PbS and PbO Thin Films via E-Beam Evaporation: Morphology, Structure, and Electrical Properties
Abstract
1. Introduction
2. Materials and Methods
3. Results and Discussion
4. Conclusions
- The films were deposited with an intended thickness of 150 nm and were physically stable, pinhole-free, and typically had better adhesion property with good uniformity.
- A sufficient amount of impurity was instinctively added due to annealing the film in an open atmosphere. As a result, an insignificant amount of oxygen in annealed samples reacted with lead and became lead oxide, which was confirmed by XRD.
- Rutherford backscattering spectrometry confirmed the thickness of film (172 nm) was slightly greater than the intended thickness of 150 nm with lead and sulfur in the films (along with substrate concentration). The diffused oxygen due to annealing shows that the concentration of lead decreases with the increase in the amount of oxygen. In contrast, a comparatively slight difference was found in EDS results due to the limitations of SEM. Both results confirmed that the oxygen amount increases with an increase in annealing temperature.
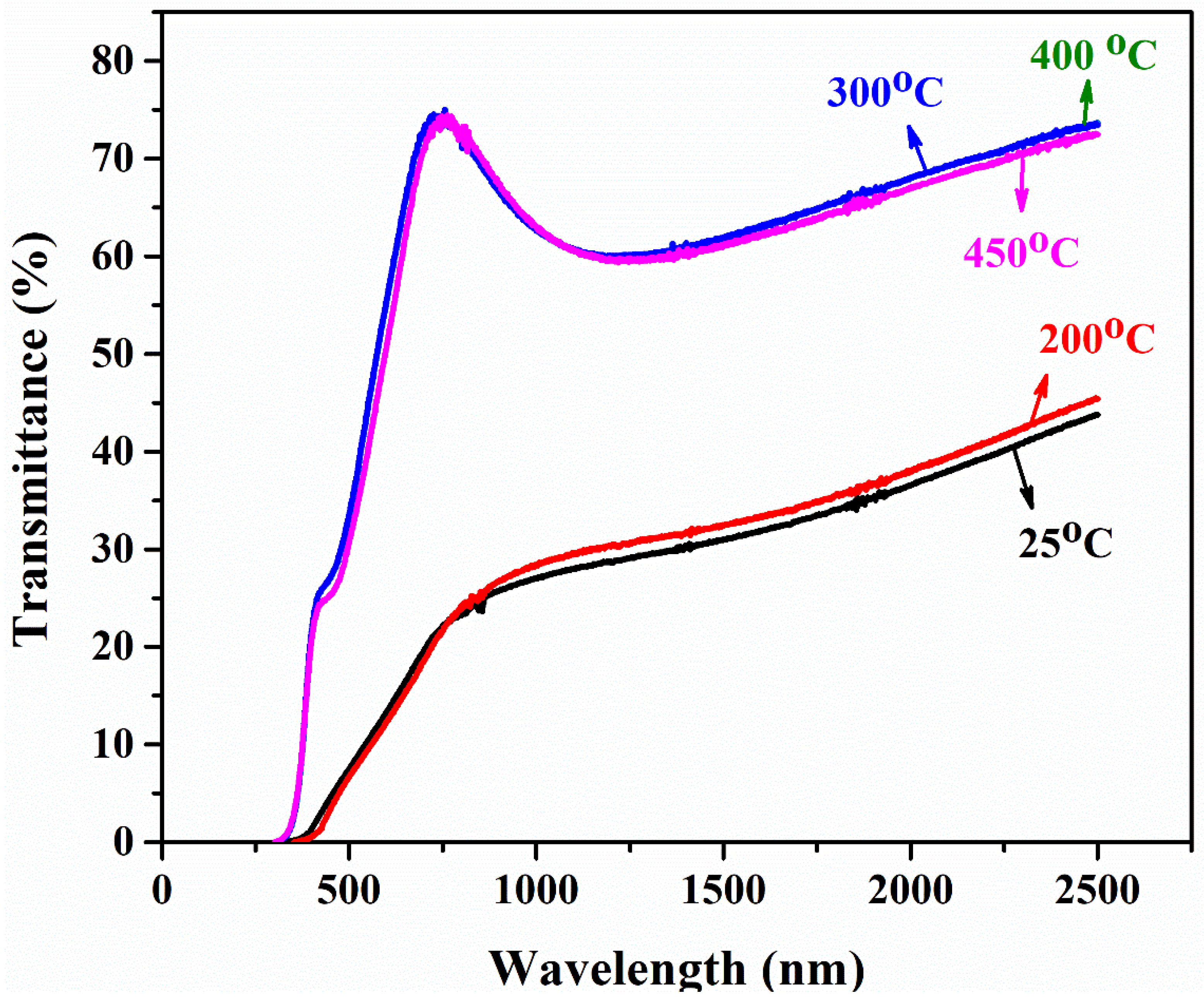
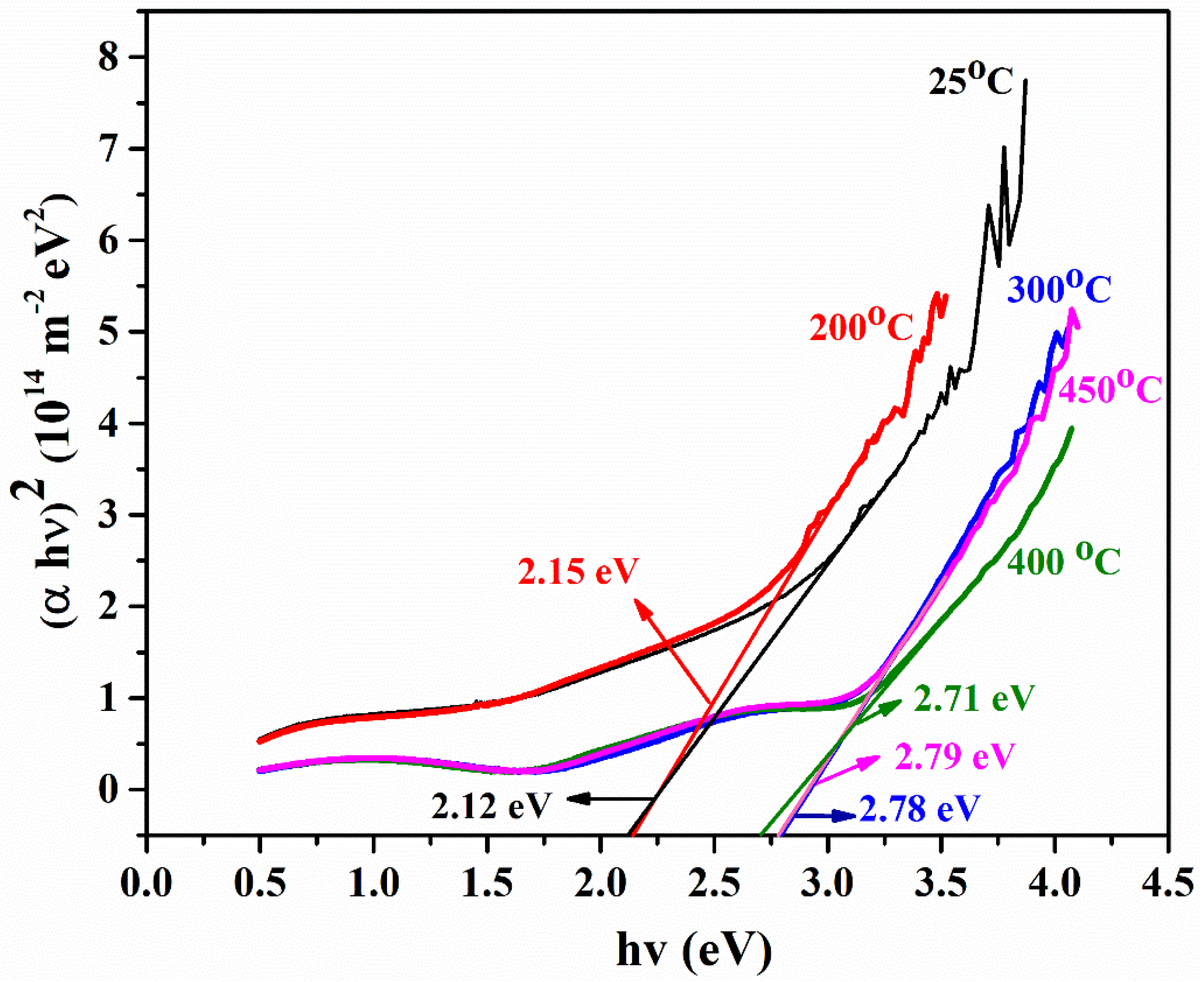
- The transmittance of as-deposited film is lower, as the annealing temperature increases; similarly, the transmittance of the film drastically increases at a shorter wavelength. This is due to the reduced light scattering at a higher temperature. A band gap of PbS film is highly influenced by temperature. The Eg of PbS films was determined to be in the 2.12–2.75 eV range. Hence, band gap can be tuned in order with the respective application for IR applications.
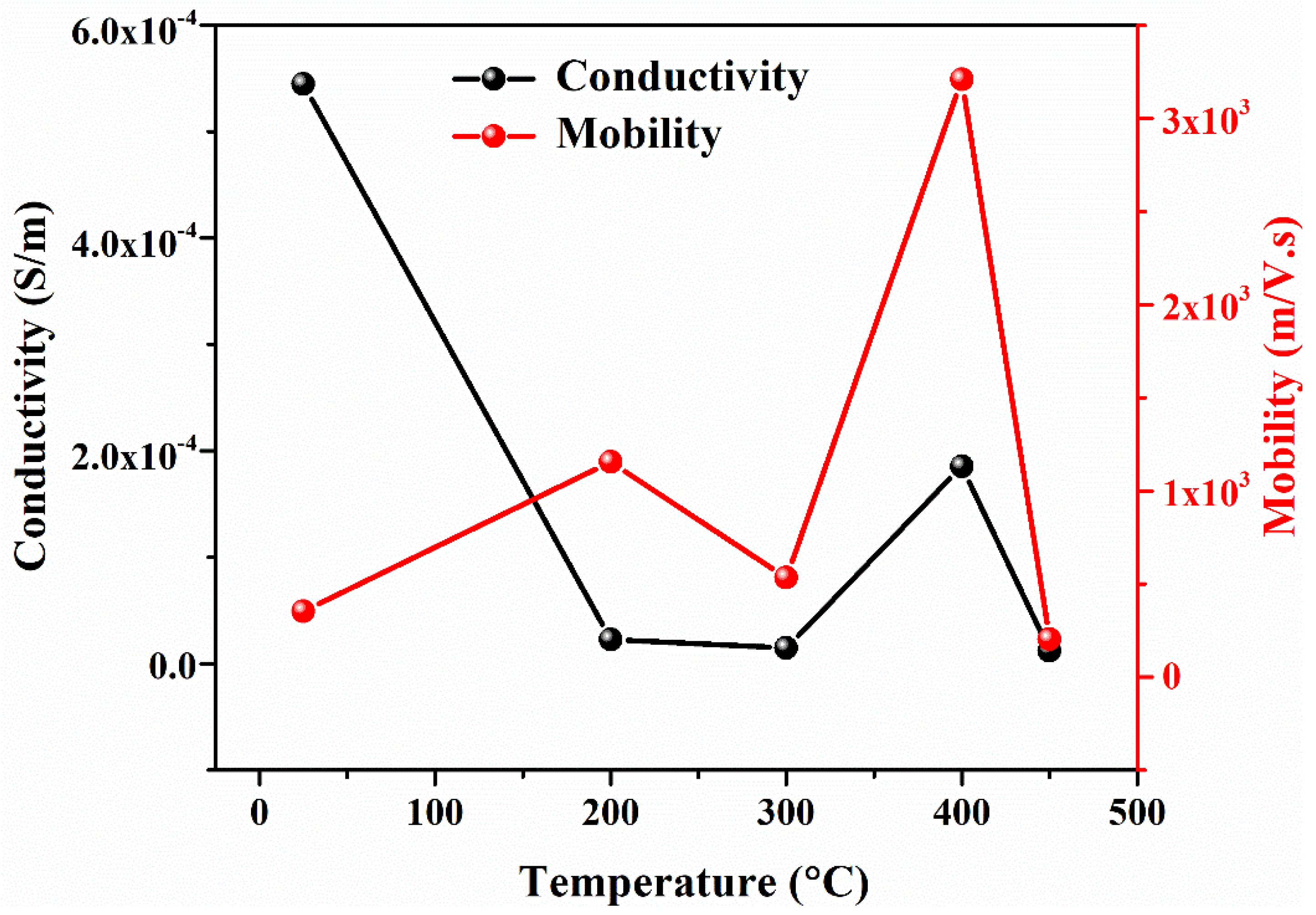
- PbS is a p-type semiconducting material determined by Hall measurements. The film’s sheet resistance is greatly increased with an increase in annealing temperature; alternately, a larger reduction in career concentration and high mobility has been found. As-deposited PbS thin film shows good conductivity, which is suitable for optoelectronic applications.
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Saleem, M.S.; Hanif, M.B.; Gregor, M.; Motola, M.; Khan, A.F. Nanostructured multi-layer MgF2/ITO coatings prepared via e-beam evaporation for efficient electromagnetic interference shielding performance. Appl. Surf. Sci. 2022, 596, 153584. [Google Scholar] [CrossRef]
- Afsar, M.F.; Jamil, A.; Rafiq, M.A. Ferroelectric, dielectric and electrical behavior of two-dimensional lead sulphide nanosheets. Adv. Nat. Sci. Nanosci. Nanotechnol. 2017, 8, 045010. [Google Scholar] [CrossRef]
- Jahromi, H.D.; Moaddeli, M. Lead sulfide mathsemicolon: A new candidate for optoelectronics applications in the ultra violet spectral range. Mater. Res. Express 2019, 6, 116220. [Google Scholar] [CrossRef]
- Torimoto, T.; Takabayashi, S.; Mori, H.; Kuwabata, S. Photoelectrochemical activities of ultrathin lead sulfide films prepared by electrochemical atomic layer epitaxy. J. Electroanal. Chem. 2002, 522, 33–39. [Google Scholar] [CrossRef]
- Seghaier, S.; Kamoun, N.; Brini, R.; Amara, A.B. Structural and optical properties of PbS thin films deposited by chemical bath deposition. Mater. Chem. Phys. 2006, 97, 71–80. [Google Scholar] [CrossRef]
- Preetha, K.C. Structural, electronic transport and optical properties of Cr doped PbS thin film by chemical bath deposition. AIP Conf. Proc. 2017, 1849, 020018. [Google Scholar]
- Hanif, M.B.; Motola, M.; Qayyum, S.; Rauf, S.; Khalid, A.; Li, C.-J.; Li, C.-X. Recent advancements, doping strategies, and the future perspective of perovskite-based Solid oxide fuel cells for energy conversion. Chem. Eng. J. 2022, 428, 132603. [Google Scholar] [CrossRef]
- Hanif, M.B.; Rauf, S.; Motola, M.; Babar, Z.U.D.; Li, C.-J.; Li, C.-X. Recent progress of perovskite-based electrolyte materials for solid oxide fuel cells and performance optimizing strategies for energy storage applications. Mater. Res. Bull. 2022, 146, 111612. [Google Scholar] [CrossRef]
- Dasgupta, N.P.; Lee, W.; Prinz, F.B. Atomic Layer Deposition of Lead Sulfide Thin Films for Quantum Confinement. Chem. Mater. 2009, 21, 3973–3978. [Google Scholar] [CrossRef]
- Obaid, A.S.; Mahdi, M.A.; Zainuriah, H. Growth of Nanocrystalline PbS Thin Films by Solid-Vapor Deposition. Adv. Mater. Res. 2013, 620, 1–6. [Google Scholar] [CrossRef]
- Cheema, D.A.; Danial, M.O.; Hanif, M.B.; Alghamdi, A.S.; Ramadan, M.; Khaliq, A.; Khan, A.F.; Subhani, T.; Motola, M. Intrinsic Properties and Future Perspective of HfO2/V2O2/HfO2 Multi-Layer Thin Films via E-Beam Evaporation as a Transparent Heat Mirror. Coatings 2022, 12, 448. [Google Scholar] [CrossRef]
- Rehman, I.; Hanif, M.B.; Alghamdi, A.S.; Khaliq, A.; Halim, K.A.; Subhani, T.; Khan, A.F. Intrinsic Properties of Multi-Layer TiO2/V2O5/TiO2 Coatings Prepared via E-Beam Evaporation. Materials 2022, 15, 3933. [Google Scholar] [CrossRef] [PubMed]
- Abrar, S.; Hanif, M.B.; Alghamdi, A.S.; Khaliq, A.; Abdel Halim, K.S.; Subhani, T.; Khan, A.F. Synthesis and Characterization of Nanostructured Multi-Layer Cr/SnO2/NiO/Cr Coatings Prepared via E-Beam Evaporation Technique for Metal-Insulator-Insulator-Metal Diodes. Materials 2022, 15, 3906. [Google Scholar] [CrossRef] [PubMed]
- Le, N.V.; Ha, T.T.; Nguyen, H.T.; Nguyen, T.T.P. Electrodeposition of Lead Sulfide Thin Film and Application as Counter Electrode for Quantum Dot Solar Cells. Meet. Abstr. 2013, 28, 2046. [Google Scholar] [CrossRef]
- Gaiduk, A.; Gaiduk, P.; Larsen, A. Chemical bath deposition of PbS nanocrystals: Effect of substrate. Thin Solid Film 2008, 516, 3791–3795. [Google Scholar] [CrossRef]
- Babar, Z.U.D.; Hanif, M.B.; Gao, J.T.; Li, C.J.; Li, C.X. Sintering behavior of BaCe0.7Zr0.1Y0.2O3-δ electrolyte at 1150 °C with the utilization of CuO and Bi2O3 as sintering aids and its electrical performance. Int. J. Hydrog. Energy 2022, 47, 7403–7414. [Google Scholar] [CrossRef]
- Dong, C.; Liu, S.; Barange, N.; Lee, J.; Pardue, T.; Yi, X.; So, F. Long-wavelength lead sulfide quantum dots sensing up to 2600 nm for short-wavelength infrared photodetectors. ACS Appl. Mater. Interfaces 2019, 47, 44451–44457. [Google Scholar] [CrossRef]
- Xie, L.; Zhang, T.; Zhao, Y. Stabilizing the MAPbI3 perovksite via the in-situ formed lead sulfide layer for efficient and robust solar cells. J. Energy Chem. 2020, 47, 62–65. [Google Scholar] [CrossRef]
- Zarubin, I.V.; Markov, V.F.; Maskaeva, L.N.; Zarubina, N.V.; Kuznetsov, M.V. Chemical sensors based on a hydrochemically deposited lead sulfide film for the determination of lead in aqueous solutions. J. Anal. Chem. 2017, 72, 327–332. [Google Scholar] [CrossRef]
- Rajashree, C.; Balu, A.R. Tuning the physical properties of PbS thin films towards optoelectronic applications through Ni doping. Optik 2016, 127, 8892–8898. [Google Scholar] [CrossRef]
- Thirumavalavan, S.; Mani, K.; Suresh, S. Investigation on structural, optical, morphological and electrical properties of lead sulphide (PbS) thin films. J. Ovonic Res. 2015, 11, 123–130. [Google Scholar]
- Said, M.S.; Zemel, J.N. Properties of PbS-PbO crystalline films. I. Preparation and physical structure. J. Appl. Phys. 1976, 47, 866–870. [Google Scholar] [CrossRef]
- Mayer, J.W.; Rimini, E. Ion Beam Handbook for Material Analysis; Elsevier: Amsterdam, The Netherlands, 2012. [Google Scholar]
- Rosario, S.R.; Kulandaisamy, I.; Arulanantham, A.M.S.; Kumar, K.D.A.; Valanarasu, S.; Shkir, M.; AlFaify, S. Fabrication and characterization of lead sulfide (PbS) thin film based heterostructure (FTO/CdS/PbS/Ag) solar cell by nebulizer spray method. Mater. Res. Express 2019, 6, 056416. [Google Scholar] [CrossRef]
- Kotadiya, N.B.; Kothari, A.J.; Tiwari, D.; Chaudhuri, T.K. Photoconducting nanocrystalline lead sulphide thin films obtained by chemical bath deposition. Appl. Phys. A 2012, 108, 819–824. [Google Scholar] [CrossRef]
- Poelman, D.; Smet, P.F. Methods for the determination of the optical constants of thin films from single transmission measurements: A critical review. J. Phys. D Appl. Phys. 2003, 36, 1850. [Google Scholar] [CrossRef]
- Kim, S.Y. Simultaneous determination of refractive index, extinction coefficient, and void distribution of titanium dioxide thin film by optical methods. Appl. Opt. 1996, 35, 6703–6707. [Google Scholar] [CrossRef] [PubMed]
- Preetha, K.C.; Remadevi, T.L. Behavior of chemically deposited PbS thin films subjected to two different routes of post deposition annealing. Mater. Sci. Semicond. Process. 2013, 16, 605–611. [Google Scholar] [CrossRef]
- Motola, M.; Hromadko, L.; Prikryl, J.; Sopha, H.; Krbal, M.; Macak, J.M. Intrinsic properties of high-aspect ratio single-and double-wall anodic TiO2 nanotube layers annealed at different temperatures. Electrochim. Acta 2020, 352, 136479. [Google Scholar] [CrossRef]
- Sihor, M.; Hanif, M.B.; Thirunavukkarasu, G.K.; Liapun, V.; Edelmannova, M.F.; Roch, T.; Motola, M. Anodization of large area Ti: A versatile material for caffeine photodegradation and hydrogen production. Catal. Sci. Technol. 2022, 12, 5045–5052. [Google Scholar] [CrossRef]
- Hanif, M.B.; Sihor, M.; Liapun, V.; Makarov, H.; Monfort, O.; Motola, M. Porous vs. Nanotubular Anodic TiO2: Does the Morphology Really Matters for the Photodegradation of Caffeine? Coatings 2022, 12, 1002. [Google Scholar] [CrossRef]
- Ahmed, S.M.; Mohammed, R.Y.; Abdulrahman, A.F.; Ahmed, F.K.; Hamad, S.M. Synthesis and characterization of lead oxide nanostructures for radiation attenuation application. Mater. Sci. Semicond. Process. 2021, 130, 105830. [Google Scholar] [CrossRef]
- Hanif, M.B.; Thirunavukkarasu, G.K.; Liapun, V.; Makarov, H.; Gregor, M.; Roch, T.; Motola, M. Fluoride-free synthesis of anodic TiO2 nanotube layers: A promising environmentally friendly method for efficient photocatalysts. Nanoscale 2022, 14, 11703–11709. [Google Scholar] [CrossRef] [PubMed]
- EYA, D. Deposition and characterization of PbO--PbS multilayer thin films by solution growth technique. Turk. J. Phys. 2014, 38, 118–124. [Google Scholar] [CrossRef]
- Mahmood, W.; Ali, J.; Zahid, I.; Thomas, A.; ul Haq, A. Optical and electrical studies of CdS thin films with thickness variation. Optik 2018, 158, 1558–1566. [Google Scholar] [CrossRef]
- Mahmood, W.; Shah, N.A. Effects of metal doping on the physical properties of ZnTe thin films. Curr. Appl. Phys. 2014, 14, 282–286. [Google Scholar] [CrossRef]
- Aziz, A.A.; Lim, C.O.; Hassan, Z.; Jamal, Z. CoSi2 formation with a thin Ti interlayer-Ti capping layer and Ti capping layer. In Recent Advances in Multidisciplinary Applied Physics; Elsevier Science Ltd.: Amsterdam, The Netherlands, 2005; pp. 337–341. [Google Scholar]
- Mohammadigharehbagh, R.; Pat, S.; Özen, S.; Yudar, H.H.; Korkmaz, Ş. Investigation of the optical properties of the indium-doped ZnO thin films deposited by a thermionic vacuum arc. Optik 2018, 157, 667–674. [Google Scholar] [CrossRef]
- Popescu, V.; Jumate, N.; Popescu, G.; Moldovan, M.; Prejmeriean, C. Studies of some electrical and photoelectrical properties of PbS films obtained by sonochemical methods. Chalcogenide Lett. 2010, 7, 95–100. [Google Scholar]
- Smith, A.; Dutton, D. Behavior of lead sulfide photocells in the ultraviolet. JOSA 1958, 48, 1007–1009. [Google Scholar] [CrossRef]
- Humphrey, J.N. Optimum utilization of lead sulfide infrared detectors under diverse operating conditions. Appl. Opt. 1965, 4, 665–675. [Google Scholar] [CrossRef]


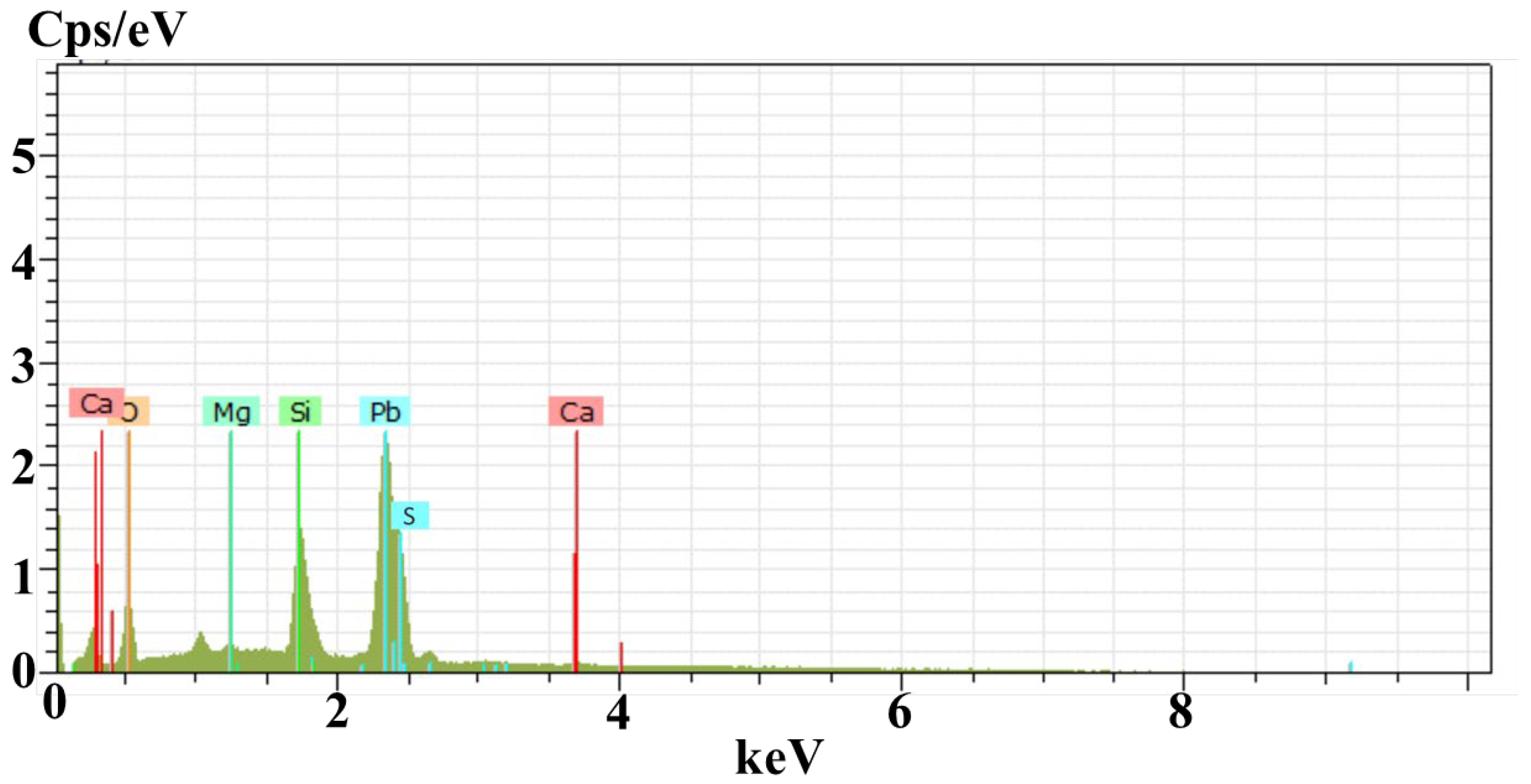


| Sample 1 PbS at 25 °C | Elements | Weight % | Atomic % |
| O | 6.45 | 29.0 | |
| Si | 11.3 | 29.0 | |
| Mg | 0.56 | 1.54 | |
| S | 5.45 | 12.2 | |
| Ca | 1.14 | 2.16 | |
| Pb | 75.1 | 26.1 | |
| Total | 100.00 | 100.00 | |
| Sample 2 PbS at 200 °C | Elements | Weight % | Atomic % |
| O | 7.01 | 31.0 | |
| Si | 11.9 | 30.0 | |
| Mg | 0.41 | 1.05 | |
| S | 4.38 | 9.7 | |
| Ca | 1.5 | 2.65 | |
| Pb | 74.8 | 25.6 | |
| Total | 100.00 | 100.00 | |
| Sample 3 PbS at 300 °C | Elements | Weight % | Atomic % |
| O | 12.8 | 46.8 | |
| Si | 12.9 | 26.9 | |
| Mg | 0.5 | 1.21 | |
| S | 1.3 | 2.38 | |
| Ca | 1.90 | 2.71 | |
| Pb | 70.6 | 20.0 | |
| Total | 100 | 100 | |
| Sample 4 PbS at 400 °C | Elements | Weight % | Atomic % |
| O | 8.46 | 35.45 | |
| Si | 12.72 | 30.36 | |
| Mg | 0.44 | 1.21 | |
| S | 3.1 | 6.48 | |
| Ca | 1.59 | 2.66 | |
| Pb | 73.69 | 23.84 | |
| Total | 100.00 | 100.00 | |
| Sample 5 PbS at 450 °C | Elements | Weight % | Atomic % |
| O | 11.51 | 43.78 | |
| Si | 13.15 | 28.49 | |
| Mg | 0.47 | 1.19 | |
| S | 1.48 | 2.81 | |
| Ca | 1.78 | 2.70 | |
| Pb | 71.61 | 21.03 | |
| Total | 100.00 | 100.00 | |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Akhtar, S.; Saeed, N.; Hanif, M.B.; Zia-ur-Rehman; Dogar, S.; Mahmood, W.; Mosiałek, M.; Napruszewska, B.D.; Ashraf, M.; Motola, M.; et al. PbS and PbO Thin Films via E-Beam Evaporation: Morphology, Structure, and Electrical Properties. Materials 2022, 15, 6884. https://doi.org/10.3390/ma15196884
Akhtar S, Saeed N, Hanif MB, Zia-ur-Rehman, Dogar S, Mahmood W, Mosiałek M, Napruszewska BD, Ashraf M, Motola M, et al. PbS and PbO Thin Films via E-Beam Evaporation: Morphology, Structure, and Electrical Properties. Materials. 2022; 15(19):6884. https://doi.org/10.3390/ma15196884
Chicago/Turabian StyleAkhtar, Saad, Nimra Saeed, Muhammad Bilal Hanif, Zia-ur-Rehman, Salahuddin Dogar, Waqar Mahmood, Michał Mosiałek, Bogna Daria Napruszewska, Muhammad Ashraf, Martin Motola, and et al. 2022. "PbS and PbO Thin Films via E-Beam Evaporation: Morphology, Structure, and Electrical Properties" Materials 15, no. 19: 6884. https://doi.org/10.3390/ma15196884
APA StyleAkhtar, S., Saeed, N., Hanif, M. B., Zia-ur-Rehman, Dogar, S., Mahmood, W., Mosiałek, M., Napruszewska, B. D., Ashraf, M., Motola, M., & Khan, A. F. (2022). PbS and PbO Thin Films via E-Beam Evaporation: Morphology, Structure, and Electrical Properties. Materials, 15(19), 6884. https://doi.org/10.3390/ma15196884













