Graphene Oxide-Modified Epoxy Asphalt Bond Coats with Enhanced Bonding Properties
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
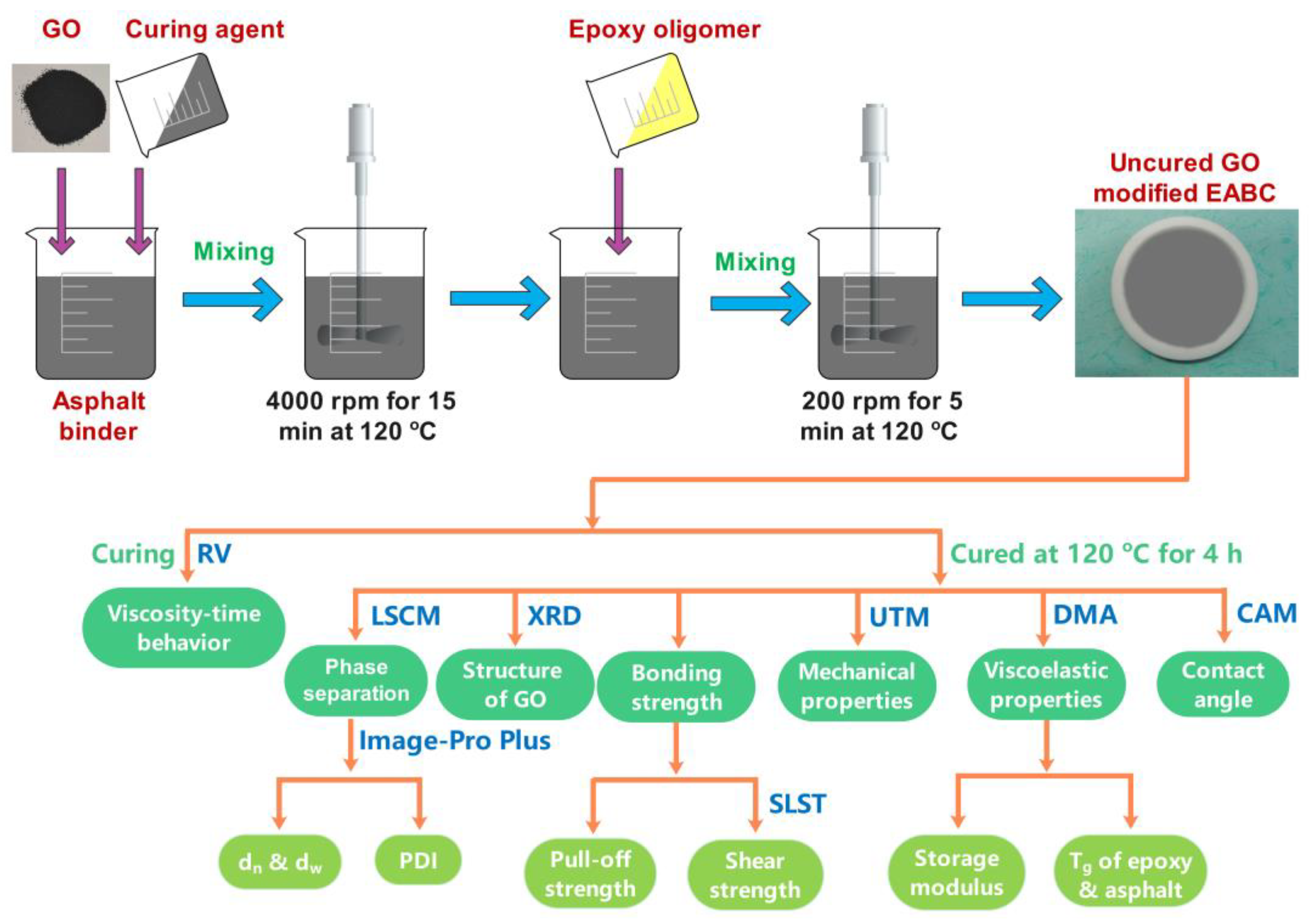
2.2. Preparation of GO-Modified EABCs
2.3. Methods
2.3.1. X-ray Diffraction (XRD) Analysis
2.3.2. Morphology
2.3.3. Rotational Viscosity
2.3.4. Hydrophobicity
2.3.5. Viscoelastic Properties
2.3.6. Pull-Off Adhesion Test
2.3.7. Single-Lap Shear Test (SLST)
2.3.8. Tensile Test
3. Results and Discussion
3.1. X-ray Diffraction Analysis
3.2. Hydrophobicity
3.3. Viscosity–Time Behavior
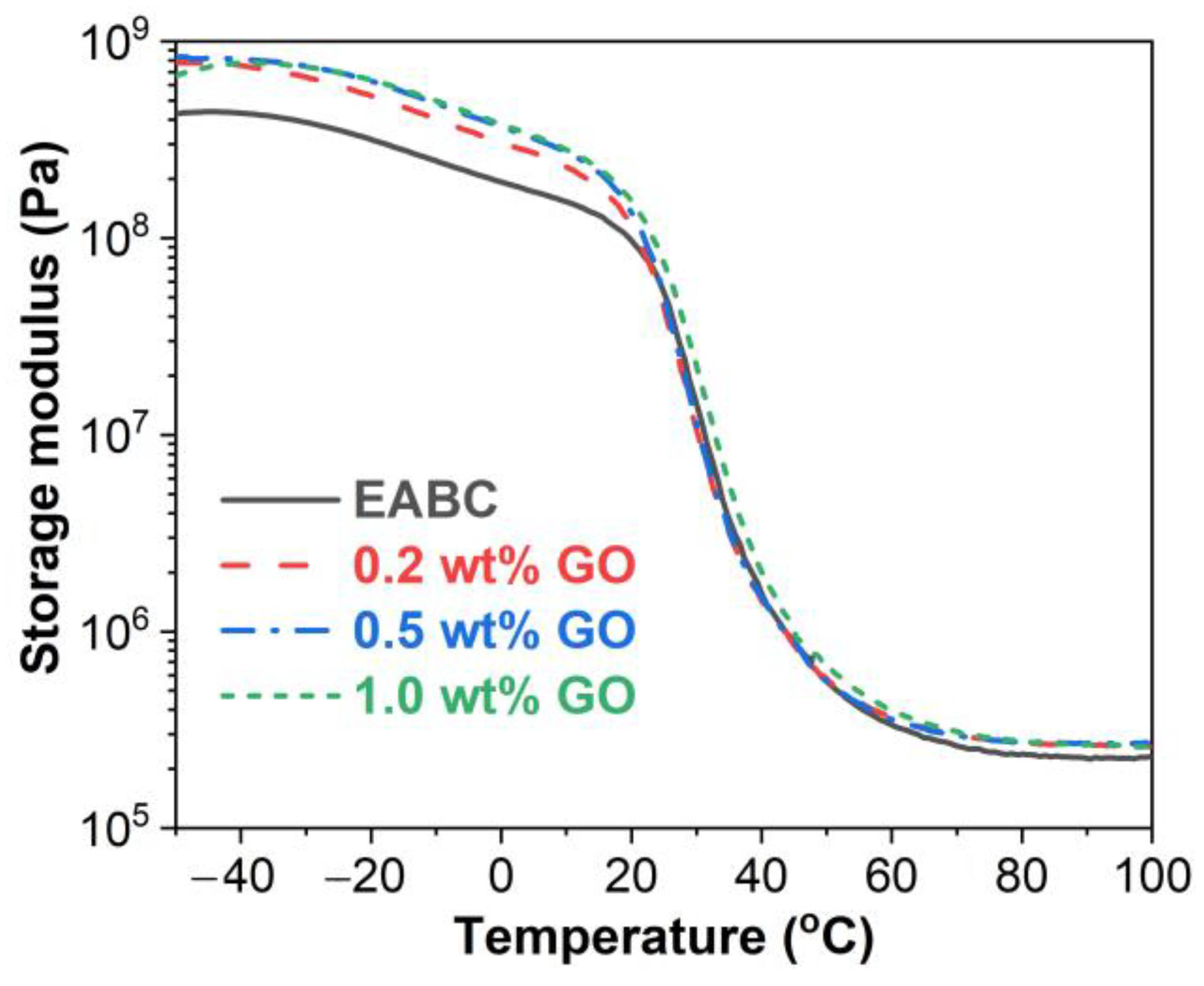
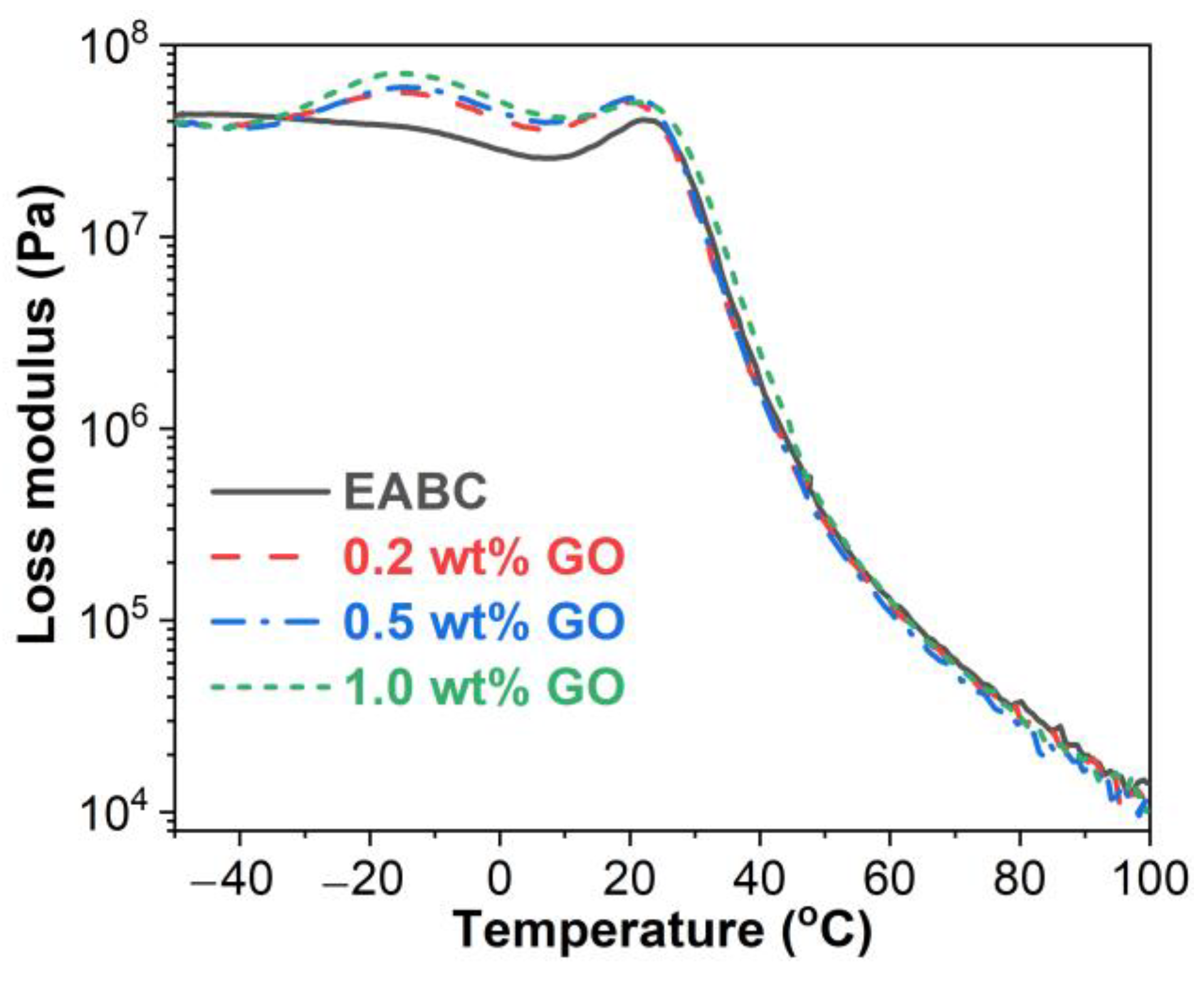
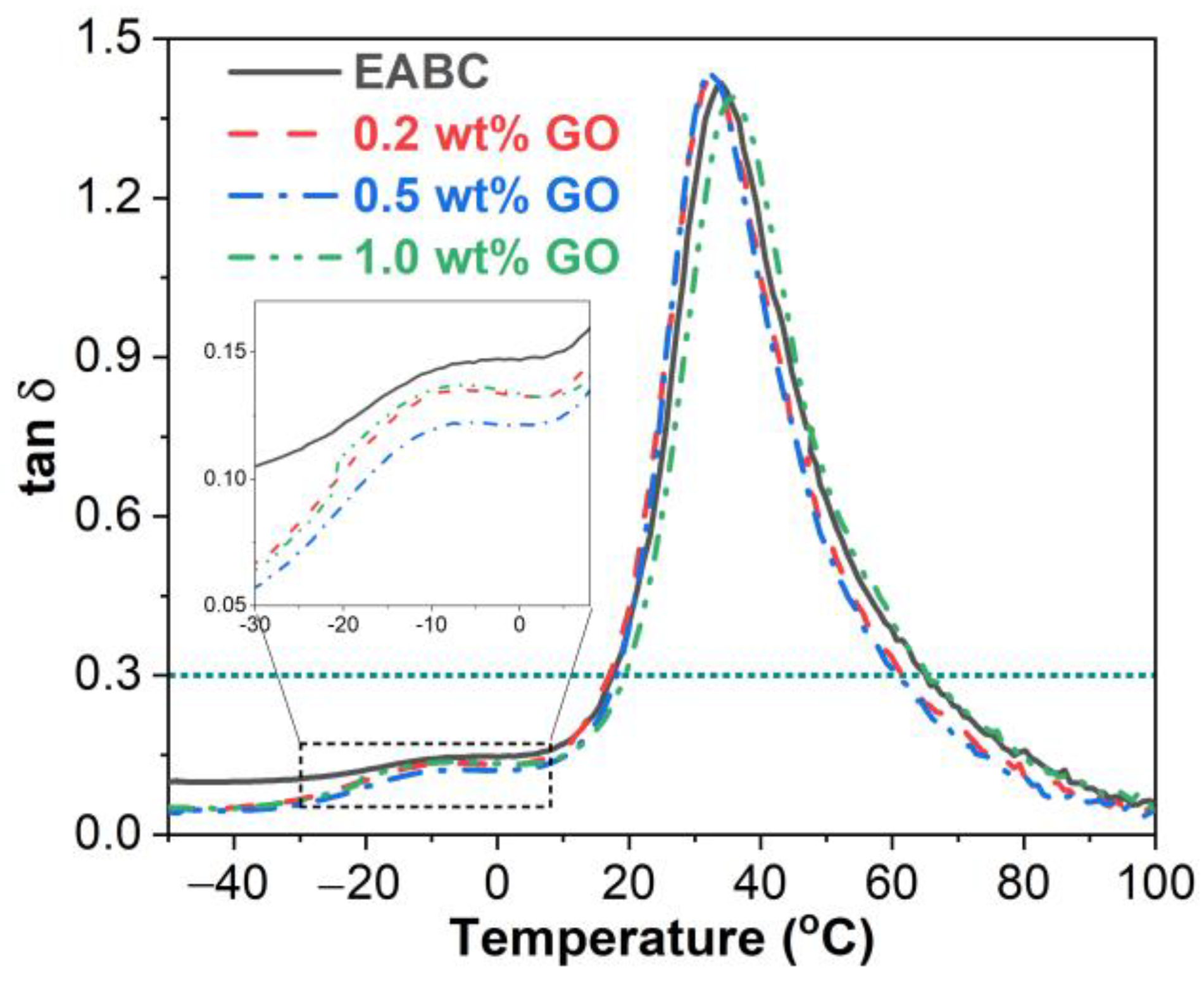
3.4. Dynamic Mechanical Properties
3.4.1. Storage Modulus (E’)
3.4.2. Glass Transitions
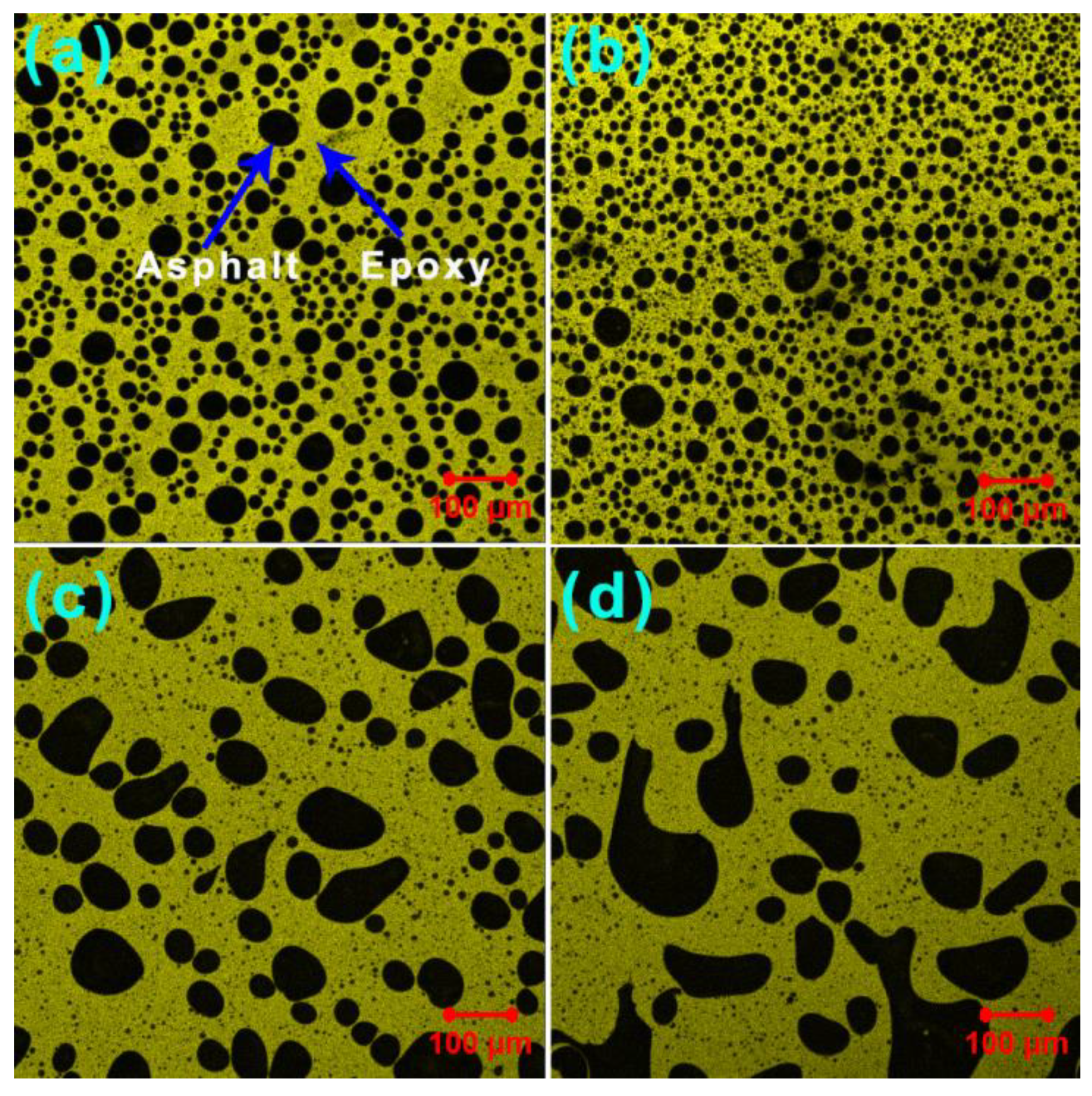
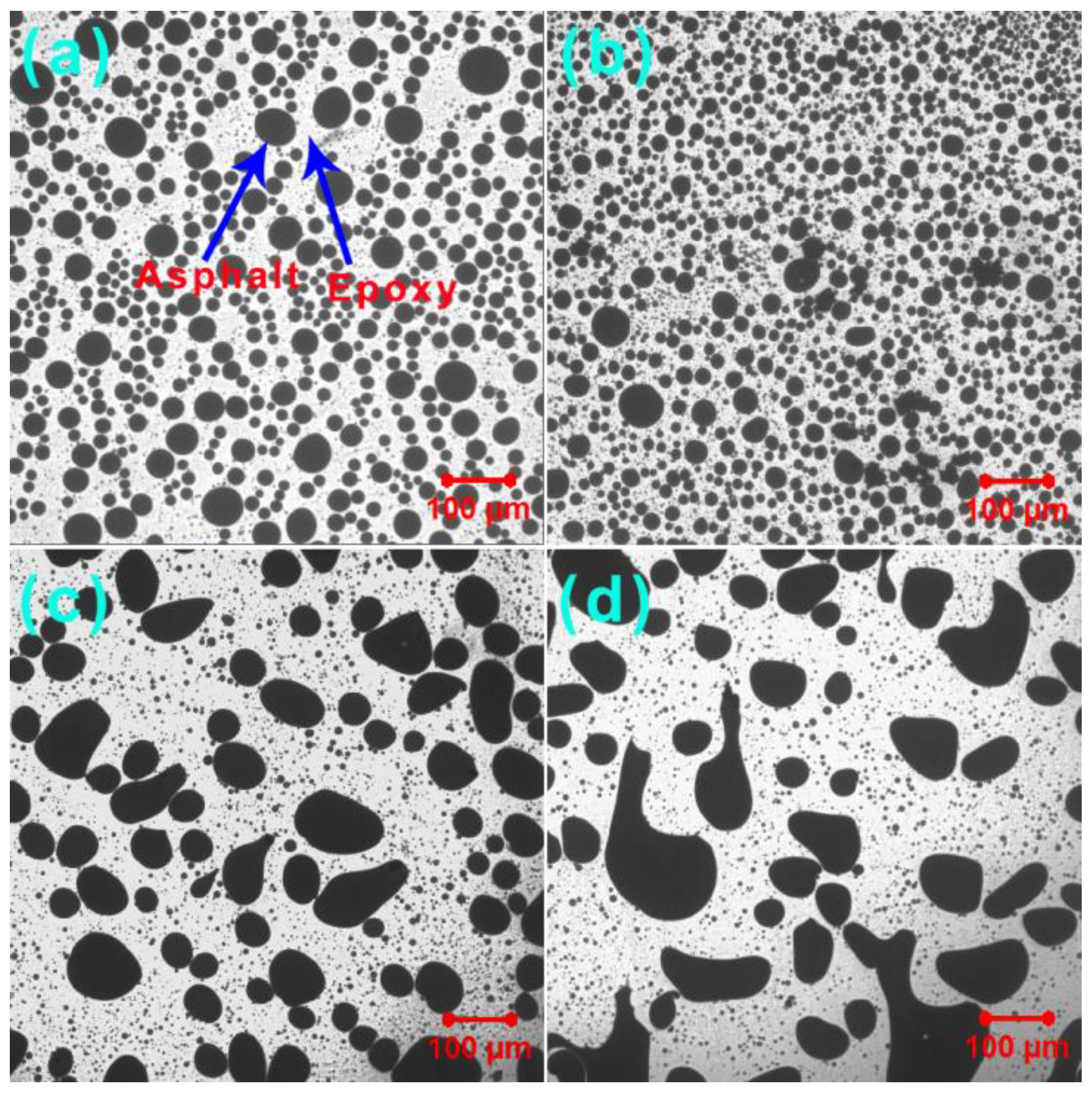
3.5. Phase-Separated Microstructures
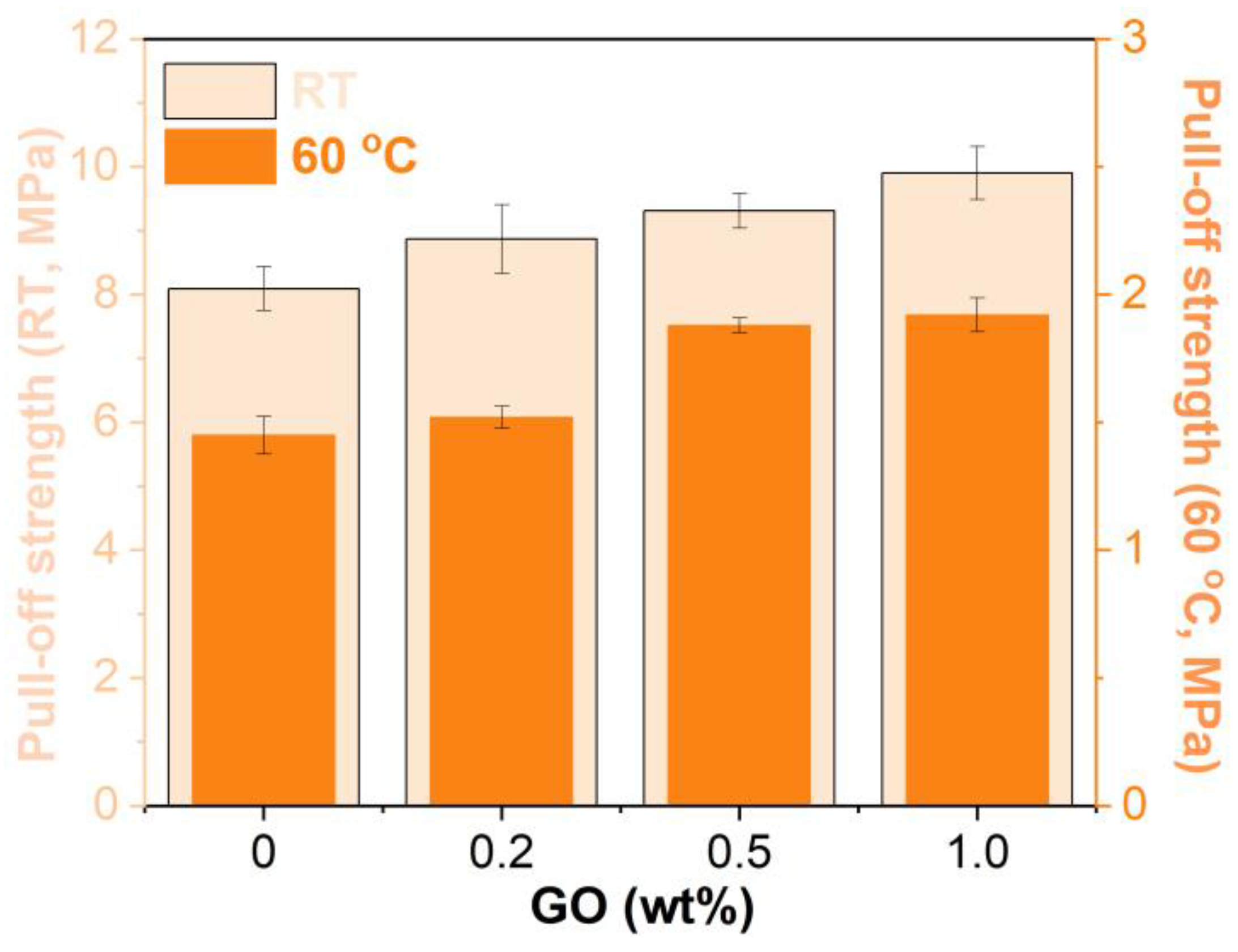
3.6. Pull-Off Strength
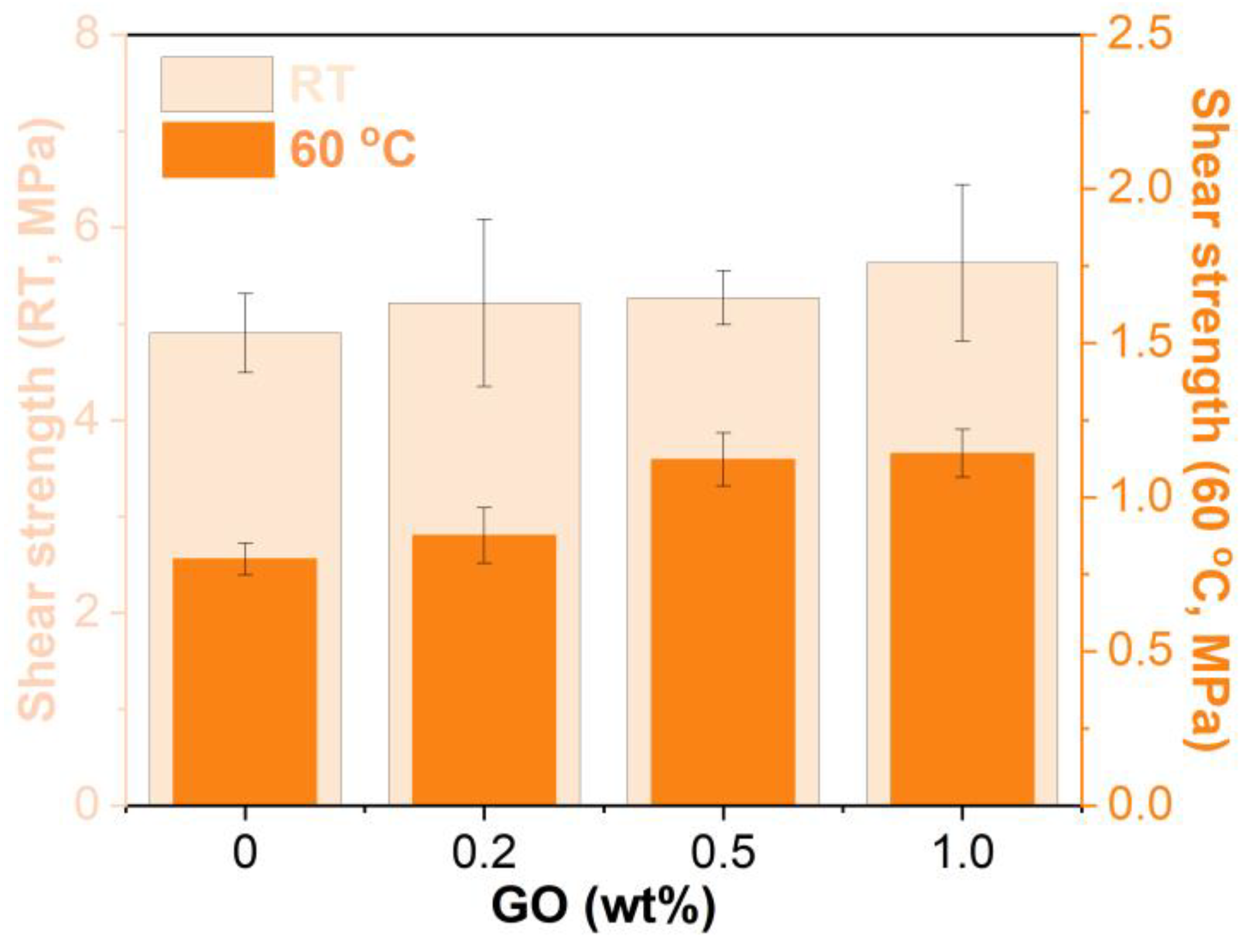
3.7. Shear Strength
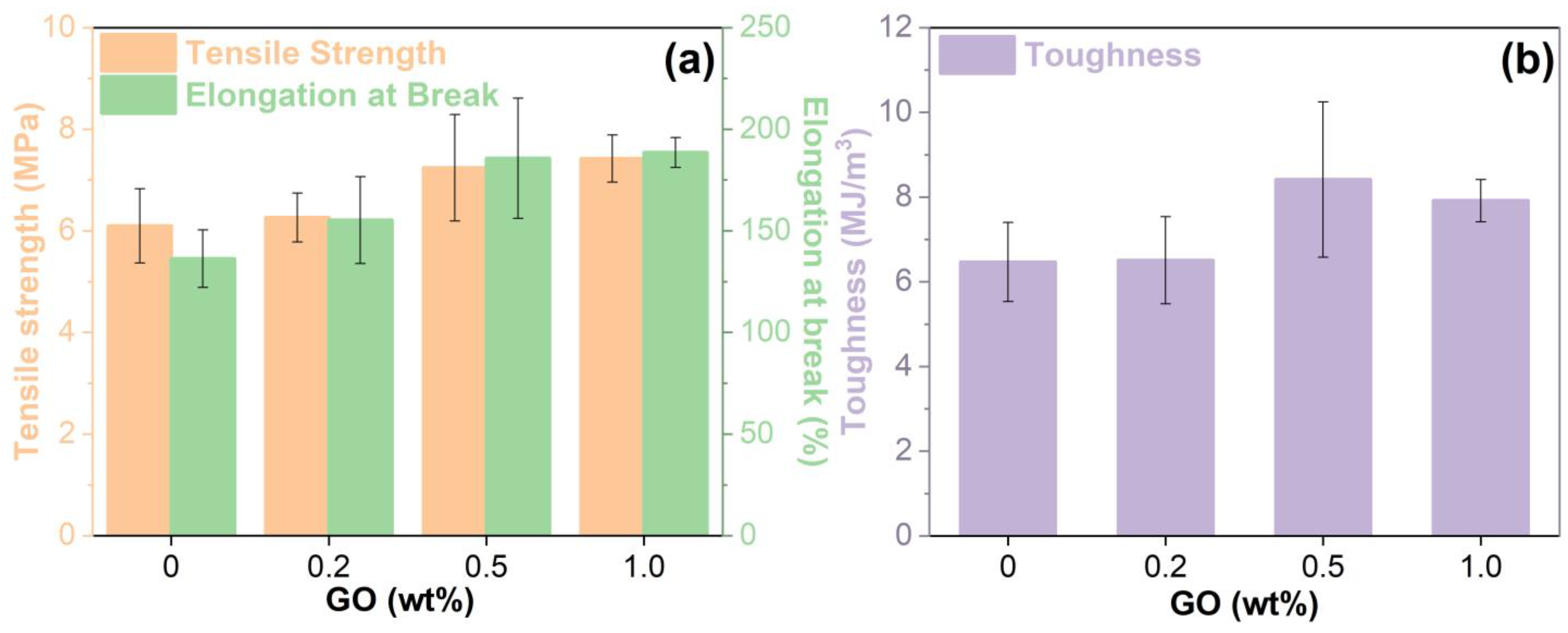
3.8. Mechanical Properties
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Mangus, A. Orthotropic steel decks. In Bridge Engineering Handbook: Superstructure Design, 2nd ed.; Chen, W.-F., Duan, L., Eds.; CRC Press: Boca Raton, FL, USA, 2014; pp. 589–646. [Google Scholar]
- Hulsey, J.L.; Yang, L.; Raad, L. Wearing surfaces for orthotropic steel bridge decks. Transp. Res. Rec. 1999, 1654, 141–150. [Google Scholar] [CrossRef]
- Seim, C.; Ingham, T. Influence of wearing surfacing on performance of orthotropic steel plate decks. Transp. Res. Rec. 2004, 1892, 98–106. [Google Scholar] [CrossRef]
- Connor, R.J.; Fisher, J.; Gatti, W.; Gopalaratnam, V.; Kozy, B.; Leshko, B.; McQuaid, D.L.; Medlock, R.; Mertz, D.; Murphy, T.; et al. Manual for Design, Construction, and Maintenance of Orthotropic Steel Deck Bridges; FHWA-IF-12-027; Federal Highway Administration: Washington, DC, USA, 2012.
- Hicks, R.; Dussek, I.; Seim, C. Asphalt surfaces on steel bridge decks. Transp. Res. Rec. 2000, 1740, 135–142. [Google Scholar] [CrossRef]
- Yao, B.; Li, F.; Wang, X.; Cheng, G. Evaluation of the shear characteristics of steel–asphalt interface by a direct shear test method. Int. J. Adhes. Adhes. 2016, 68, 70–79. [Google Scholar] [CrossRef]
- Xie, H.; Li, C.; Wang, Q. A critical review on performance and phase separation of thermosetting epoxy asphalt binders and bond coats. Constr. Build. Mater. 2022, 326, 126792. [Google Scholar] [CrossRef]
- Xie, H.; Zhao, R.; Wang, R.; Xi, Z.; Yuan, Z.; Zhang, J.; Wang, Q. Influence of thermal shock on the performance of B-staged epoxy bond coat for orthotropic steel bridge pavements. Constr. Build. Mater. 2021, 294, 123598. [Google Scholar] [CrossRef]
- Sun, Y.; Liu, Y.; Gong, J.; Han, X.; Xi, Z.; Zhang, J.; Wang, Q.; Xie, H. Thermal and bonding properties of epoxy asphalt bond coats. J. Therm. Anal. Calorim. 2022, 147, 2013–2025. [Google Scholar] [CrossRef]
- Li, C.; Han, X.; Gong, J.; Su, W.; Xi, Z.; Zhang, J.; Wang, Q.; Xie, H. Impact of waste cooking oil on the viscosity, microstructure and mechanical performance of warm-mix epoxy asphalt binder. Constr. Build. Mater. 2020, 251, 118994. [Google Scholar] [CrossRef]
- Su, W.; Zhao, R.; Wang, R.; Xi, Z.; Cai, J.; Zhang, J.; Wang, Q.; Xie, H. Microstructure and performance of epoxy asphalt binders modified by core-shell rubbers containing different core polymers. Constr. Build. Mater. 2021, 304, 124689. [Google Scholar] [CrossRef]
- Gong, J.; Liu, Y.; Jiang, Y.; Wang, Q.; Xi, Z.; Cai, J.; Xie, H. Performance of epoxy asphalt binder containing warm-mix asphalt additive. Int. J. Pavement Eng. 2021, 22, 223–232. [Google Scholar] [CrossRef]
- Gong, J.; Han, X.; Su, W.; Xi, Z.; Cai, J.; Wang, Q.; Li, J.; Xie, H. Laboratory evaluation of warm-mix epoxy SBS modified asphalt binders containing Sasobit. J. Build. Eng. 2020, 32, 101550. [Google Scholar] [CrossRef]
- Liu, Y.; Zhang, J.; Jiang, Y.; Li, C.; Xi, Z.; Cai, J.; Xie, H. Investigation of secondary phase separation and mechanical properties of epoxy SBS-modified asphalts. Constr. Build. Mater. 2018, 165, 163–172. [Google Scholar] [CrossRef]
- Li, X.; Shen, J.; Ling, T.; Mei, Q. Pyrolysis combustion characteristics of epoxy asphalt based on TG-MS and cone calorimeter test. Materials 2022, 15, 4973. [Google Scholar] [CrossRef]
- Gong, J.; Liu, Y.; Wang, Q.; Xi, Z.; Cai, J.; Ding, G.; Xie, H. Performance evaluation of warm mix asphalt additive modified epoxy asphalt rubbers. Constr. Build. Mater. 2019, 204, 288–295. [Google Scholar] [CrossRef]
- Su, W.; Han, X.; Gong, J.; Xi, Z.; Zhang, J.; Wang, Q.; Xie, H. Toughening epoxy asphalt binder using core-shell rubber nanoparticles. Constr. Build. Mater. 2020, 258, 119716. [Google Scholar] [CrossRef]
- Zhang, J.; Su, W.; Liu, Y.; Gong, J.; Xi, Z.; Zhang, J.; Wang, Q.; Xie, H. Laboratory investigation on the microstructure and performance of SBS modified epoxy asphalt binder. Constr. Build. Mater. 2021, 270, 121378. [Google Scholar] [CrossRef]
- Jiang, Y.; Zhao, R.; Xi, Z.; Cai, J.; Yuan, Z.; Zhang, J.; Wang, Q.; Xie, H. Improving toughness of epoxy asphalt binder with reactive epoxidized SBS. Mater. Struct. 2021, 54, 145. [Google Scholar] [CrossRef]
- Sun, Y.; Gong, J.; Liu, Y.; Jiang, Y.; Xi, Z.; Cai, J.; Xie, H. Viscous, damping, and mechanical properties of epoxy asphalt adhesives containing different penetration-grade asphalts. J. Appl. Polym. Sci. 2019, 136, 47027. [Google Scholar] [CrossRef]
- Sun, Y.; Han, X.; Su, W.; Gong, J.; Xi, Z.; Zhang, J.; Wang, Q.; Xie, H. Mechanical and bonding properties of pristine montmorillonite reinforced epoxy asphalt bond coats. Polym. Compos. 2020, 41, 3034–3042. [Google Scholar] [CrossRef]
- Sun, Y.; Liu, Y.; Jiang, Y.; Xu, K.; Xi, Z.; Xie, H. Thermal and mechanical properties of natural fibrous nanoclay reinforced epoxy asphalt adhesives. Int. J. Adhes. Adhes. 2018, 85, 308–314. [Google Scholar] [CrossRef]
- Huang, Q.; Qian, Z.; Chen, L.; Zhang, M.; Zhang, X.; Sun, J.; Hu, J. Evaluation of epoxy asphalt rubber with silane coupling agent used as tack coat for seasonally frozen orthotropic steel bridge decks. Constr. Build. Mater. 2020, 241, 117957. [Google Scholar] [CrossRef]
- Keyte, J.; Pancholi, K.; Njuguna, J. Recent developments in graphene oxide/epoxy carbon fiber-reinforced composites. Front. Mater. 2019, 6, 224. [Google Scholar] [CrossRef]
- Shi, G.; Araby, S.; Gibson, C.T.; Meng, Q.; Zhu, S.; Ma, J. Graphene platelets and their polymer composites: Fabrication, structure, properties, and applications. Adv. Funct. Mater. 2018, 28, 1706705. [Google Scholar] [CrossRef]
- Chen, D.; Feng, H.; Li, J. Graphene oxide: Preparation, functionalization, and electrochemical applications. Chem. Rev. 2012, 112, 6027–6053. [Google Scholar] [CrossRef] [PubMed]
- Dreyer, D.R.; Park, S.; Bielawski, C.W.; Ruoff, R.S. The chemistry of graphene oxide. Chem. Soc. Rev. 2010, 39, 228–240. [Google Scholar] [CrossRef]
- Gao, W. The chemistry of graphene oxide. In Graphene Oxide: Reduction Recipes, Spectroscopy, and Applications; Gao, W., Ed.; Springer International Publishing: Cham, Switzerland, 2015; pp. 61–95. [Google Scholar]
- Konios, D.; Stylianakis, M.M.; Stratakis, E.; Kymakis, E. Dispersion behaviour of graphene oxide and reduced graphene oxide. J. Colloid Interface Sci. 2014, 430, 108–112. [Google Scholar] [CrossRef]
- Adnan, A.M.; Lü, C.; Luo, X.; Wang, J. Impact of graphene oxide on zero shear viscosity, fatigue life and low-temperature properties of asphalt binder. Materials 2021, 14, 3073. [Google Scholar] [CrossRef]
- Zeng, W.; Wu, S.; Pang, L.; Sun, Y.; Chen, Z. The utilization of graphene oxide in traditional construction materials: Asphalt. Materials 2017, 10, 48. [Google Scholar] [CrossRef]
- Hou, W.; Gao, Y.; Wang, J.; Blackwood, D.J.; Teo, S. Recent advances and future perspectives for graphene oxide reinforced epoxy resins. Mater. Today Commun. 2020, 23, 100883. [Google Scholar] [CrossRef]
- Zhao, Y.; Jia, X.; Li, L.; Jiang, X.; Xu, R. Effects of surface modified graphene oxide on the cure kinetics of warm-mixed epoxy-asphalt. Polym. Sci. Ser. B 2022, 64, 229–239. [Google Scholar] [CrossRef]
- Zhao, R.; Jing, F.; Li, C.; Wang, R.; Xi, Z.; Cai, J.; Wang, Q.; Xie, H. Viscosity-curing time behavior, viscoelastic properties, and phase separation of graphene oxide/epoxy asphalt composites. Polym. Compos. 2022, 43, 5454–5464. [Google Scholar] [CrossRef]
- Si, J.; Wang, J.; Li, Y.; Ma, J.; Ruan, W.; Yu, X.; Jiang, R. Enhanced mechanical performances of epoxy asphalt adhesives modified by graphene oxide. Road Mater. Pavement Des. 2022, 1–15. [Google Scholar] [CrossRef]
- Zhao, R.; Jing, F.; Wang, R.; Cai, J.; Zhang, J.; Wang, Q.; Xie, H. Influence of oligomer content on viscosity and dynamic mechanical properties of epoxy asphalt binders. Constr. Build. Mater. 2022, 338, 127524. [Google Scholar] [CrossRef]
- Jiang, Y.; Liu, Y.; Gong, J.; Li, C.; Xi, Z.; Cai, J.; Xie, H. Microstructures, thermal and mechanical properties of epoxy asphalt binder modified by SBS containing various styrene-butadiene structures. Mater. Struct. 2018, 51, 86. [Google Scholar] [CrossRef]
- Asphalt Institute. Performance Graded Asphalt Binder Specification and Testing Superpave; Asphalt Institute: Lexington, KY, USA, 2003. [Google Scholar]
- Jiang, Y.; Han, X.; Gong, J.; Xi, Z.; Cai, J.; Wang, Q.; Ding, G.; Xie, H. Laboratory investigation of epoxy asphalt binder modified by brominated SBS. Constr. Build. Mater. 2019, 228, 116733. [Google Scholar] [CrossRef]
- GB/T 30598; General Specifications of Epoxy Asphalt Materials for Paving Roads and Bridges. Chinese Standard: Beijing, China, 2014.
- Qiu, S.L.; Wang, C.S.; Wang, Y.T.; Liu, C.G.; Chen, X.Y.; Xie, H.F.; Huang, Y.A.; Cheng, R.S. Effects of graphene oxides on the cure behaviors of a tetrafunctional epoxy resin. eXPRESS Polym. Lett. 2011, 5, 809–818. [Google Scholar] [CrossRef]
- Xie, H.; Liu, B.; Yuan, Z.; Shen, J.; Cheng, R. Cure kinetics of carbon nanotube/tetrafunctional epoxy nanocomposites by isothermal differential scanning calorimetry. J. Polym. Sci. Part B: Polym. Phys. 2004, 42, 3701–3712. [Google Scholar] [CrossRef]
- Duncan, J. Principles and applications of mechanical thermal analysis. In Principles and Applications of Thermal Analysis; Gabbott, P., Ed.; Blackwell Publishing: Oxford, UK, 2008; pp. 119–163. [Google Scholar]
- Jing, F.; Zhao, R.; Li, C.; Xi, Z.; Wang, Q.; Xie, H. Influence of the Epoxy/Acid Stoichiometry on the Cure Behavior and Mechanical Properties of Epoxy Vitrimers. Molecules 2022, 27, 6335. [Google Scholar] [CrossRef]
- Menard, K.P.; Menard, N.R. Dynamic Mechanical Analysis, 3rd ed.; CRC Press: Boca Raton, FL, USA, 2020. [Google Scholar]
- Liu, Y.; Zhang, J.; Chen, R.; Cai, J.; Xi, Z.; Xie, H. Ethylene vinyl acetate copolymer modified epoxy asphalt binders: Phase separation evolution and mechanical properties. Constr. Build. Mater. 2017, 137, 55–65. [Google Scholar] [CrossRef]
- Yin, H.; Jin, H.; Wang, C.; Sun, Y.; Yuan, Z.; Xie, H.; Wang, Z.; Cheng, R. Thermal, damping, and mechanical properties of thermosetting epoxy-modified asphalts. J. Therm. Anal. Calorim. 2014, 115, 1073–1080. [Google Scholar] [CrossRef]
- Yin, H.; Zhang, Y.; Sun, Y.; Xu, W.; Yu, D.; Xie, H.; Cheng, R. Performance of hot mix epoxy asphalt binder and its concrete. Mater. Struct. 2015, 48, 3825–3835. [Google Scholar] [CrossRef]
- Sharifnabi, A.; Fathi, M.H.; Yekta, B.E.; Hossainalipour, M. The structural and bio-corrosion barrier performance of Mg-substituted fluorapatite coating on 316L stainless steel human body implant. Appl. Surf. Sci. 2014, 288, 331–340. [Google Scholar] [CrossRef]
- Chen, R.; Gong, J.; Jiang, Y.; Wang, Q.; Xi, Z.; Xie, H. Halogen-free flame retarded cold-mix epoxy asphalt binders: Rheological, thermal and mechanical characterization. Constr. Build. Mater. 2018, 186, 863–870. [Google Scholar] [CrossRef]
- Chen, R.; Zhao, R.; Liu, Y.; Cai, J.; Xi, Z.; Zhang, J.; Wang, Q.; Xie, H. Development of eco-friendly fire-retarded warm-mix epoxy asphalt binders using reactive polymeric flame retardants for road tunnel pavements. Constr. Build. Mater. 2021, 284, 122752. [Google Scholar] [CrossRef]
- Li, C.; Gong, J.; Zhao, R.; Xi, Z.; Wang, Q.; Xie, H. Laboratory performance of recycled polyethylene modified epoxy asphalt binders. Int. J. Pavement Eng. 2022. [Google Scholar] [CrossRef]






| Property | Standard | Value |
|---|---|---|
| Penetration (25 °C, 0.1 mm) | ASTM D5-06 | 91.0 |
| Ductility (10 °C, cm) | ASTM D113-07 | 93.0 |
| Softening point (°C) | ASTM D36-06 | 46.3 |
| Viscosity (120 °C, mPa·s) | ASTM D4402-06 | 787 |
| Saturates (%) | ASTM D4124-09 | 16.7 |
| Aromatics (%) | 33.9 | |
| Resins (%) | 44.7 | |
| Asphaltenes (%) | 4.7 |
| Property | Value |
|---|---|
| Number of layers | 1~2 |
| Diameter (µm) | 10~50 |
| Thickness (nm) | ~1 |
| Carbon content (%) | <50 |
| Oxygen content (%) | >42 |
| Sulfur content (%) | <4 |
| Purity (%) | 95 |
| GO (wt%) | Tg of asphalt (°C) | Tg of epoxy (°C) | ||
|---|---|---|---|---|
| E” | tan δ | E” | tan δ | |
| 0 | −13.7 | −8.6 | 22.1 | 34.0 |
| 0.2 | −14.4 | −10.2 | 20.6 | 32.5 |
| 0.5 | −14.4 | −10.9 | 20.8 | 31.8 |
| 1.0 | −16.2 | −9.4 | 21.4 | 35.9 |
| GO (wt%) | dn (mm) | dw (mm) | PDI |
|---|---|---|---|
| 0 | 23.1 | 34.0 | 1.47 |
| 0.2 | 15.6 | 18.9 | 1.21 |
| 0.5 | 17.3 | 51.0 | 2.95 |
| 1.0 | 17.3 | 56.6 | 3.27 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhang, J.; Wang, R.; Zhao, R.; Jing, F.; Li, C.; Wang, Q.; Xie, H. Graphene Oxide-Modified Epoxy Asphalt Bond Coats with Enhanced Bonding Properties. Materials 2022, 15, 6846. https://doi.org/10.3390/ma15196846
Zhang J, Wang R, Zhao R, Jing F, Li C, Wang Q, Xie H. Graphene Oxide-Modified Epoxy Asphalt Bond Coats with Enhanced Bonding Properties. Materials. 2022; 15(19):6846. https://doi.org/10.3390/ma15196846
Chicago/Turabian StyleZhang, Junsheng, Rui Wang, Ruikang Zhao, Fan Jing, Chenxuan Li, Qingjun Wang, and Hongfeng Xie. 2022. "Graphene Oxide-Modified Epoxy Asphalt Bond Coats with Enhanced Bonding Properties" Materials 15, no. 19: 6846. https://doi.org/10.3390/ma15196846





