; Gaytan, S.M.; Hernandez, J.; Ramirez, D.A.; Shindo, P.W.; Medina, F.; Wicker, R.B. Fabrication of Metal and Alloy Components by Additive Manufacturing: Examples of 3D Materials Science. J. Mater. Res. Technol. 2012, 1, 42–54. [Google Scholar] [CrossRef]
- Herzog, D.; Seyda, V.; Wycisk, E.; Emmelmann, C. Additive Manufacturing of Metals. Acta Mater. 2016, 117, 371–392. [Google Scholar] [CrossRef]
- Sames, W.J.; List, F.A.; Pannala, S.; Dehoff, R.R.; Babu, S.S. The Metallurgy and Processing Science of Metal Additive Manufacturing. Int. Mater. Rev. 2016, 61, 315–360. [Google Scholar] [CrossRef]
- Saeidi, K.; Gao, X.; Lofaj, F.; Kvetková, L.; Shen, Z.J. Transformation of Austenite to Duplex Austenite-Ferrite Assembly in Annealed Stainless Steel 316L Consolidated by Laser Melting. J. Alloys Compd. 2015, 633, 463–469. [Google Scholar] [CrossRef]
- Saeidi, K.; Gao, X.; Zhong, Y.; Shen, Z.J. Hardened Austenite Steel with Columnar Sub-Grain Structure Formed by Laser Melting. Mater. Sci. Eng. A 2015, 625, 221–229. [Google Scholar] [CrossRef]
- Montero-Sistiaga, M.L.; Godino-Martinez, M.; Boschmans, K.; Kruth, J.P.; Van Humbeeck, J.; Vanmeensel, K. Microstructure Evolution of 316L Produced by HP-SLM (High Power Selective Laser Melting). Addit. Manuf. 2018, 23, 402–410. [Google Scholar] [CrossRef]
- Kurzynowski, T.; Gruber, K.; Stopyra, W.; Kuźnicka, B.; Chlebus, E. Correlation between Process Parameters, Microstructure and Properties of 316 L Stainless Steel Processed by Selective Laser Melting. Mater. Sci. Eng. A 2018, 718, 64–73. [Google Scholar] [CrossRef]
- Tucho, W.M.; Lysne, V.H.; Austbø, H.; Sjolyst-Kverneland, A.; Hansen, V. Investigation of Effects of Process Parameters on Microstructure and Hardness of SLM Manufactured SS316L. J. Alloys Compd. 2018, 740, 910–925. [Google Scholar] [CrossRef]
- Cherry, J.A.; Davies, H.M.; Mehmood, S.; Lavery, N.P.; Brown, S.G.R.; Sienz, J. Investigation into the Effect of Process Parameters on Microstructural and Physical Properties of 316L Stainless Steel Parts by Selective Laser Melting. Int. J. Adv. Manuf. Technol. 2015, 76, 869–879. [Google Scholar] [CrossRef]
- Sun, Z.; Tan, X.; Tor, S.B.; Yeong, W.Y. Selective Laser Melting of Stainless Steel 316L with Low Porosity and High Build Rates. Mater. Des. 2016, 104, 197–204. [Google Scholar] [CrossRef]
- Zhong, Y.; Liu, L.; Wikman, S.; Cui, D.; Shen, Z. Intragranular Cellular Segregation Network Structure Strengthening 316L Stainless Steel Prepared by Selective Laser Melting. J. Nucl. Mater. 2016, 470, 170–178. [Google Scholar] [CrossRef]
- Eo, D.R.; Park, S.H.; Cho, J.W. Inclusion Evolution in Additive Manufactured 316L Stainless Steel by Laser Metal Deposition Process. Mater. Des. 2018, 155, 212–219. [Google Scholar] [CrossRef]
- Chao, Q.; Cruz, V.; Thomas, S.; Birbilis, N.; Collins, P.; Taylor, A.; Hodgson, P.D.; Fabijanic, D. On the Enhanced Corrosion Resistance of a Selective Laser Melted Austenitic Stainless Steel. Scr. Mater. 2017, 141, 94–98. [Google Scholar] [CrossRef]
- Laleh, M.; Hughes, A.E.; Xu, W.; Gibson, I.; Tan, M.Y. A Critical Review of Corrosion Characteristics of Additively Manufactured Stainless Steels. Int. Mater. Rev. 2021, 66, 563–599. [Google Scholar] [CrossRef]
- Laleh, M.; Hughes, A.E.; Xu, W.; Cizek, P.; Tan, M.Y. Unanticipated Drastic Decline in Pitting Corrosion Resistance of Additively Manufactured 316L Stainless Steel after High-Temperature Post-Processing. Corros. Sci. 2020, 165, 108412. [Google Scholar] [CrossRef]
- Li, C.; Liu, Z.Y.; Fang, X.Y.; Guo, Y.B. Residual Stress in Metal Additive Manufacturing. Procedia CIRP 2018, 71, 348–353. [Google Scholar] [CrossRef]
- Wu, A.S.; Brown, D.W.; Kumar, M.; Gallegos, G.F.; King, W.E. An Experimental Investigation into Additive Manufacturing-Induced Residual Stresses in 316L Stainless Steel. Metall. Mater. Trans. A Phys. Metall. Mater. Sci. 2014, 45, 6260–6270. [Google Scholar] [CrossRef]
- Cruz, V.; Chao, Q.; Birbilis, N.; Fabijanic, D.; Hodgson, P.D.; Thomas, S. Electrochemical Studies on the Effect of Residual Stress on the Corrosion of 316L Manufactured by Selective Laser Melting. Corros. Sci. 2020, 164, 108314. [Google Scholar] [CrossRef]
- Kong, D.; Ni, X.; Dong, C.; Zhang, L.; Man, C.; Yao, J.; Xiao, K.; Li, X. Heat Treatment Effect on the Microstructure and Corrosion Behavior of 316L Stainless Steel Fabricated by Selective Laser Melting for Proton Exchange Membrane Fuel Cells. Electrochim. Acta 2018, 276, 293–303. [Google Scholar] [CrossRef]
- Kong, D.; Dong, C.; Ni, X.; Zhang, L.; Yao, J.; Man, C.; Cheng, X.; Xiao, K.; Li, X. Mechanical Properties and Corrosion Behavior of Selective Laser Melted 316L Stainless Steel after Different Heat Treatment Processes. J. Mater. Sci. Technol. 2019, 35, 1499–1507. [Google Scholar] [CrossRef]
- Pinto, F.C.; Aota, L.S.; Souza Filho, I.R.; Raabe, D.; Sandim, H.R.Z. Recrystallization in Non-Conventional Microstructures of 316L Stainless Steel Produced via Laser Powder-Bed Fusion: Effect of Particle Coarsening Kinetics. J. Mater. Sci. 2022, 57, 9576–9598. [Google Scholar] [CrossRef]
- Andreatta, F.; Lanzutti, A.; Vaglio, E.; Totis, G.; Sortino, M.; Fedrizzi, L. Corrosion Behaviour of 316L Stainless Steel Manufactured by Selective Laser Melting. Mater. Corros. 2019, 70, 1633–1645. [Google Scholar] [CrossRef]
- Sander, G.; Thomas, S.; Cruz, V.; Jurg, M.; Birbilis, N.; Gao, X.; Brameld, M.; Hutchinson, C.R. On The Corrosion and Metastable Pitting Characteristics of 316L Stainless Steel Produced by Selective Laser Melting. J. Electrochem. Soc. 2017, 164, C250–C257. [Google Scholar] [CrossRef]
- Sun, S.H.; Ishimoto, T.; Hagihara, K.; Tsutsumi, Y.; Hanawa, T.; Nakano, T. Excellent Mechanical and Corrosion Properties of Austenitic Stainless Steel with a Unique Crystallographic Lamellar Microstructure via Selective Laser Melting. Scr. Mater. 2019, 159, 89–93. [Google Scholar] [CrossRef]
- Zhang, Y.; Zhang, J.; Yan, Q.; Zhang, L.; Wang, M.; Song, B.; Shi, Y. Amorphous Alloy Strengthened Stainless Steel Manufactured by Selective Laser Melting: Enhanced Strength and Improved Corrosion Resistance. Scr. Mater. 2018, 148, 20–23. [Google Scholar] [CrossRef]
- Lodhi, M.J.K.; Deen, K.M.; Greenlee-Wacker, M.C.; Haider, W. Additively Manufactured 316L Stainless Steel with Improved Corrosion Resistance and Biological Response for Biomedical Applications. Addit. Manuf. 2019, 27, 8–19. [Google Scholar] [CrossRef]
- Lodhi, M.J.K.; Deen, K.M.; Haider, W. Corrosion Behavior of Additively Manufactured 316L Stainless Steel in Acidic Media. Materialia 2018, 2, 111–121. [Google Scholar] [CrossRef]
- Yue, T.M.; Yu, J.K.; Man, H.C. The Effect of Excimer Laser Surface Treatment on Pitting Corrosion Resistance of 316LS Stainless Steel. Surf. Coat. Technol. 2001, 137, 65–71. [Google Scholar] [CrossRef]
- Revilla, R.I.; Wouters, B.; Andreatta, F.; Lanzutti, A.; Fedrizzi, L.; De Graeve, I. EIS Comparative Study and Critical Equivalent Electrical Circuit (EEC) Analysis of the Native Oxide Layer of Additive Manufactured and Wrought 316L Stainless Steel. Corros. Sci. 2020, 167, 108480. [Google Scholar] [CrossRef]
- Revilla, R.I.; Van Calster, M.; Raes, M.; Arroud, G.; Andreatta, F.; Pyl, L.; Guillaume, P.; De Graeve, I. Microstructure and Corrosion Behavior of 316L Stainless Steel Prepared Using Different Additive Manufacturing Methods: A Comparative Study Bringing Insights into the Impact of Microstructure on Their Passivity. Corros. Sci. 2020, 176, 108914. [Google Scholar] [CrossRef]
- Kong, D.; Dong, C.; Ni, X.; Zhang, L.; Luo, H.; Li, R.; Wang, L.; Man, C.; Li, X. The Passivity of Selective Laser Melted 316L Stainless Steel. Appl. Surf. Sci. 2020, 504, 144495. [Google Scholar] [CrossRef]
- Zhou, C.; Wang, J.; Hu, S.; Tao, H.; Fang, B.; Li, L.; Zheng, J.; Zhang, L. Enhanced Corrosion Resistance of Additively Manufactured 316L Stainless Steel After Heat Treatment. J. Electrochem. Soc. 2020, 167, 141504. [Google Scholar] [CrossRef]
- Vignal, V.; Voltz, C.; Thiébaut, S.; Demésy, M.; Heintz, O.; Guerraz, S. Pitting Corrosion of Type 316L Stainless Steel Elaborated by the Selective Laser Melting Method: Influence of Microstructure. J. Mater. Eng. Perform. 2021, 30, 5050–5058. [Google Scholar] [CrossRef]
- Lanzutti, A.; Marin, E.; Tamura, K.; Morita, T.; Magnan, M.; Vaglio, E.; Andreatta, F.; Sortino, M.; Totis, G.; Fedrizzi, L. High Temperature Study of the Evolution of the Tribolayer in Additively Manufactured AISI 316L Steel. Addit. Manuf. 2020, 34, 101258. [Google Scholar] [CrossRef]


| Cr [wt%] | Ni [wt%] | Mo [wt%] | Mn [wt%] | Si [wt%] | C [wt%] | S [wt%] | N [wt%] | 0 [ppm] | |
|---|---|---|---|---|---|---|---|---|---|
| wrought | 17.4 | 12.1 | 2.1 | 1.6 | 0.3 | 0.020 | 0.002 | 0.036 | 48 |
| L-PBF | 17.6 | 12.8 | 2.3 | 1.0 | 0.7 | 0.023 | 0.004 | 0.083 | 238 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Andreatta, F.; Lanzutti, A.; Revilla, R.I.; Vaglio, E.; Totis, G.; Sortino, M.; de Graeve, I.; Fedrizzi, L. Effect of Thermal Treatment on Corrosion Behavior of AISI 316L Stainless Steel Manufactured by Laser Powder Bed Fusion. Materials 2022, 15, 6768. https://doi.org/10.3390/ma15196768
Andreatta F, Lanzutti A, Revilla RI, Vaglio E, Totis G, Sortino M, de Graeve I, Fedrizzi L. Effect of Thermal Treatment on Corrosion Behavior of AISI 316L Stainless Steel Manufactured by Laser Powder Bed Fusion. Materials. 2022; 15(19):6768. https://doi.org/10.3390/ma15196768
Chicago/Turabian StyleAndreatta, Francesco, Alex Lanzutti, Reynier I. Revilla, Emanuele Vaglio, Giovanni Totis, Marco Sortino, Iris de Graeve, and Lorenzo Fedrizzi. 2022. "Effect of Thermal Treatment on Corrosion Behavior of AISI 316L Stainless Steel Manufactured by Laser Powder Bed Fusion" Materials 15, no. 19: 6768. https://doi.org/10.3390/ma15196768






