Effect of Bi and Ca on the Solidification Parameters of Sr-Modified Al-S-Cu (Mg) Alloys
Abstract
:1. Introduction
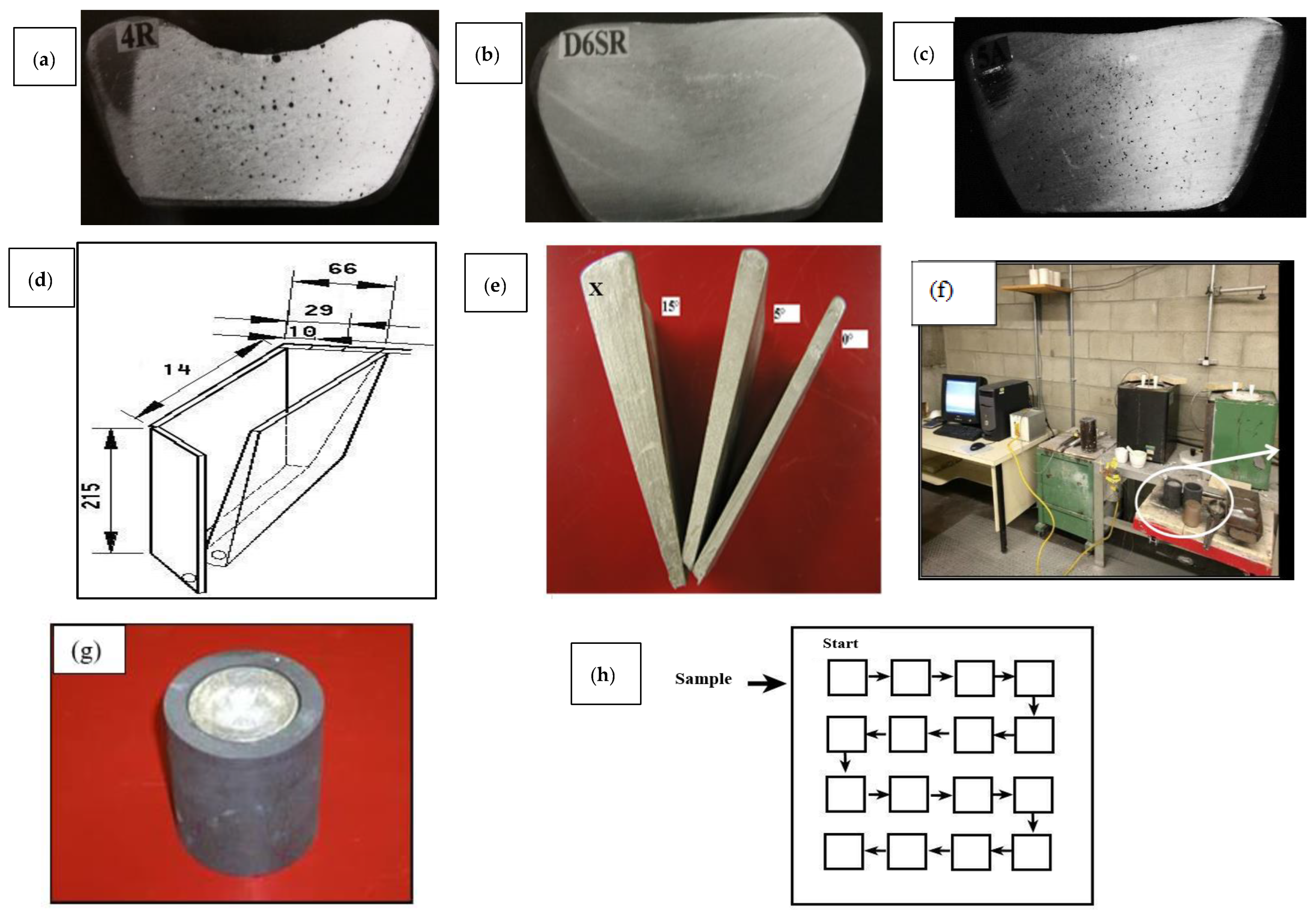
2. Experimental Procedures
3. Results and Discussion
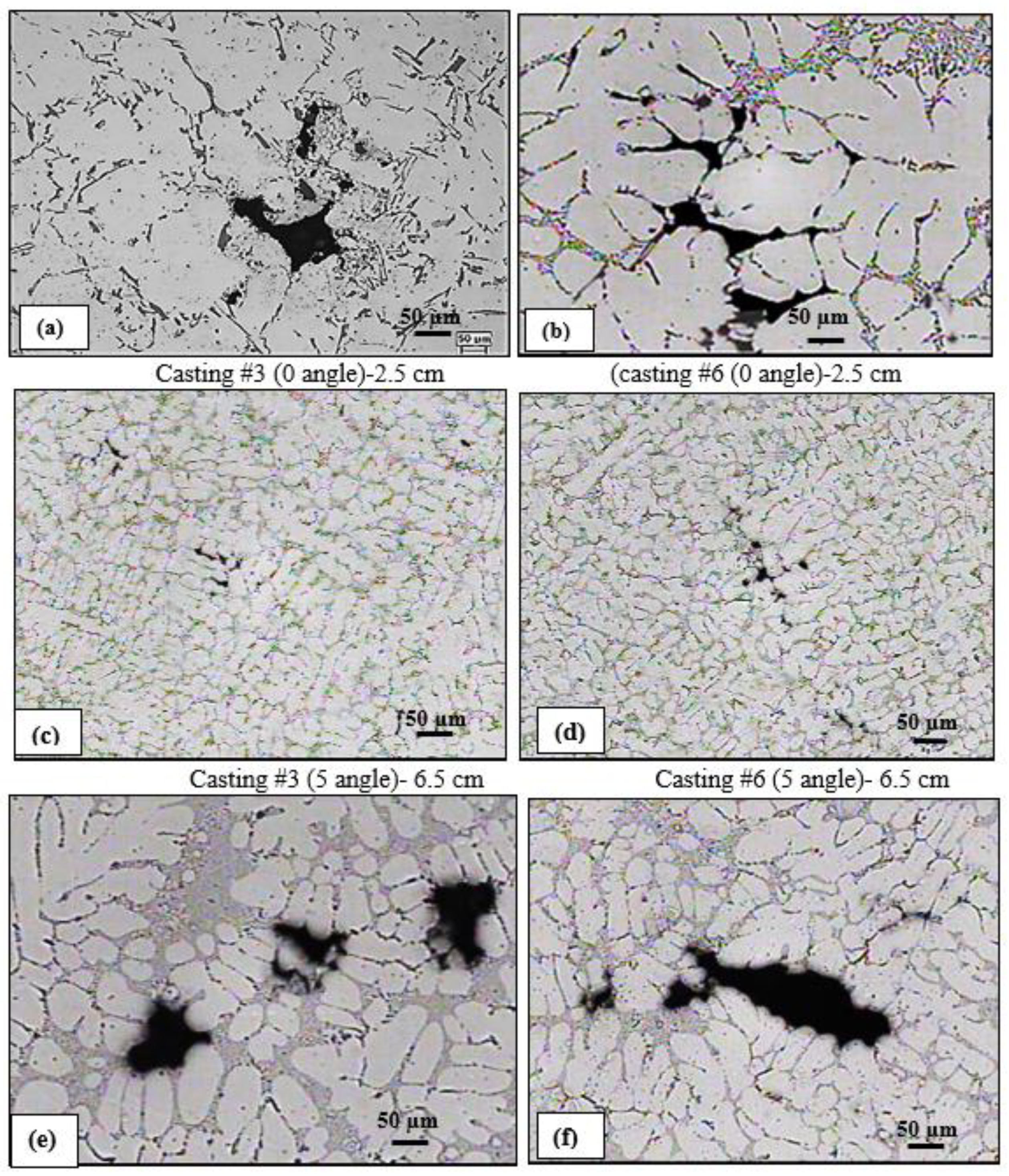
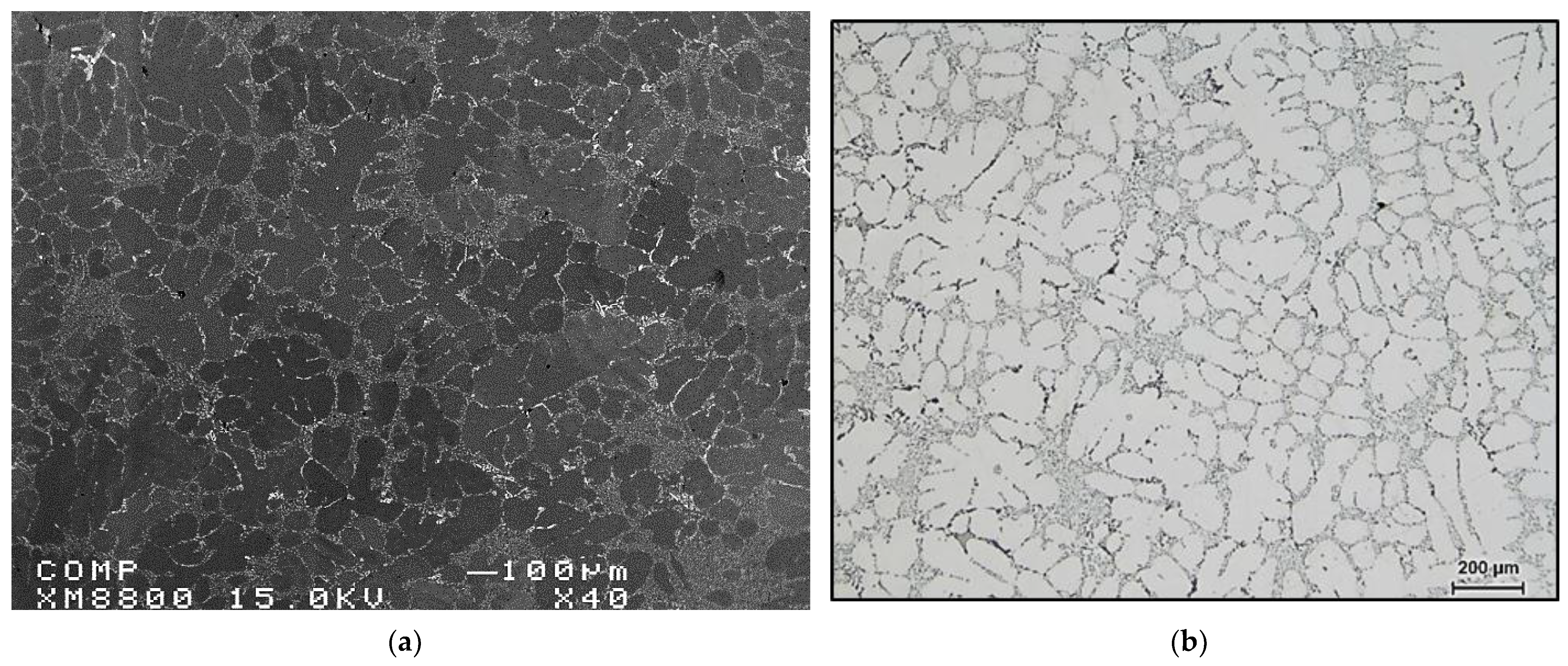
3.1. Porosity Formation
3.2. Solidification Curves
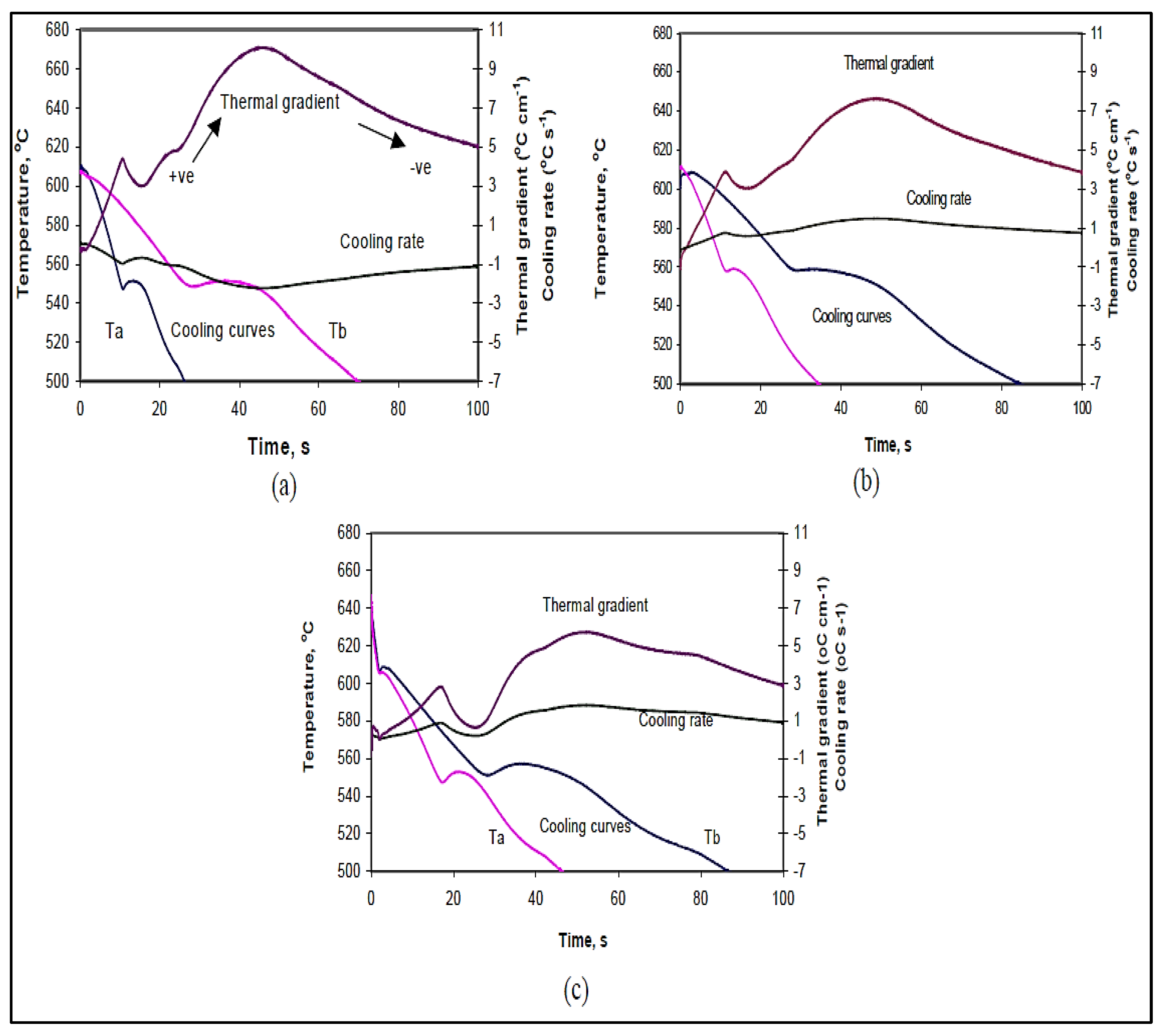
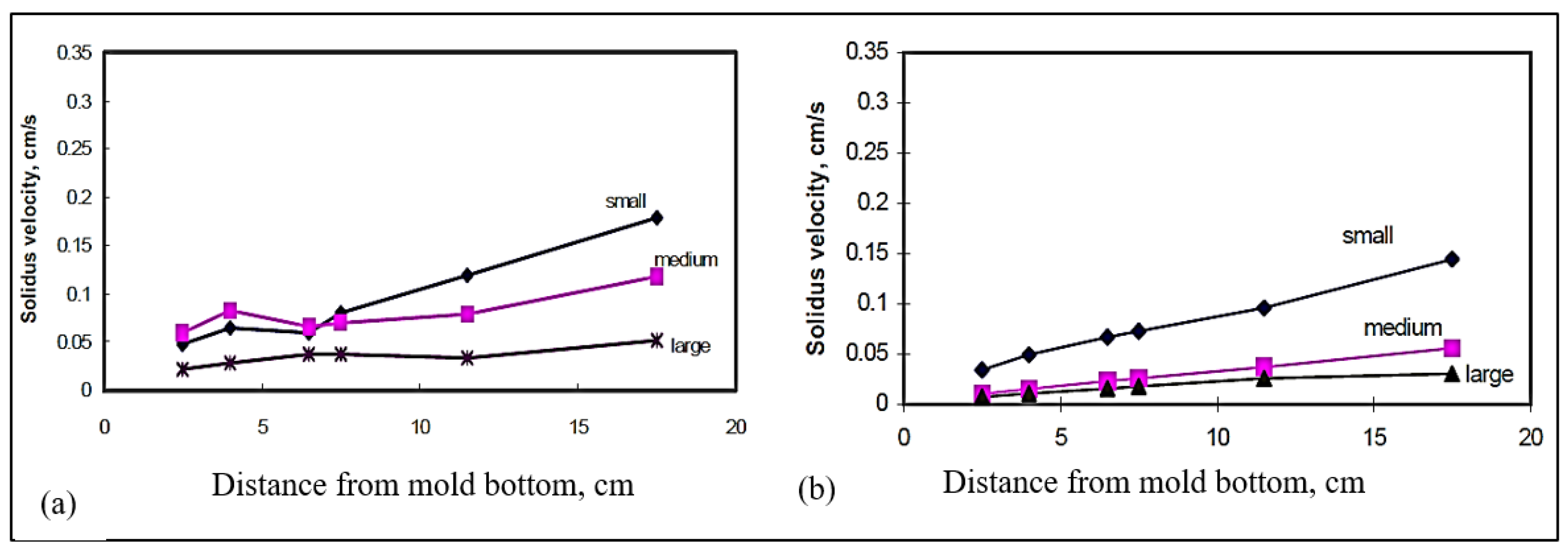
3.3. Thermal Gradient
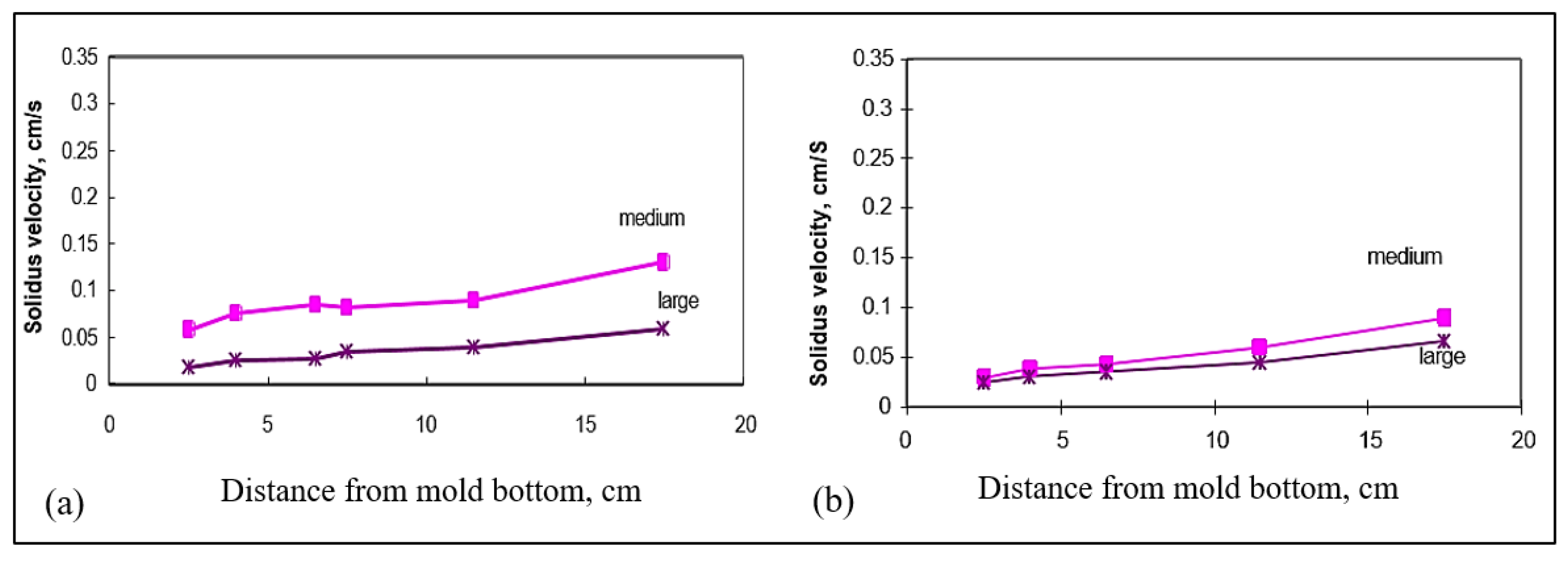
3.4. Interface Velocity
4. Conclusions
- The addition of Bi increases the Si-eutectic temperature, while no noticeable effect on the eutectic temperature is observed when Ca is added, indicating that Bi has a higher affinity to react with Sr than does Ca.
- The additions of Bi and Ca lower the thermal gradient in the mushy zone during solidification. In addition, Bi and Ca lower the solidus velocities.
- The solidus interface velocity is affected by the rejection of solute atoms in front of the moving solid/liquid interface. This observation is more obvious in the small mold (0° angle) section than in the large mold (15° angle) section, due to the much higher velocity obtained in the former case.
- In the absence of oxides, the effect of Bi and Ca additions on the solidification thermal parameters constitutes an important reason for the observed changes in Si morphology and porosity volume fraction.
- Both BiO and CaO form very fine microporosity (less than 1µm). Their fine size and also the concentration of such microporosity in/near the hot spots, particularly in the case of the small mold castings, explains the low percentage porosity values obtained in the B and C alloys with Bi and Ca additions.
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- El-Hadad, S.; Samuel, A.M.; Samuel, F.H.; Doty, H.W.; Valtierra, S. Role of Bi and Ca additions in controlling the microstructure of Sr-modified 319 alloys. In Proceedings of the 10th International Conference on Aluminum Alloys, Vancouver, BC, Canada, 11–14 July 2007. [Google Scholar]
- Cho, J.I.; Loper, C.R. Limitations of Bismuth Residual in A356.2 Al. AFS Trans. 2000, 108, 359–367. [Google Scholar]
- Kaya, H.; Aker, A. Effect of alloying elements and growth rates on microstructure and mechanical properties in the directionally solidified Al–Si–X alloys. J. Alloy. Compd. 2017, 694, 145–154. [Google Scholar] [CrossRef]
- Samuel, A.M.; Ammar, H.R.; Zedan, Y.; Doty, H.W.; Samuel, F.H. Effect of Ca–Sr–Mg and Bi–Sr–Mg Interactions on the Microstructural Characterization and Tensile Properties of B319 Alloy. Inter. Met. 2022, 1–20. [Google Scholar] [CrossRef]
- Sigworth, G.K. Theoretical and Practical Aspects of Modification of Al-Si Alloys. AFS Trans. 1983, 91, 7–15. [Google Scholar]
- Farahany, S.; Ourdjini, A.; Idris, M.H.; Shabestari, S.G. Evaluation of the effect of Bi, Sb, Sr and solidification condition on eutectic phases in an Al–Si–Cu alloy (ADC12) by in situ thermal analysis. Thermochim. Acta 2013, 559, 59–68. [Google Scholar] [CrossRef]
- Farahany, S.; Ourdjini, A.; Abu Bakar, T.A.; Idris, M.H. Role of bismuth on solidification, microstructure and mechanical properties of a near eutectic Al-Si alloy. Met. Mater. Int. 2014, 20, 929–938. [Google Scholar] [CrossRef]
- Loper, C.R.; Cho, J. Influence of Trace Amounts of Calcium in Aluminum Castings. AFS Trans. 2000, 108, 585–592. [Google Scholar]
- Dias, M.; Oliveira, R.; Kakitani, R.; Cheung, N.; Henein, H.; Spinelli, J.E.; Garcia, A. Effects of solidification thermal parameters and Bi doping on silicon size, morphology and mechanical properties of Al-15wt.% Si-3.2wt.% Bi and Al-18wt.% Si-3.2wt.% Bi alloys. J. Mater. Res. Technol. 2022, 9, 3460–3470. [Google Scholar] [CrossRef]
- Hosseini, V.A.; Shabestari, S.G.; Gholizadeh, R. Study on the Effect of Solidification Rate on the Solidification Parameters Microstructure and Mechanical Properties of LM13 Alloy Using Solidification Curve Thermal Analysis Technique. Mater. Des. 2013, 50, 7–14. [Google Scholar] [CrossRef]
- Chen, R.; Shi, Y.; Xu, Q.; Liu, B.U. Effect of solidification rate on solidification parameters and microstructure of Al-7Si-0.3Mg-0.15Fe alloy. Trans. Nonferrous Met. Soc. China 2014, 24, 1645–1652. [Google Scholar] [CrossRef]
- Biswas, P.; Prasadu, K.D.; Mondal, M.K. Effect of Bi addition on microstructure and mechanical properties of hypereutectic Al-17.6 Si alloy. Mater. Res. Express 2019, 6, 1165b9. [Google Scholar] [CrossRef]
- Barrirero, J.; Engstler, M.; Ghafoor, N.; de Jonge, N.; Odén, M.; Mücklich, F. Comparison of segregations formed in unmodified and Sr-modified Al–Si alloys studied by atom probe tomography and transmission electron microscopy. J. Alloy. Compd. 2014, 611, 410–421. [Google Scholar] [CrossRef] [Green Version]
- Okayasu, M.; Takeuchi, S.; Wu, S.; Ochi, T. Effects of Sb, Sr, and Bi on the material properties of cast Al-Si-Cu alloys produced through heated mold continuous casting. J. Mech. Sci. Technol. 2016, 30, 1139–1147. [Google Scholar] [CrossRef]
- Ellingham, H.J.T. Reducibility of oxides and sulphides in metallurgical processes. J. Soc. Chem. Ind. Lond. 1944, 63, 125. [Google Scholar] [CrossRef]
- Gleeson, B. 1.09-Thermodynamics and Theory of External and Internal Oxidation of Alloys. Shreir’s Corros. 2010, 1, 180–194. [Google Scholar] [CrossRef]
- Jeon, M.K.; Kim, S.W.; Lee, S.K.; Choi, E.Y. Thermodynamic Calculations on the Chemical Behavior of SrO During Electrolytic Oxide Reduction. JNFCWT 2020, 18, 415–420. [Google Scholar] [CrossRef]
- Ambhorn, S.C.; Thublaor, T.; Pascal, C. Thermodynamics and Kinetics of the High Temperature Oxidation of Stainless Steels. Solid State Phenom. 2019, 300, 1–24. [Google Scholar] [CrossRef] [Green Version]
- El-Hadad, S.; Samuel, A.M.; Samuel, F.H.; Doty, H.W.; Valtierra, S. Effects of Bi and Ca Addition on the Characteristics of Eutectic Si Particles in Sr-modified 319 Alloys. Int. J. Cast Met. Res. 2003, 15, 551–564. [Google Scholar] [CrossRef]
- Kucharčík, L.; Brůna, M.; Sládek, A. Influence of Chemical Composition on Porosity in Aluminium Alloys. Arch. Foundry Eng. 2014, 14, 5–8. [Google Scholar] [CrossRef]
- Nguyen, T.; Bonamy, D. Role of crystal lattice structure in predicting fracture toughness. Phys. Rev. Lett. Am. Phys. Soc. 2019, 123, 205503. [Google Scholar] [CrossRef] [Green Version]
- Battle, T.P.; Pehlke, R.D. Equilibrium partition coefficients in iron-based alloys. Met. Mater. Trans. B 1989, 20, 149–160. [Google Scholar] [CrossRef]
- Valdes, J.; Shang, S.L.; Liu, Z.-K.; King, P.; Liu, X. Quenching differential thermal analysis and thermodynamic calculation to determine partition coefficients of solute elements in simplified Ni-base superalloys. Met. Mater. Trans. A 2010, 41, 487. [Google Scholar] [CrossRef]
- Fuller, C.S.; Ditzenberger, J.A. Diffusion of Donor and Acceptor Elements in Silicon. J. Appl. Phys. 1956, 27, 544. [Google Scholar] [CrossRef]
- Cho, J.I.; Kim, C.W. The Relationship between Dendrite Arm Spacing and Cooling Rate of Al-Si Casting Alloys in High Pressure Die Casting. Inter. Met. 2014, 8, 49–55. [Google Scholar] [CrossRef]
- Gowri, S.; Samuel, F.H. Effect of cooling rate on the solidification behavior of Al-7 Pct Si-SiCp metal-matrix composites. Met. Mater. Trans. A 1992, 23, 3369–3376. [Google Scholar] [CrossRef]
- Doran, H. Organic Crystal Nucleation in Ultrathin Liquid Films: Applications of Computational and Experimental Methods for the Exploration of Dynamic Spatial Relationships and Controlled Growth. Master’s Thesis, Western Washington University, Bellingham, WA, USA, 2021. Available online: https://cedar.wwu.edu/wwuet/1009 (accessed on 25 September 2022).
- Ibragimov, V.E.; Bazhin, V.Y. Remelting of highly polluted metallic aluminium scrap with ecological refining reagents. IOP Conf. Ser. Mater. Sci. Eng. 2019, 537, 062087. [Google Scholar] [CrossRef]
- Bäckerud, L.; Chai, G.; Tamminen, J. Solidification Characteristics of Aluminum Alloys; Volume 2: Foundry Alloys; AFS/Skanaluminium: Des Plaines, IL, USA, 1990; pp. 71–84. [Google Scholar]
- Speight, J.G. Molecular interactions, partitioning, and thermodynamics. In Reaction Mechanisms in Environmental Engineering Analysis and Prediction, 1st ed.; Butterworth-Heinemann: Oxford, UK, 2018; Chapter 9; pp. 307–336. [Google Scholar]
- Patten, C.; Barnes, S.J.; Mathez, E.A.; Jenner, F.E. Partition coefficients of chalcophile elements between sulfide and silicate melts and the early crystallization history of sulfide liquid. Chem. Geol. 2013, 358, 170–188. [Google Scholar] [CrossRef]
- Tiedje, N.S.; Taylor, J.A.; Easton, M.A. Feeding and Distribution of Porosity in Cast Al-Si Alloys as Function of Alloy Composition and Modification. Met. Mater. Trans. A 2012, 43, 4846–4858. [Google Scholar] [CrossRef]











| Alloy | Si | Cu | Fe | Mg | Mn | Ti | Zn | Sr | Al |
|---|---|---|---|---|---|---|---|---|---|
| B * | 6.14 | 3.63 | 0.11 | 0.048 | <0.0005 | 0.14 | <0.0017 | 0.0152 | Bal |
| C ** | 6.16 | 3.48 | 0.10 | 0.635 | <0.0015 | 0.15 | <0.0017 | 0.0118 | Bal |
| Alloy Code | Alloy Used + Addition | Alloy Code | Alloy Used + Addition |
|---|---|---|---|
| BB | B + 0621 ppm Bi | CB6 | C + 3020 ppm Bi |
| BB2 | B + 2186 ppm Bi | BC | B + 0052 ppm Ca |
| BB4 | B + 3785 ppm Bi | BC2 | B + 0103 ppm Ca |
| BB6 | B + 4060 ppm Bi | BC5 | B + 0465 ppm Ca |
| CB | C + 0464 ppm Bi | CC | C + 0063 ppm Ca |
| CB1 | C + 0874 ppm Bi | CC2 | C + 0094 ppm Ca |
| CB2 | C + 1034 ppm Bi | CC5 | C + 0486 ppm Ca |
| CB4 | C + 2264 ppm Bi | ||
| (a) | ||||||||
|---|---|---|---|---|---|---|---|---|
| Alloy Code | Area Percentage Porosity | Pore Characteristics | ||||||
| Area (µm2) | Length (µm) | Density (#pores/mm2) | ||||||
| Average | S. D. | Average | S. D. | Average | S. D. | |||
| BB | 0.065 | 0.047 | 152.5 | 58.6 | 08.5 | 11.6 | 162 | |
| BB2 | 0.078 | 0.016 | 119.4 | 29.3 | 13.5 | 13.6 | 152 | |
| BB4 | 0.092 | 0.054 | 506.1 | 87.4 | 33.9 | 31.3 | 179 | |
| BB6 | 0.062 | 0.025 | 320.4 | 33.3 | 32.1 | 25.9 | 75 | |
| CB | 0.129 | 0.245 | 929.8 | 321.3 | 60.3 | 38.2 | 240 | |
| CB1 | 0.231 | 0.220 | 833.0 | 371.9 | 48.7 | 40.3 | 275 | |
| CB2 | 0.210 | 0.151 | 927.1 | 83.2 | 48.1 | 42.7 | 222 | |
| CB4 | 0.192 | 0.128 | 590.6 | 29.8 | 39.6 | 24.6 | 155 | |
| BC | 0.086 | 0.084 | 455.8 | 67.1 | 28.0 | 22.7 | 184 | |
| BC2 | 0.008 | 0.008 | 104.7 | 24.4 | 11.7 | 12.1 | 81 | |
| BC5 | 0.177 | 0.178 | 655.7 | 44.3 | 47.4 | 31.2 | 163 | |
| CC5 | 0.258 | 0.223 | 562.9 | 27.6 | 38.3 | 25.9 | 410 | |
| (b) | ||||||||
| Alloy Code | Pore Characteristics | |||||||
| Area (µm2) | Length (µm) | Density (#pores/mm2) | ||||||
| Average | S. D. | Average | S. D. | |||||
| BB | 160.2 | 82.0 | 17.8 | 09.3 | 155 | |||
| BB2 | 174.7 | 28.4 | 22.5 | 13.2 | 468 | |||
| BB4 | 123.1 | 69.6 | 18.8 | 10.2 | 652 | |||
| BB6 | 184.3 | 25.7 | 22.5 | 10.2 | 575 | |||
| CB | 244.6 | 35.0 | 25.6 | 18.8 | 237 | |||
| CB1 | 343.0 | 78.1 | 18.4 | 16.4 | 749 | |||
| CB2 | 243.2 | 78.9 | 14.7 | 8.1 | 822 | |||
| CB4 | 287.0 | 53.6 | 35.0 | 19.5 | 474 | |||
| BC | 182.8 | 88.5 | 12.4 | 11.2 | 142 | |||
| BC2 | 153.1 | 57.7 | 15.6 | 17.1 | 276 | |||
| BC5 | 146.2 | 63.1 | 22.2 | 12.4 | 550 | |||
| CC5 | 266.7 | 67.8 | 16.4 | 11.7 | 478 | |||
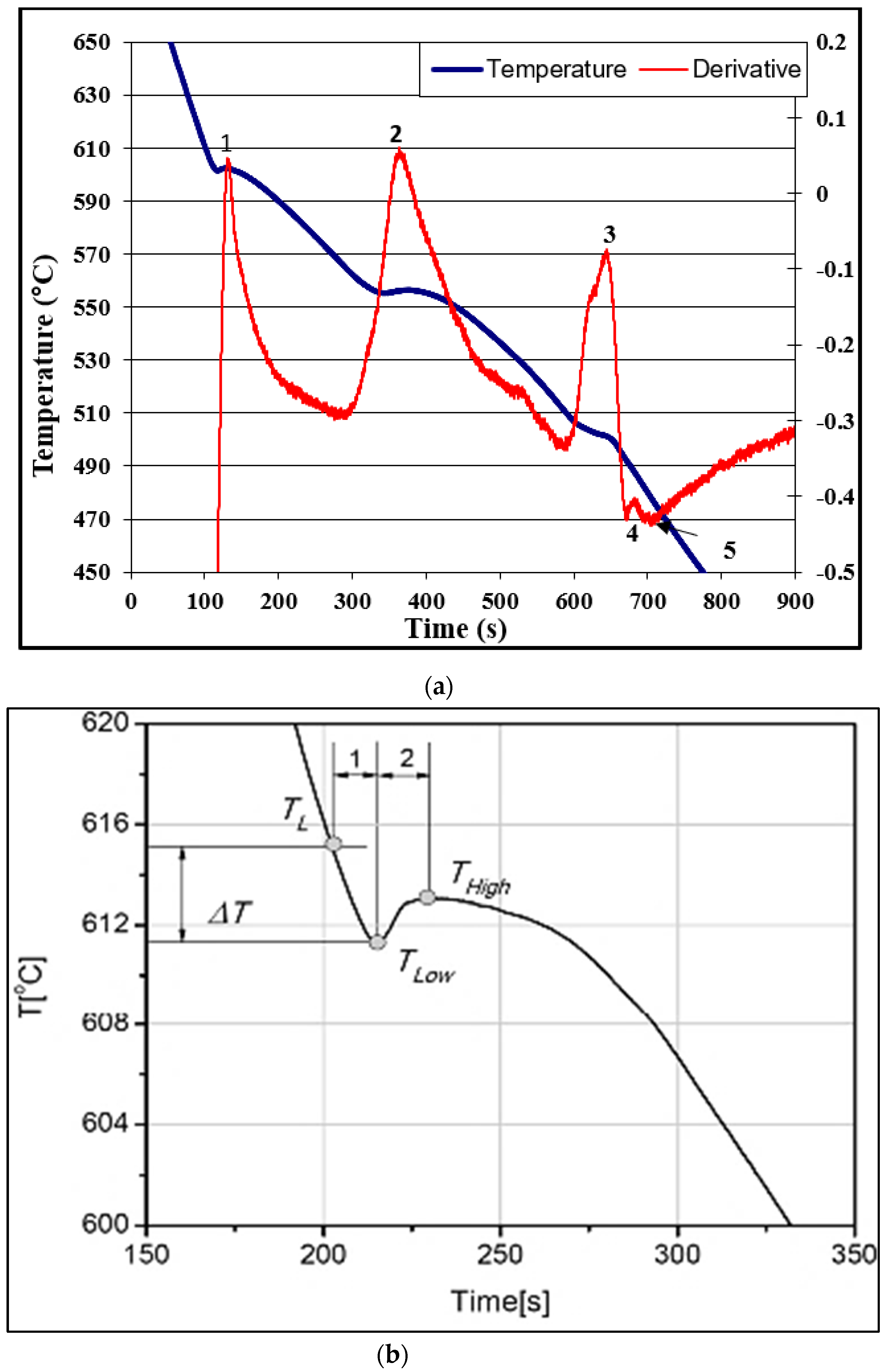
| Reaction # | Temperature (°C) | Type of Reaction |
|---|---|---|
| 1 | 601 | Precipitation of α-Al network |
| 2 | 561.2 | Al-Si eutectic reaction |
| 3 | 504.2 | Al-Al2Cu eutectic reaction |
| 4 | 498.1 | Precipitation of Al5Mg8Cu2Si6 |
| 5 | 483 | End of solidification |
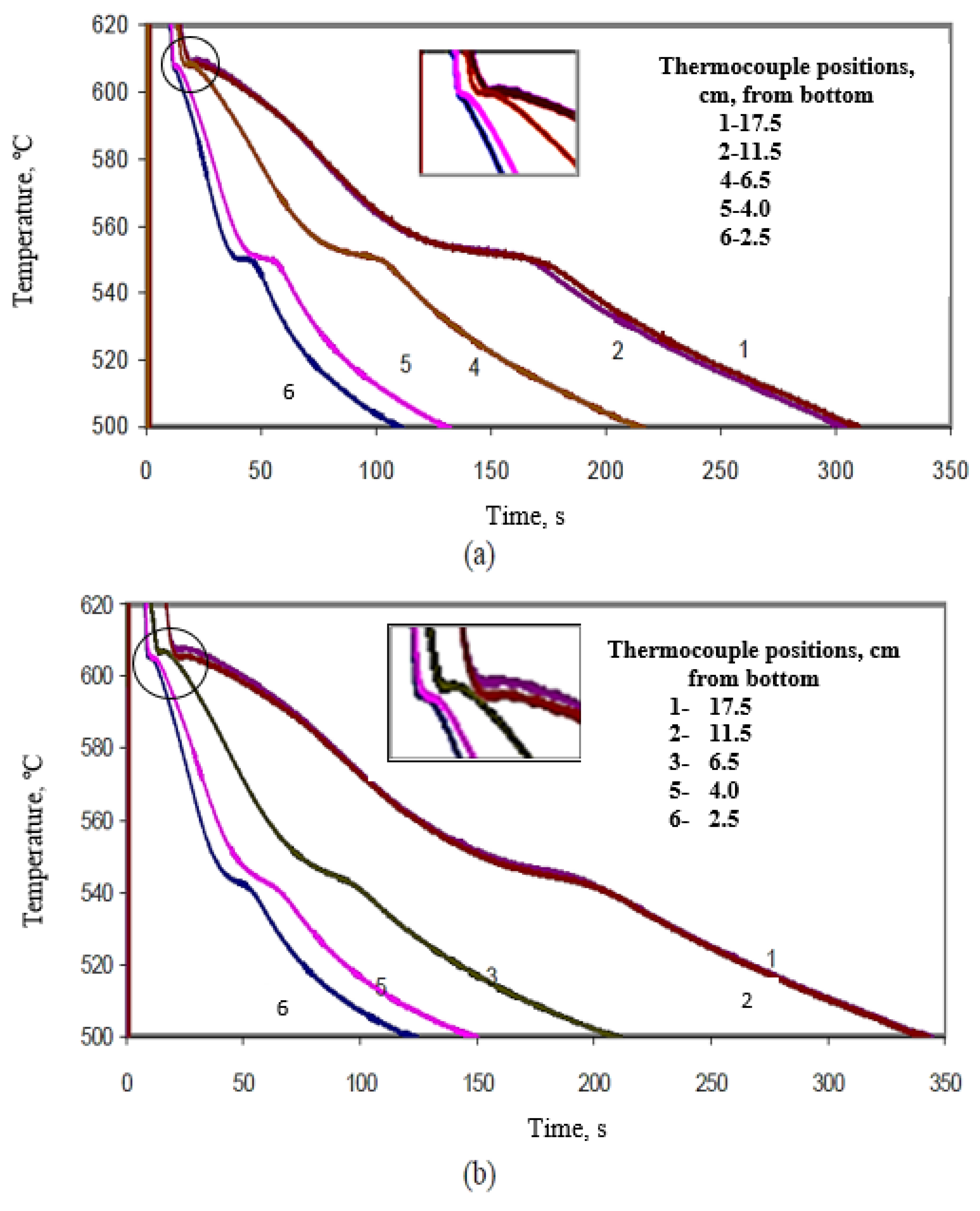
| Figure # | Alloy Composition | Thermocouple Position (cm) | Solidification Time (s) |
|---|---|---|---|
| 12a | B | 17.5 | 300 |
| 11.5 | 300 | ||
| 6.5 | 210 | ||
| 4.0 | 120 | ||
| 2.5 | 100 | ||
| 12b | C | 17.5 | 330 |
| 11.5 | 330 | ||
| 6.5 | 200 | ||
| 4.0 | 130 | ||
| 2.5 | 115 | ||
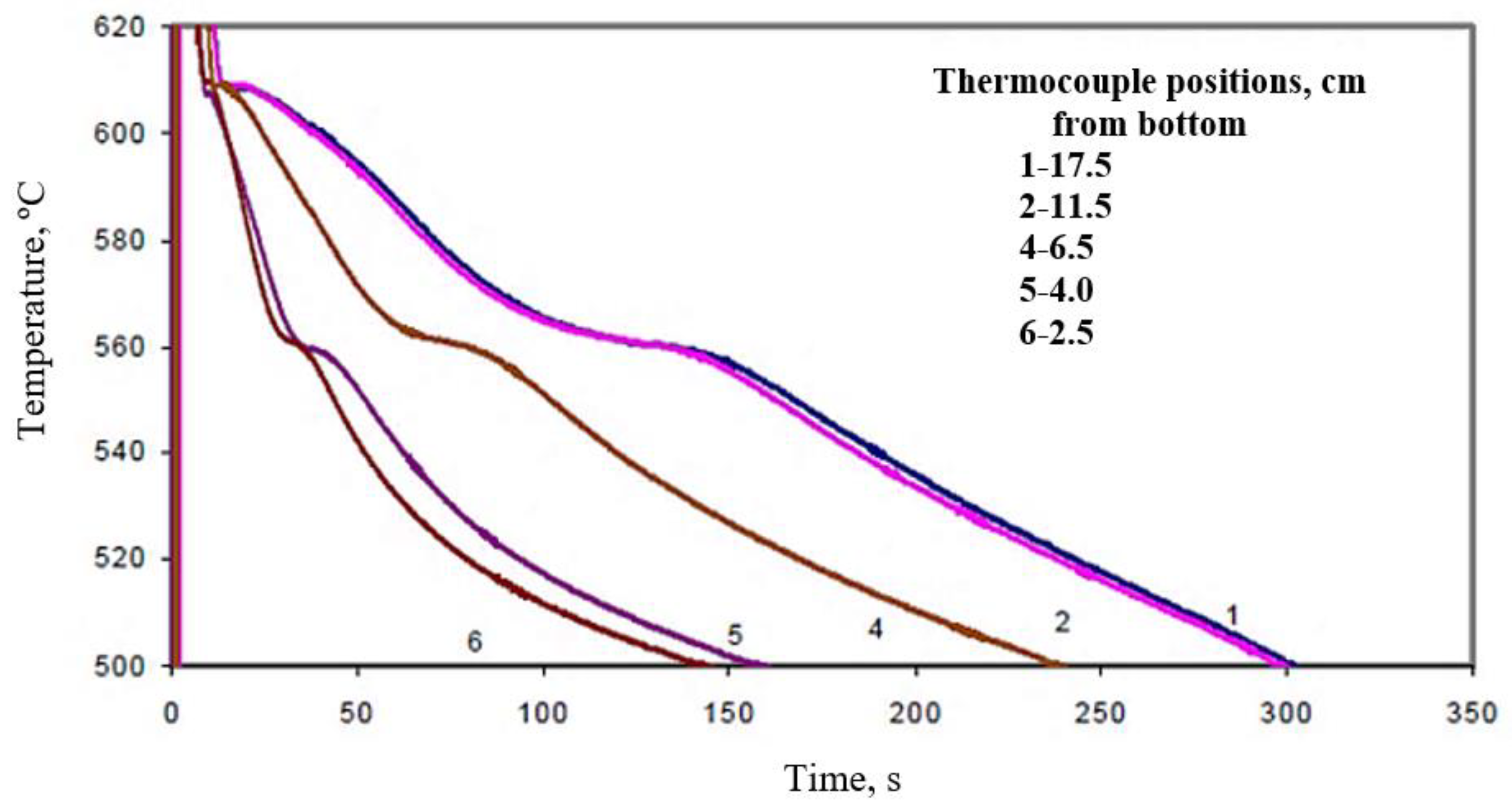
| 13 | BB2 | 17.5 | 280 |
| 11.5 | 330 | ||
| 6.5 | 200 | ||
| 4.0 | 130 | ||
| 2.5 | 115 | ||
| 14 | CC2 | 17.5 | 250 |
| 11.5 | 250 | ||
| 4.0 | 110 | ||
| 2.5 | 90 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
El-Hadad, S.; Samuel, A.M.; Samuel, F.H.; Doty, H.W.; Songmene, V. Effect of Bi and Ca on the Solidification Parameters of Sr-Modified Al-S-Cu (Mg) Alloys. Materials 2022, 15, 6903. https://doi.org/10.3390/ma15196903
El-Hadad S, Samuel AM, Samuel FH, Doty HW, Songmene V. Effect of Bi and Ca on the Solidification Parameters of Sr-Modified Al-S-Cu (Mg) Alloys. Materials. 2022; 15(19):6903. https://doi.org/10.3390/ma15196903
Chicago/Turabian StyleEl-Hadad, Shimaa, Agnes M. Samuel, Fawzy H. Samuel, Herbert W. Doty, and Victor Songmene. 2022. "Effect of Bi and Ca on the Solidification Parameters of Sr-Modified Al-S-Cu (Mg) Alloys" Materials 15, no. 19: 6903. https://doi.org/10.3390/ma15196903





