Diatomaceous Earth—Lightweight Pozzolanic Admixtures for Repair Mortars—Complex Chemical and Physical Assessment
Abstract
:1. Introduction
2. Materials and Methods
2.1. Raw Materials
2.2. Chemical and Physical Analysis of Raw Materials
2.3. Blended Pastes—Preparation and Characterization
3. Results and Discussion
3.1. Properties of Fresh Pastes
3.2. Properties of 28-Days and 90-Days Pastes
4. Conclusions
- (i)
- Three basic factors influence the contribution of diatomaceous earth to cement mixture strength when partially replacing Portland cement. These are the filler effect, the pertinent acceleration of ordinary Portland cement hydration, and the pozzolanic reaction of diatomite with cement hydrates.
- (ii)
- The filler effect is immediate. The acceleration of ordinary Portland cement hydration has a significant impact within the first 24 h, leading to the shortening of both initial and final setting time.
- (iii)
- Due to the chemical and phase composition, particle size, and morphology of diatomaceous particles, the examined blended pastes exhibited distinct rheology, heat release, the time evolution of hydration and pozzolanic reactions, structural parameters, and, consequently, mechanical strength.
- (iv)
- The progress in hydration and pozzolanic reactions was apparent from assessing 28-day and 90-day hardened samples.
- (v)
- Based on received SAI values, the researched mineral admixtures were classified as highly reactive pozzolans, which can be beneficially applied in the design and production of potentially more durable repair mortars applicable also for historical masonry with excessive moisture presence. However, the analyzed diatomaceous earth can also find use in the production of blended cement, cement-based composites, or as a part of multi-component binders. The ability to improve the reactivity of diatomite by additional grinding must also be considered in the preparation of blended binders.
- (vi)
- The optimum replacement level of ordinary Portland cement by diatomaceous earth to give maximum long-term strength enhancement is about 10 wt.%. However, an even higher dosage of diatomaceous earth provides materials of sufficient strength and Young’s modulus. For example, in the case of ENO7, the improvement in mechanical parameters was found to be up to 15% Portland cement replacement. With respect to identified SAI, the safe value of Portland cement substitution is 20 wt.%.
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Vyšvařil, M.; Pavlíková, M.; Záleská, M.; Pivák, A.; Žižlavský, T.; Rovnaníková, P.; Bayer, P.; Pavlík, Z. Non-hydrophobized perlite renders for repair and thermal insulation purposes: Influence of different binders on their properties and durability. Constr. Build. Mater. 2020, 263, 120617. [Google Scholar] [CrossRef]
- Pavlík, Z.; Pokorný, J.; Pavlíková, M.; Zemanová, L.; Záleská, M.; Vyšvăril, M.; Žižlavský, T. Mortars with crushed lava granulate for repair of damp historical buildings. Materials 2019, 12, 3557. [Google Scholar] [CrossRef] [PubMed]
- Loureiro, A.M.S.; Paz, S.P.A.; Veiga, M.R.; Angélica, R.S. Assessment of compatibility between historic mortars and lime-METAKAOLIN restoration mortars made from amazon industrial waste. Appl. Clay Sci. 2020, 198, 105843. [Google Scholar] [CrossRef]
- Callebaut, K.; Elsen, J.; van Balen, K.; Viaene, W. Nineteenth century hydraulic restoration mortars in the Saint Michael’s Church (Leuven, Belgium): Natural hydraulic lime or cement? Cem. Concr. Res. 2001, 31, 397–403. [Google Scholar] [CrossRef]
- Ayati, B.; Newport, D.; Wong, H.; Cheeseman, C. Low-carbon cements: Potential for low-grade calcined clays to form supplementary cementitious materials. Clean. Mater. 2022, 5, 100099. [Google Scholar] [CrossRef]
- Scrivener, K.L.; John, V.M.; Gartner, E.M. UN Environment. Eco-efficient cements: Potential economically viable solutions for a low-CO2 cement-based materials industry. Cem. Concr. Res. 2018, 114, 2–26. [Google Scholar] [CrossRef]
- Athira, G.; Bahurudeen, A. Rheological properties of cement paste blended with sugarcane bagasse ash and rice straw ash. Constr. Build. Mater. 2022, 332, 127377. [Google Scholar] [CrossRef]
- Fediuk, R.; Smoliakov, A.; Stoyushko, N. Increase in composite binder activity. IOP Conf. Ser. Mater. Sci. Eng. 2016, 156, 012042. [Google Scholar] [CrossRef]
- Siddique, S.; Jang, J.G.; Gupta, T. Developing marble slurry as supplementary cementitious material through calcination: Strength and microstructure study. Constr. Build. Mater. 2021, 293, 123474. [Google Scholar] [CrossRef]
- Biajawi, M.I.A.; Embong, R.; Muthusamy, K.; Ismail, N.; Obianyo, I. Recycled coal bottom ash as sustainable materials for cement replacement in cementitious Composites: A review. Constr. Build. Mater. 2022, 338, 127624. [Google Scholar] [CrossRef]
- Shi, C.; Fernández-Jimenez, A.; Palomo, A. New cements for 21st century: The pursuit of an alternative to Portland cement. Cem. Concr. Res. 2011, 41, 750–763. [Google Scholar] [CrossRef]
- Zaleska, M.; Pavlikova, M.; Pavlik, Z.; Jankovsky, O.; Pokorny, J.; Tydlitat, V.; Svora, P.; Cerny, R. Physical and chemical characterization of technogenic pozzolans for the application in blended cements. Constr. Build. Mater. 2018, 160, 106–116. [Google Scholar] [CrossRef]
- Barabanshchikov, Y.; Usanova, K. Influence of silica fume on high-calcium fly ash expansion during hydration. Materials 2022, 15, 3544. [Google Scholar] [CrossRef]
- Amran, M.; Fediuk, R.; Murali, G.; Avudaiappan, S.; Ozbakkaloglu, T.; Vatin, N.; Karelina, M.; Klyuev, S.; Cholampour, A. Flay ash-based eco-efficient concretes: A comprehensive review of the short-term properties. Materials 2021, 14, 4264. [Google Scholar] [CrossRef]
- Amran, M.; Murali, G.; Khalid, N.H.A.; Fediuk, R.; Ozbakkalogiu, T.; Lee, Y.H.; Haruna, S.; Lee, Y.Y. Slag uses in making an ecofriendly and sustainable concrete: A review. Constr. Build. Mater. 2021, 272, 121942. [Google Scholar] [CrossRef]
- Zhang, Y.; Schlangen, E.; Çopuroğlu, O. Effect of slags of different origins and the role of sulfur in slag on the hydration characteristics of cement-slag systems. Constr. Build. Mater. 2022, 316, 125266. [Google Scholar] [CrossRef]
- Siddique, S.; Kim, H.; Jang, J.G. Properties of high-volume slag cement mortar incorporating circulating fluidized bed combustion fly ash and bottom ash. Constr. Build. Mater. 2021, 289, 123150. [Google Scholar] [CrossRef]
- Kocak, Y. Effects of metakaolin on the hydration development of Portland-composite cement. J. Build. Eng. 2020, 31, 101419. [Google Scholar] [CrossRef]
- Bucher, R.; Cyr, M.; Escadeillas, G. Performance-based evaluation of flash-metakaolin as cement replacement in marine structures-Case of chloride migration and corrosion. Constr. Build. Mater. 2021, 267, 120926. [Google Scholar] [CrossRef]
- Izadifard, R.A.; Moghadam, M.A.; Sepahi, M.M. Influence of metakaolin as a partial replacement of cement on characteristics of concrete exposed to high temperatures. J. Sust. Cement-Based Mater. 2021, 10, 336–352. [Google Scholar] [CrossRef]
- Jankovsky, O.; Pavlikova, M.; Sedmidubsky, D.; Bousa, D.; Lojka, M.; Pokorny, J.; Zaleska, M.; Pavlik, Z. Study on pozzolana activity of wheat straw ash as potential admixture for blended cements. Ceramics-Silikaty 2017, 61, 327–339. [Google Scholar] [CrossRef] [Green Version]
- Sierra, E.J.; Miller, A.; Sakulich, A.R.; MacKenzie, K.; Barsoum, M.W. Pozzolanic activity of diatomaceous earth. J. Am. Ceram. Soc. 2010, 93, 3406–3410. [Google Scholar] [CrossRef]
- Flower, R.J. Diatoms in Ancient Building Materials: Application of Diatom Analysis to Egyptian Mud Bricks. Nova Hedwigia 2006, 130, 245–264. [Google Scholar]
- Paschen, S. Diatomaceous earth extraction, processing and application. Erzmetall 1986, 39, 158–161. [Google Scholar]
- Reka, A.A.; Pavlovski, B.; Fazlija, E.; Berisha, A.; Pacarizi, M.; Daghmehchi, M.; Sacalis, C.; Jovanovski, G.; Makreski, P.; Oral, A. Diatomaceous Earth: Characterization, thermal modification, and application. Open Chem. 2021, 19, 451–461. [Google Scholar] [CrossRef]
- Lemons, J.F. Diatomite. Am. Ceramic Soc. Bull. 1997, 76, 92–95. [Google Scholar]
- Dolley, T.P. Diatomite. Ceramic Bull. 1991, 70/5, 860. [Google Scholar]
- Rodriguez, C.; Minamo, I.; Para, C.; Pujante, P.; Benito, F. Properties of Precast Concrete Using Food Industry-Filtered Recycled Diatoms. Sustainability 2021, 13, 3137. [Google Scholar] [CrossRef]
- Goren, R.; Bazkara, T.; Marsoglu, M. A study on the purification of diatomite in hydrochloric acid. Scand. J. Metall. 2002, 31, 115–119. [Google Scholar] [CrossRef]
- Pimraksa, K.; Chindaprasirt, P. Lightweight bricks made of diatomaceous earth, lime and gypsum. Ceram. Int. 2009, 35, 471–478. [Google Scholar] [CrossRef]
- Man, J.; Gao, W.; Yan, S.; Liu, S.; Hao, H. Preparation of porous brick from diatomite and sugar filter mud at lower temperature. Constr. Build. Mater. 2017, 156, 135–1042. [Google Scholar] [CrossRef]
- Jiang, F.; Ling, X.; Zhang, L.; Cang, D.; Ding, Y. Modified diatomite-based porous ceramic to develop shape-stabilized NaNO3 salt with enhanced thermal conductivity for thermal energy storage. Sol. Energy Mater. Sol. Cells 2021, 231, 111328. [Google Scholar] [CrossRef]
- Han, L.; Li, F.; Deng, X.; Wang, J.; Zhang, H.; Zhang, S. Foam-gelcasting preparation, microstructure and thermal insulation performance of porous diatomite ceramics with hierarchical pore structures. J. Eur. Ceram. Soc. 2017, 37, 2717–2725. [Google Scholar] [CrossRef]
- Sedlacik, M.; Nguyen, M.; Opravil, T.; Sokolar, R. Preparation and characterization of glass-ceramic foam from clay-rich waste diatomaceous earth. Materials 2022, 15, 1384. [Google Scholar] [CrossRef]
- Lauermannova, A.-M.; Lojka, M.; Jankovsky, O.; Faltysova, I.; Pavlikova, M.; Pivak, A.; Zaleska, M.; Pavlik, Z. High-performance magnesium oxychloride composites with silica sand and diatomite. J. Mater. Res. Technol. 2021, 11, 957–969. [Google Scholar] [CrossRef]
- Xu, T.; Wu, F.; Zou, T.; Li, J.; Yang, J.; Zhou, X.; Liu, D.; Bie, Y. Development of diatomite-based shape-stabilized composite phase change material for use in floor radiant heating. J. Mol. Liq. 2022, 348, 118372. [Google Scholar] [CrossRef]
- Li, M. Mechanical and thermal performance assessment of paraffin/expanded vermiculite-diatomite composite phase change materials integrated mortar: Experimental and numerical approach. Sol. Energy 2021, 227, 343–353. [Google Scholar] [CrossRef]
- Kadscheev, I.D.; Popov, A.G.; Ivanov, S.E. Improving the thermal insulation of high-temperature furnaces by the use of diatomite. Refract. Ind. Ceram. 2009, 50, 98–100. [Google Scholar] [CrossRef]
- Li, X.Q.; Yu, T.Y.; Park, S.J.; Kim, Y.H. Reinforcing effects of gypsum composite with basalt fiber and diatomite for improvement of high-temperature endurance. Constr. Build. Mater. 2022, 325, 126762. [Google Scholar] [CrossRef]
- Taoukil, D.; El meski, Y.; Lahlaouti, M.I.; Djedjig, R.; El bouardi, A. Effect of the use of diatomite as partial replacement of sand on thermal and mechanical properties of mortars. J. Build. Eng. 2021, 42, 103038. [Google Scholar] [CrossRef]
- Gunduz, L.; Kalkan, S.O. The effect of different natural porous aggregates on thermal characteristic feature in cementitious lightweight mortars for sustainable buildings. Iranian J. Sci. Technol. Trans. Civ. Eng. 2022. [Google Scholar] [CrossRef]
- Aruntas, H.Y.; Tokyay, M. The use of diatomite as pozzolanic material in blended cement production. Cem. Concr. World 1996, 1, 3–41. [Google Scholar]
- Yilmaz, B.; Ediz, N. The use of raw and calcined diatomite in cement production. Cem. Concr. Compos. 2008, 30, 202–211. [Google Scholar] [CrossRef]
- Li, J.; Zhang, W.; Chen, L.; Monteiro, P.J.M. Green concrete containing diatomaceous earth and limestone: Workability, mechanical properties, and life-cycle assessment. J. Clean. Prod. 2019, 223, 662–679. [Google Scholar] [CrossRef]
- Kastis, D. Properties and hydration of blended cements with calcareous diatomite. Cem. Concr. Res. 2006, 36, 1821–1826. [Google Scholar] [CrossRef]
- dos Santos, A.A.M.; Cordeiro, G.C. Investigation of particle characteristics and enhancing the pozzolanic activity of diatomite by grinding. Mater. Chem. Phys. 2021, 270, 124799. [Google Scholar] [CrossRef]
- Aydin, A.C.; Gül, R. Influence of volcanic originated natural materials as additive on the setting time and some mechanical properties of concrete. Constr. Build. Mater. 2007, 21, 1277–1281. [Google Scholar] [CrossRef]
- Lim, N.W.; Sabaa, B.A. The Pozzolanic Activity of Calcined Diatomite as a Factor of Temperature and Time. Key Eng. Mater. 1991, 53, 530–535. [Google Scholar] [CrossRef]
- Fragoulis, D.; Stamatkis, M.G.; Papageorgiou, D.; Chaniotakis, E. The physical and mechanical properties of composite cements manufactured with calacareous and clayey Greek diatomite mixtures. Cem. Concr. Compos. 2005, 27, 205–209. [Google Scholar] [CrossRef]
- Starnatakis, M.G.; Fragoulls, D.; Csirik, G.; Bedelean, I.; Pedersen, S. The influence of biogenic micro-silica-rich rocks on the properties of blended cements. Cem. Concr. Compos. 2003, 25, 177–184. [Google Scholar] [CrossRef]
- Değimenci, N.; Yilmaz, A. Use of diatomite as partial replacement for Portland cement in cement mortars. Constr. Build. Mater. 2009, 23, 284–288. [Google Scholar] [CrossRef]
- Yilmaz, B. A study on the effects of diatomite blend in natural pozzolan-blended cements. Adv. Cem. Res. 2008, 20, 13–21. [Google Scholar] [CrossRef]
- EN 197-1; Cement–Part 1: Composition, Specifications and Conformity Criteria for Common Cements. European Committee for Standardization: Brussels, Belgium, 2011.
- Bogue, R.H. Calculation of the Compounds in Portland Cement. Ind. Eng. Chem. Anal. 1929, 1, 192–197. [Google Scholar] [CrossRef]
- ASTM C618; Standard Specification for Coal Fly Ash and Raw or Calcined Natural Pozzolan for Use as a Mineral Admixture in Concrete. ASTM: Wet Conshohocken, PA, USA, 1998.
- Rietveld, H.M. Line profiles of neutron powder-diffraction peaks for structure refinement. Acta Cryst. 1967, 22, 151–152. [Google Scholar] [CrossRef]
- Fernández-Carrasco, L.; Torrens-Martín, D.; Morales, L.M.; Martínez-Ramírez, S. Chapter 19 Infrared Spectroscopy in the Analysis of Building and Construction Materials. In Infrared Spectroscopy-Materials Science, Engineering and Technology; Theophanides, T., Ed.; BoD–Books on Demand: Norderstedt, Germany, 2012. [Google Scholar]
- Ylmén, R.; Jäglid, U.; Steenari, B.-M.; Panas, I. Early hydration and setting of Portland cement monitored by IR, SEM and Vicat techniques. Cem. Concr. Res. 2009, 39, 433–439. [Google Scholar] [CrossRef]
- Ilia, I.K.; Stamatakis, M.G.; Perraki, T.S. Mineralogy and technical properties of clayey diatomites from north and central Greece. Cent. Eur. J. Geosci. 2009, 1/4, 393–403. [Google Scholar] [CrossRef]
- Madejova, J.; Jnek, M.; Komadel, P.; Herbert, H.J.; Moog, H.C. FTIR analyses of water in MX-80 bentonite compacted from high salinary salt solution systems. Appl. Clay Sci. 2002, 20, 255–271. [Google Scholar] [CrossRef]
- Fidalgo, A.; Ilhrco, L.M. The defect structure of sol–gel-derived silica/polytetrahydrofuran hybrid films by FTIR. J. Non-Cryst. Solids 2001, 283, 144–154. [Google Scholar] [CrossRef]
- Swan, G.E.A.; Patwarhan, S.V. Application of Transform Infrared Spectroscopy (FTIR) for assessing biogenic silica sample purity in geochemical analyses and palaeoenvironmental research. Clim. Past 2011, 7, 65–74. [Google Scholar] [CrossRef] [Green Version]
- Kaczmarska, I.; Ehrman, J.M.; Bates, S.S. A review of auxospore structure, ontogeny and diatom phylogeny. In Proceedings of the 16th International Diatom Symposium, Athens, Greece, 25 August–1 September 2000. [Google Scholar]
- Cavalier-Smith, T. Kingdoms Protozoa and Chromista and the eozoan root of the eukaryotic tree. Biol. Lett. 2010, 6/3, 342–345. [Google Scholar] [CrossRef] [Green Version]
- Werner, D. The Biology of Diatoms; Blackwell: Oxford, UK, 1977. [Google Scholar]
- Round, F.M.; Crawford, R.M.; Mann, D.G. The Diatoms–Biology and Morphology of the Genera; Cambridge University Press: Cambridge, UK, 1990. [Google Scholar]
- EN 196-6; Methods of Testing Cement–Part 6: Determination of Fineness. European Committee for Standardization: Brussels, Belgium, 2010.
- Neville, A.M.; Brooks, J.J. Concrete Technology; Longman Scientific and Technical: New York, NY, USA, 1987. [Google Scholar]
- Binici, H.; Aksogan, O.; Cagatay, I.H.; Tokyay, M.; Emsen, E. The effect of particle size distribution on the properties of blended cements incorporating GGBFS and natural pozzolan (NP). Powder Technol. 2007, 177, 140–147. [Google Scholar] [CrossRef]
- Taylor, H.F.W. Cement Chemistry, 2nd ed.; Thomas Telford: London, UK, 1997. [Google Scholar]
- EN 12457-2; Characterisation of Waste-Leaching-Compliance Test for Leaching of Granular Waste Materials and Sludges-Part 2: One stage Batch Test at a Liquid to Solid Ratio of 10 l/kg for Materials with Particle Size below 4 mm (without or with Size Reduction). European Committee for Standardization: Brussels, Belgium, 2002.
- EN 196-5; Methods of Testing Cement-Part 5: Pozzolanicity Test for Pozzolanic Cement. European Committee for Standardization: Brussels, Belgium, 2011.
- NF P18-513; Pozzolanic Addition for Concrete-Metakaolin-Definitions, Specifications And Conformity Criteria. Association Française de Normalisation: La Plaine Saint-Denis, France, 2010.
- Raverdy, M.; Brivot, F.; Paillere, A.M.; Dron, R. Appreciation de L’activite Pouzzolanique des Constituants Secondaires; 7 th International Congress Chemical Cement: Paris, France, 1980; Volume 3, pp. 36–41. [Google Scholar]
- Jun-Yuan, H.; Scheetz, B.E.; Roy, D.M. Hydration of fly ash-portland cement. Cem. Concr. Res. 1984, 14, 505–511. [Google Scholar] [CrossRef]
- EN 1015-3; Methods of Test for Mortar for Masonry-Part 3: Determination of Consistence of Fresh Mortar (by Flow Table). European Committee for Standardization: Brussels, Belgium, 1999.
- EN 196-3; Methods of Testing Cement-Part 3: Determination of Setting Times and Soundness. European Committee for Standardization: Brussels, Belgium, 1997.
- EN 1015-10; Methods of Test for Mortar for Masonry—Part 10: Determination of Dry Bulk Density of Hardened Mortar. European Committee for Standardization: Brussels, Belgium, 1999.
- EN 1015-11; Methods of Test for Mortar for Masonry—Part 11: Determination of Flexural and Compressive Strength of Hardened Mortar. European Committee for Standardization: Brussels, Belgium, 2020.
- Mindess, S.; Young, J.F.; Darwin, D. Concrete, 2nd ed.; Pearson Education Inc.: Upper Saddle River, NJ, USA, 2003. [Google Scholar]
- Bhaskar, J.S.; Parthasarathy, G. Fourier Transform Infrared Spectroscopic Characterization of Kaolinite from Assam and Meghalaya, Northeastern India. J. Mod. Phys. 2010, 1, 206–210. [Google Scholar] [CrossRef] [Green Version]
- Hughes, T.L.; Methven, C.M.; Jones, T.G.J.; Pelham, S.E.; Fletcher, P.; Hall, C. Determining cement composition by Fourier transform infrared spectroscopy. Adv. Cem. Bas. Mat. 1995, 2, 91–104. [Google Scholar] [CrossRef]
- Richard, T.; Mercurz, L.; Poulet, F.; d’Hendecourt, L. Diffuse reflectance infrared Fourier transform spectroscopy as a tool to characterise water in adsorption/confinement situations. J. Colloid Interface Sci. 2006, 304, 125–136. [Google Scholar] [CrossRef]
- Trezza, M.A.; Lavat, A.E. Analysis of the system 3CaO.Al2O3.CaSO4.2H2O-CaCO3-H2O by FT-IR spectroscopy. Cem Concr. Res. 2001, 31, 869–872. [Google Scholar] [CrossRef]
- Silva, D.A.; Roman, H.R.; Gleize, P.J.P. Evidences of chemical interaction between EVA and hydrating Portland cement. Cem. Concr. Res. 2002, 32, 1383–1390. [Google Scholar] [CrossRef]
- Horgnies, M.; Chen, J.J.; Bouillon, C. Overview about the use of Fourier Transform Infrared spectroscopy to study cementitious materials. WIT Transactions on Engineering Sciences 2013, 77, 251–262. [Google Scholar] [CrossRef] [Green Version]
- Delgado, A.H.; Paroli, R.M.; Beaudoin, J.J. Comparison of IR Techniques for the Characterization of Construction Cement Minerals and Hydrated Products. Appl. Spectr. 1996, 60/8, 970–976. [Google Scholar] [CrossRef]
- Liang, T.; Nanru, Y. Hydration products of calcium aluminoferrite in the presence of gypsum. Cem. Concr. Res. 1994, 24, 150–158. [Google Scholar] [CrossRef]
- Payá, J.; Monzo, J.M.; Borrachero, M.V.; Velazquez, S. Pozzolanic reaction rate of fluid catalytic cracking catalyst residue (FC3R) in cement paste. Adv. Cem Res. 2013, 25, 112–118. [Google Scholar] [CrossRef] [Green Version]
- Pavlíková, M.; Zemanová, L.; Záleská, M.; Pokorný, J.; Lojka, M.; Jankovský, O.; Pavlík, Z. Ternary blended binder for production of a novel type of lightweight repair mortar. Materials 2019, 12, 996. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Jang-Hyun, P.; Chang-Bok, Y. Properties and durability of cement mortar using calcium stearate and natural pozzolan for concrete surface treatment. Materials 2022, 15, 5762. [Google Scholar] [CrossRef]


| Parameter, Substance | CEM I 42.5 R | ENO3 | ENO7 | BOR |
|---|---|---|---|---|
| Content (Mass %) | ||||
| Annealing loss at 105 °C | 0.40 | 0.05 | 82.58 | 3.30 |
| Insoluble fraction | 1.63 | 2.00 | 1.20 | 2.48 |
| SiO2 | 19.00 | 89.70 | 90.90 | 82.90 |
| Al2O3 | 4.31 | 3.40 | 3.20 | 13.20 |
| Fe2O3 | 2.40 | 2.10 | 3.10 | 1.50 |
| TiO2 | 0.28 | 0.60 | 0.60 | 0.60 |
| CaO | 62.90 | 0.60 | 0.57 | 0.30 |
| MgO | 1.80 | 0.20 | 0.20 | 0.20 |
| K2O | 0.82 | 0.30 | 0.30 | 0.90 |
| Na2O | 0.14 | 2.70 | 0.20 | 0.10 |
| P2O5 | 0.16 | 0.10 | 0.80 | 0.10 |
| SO3 | 3.24 | 0.00 | 0.00 | 0.10 |
| Substance | ENO3 | ENO7 | BOR |
|---|---|---|---|
| Content (wt.%) | |||
| amorphous phase | 37.70 | 66.10 | 53.90 |
| Opal CT | 22.05 | 0.00 | 0.00 |
| SiO2 quartz | 0.00 | 17.00 | 6.00 |
| SiO2 tridymit | 1.10 | 0.00 | 0.00 |
| SiO2 cristobalite | 39.20 | 16.10 | 0.00 |
| Al2(Si2O5)(OH)4 Kaolinite | 0.00 | 0.00 | 32.70 |
| K2(Al4FeO)(Si6Al2O20)(OH)4 illite | 0.00 | 0.00 | 7.30 |
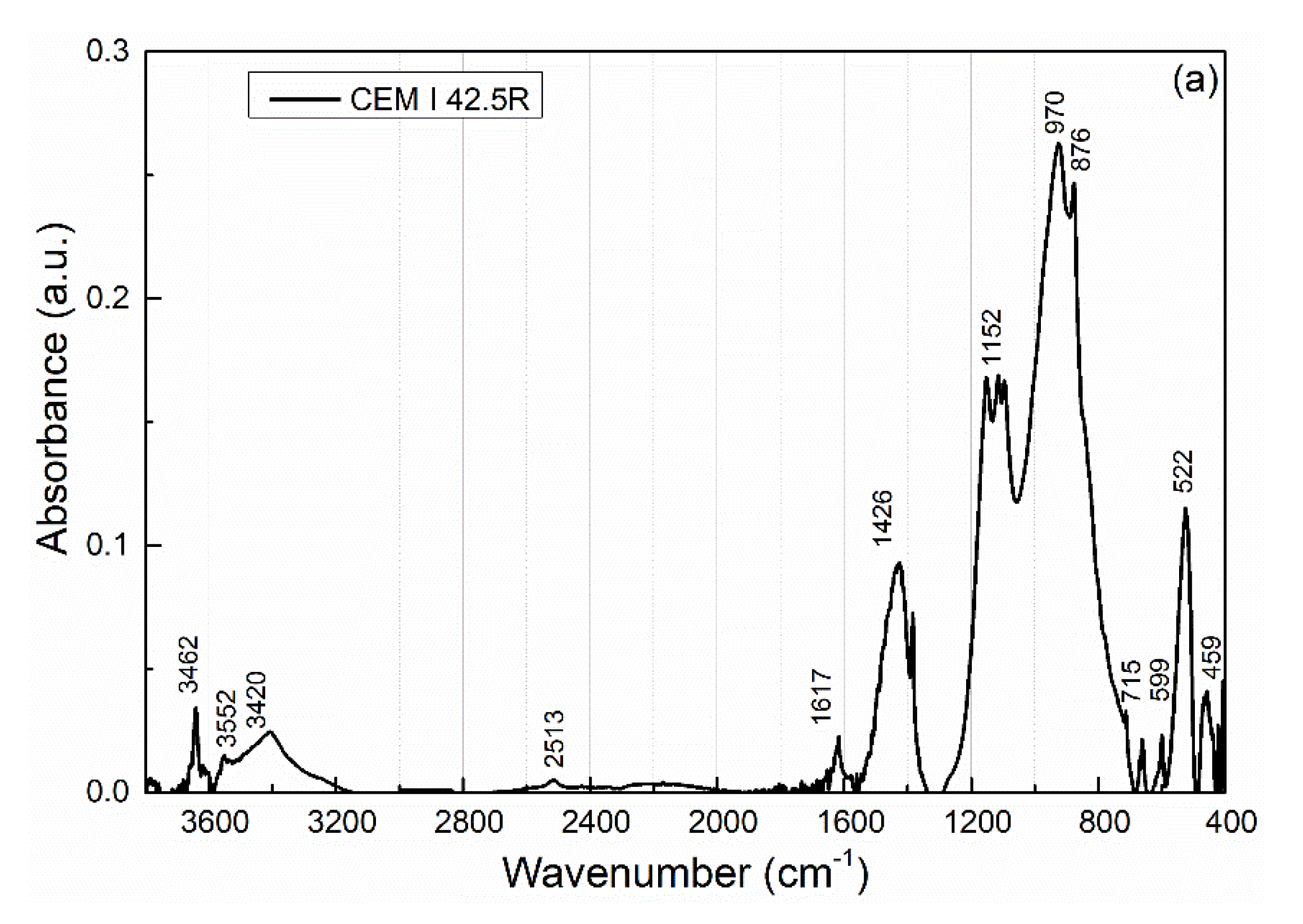
| Wavenumbers (cm−1) | Assignment |
|---|---|
| 3641 | ν (O-H) in portlandite (Ca(OH)2) |
| 3552 | ν (O-H) in water, chemisorption of water on the surface of molecules |
| 3420 | ν (H-O-H) in water, chemisorption of water on the surface of molecules |
| 2513, 1426, 876, 715 | ν (C=O) in calcite (CaCO3) |
| 1617 | ν2 (O-H) in water |
| 1384 1152, 1114, 1095 | ν (C=O) in CO32− compounds ν3 (SO42−) in gypsum, basanite, and anhydrite [58] |
| 970, 924 | ν3 (Si-O) in C3S |
| 902, 780, 516, 509, 421 | ν (Al-O) in C3A |
| 845 | ν4 (Si-O) in β−C2S |
| 522 | ν4 (Si-O) in C3S |
| 700–500 459 | ν (Fe-O) in C4AF ν4 (Si-O-Si) |
| Wavenumbers (cm−1) | Assignment |
|---|---|
| 3696 3651 3620 | ν (H-O-H) free ν (O-H) inner surface [60] ν (O-H) in crystalline molecules |
| ~3436 1631 | ν (H-O-H) in water, chemisorption of water on the surface of molecules ν (H-O-H) in water in minerals |
| 1385 | ν (C=O) in CO32− compounds |
| 1099 | ν (Si-O-Si) asymmetric in plane vibration |
| 950 797, 695 650–500 538 470 | ν (Si-OH) ν (Si-O) ν (Si-O-AlIV) ν (Fe-O) in Fe2O3 ν4 (Si-O-Si) |
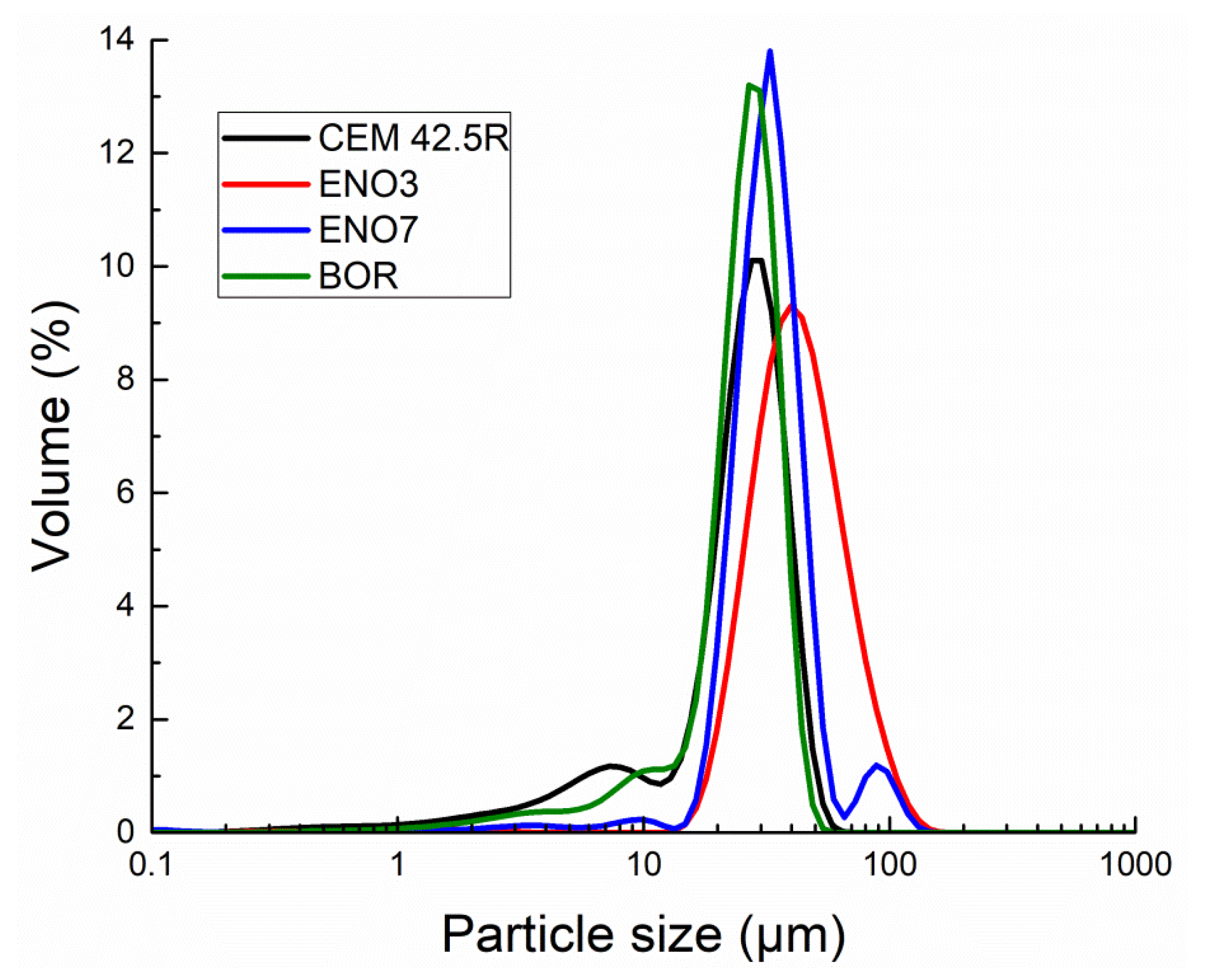
| Material | Size (µm) Freq d10 | Size (µm) Freq d50 | Size (µm) Freq d90 |
|---|---|---|---|
| CEMI 42.5R | 5.8 | 23.4 | 35.5 |
| ENO3 | 22.3 | 37.5 | 63.5 |
| ENO7 | 19.6 | 29.0 | 43.0 |
| BOR | 9.5 | 23.0 | 32.5 |
| Material | Blaine Fineness (m2·kg−1) | BET Specific Surface Area (m2·kg−1) | Specific Density (kg·m−3) |
|---|---|---|---|
| CEMI 42.5R | 360 | 5 500 | 2 860 |
| ENO3 | 785 | 2 494 | 2 334 |
| ENO7 | 1 865 | 2 007 | 2 399 |
| BOR | 2 095 | 18 312 | 2 416 |
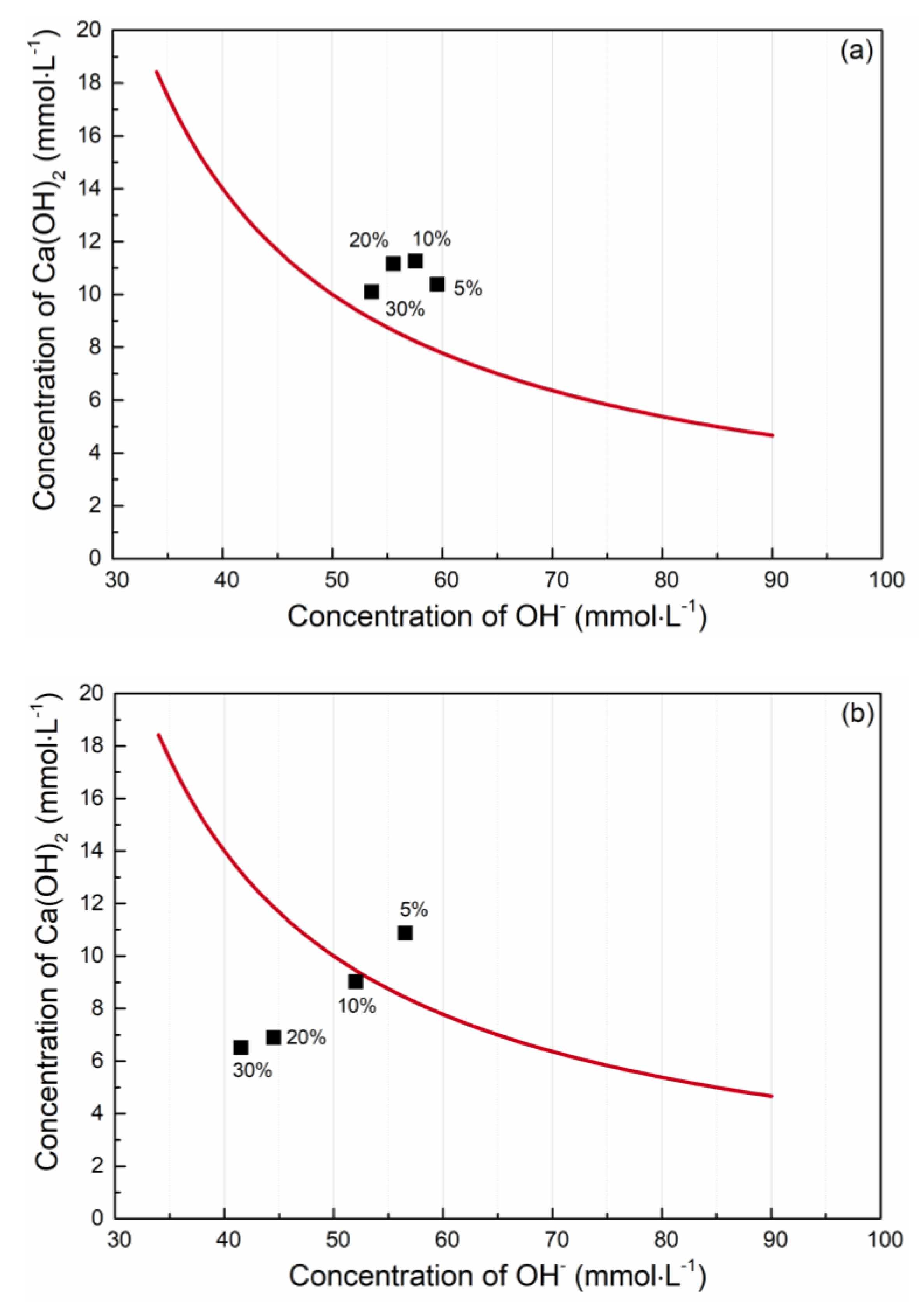
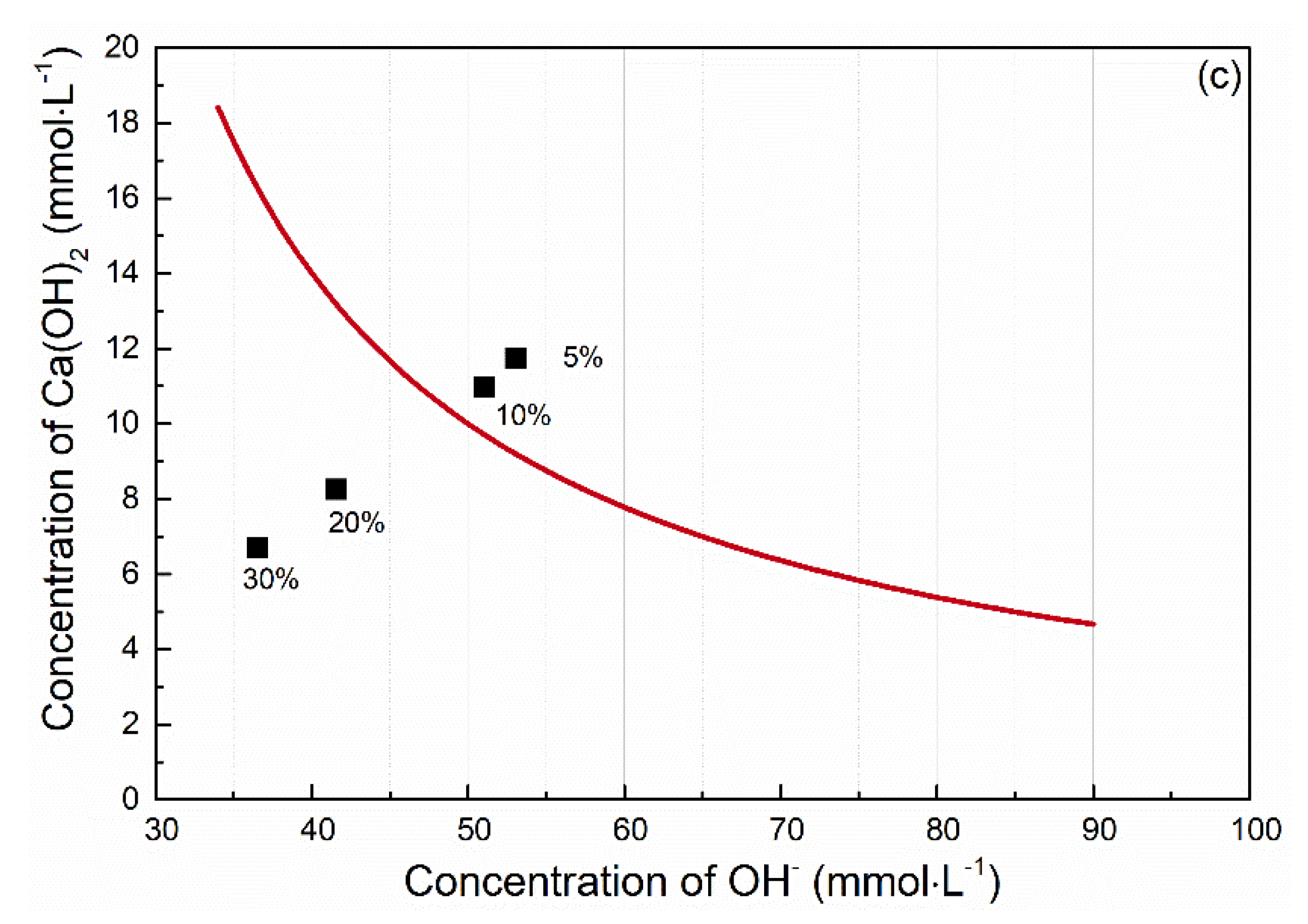
| Material | pH Value (-) | Pozzolanic Activity (mg Ca(OH)2 Fixed by 1 g) |
|---|---|---|
| CEM I 42.5R | 12.45 | 1147 |
| ENO3 | 9.34 | 246 |
| ENO7 | 7.11 | 1349 |
| BOR | 6.49 | 1614 |
| Paste Mixture | Value of Spreading (mm) | Initial Setting Time (min.) | Final Setting Time (min.) |
|---|---|---|---|
| RP | >300 | 400 | 690 |
| ENO3/5 | 280 × 280 | 355 | 635 |
| ENO3/10 | 240 × 245 | 330 | 635 |
| ENO3/15 | 210 × 210 | 280 | 500 |
| ENO3/20 | 180 × 180 | 245 | 375 |
| ENO3/30 | 150 × 145 | 240 | 350 |
| ENO7/5 | 255 × 260 | 380 | 680 |
| ENO7/10 | 210 × 210 | 315 | 640 |
| ENO7/15 | 175 × 175 | 225 | 410 |
| ENO7/20 | 140 × 140 | 215 | 395 |
| ENO7/30 | 140 × 140 | 215 | 390 |
| BOR/5 | 260 × 260 | 375 | 670 |
| BOR/10 | 210 × 210 | 350 | 640 |
| BOR/15 | 165 × 165 | 250 | 450 |
| BOR/20 | 145 × 145 | 240 | 355 |
| BOR/30 | 140 × 140 | 220 | 350 |
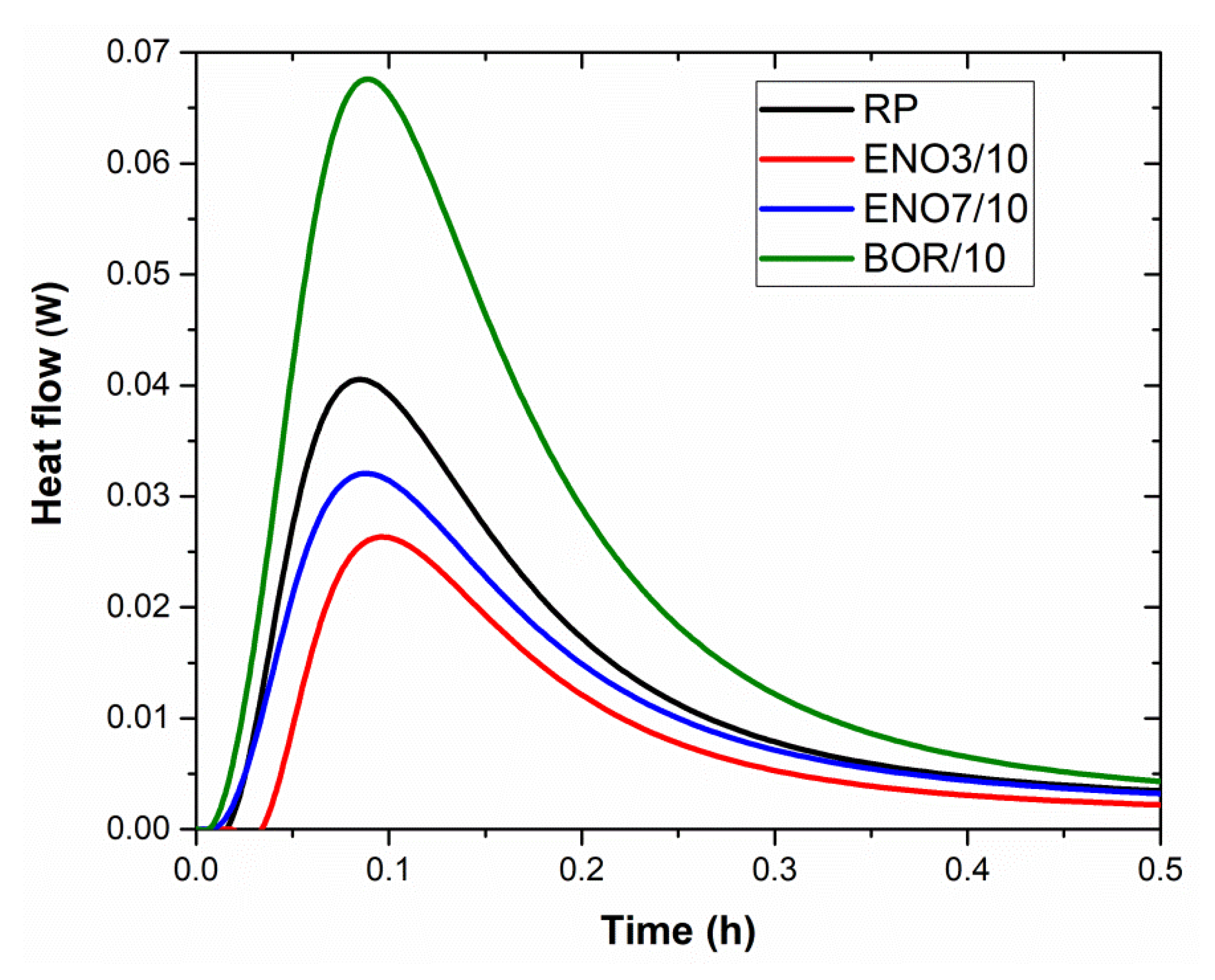
| Paste Mixture | 1 h (J·g−1) | 24 h (J·g−1) |
|---|---|---|
| RP | 7.56 | 203.15 |
| ENO3/10 | 1.04 | 161.87 |
| ENO7/10 | 1.63 | 202.08 |
| BOR/10 | 11.77 | 202.08 |
| Substance | ENO3/10 | ENO7/10 | BOR/10 |
|---|---|---|---|
| Content (Mass %) | |||
| Amorphous phase | 70.00 | 65.90 | 66.90 |
| Aluminum oxide chloride | 0.10 | 0.30 | 0.30 |
| Cristobalite | 1.30 | 0.90 | - |
| Quartz | 0.60 | 1.30 | 1.00 |
| Hatrurit | 5.40 | 3.90 | 4.60 |
| Portlandite | 13.00 | 15.80 | 16.90 |
| Ettringite | 1.00 | 2.10 | 1.10 |
| Calcium carbonate | 8.60 | 10.00 | 9.30 |
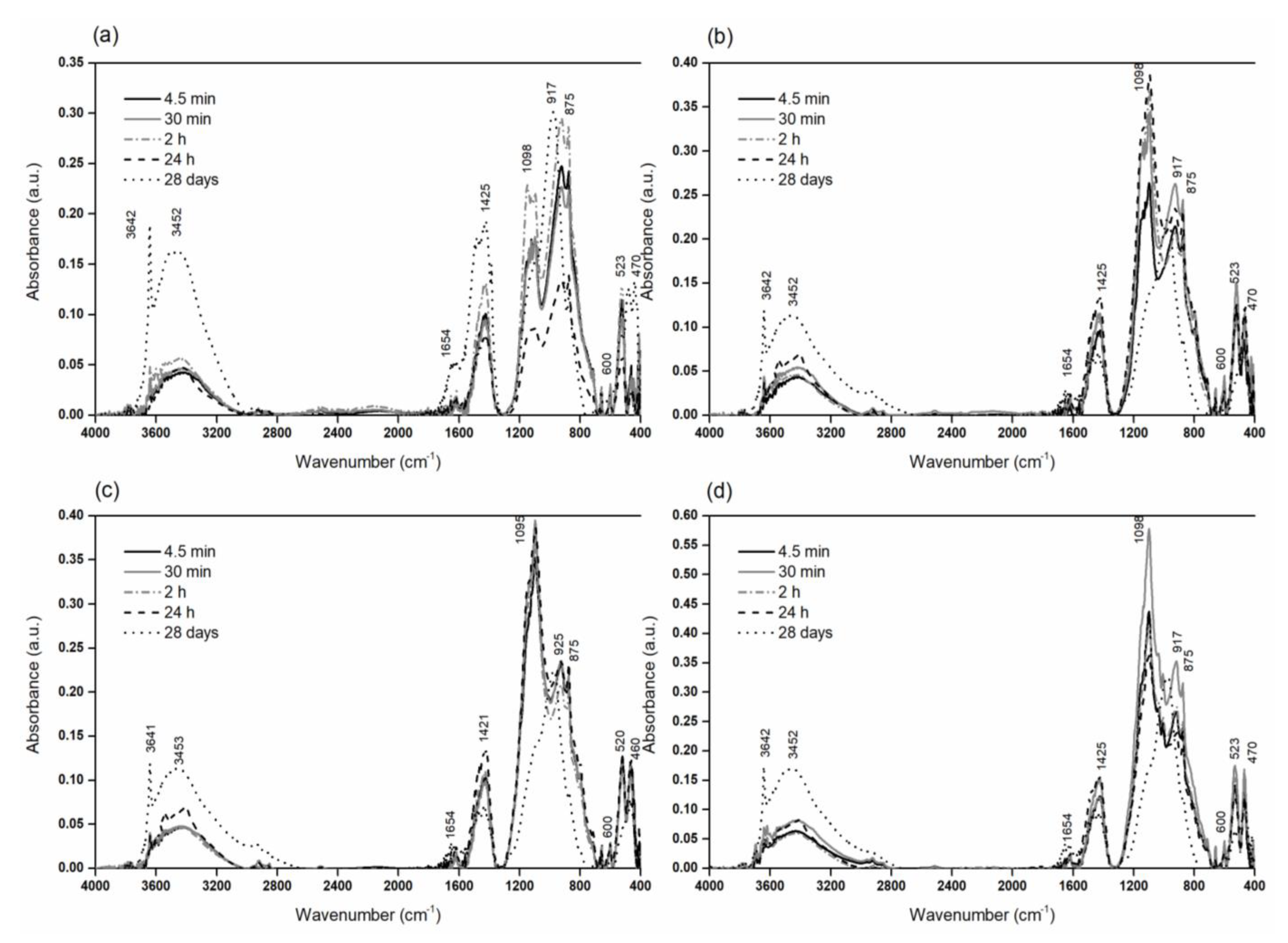
| Wavenumber (cm−1) | Assignment |
|---|---|
| 3641, 3642 | ν (O-H) crystalline hydroxyl [81], e.g., portlandite (Ca(OH)2) [82] |
| 3424–3553 | ν (H-O-H) in liquid water, chemisorption of water on the surface of molecules [83] |
| 1617–1653 | ν2 (H-O-H) in water [84] |
| 1426, 1384, 875–873 | ν (C=O) in CO32− compounds, calcite [85] |
| 1095–1098 | ν3 (SO42−) ettringite |
| 925–968 | ν3 (Si-O) in the C-S-H phase [86] |
| 917–925 | ν4 (Si-O) in the C-S-H phase [86] |
| 780–918 | δ (O-H) linked to Al3+ [81] |
| 599–601 | ν (Al-O) in C3A [85] |
| 520–523 | ν4 (Si-O) in C3S, β-C2S, C3A [87] |
| 700–500 | ν (Fe-O, Al-O) in C4AF [88] |
| 440–470 | ν4 (Si-O-Si) in the C-S-H phase [87] |
| Paste | Bulk Density (kg·m−3) | Specific Density (kg·m−3) | Open Porosity (%) | |||
|---|---|---|---|---|---|---|
| 28 Days | 90 Days | 28 Days | 90 Days | 28 Days | 90 Days | |
| RP | 1558 ± 22 | 1589 ± 22 | 2181 ± 26 | 2159 ± 26 | 28.6 ± 0.6 | 26.4 ± 0.5 |
| ENO3/5 | 1451 ± 20 | 1495 ± 21 | 2017 ± 24 | 2008 ± 24 | 28.1 ± 0.6 | 25.5 ± 0.5 |
| ENO3/10 | 1422 ± 20 | 1460 ± 20 | 2007 ± 24 | 1991 ± 24 | 29.1 ± 0.6 | 26.7 ± 0.5 |
| ENO3/15 | 1375 ± 19 | 1416 ± 20 | 1966 ± 24 | 1951 ± 23 | 30.1 ± 0.6 | 27.4 ± 0.5 |
| ENO3/20 | 1319 ± 19 | 1364 ± 19 | 1925 ± 23 | 1907 ± 23 | 31.5 ± 0.6 | 28.5 ± 0.6 |
| ENO7/5 | 1576 ± 22 | 1594 ± 22 | 2154 ± 26 | 2141 ± 26 | 26.8 ± 0.5 | 25.5 ± 0.5 |
| ENO7/10 | 1610 ± 23 | 1623 ± 23 | 2163 ± 26 | 2149 ± 26 | 25.6 ± 0.5 | 24.4 ± 0.5 |
| ENO7/15 | 1549 ± 22 | 1573 ± 22 | 2148 ± 26 | 2132 ± 26 | 27.9 ± 0.6 | 26.3 ± 0.5 |
| ENO7/20 | 1473 ± 21 | 1488 ± 21 | 2083 ± 25 | 2069 ± 25 | 29.3 ± 0.6 | 28.1 ± 0.6 |
| BOR/5 | 1492 ± 21 | 1517 ± 21 | 2078 ± 25 | 2072 ± 25 | 28.2 ± 0.6 | 26.8 ± 0.5 |
| BOR/10 | 1434 ± 20.1 | 1456 ± 20 | 2021 ± 24 | 2008 ± 24 | 28.9 ± 0.6 | 27.5 ± 0.6 |
| BOR/15 | 1411 ± 19.8 | 1425 ± 20 | 1996 ± 24 | 1981 ± 24 | 29.3 ± 0.6 | 28.1 ± 0.6 |
| BOR/20 | 1349 ± 18.9 | 1371 ± 19 | 1936 ± 23 | 1922 ± 23 | 30.3 ± 0.6 | 28.7 ± 0.6 |
| Paste | Flexural Strength (MPa) | Compressive Strength (MPa) | SAI (%) | Young’s Modulus (GPa) | ||||
|---|---|---|---|---|---|---|---|---|
| Days | ||||||||
| 28 | 90 | 28 | 90 | 28 | 90 | 28 | 90 | |
| RP | 7.3 ± 0.1 | 8.2 ± 0.1 | 52.3 ± 0.7 | 58.4 ± 0.8 | - | - | 14.5 ± 0.2 | 16.7 ± 0.2 |
| ENO3/5 | 7.5 ± 0.1 | 8.7 ± 0.1 | 52.5 ± 0.7 | 59.2 ± 0.8 | 100.4 | 101.3 | 14.5 ± 0.2 | 17.1 ± 0.2 |
| ENO3/10 | 7.2 ± 0.1 | 8.4 ± 0.1 | 50.0 ± 0.7 | 58.1 ± 0.8 | 95.6 | 99.5 | 14.2 ± 0.2 | 16.7 ± 0.2 |
| ENO3/15 | 6.6 ± 0.1 | 7.8 ± 0.1 | 48.2 ± 0.7 | 54.7 ± 0.8 | 92.1 | 93.7 | 13.6 ± 0.2 | 15.1 ± 0.2 |
| ENO3/20 | 6.0 ± 0.1 | 7.3 ± 0.1 | 43.1 ± 0.6 | 53.0 ± 0.7 | 82.4 | 90.8 | 12.1 ± 0.2 | 14.1 ± 0.2 |
| ENO7/5 | 7.8 ± 0.1 | 9.0 ± 0.1 | 55.8 ± 0.8 | 60.6 ± 0.8 | 106.7 | 103.8 | 15.8 ± 0.2 | 17.2 ± 0.2 |
| ENO7/10 | 8.4 ± 0.1 | 9.4 ± 0.1 | 59.4 ± 0.8 | 63.7 ± 0.9 | 113.6 | 109.1 | 16.8 ± 0.2 | 17.4 ± 0.2 |
| ENO7/15 | 8.0 ± 0.1 | 8.5 ± 0.1 | 54.4 ± 0.8 | 58.4 ± 0.8 | 104.0 | 100.0 | 15.9 ± 0.2 | 17.0 ± 0.2 |
| ENO7/20 | 6.9 ± 0.1 | 7.7 ± 0.1 | 50.2 ± 0.7 | 53.8 ± 0.8 | 96.0 | 92.1 | 14.0 ± 0.2 | 14.7 ± 0.2 |
| BOR/5 | 7.6 ± 0.1 | 8.5 ± 0.1 | 52.6 ± 0.7 | 59.4 ± 0.8 | 100.6 | 101.7 | 14.9 ± 0.2 | 17.1 ± 0.2 |
| BOR/10 | 7.4 ± 0.1 | 8.3 ± 0.1 | 52.3 ± 0.7 | 58.9 ± 0.8 | 100.0 | 100.9 | 14.7± 0.2 | 16.9 ± 0.2 |
| BOR/15 | 7.0 ± 0.1 | 7.8 ± 0.1 | 50.4 ± 0.7 | 53.1 ± 0.7 | 96.4 | 90.9 | 14.3 ± 0.2 | 14.7 ± 0.2 |
| BOR/20 | 6.7 ± 0.1 | 7.1 ± 0.1 | 48.6 ± 0.7 | 51.9 ± 0.7 | 92.9 | 88.9 | 13.70 ± 0.2 | 13.7 ± 0.2 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Pavlíková, M.; Rovnaníková, P.; Záleská, M.; Pavlík, Z. Diatomaceous Earth—Lightweight Pozzolanic Admixtures for Repair Mortars—Complex Chemical and Physical Assessment. Materials 2022, 15, 6881. https://doi.org/10.3390/ma15196881
Pavlíková M, Rovnaníková P, Záleská M, Pavlík Z. Diatomaceous Earth—Lightweight Pozzolanic Admixtures for Repair Mortars—Complex Chemical and Physical Assessment. Materials. 2022; 15(19):6881. https://doi.org/10.3390/ma15196881
Chicago/Turabian StylePavlíková, Milena, Pavla Rovnaníková, Martina Záleská, and Zbyšek Pavlík. 2022. "Diatomaceous Earth—Lightweight Pozzolanic Admixtures for Repair Mortars—Complex Chemical and Physical Assessment" Materials 15, no. 19: 6881. https://doi.org/10.3390/ma15196881
APA StylePavlíková, M., Rovnaníková, P., Záleská, M., & Pavlík, Z. (2022). Diatomaceous Earth—Lightweight Pozzolanic Admixtures for Repair Mortars—Complex Chemical and Physical Assessment. Materials, 15(19), 6881. https://doi.org/10.3390/ma15196881







