Influence of Crosslinking Extent on Free Volumes of Silicone Rubber and Water Diffusion after Corona Discharge
Abstract
1. Introduction
2. Materials and Experimental Method
2.1. Sample Preparation
2.2. Corona Discharge Treatment
2.3. Positron Annihilation Lifetime Spectroscopy
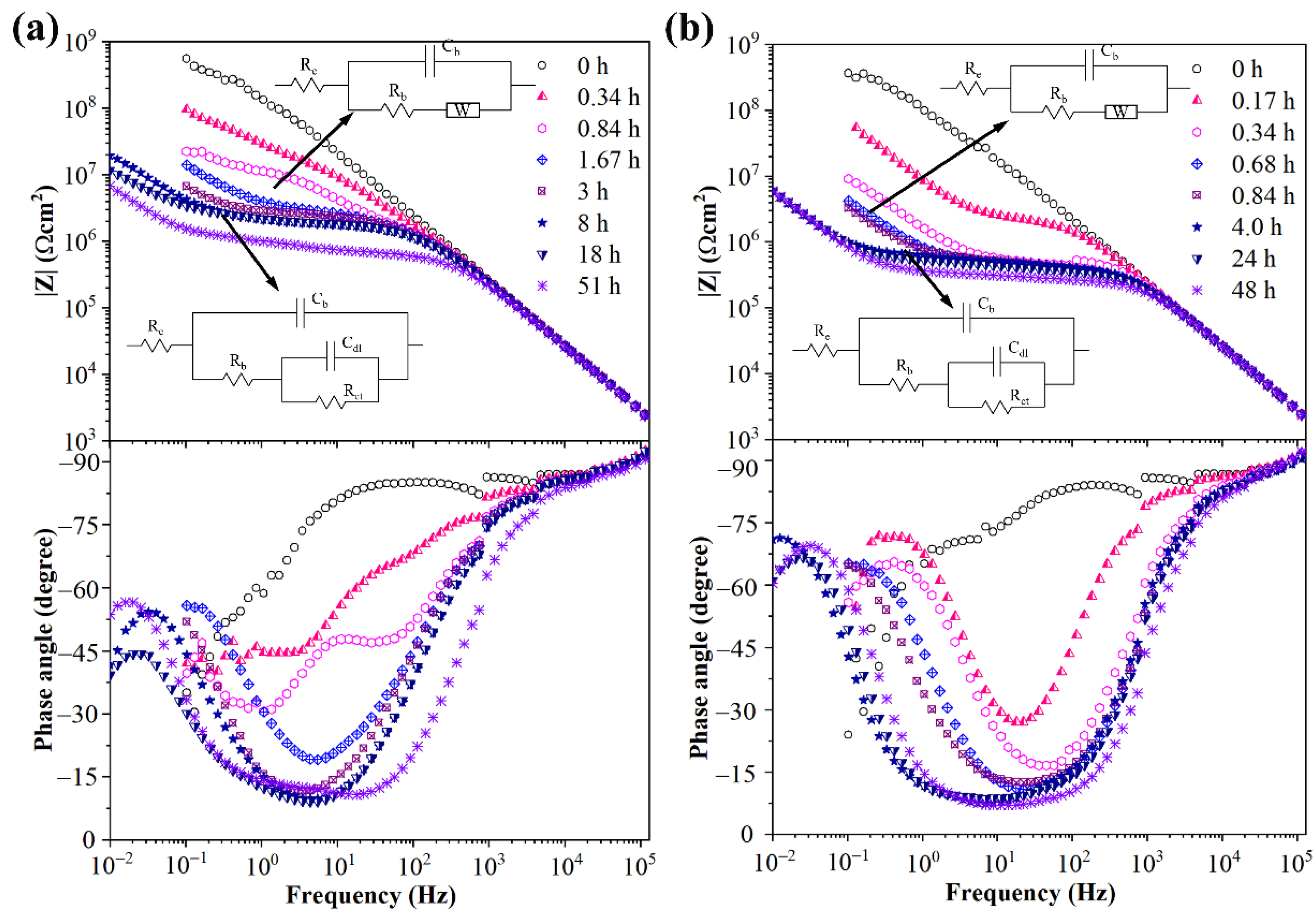
2.4. Electrochemical Impedance Spectroscopy Measurement
2.5. Characterization
3. Results and Discussion
3.1. Free Volume Holes in Silicone Rubber with Different Crosslinking Levels
3.2. Surface Chemical Structure
3.3. Surface Micromorphology
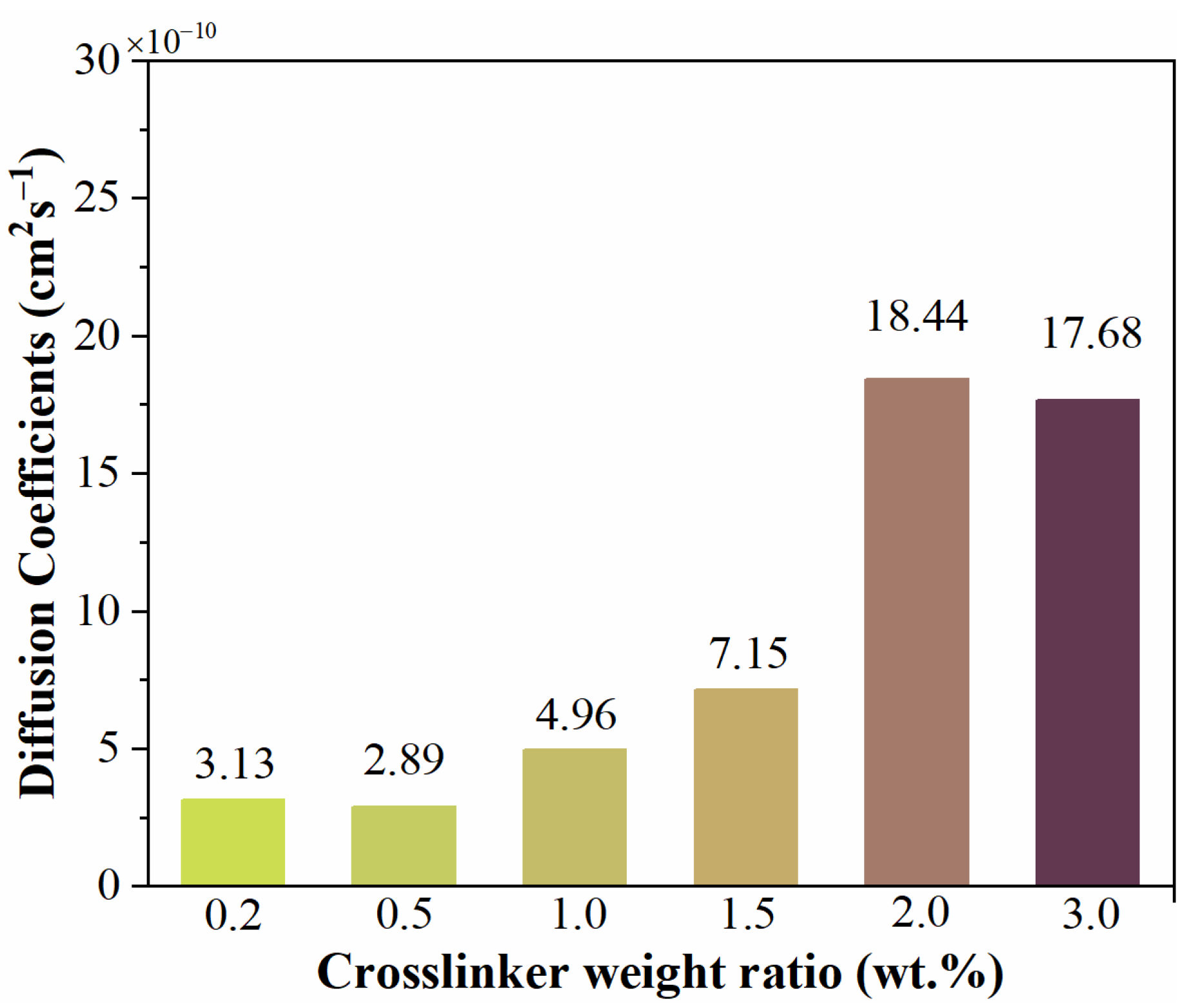
3.4. Water Diffusion Process
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Reynders, J.P.; Jandrell, I.R.; Reynders, S.M. Review of aging and recovery of silicone rubber insulation for outdoor use. IEEE Trans. Dielectr. Electr. Insul. 1999, 6, 620–631. [Google Scholar] [CrossRef]
- Shit, S.C.; Shah, P.M. A Review on Silicone Rubber. Natl. Acad. Sci. Lett. 2013, 36, 355–365. [Google Scholar] [CrossRef]
- Goudie, J.; Owen, M.; Orbeck, T. A review of possible degradation mechanisms of silicone elastomers in high voltage insulation applications. In Proceedings of the 1998 Annual Report Conference on Electrical Insulation and Dielectric Phenomena (Cat. No.98CH36257), Atlanta, GA, USA, 25–28 October 1998; Volume 1, pp. 120–127. [Google Scholar] [CrossRef]
- Akbar, M.; Ullah, R.; Alam, S. Aging of silicone rubber-based composite insulators under multi-stressed conditions: An overview. Mater. Res. Express 2019, 6, 102003. [Google Scholar] [CrossRef]
- Farhadinejad, Z.; Ehsani, M.; Ahmadi-Joneidi, I.; Shayegani, A.A.; Mohseni, H. Effects of UVC radiation on thermal, electrical and morphological behavior of silicone rubber insulators. IEEE Trans. Dielectr. Electr. Insul. 2012, 19, 1740–1749. [Google Scholar] [CrossRef]
- Kumagai, S. Influence of Algal Fouling on Hydrophobicity and Leakage Current on Silicone Rubber. IEEE Trans. Dielectr. Electr. Insul. 2007, 14, 1201–1206. [Google Scholar] [CrossRef]
- Zheng, F.; He, C.; Fang, P.; Wang, J.; Xiong, B.; Wang, K.; Liu, F.; Peng, X.; Xu, X.; Xu, Z.; et al. The surface structure of UV exposed poly-dimethylsiloxane (PDMS) insulator studied by slow positron beam. Appl. Surf. Sci. 2013, 283, 327–331. [Google Scholar] [CrossRef]
- Chakraborty, R.; Reddy, B.S. Studies on high temperature vulcanized silicone rubber insulators under arid climatic aging. IEEE Trans. Dielectr. Electr. Insul. 2017, 24, 1751–1760. [Google Scholar] [CrossRef]
- Hillborg, H.; Gedde, U. Hydrophobicity recovery of polydimethylsiloxane after exposure to corona discharges. Polymer 1998, 39, 1991–1998. [Google Scholar] [CrossRef]
- Zhu, Y. Influence of corona discharge on hydrophobicity of silicone rubber used for outdoor insulation. Polym. Test. 2018, 74, 14–20. [Google Scholar] [CrossRef]
- Wang, J.; Liang, X.; Gao, Y. Failure analysis of decay-like fracture of composite insulator. IEEE Trans. Dielectr. Electr. Insul. 2014, 21, 2503–2511. [Google Scholar] [CrossRef]
- Kumosa, M.S.; Kumosa, L.; Armentrout, D.L. Failure analyses of nonceramic insulators. Part 1: Brittle fracture characteristics. IEEE Electr. Insul. Mag. 2005, 21, 14–27. [Google Scholar] [CrossRef]
- Liu, H.; Yang, T.; Zhou, C.; Jia, R.; Zhang, Y.; Qi, Z. Analysis of 500kV composite insulator fracture fault. J. Phys. Conf. Ser. 2020, 1633, 012056. [Google Scholar] [CrossRef]
- Rowland, S.M.; Xiong, Y.; Robertson, J.; Hoffmann, S. Aging of silicone rubber composite insulators on 400 kV transmission lines. IEEE Trans. Dielectr. Electr. Insul. 2007, 14, 130–136. [Google Scholar] [CrossRef]
- Wang, Z.; Jia, Z.D.; Fang, M.H.; Guan, Z.C. Absorption and permeation of water and aqueous solutions of high-temperature vulcanized silicone rubber. IEEE Trans. Dielectr. Electr. Insul. 2015, 22, 3357–3365. [Google Scholar] [CrossRef]
- Gao, Y.; Wang, J.; Liang, X.; Yan, Z.; Liu, Y.; Cai, Y. Investigation on permeation properties of liquids into HTV silicone rubber materials. IEEE Trans. Dielectr. Electr. Insul. 2014, 21, 2428–2437. [Google Scholar] [CrossRef]
- Wang, Z.; Zhao, L.H.; Jia, Z.D.; Guan, Z.C. Water and moisture permeability of high-temperature vulcanized silicone rubber. IEEE Trans. Dielectr. Electr. Insul. 2017, 24, 2440–2448. [Google Scholar] [CrossRef]
- Gubanski, S.M. Modern outdoor insulation—Concerns and challenges. IEEE Electr. Insul. Mag. 2005, 21, 5–11. [Google Scholar] [CrossRef]
- Tokoro, T.; Nagao, M.; Kosaki, M. Effect of water absorption on the high-field dielectric property of silicone rubber. In Proceedings of the 1998 International Symposium on Electrical Insulating Materials, 1998 Asian International Conference on Dielectrics and Electrical Insulation, 30th Symposium on Electrical Insulating Ma, Toyohashi, Japan, 30 September 1998; pp. 461–464. [Google Scholar]
- Sundfors, F.; Lindfors, T.; Höfler, L.; Bereczki, R.; Gyurcsányi, R.E. FTIR-ATR Study of Water Uptake and Diffusion through Ion-Selective Membranes Based on Poly(acrylates) and Silicone Rubber. Anal. Chem. 2009, 81, 5925–5934. [Google Scholar] [CrossRef]
- Qin, Z.; Li, D.; Li, Q.; Yang, R. Effect of nano-aluminum hydroxide on mechanical properties, flame retardancy and combustion behavior of intumescent flame retarded polypropylene. Mater. Des. 2016, 89, 988–995. [Google Scholar] [CrossRef]
- Xue, Y.; Li, X.-F.; Zhang, D.-H.; Wang, H.-S.; Chen, Y.; Chen, Y.-F. Comparison of ATH and SiO2 fillers filled silicone rubber composites for HTV insulators. Compos. Sci. Technol. 2018, 155, 137–143. [Google Scholar] [CrossRef]
- Hu, S.; Tan, Z.-W.; Chen, F.; Li, J.-G.; Sheng, H.; Huang, Z.-X.; Zhang, L.-M. Flame-retardant properties and synergistic effect of ammonium polyphosphate/aluminum hydroxide/mica/silicone rubber composites. Fire Mater. 2020, 44, 673–682. [Google Scholar] [CrossRef]
- Wang, Z.; Li, J.; Zhou, Y.; Zhang, N.; He, C.; Peng, X.; Huang, Z.; Cao, X.; Wang, B.; Fang, P. Investigation of the surface microstructure evolution of silicone rubber during corona discharge via slow positron beam and electrochemical impedance spectroscopy. Plasma Process. Polym. 2019, 16, 1900057. [Google Scholar] [CrossRef]
- Wang, Z.; Yang, Y.; Peng, X.; Huang, Z.; Qian, L.; He, C.; Fang, P. Water diffusivity transition in fumed silica-filled polydimethylsiloxane composite: Correlation with the interfacial free volumes characterized by positron annihilation lifetime spectroscopy. J. Mater. Sci. 2020, 56, 3095–3110. [Google Scholar] [CrossRef]
- Frounchi, M.; Dadbin, S.; Panahinia, F. Comparison between electron-beam and chemical crosslinking of silicone rubber. Nucl. Instrum. Methods Phys. Res. Sect. B Beam Interact. Mater. At. 2005, 243, 354–358. [Google Scholar] [CrossRef]
- Gan, L.; Shang, S.; Jiang, S.-X. Impact of vinyl concentration of a silicone rubber on the properties of the graphene oxide filled silicone rubber composites. Compos. Part B Eng. 2015, 84, 294–300. [Google Scholar] [CrossRef]
- Xu, C.; Wang, Y.; Lin, B.; Liang, X.; Chen, Y. Thermoplastic vulcanizate based on poly(vinylidene fluoride) and methyl vinyl silicone rubber by using fluorosilicone rubber as interfacial compatibilizer. Mater. Des. 2015, 88, 170–176. [Google Scholar] [CrossRef]
- Zhang, J.; Feng, S. Effect of crosslinking on the conductivity of conductive silicone rubber. J. Appl. Polym. Sci. 2003, 89, 3471–3475. [Google Scholar] [CrossRef]
- Warley, R.L.; Feke, D.L.; Manas-Zloczower, I. Effect of peroxide crosslinking on the dynamic modulus of silicone rubber. J. Appl. Polym. Sci. 2005, 97, 1504–1512. [Google Scholar] [CrossRef]
- He, C.; Li, B.; Ren, Y.; Lu, W.; Zeng, Y.; He, W.; Feng, A. How the Crosslinking Agent Influences the Thermal Stability of RTV Phenyl Silicone Rubber. Materials 2018, 12, 88. [Google Scholar] [CrossRef]
- Jean, Y. Positron annihilation spectroscopy for chemical analysis: A novel probe for microstructural analysis of polymers. Microchem. J. 1990, 42, 72–102. [Google Scholar] [CrossRef]
- Tao, S.J. Positronium Annihilation in Molecular Substances. J. Chem. Phys. 1972, 56, 5499–5510. [Google Scholar] [CrossRef]
- Li, C.; Qi, N.; Liu, Z.; Zhou, B.; Chen, Z.; Wang, Z. Effect of synthesis temperature on the ordered pore structure in mesoporous silica studied by positron annihilation spectroscopy. Appl. Surf. Sci. 2015, 363, 445–450. [Google Scholar] [CrossRef]
- Rasband, P.; Hecker, W. Catalyst Characterization Using Quantitative FTIR: CO on Supported Rh. J. Catal. 1993, 139, 551–560. [Google Scholar] [CrossRef]
- Rege, S.U.; Yang, R.T. A novel FTIR method for studying mixed gas adsorption at low concentrations: H2O and CO2 on NaX zeolite and γ-alumina. Chem. Eng. Sci. 2001, 56, 3781–3796. [Google Scholar] [CrossRef]
- Armstrong, R.; Wright, D. Polymer protective coatings—The distinction between coating porosity and the wetted metal area. Electrochim. Acta 1993, 38, 1799–1801. [Google Scholar] [CrossRef]
- Brasher, D.; Kingsbury, A. Electrical measurements in the study of immersed paint coatings on metal. I. Comparison between capacitance and gravimetric methods of estimating water-uptake. J. Appl. Chem. 1954, 4, 62–72. [Google Scholar] [CrossRef]
- Bellucci, F.; Nicodemo, L. Water Transport in Organic Coatings. Corrosion 1993, 49, 235–247. [Google Scholar] [CrossRef]
- Perera, D.; Selier, P. Water transport in organic coatings. Prog. Org. Coatings 1973, 2, 57–80. [Google Scholar] [CrossRef]
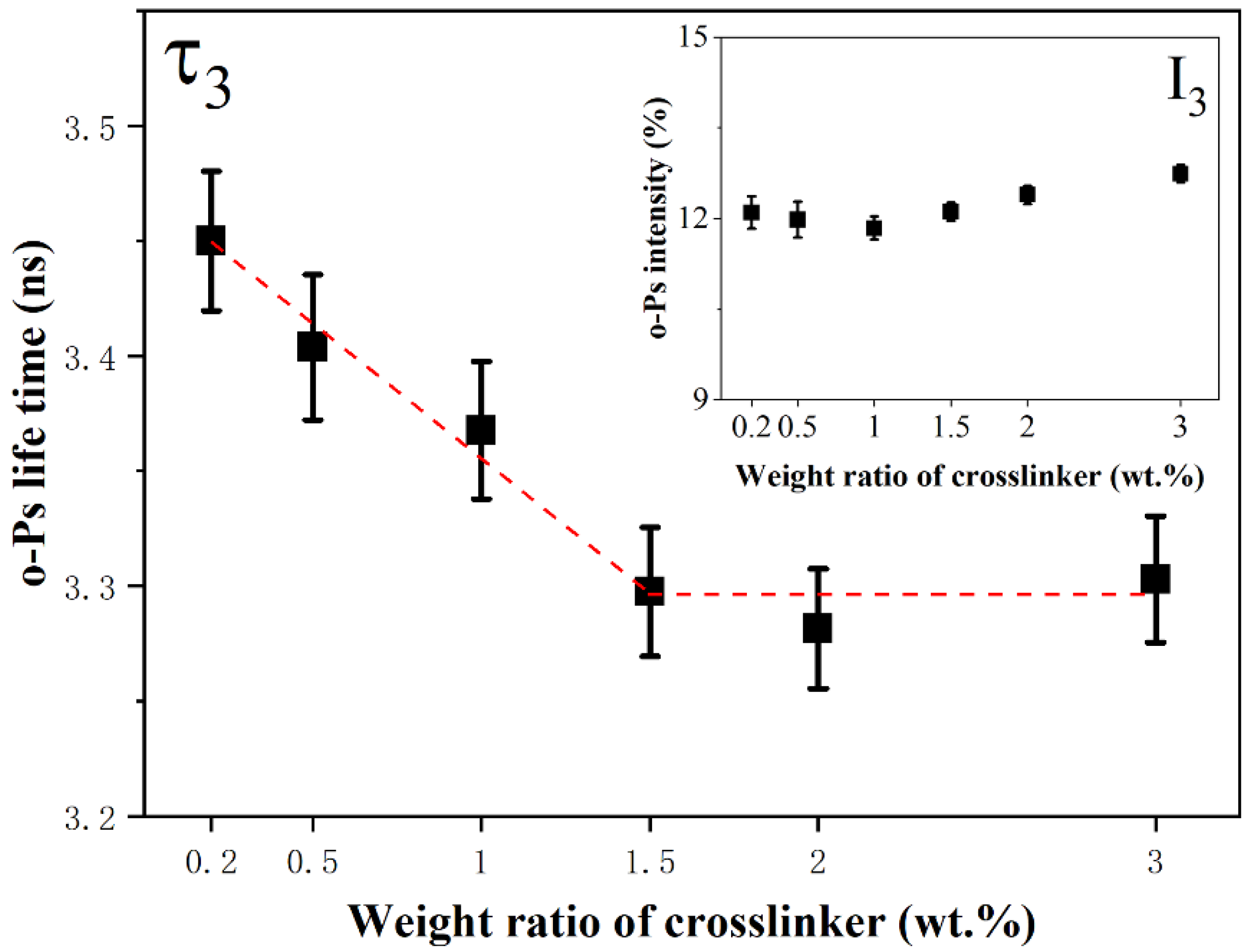
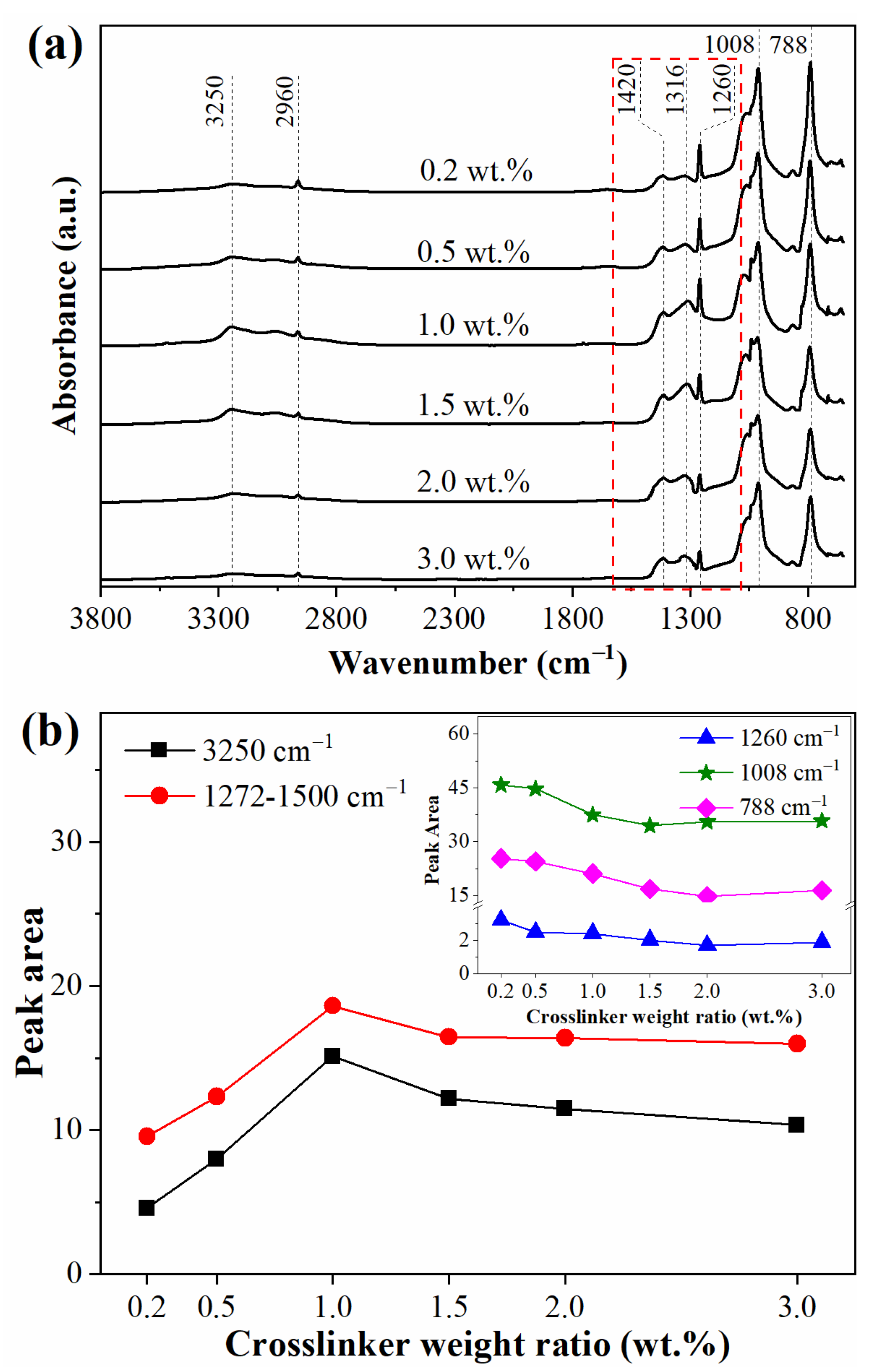
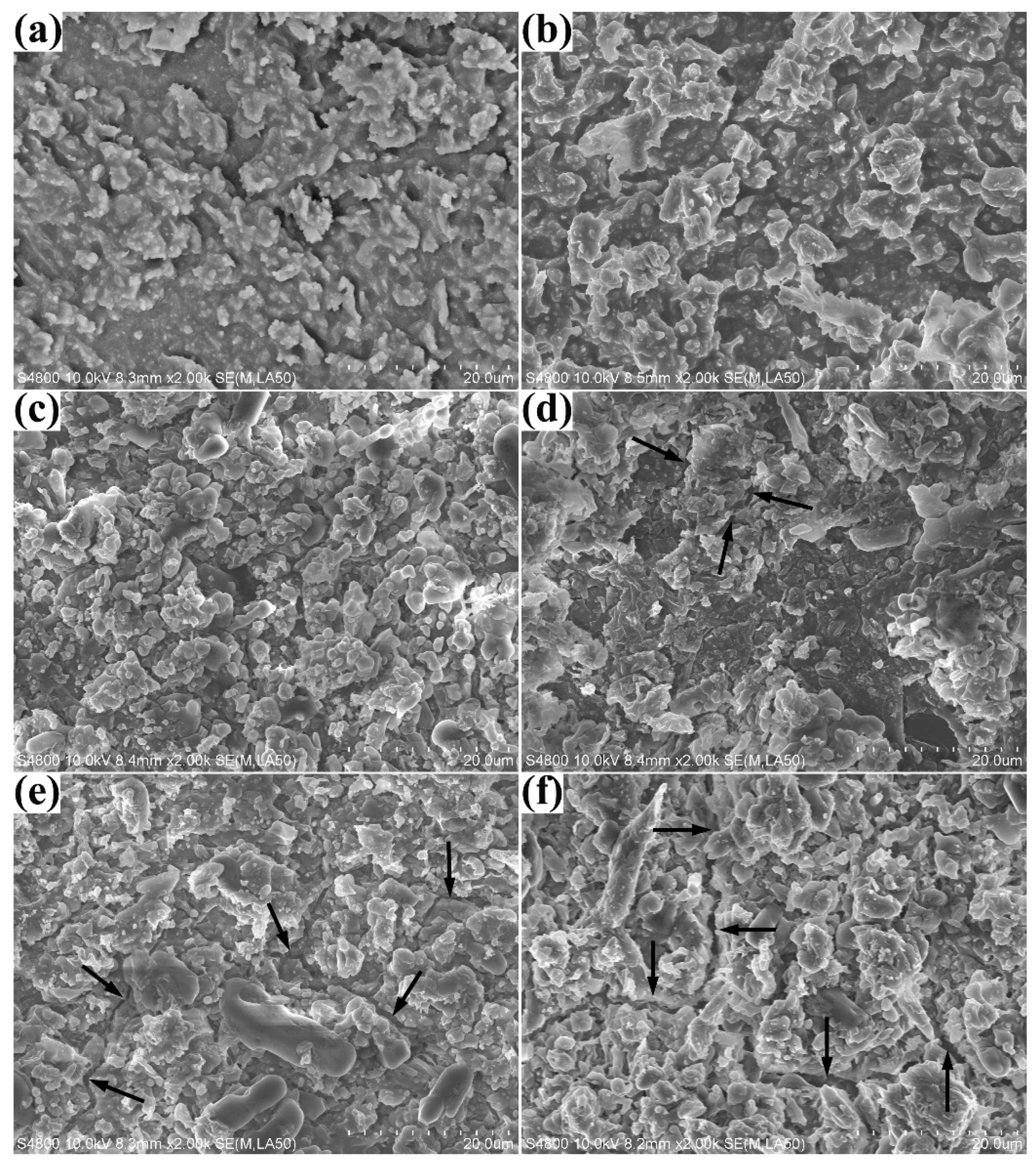
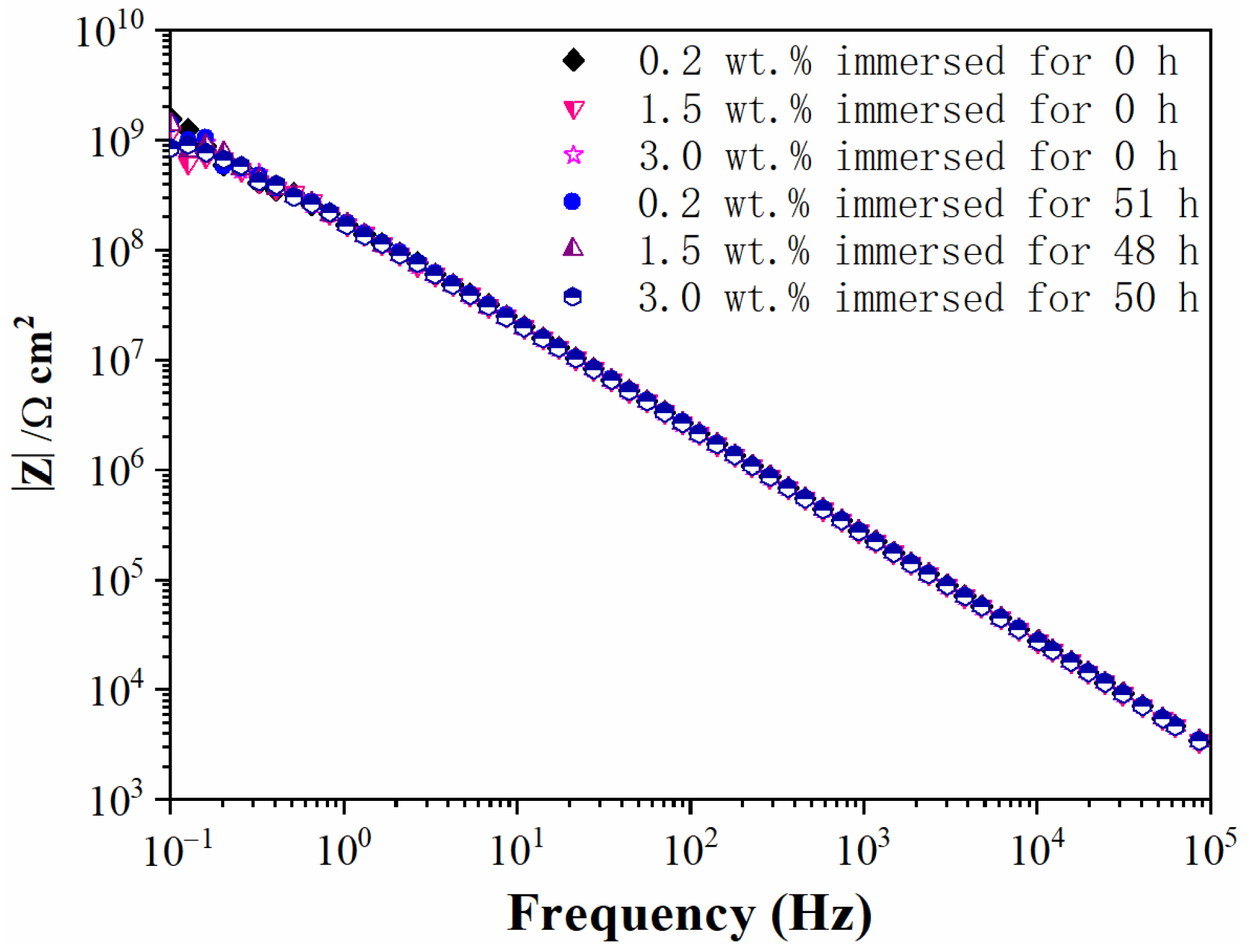



| Weight Ratio (wt.%) | τ3 (ns) | I3 (%) | Rf (Å) | Vf (Å3) |
|---|---|---|---|---|
| 0.2 | 3.45 ± 0.03 | 12.1 ± 0.3 | 3.92 | 252 |
| 0.5 | 3.40 ± 0.03 | 12.0 ± 0.3 | 3.89 | 246 |
| 1.0 | 3.37 ± 0.03 | 11.8 ± 0.2 | 3.87 | 242 |
| 1.5 | 3.30 ± 0.03 | 12.1 ± 0.2 | 3.82 | 234 |
| 2.0 | 3.28 ± 0.03 | 12.5 ± 0.2 | 3.81 | 232 |
| 3.0 | 3.30 ± 0.03 | 12.7 ± 0.2 | 3.83 | 235 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yang, Y.; Wang, Z.; Peng, X.; Huang, Z.; Fang, P. Influence of Crosslinking Extent on Free Volumes of Silicone Rubber and Water Diffusion after Corona Discharge. Materials 2022, 15, 6833. https://doi.org/10.3390/ma15196833
Yang Y, Wang Z, Peng X, Huang Z, Fang P. Influence of Crosslinking Extent on Free Volumes of Silicone Rubber and Water Diffusion after Corona Discharge. Materials. 2022; 15(19):6833. https://doi.org/10.3390/ma15196833
Chicago/Turabian StyleYang, Yue, Zheng Wang, Xiangyang Peng, Zhen Huang, and Pengfei Fang. 2022. "Influence of Crosslinking Extent on Free Volumes of Silicone Rubber and Water Diffusion after Corona Discharge" Materials 15, no. 19: 6833. https://doi.org/10.3390/ma15196833
APA StyleYang, Y., Wang, Z., Peng, X., Huang, Z., & Fang, P. (2022). Influence of Crosslinking Extent on Free Volumes of Silicone Rubber and Water Diffusion after Corona Discharge. Materials, 15(19), 6833. https://doi.org/10.3390/ma15196833







