Essential Oils as Antimicrobial Active Substances in Wound Dressings
Abstract
:1. Introduction
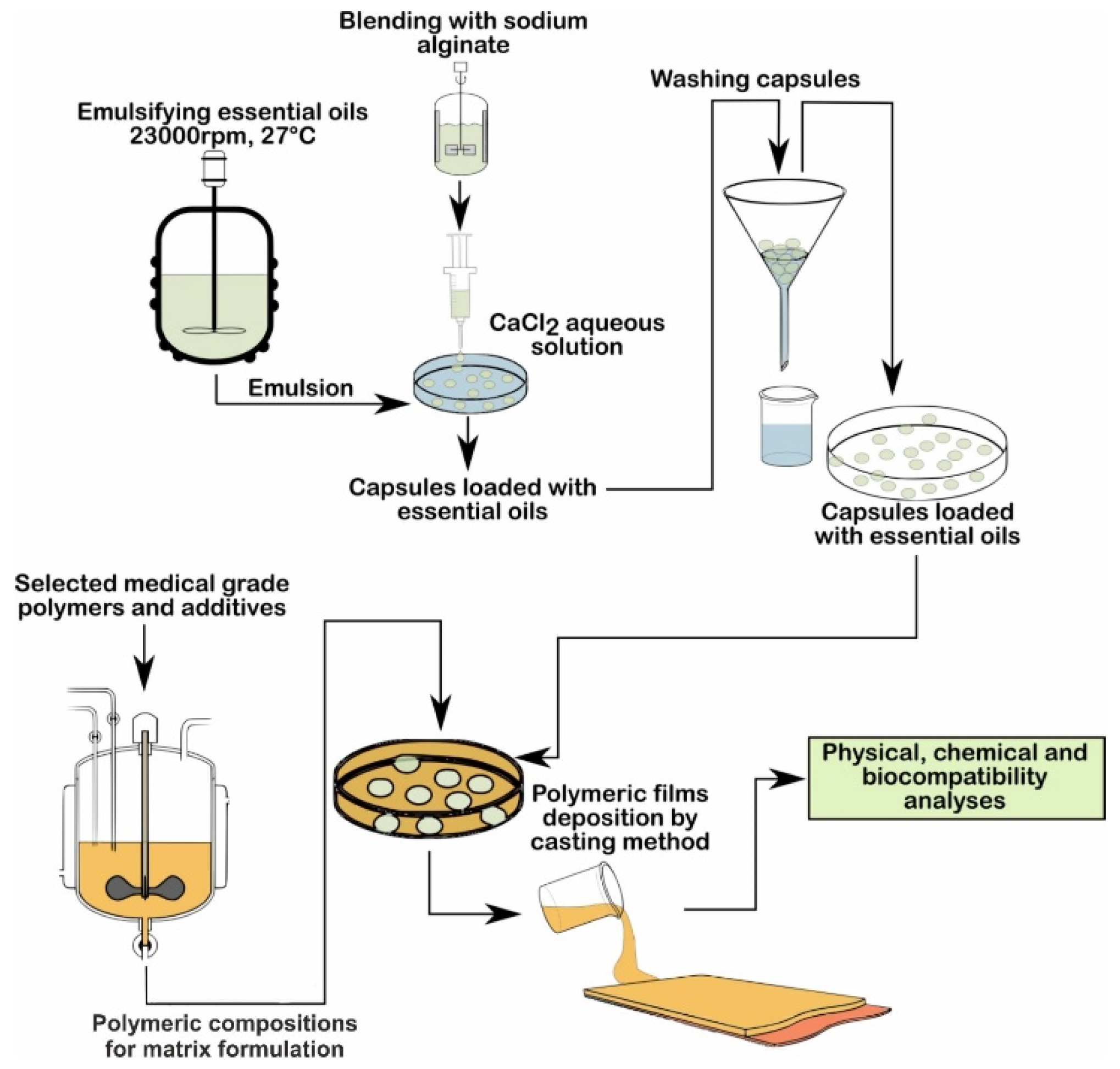
2. Materials and Methods
2.1. Contact Angle
2.2. Optical Microscopy
2.3. Film Thickness
2.4. Gel Fraction
2.5. Swelling Ability
2.6. Water Solubility
2.7. Water Vapor Permeability
2.8. Fourier-Transform Infrared Spectroscopy Analyses (FTIR)
2.9. Scanning Electron Microscopy and Energy Dispersive X-ray Analysis
2.10. Microbiological Analyses
2.11. MTT Assay
2.12. Statistical Analyses
3. Results and Discussions
3.1. Contact Angle
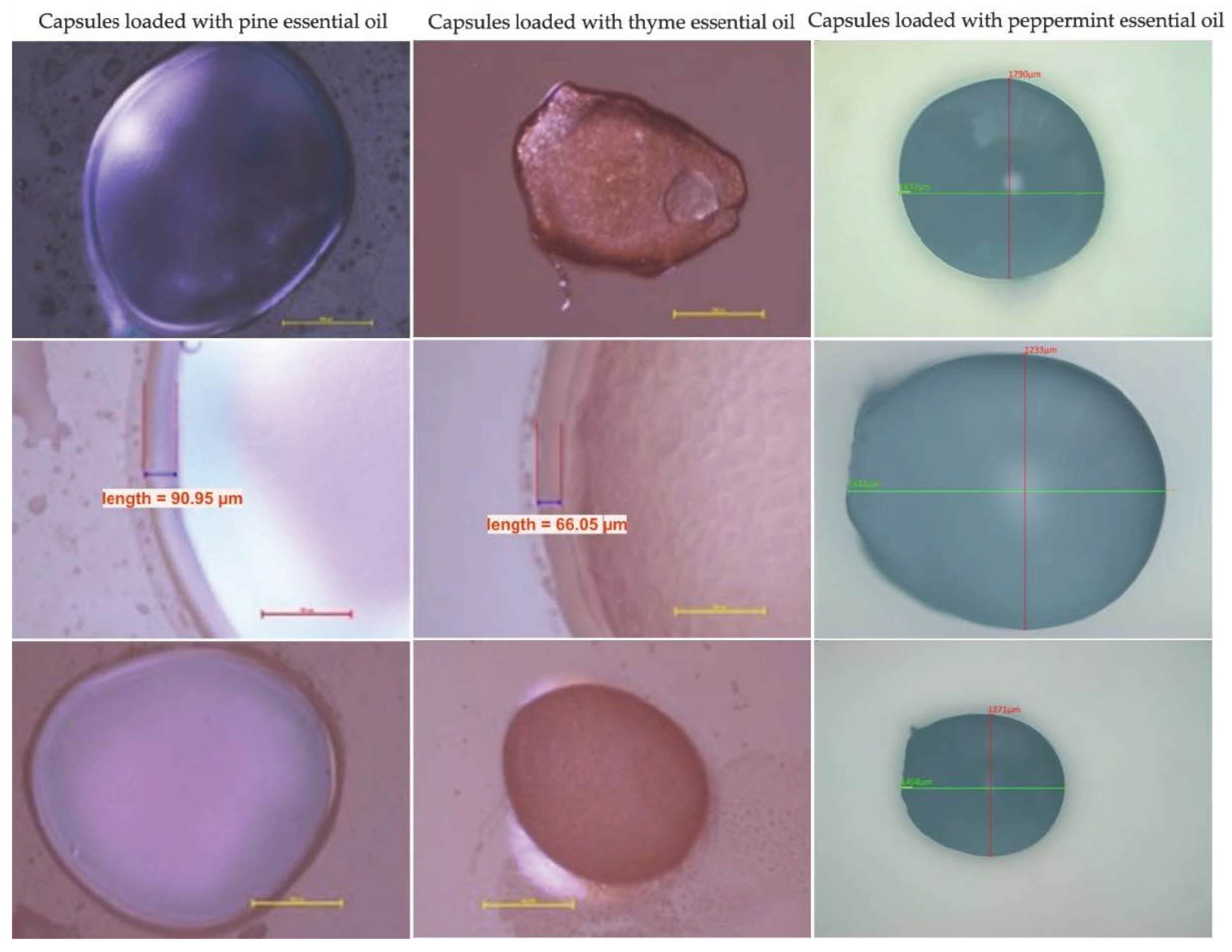
3.2. Optical Microscopy
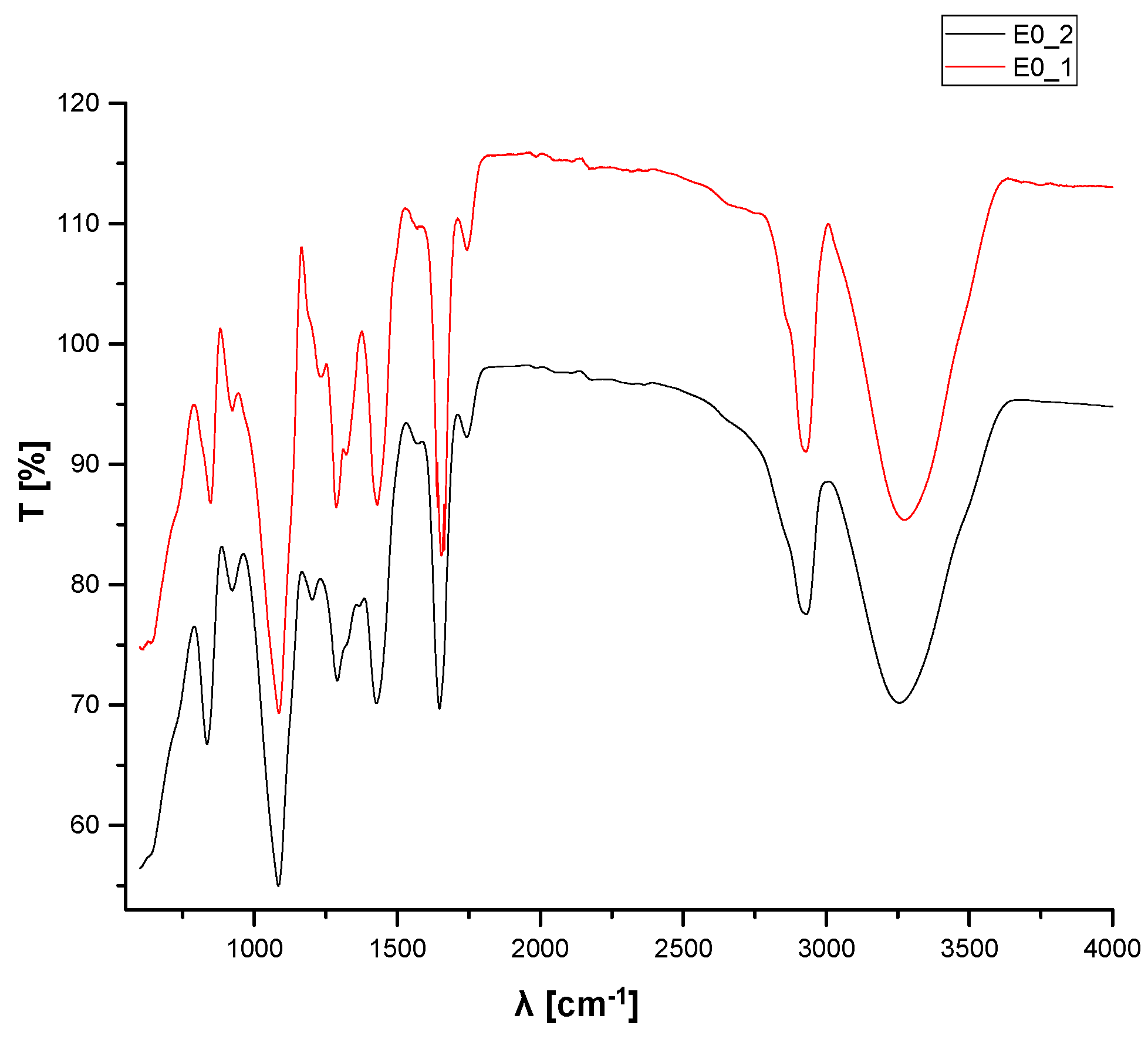
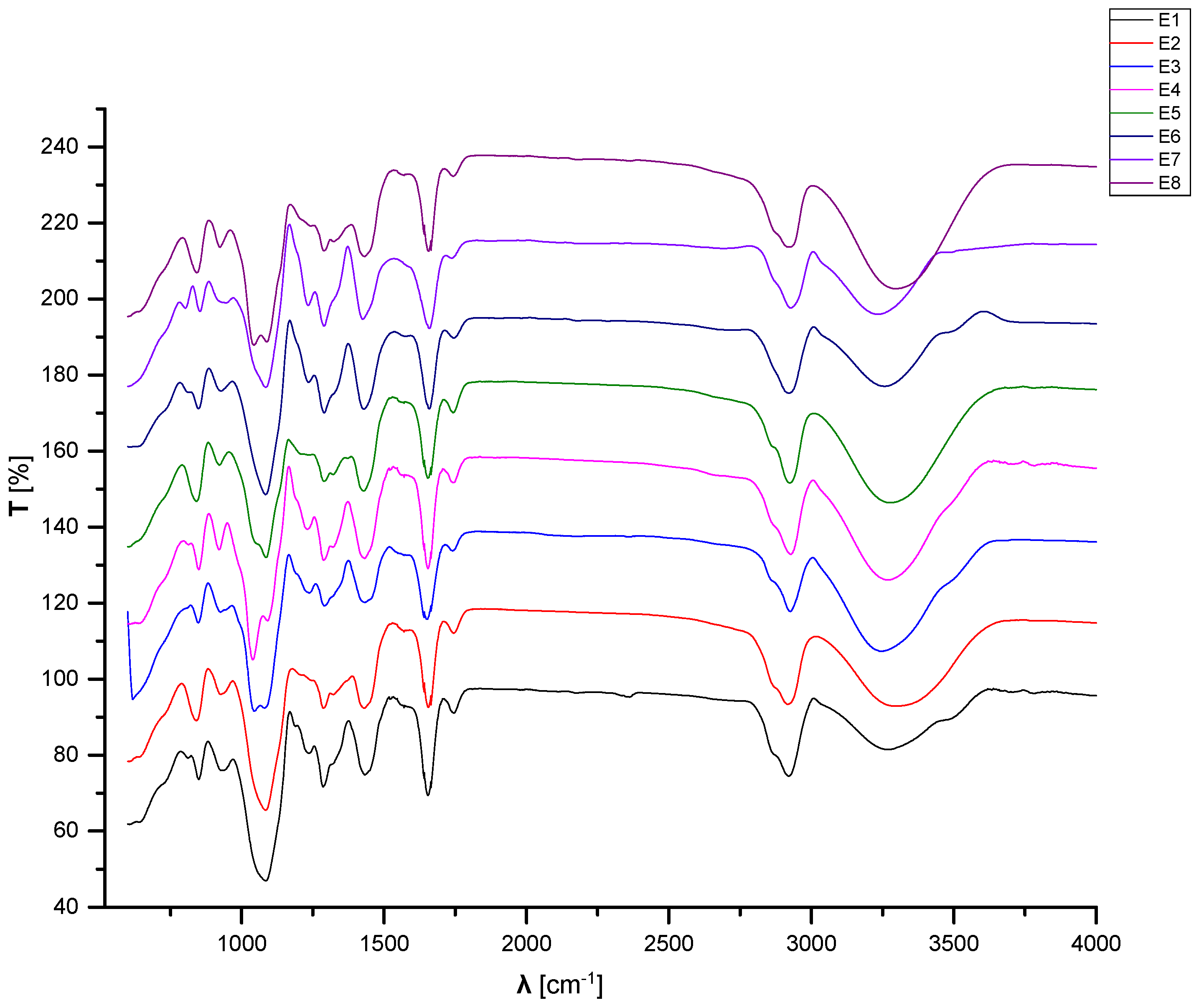
3.3. FTIR Analyses
3.4. Gel Fraction
3.5. Scanning Electron Microscopy and Energy Dispersive X-ray Analysis
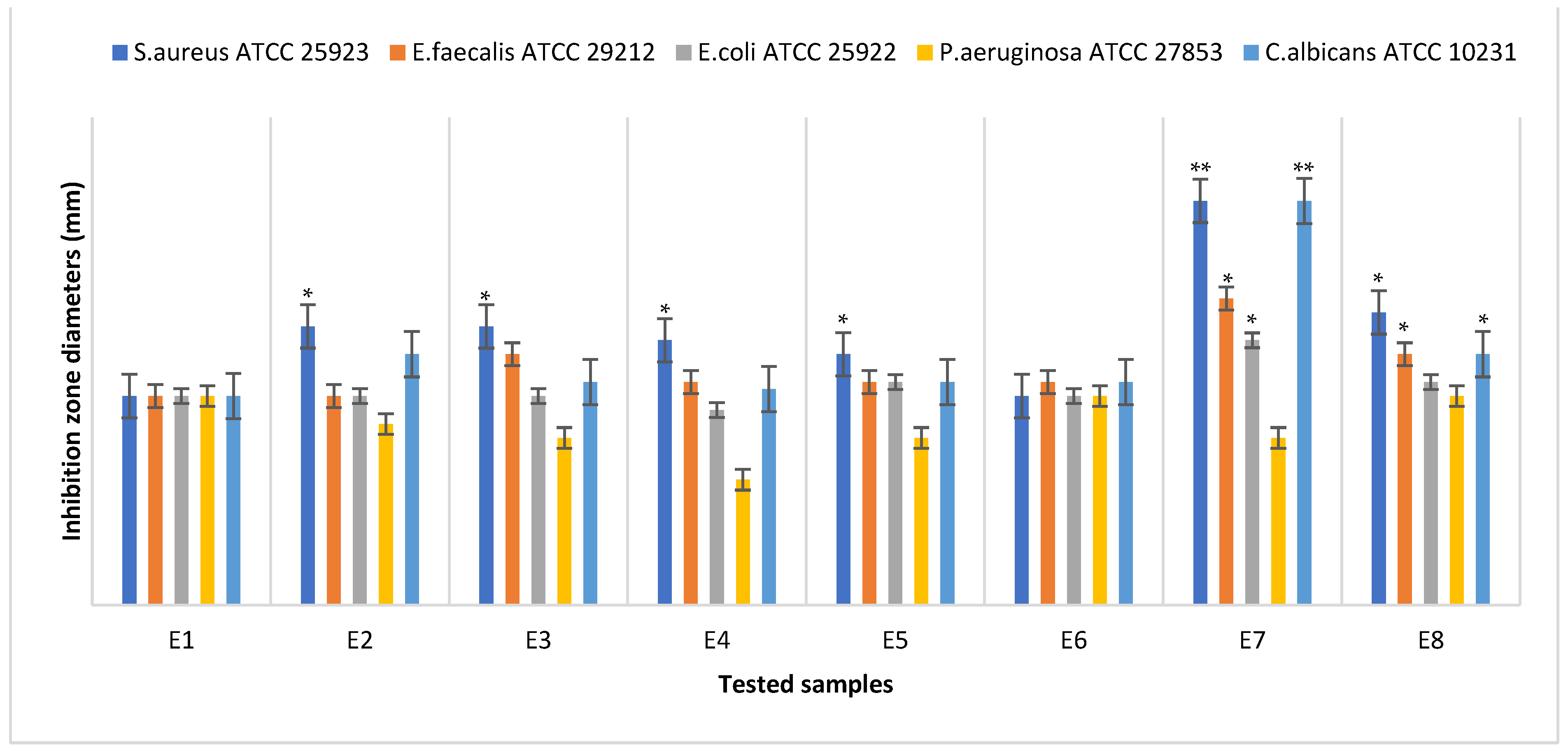
3.6. Antimicrobial Assays
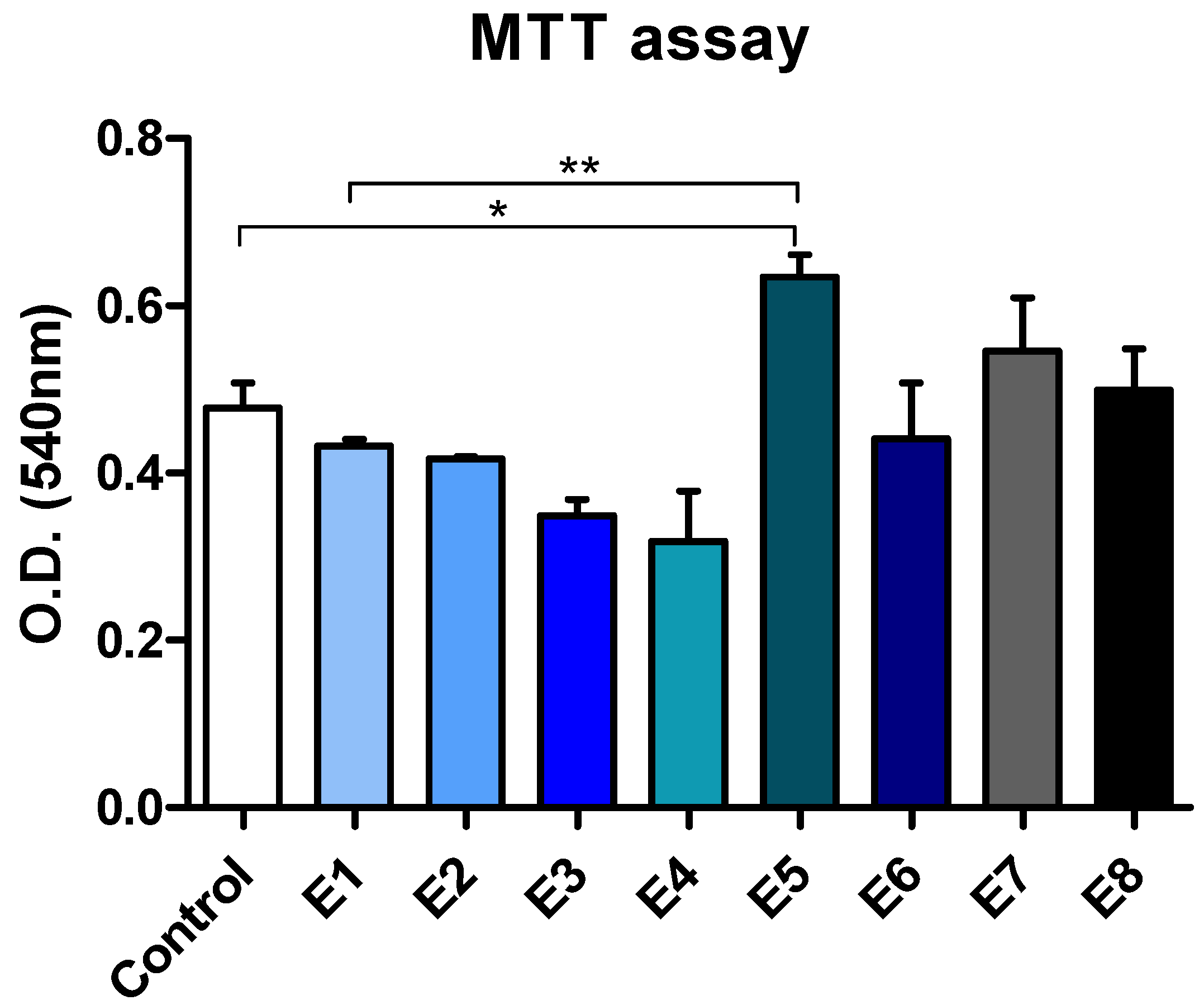
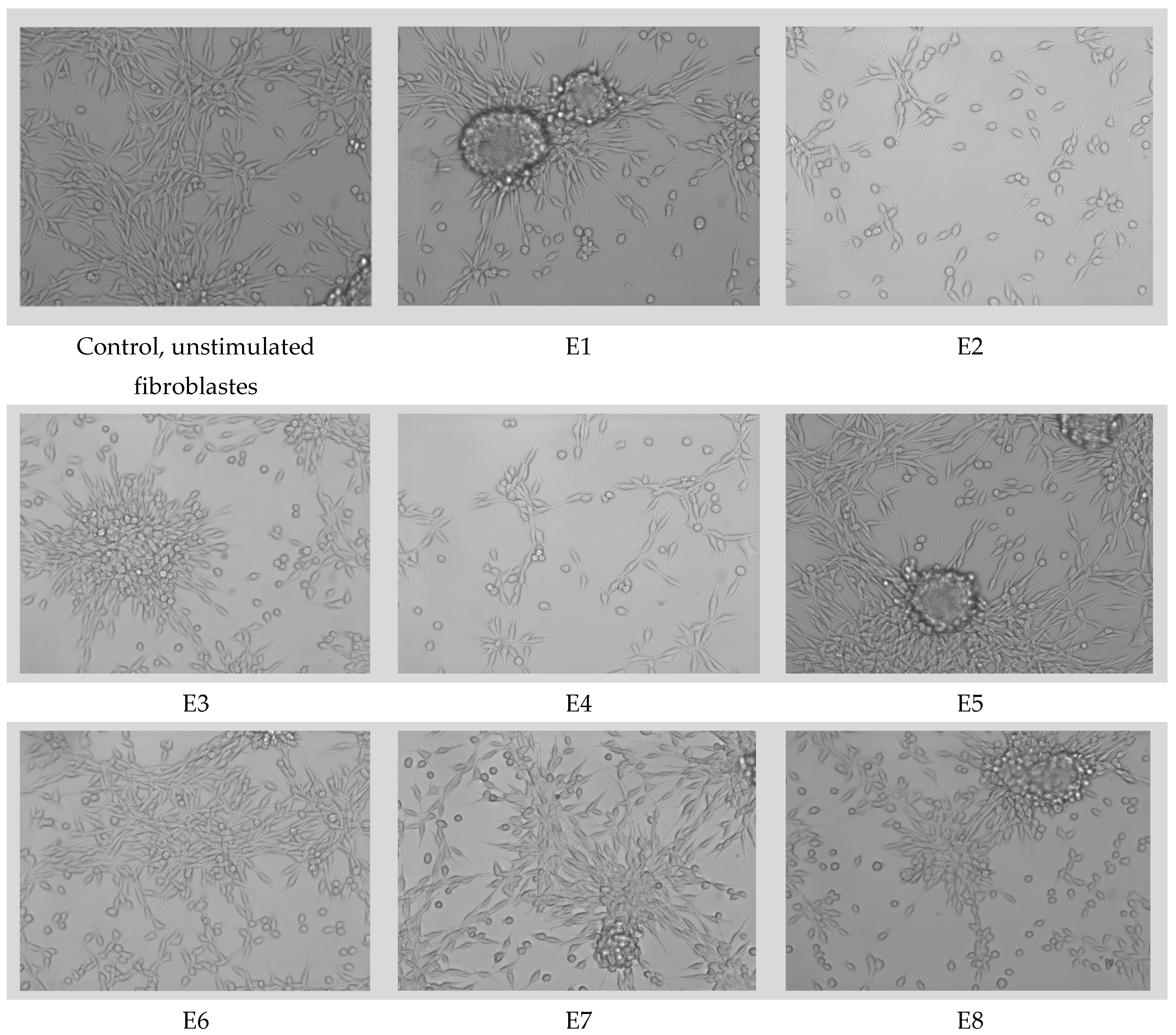
3.7. MTT Assay
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Antoniac, I.; Negrusoiu, M.; Mardare, M.; Socoliuc, C.; Zazgyva, A.; Niculescu, M. Adverse Local Tissue Reaction after 2 Revision Hip Replacements for Ceramic Liner Fracture. Medicine 2017, 96, e6687. [Google Scholar] [CrossRef] [PubMed]
- Rau, J.V.; Fadeeva, I.V.; Forysenkova, A.A.; Davydova, G.A.; Fosca, M.; Filippov, Y.Y.; Antoniac, I.V.; Antoniac, A.; D’Arco, A.; di Fabrizio, M.; et al. Strontium Substituted Tricalcium Phosphate Bone Cement: Short and Long-Term Time-Resolved Studies and In Vitro Properties. Adv. Mater. Interfaces 2022, 9, 2200803. [Google Scholar] [CrossRef]
- Robu, A.; Ciocoiu, R.; Antoniac, A.; Antoniac, I.; Raiciu, A.D.; Dura, H.; Forna, N.; Cristea, M.B.; Carstoc, I.D. Bone Cements Used for Hip Prosthesis Fixation: The Influence of the Handling Procedures on Functional Properties Observed during In Vitro Study. Materials 2022, 15, 2967. [Google Scholar] [CrossRef] [PubMed]
- Marinescu, R.; Antoniac, I.; Laptoiu, D.; Antoniac, A.; Grecu, D. Complications Related to Biocomposite Screw Fixation in ACL Reconstruction Based on Clinical Experience and Retrieval Analysis. Mater. Plast. 2015, 52, 340–344. [Google Scholar]
- Antoniac, I.; Stoia, D.; Ghiban, B.; Tecu, C.; Miculescu, F.; Vigaru, C.; Saceleanu, V. Failure Analysis of a Humeral Shaft Locking Compression Plate—Surface Investigation and Simulation by Finite Element Method. Materials 2019, 12, 1128. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Antoniac, I.v.; Antoniac, A.; Vasile, E.; Tecu, C.; Fosca, M.; Yankova, V.G.; Rau, J.v. In Vitro Characterization of Novel Nanostructured Collagen-Hydroxyapatite Composite Scaffolds Doped with Magnesium with Improved Biodegradation Rate for Hard Tissue Regeneration. Bioact. Mater. 2021, 6, 3383–3395. [Google Scholar] [CrossRef] [PubMed]
- Corobea, M.S.; Albu, M.G.; Ion, R.; Cimpean, A.; Miculescu, F.; Antoniac, I.V.; Raditoiu, V.; Sirbu, I.; Stoenescu, M.; Voicu, S.I.; et al. Modification of Titanium Surface with Collagen and Doxycycline as a New Approach in Dental Implants. J. Adhes. Sci. Technol. 2015, 29, 2537–2550. [Google Scholar] [CrossRef]
- Sarosi, C.; Biris, A.R.; Antoniac, A.; Boboia, S.; Alb, C.; Antoniac, I.; Moldovan, M. The Nanofiller Effect on Properties of Experimental Graphene Dental Nanocomposites. J. Adhes. Sci. Technol. 2016, 30, 1779–1794. [Google Scholar] [CrossRef]
- Cavalu, S.; Antoniac, I.V.; Mohan, A.; Bodog, F.; Doicin, C.; Mates, I.; Ulmeanu, M.; Murzac, R.; Semenescu, A. Nanoparticles and Nanostructured Surface Fabrication for Innovative Cranial and Maxillofacial Surgery. Materials 2020, 13, 5391. [Google Scholar] [CrossRef] [PubMed]
- Rivis, M.; Pricop, M.; Talpos, S.; Ciocoiu, R.; Antoniac, I.; Gheorghita, D.; Trante, O.; Moldovan, H.; Grigorescu, G.; Seceleanu, V.; et al. Influence of the Bone Cements Processing on the Mechanical Properties in Cranioplasty. Rev. De Chim. 2018, 69, 990–993. [Google Scholar] [CrossRef]
- George, P.; Antoniac, I.V. Abdominal Compartment Syndrome—A Major Complication of Large Incisional Hernia Surgery. Chirurgia 2013, 108, 414–417. [Google Scholar]
- Pariza, G.; Mavrodin, C.I.; Antoniac, I. Dependency Between the Porosity and Polymeric Structure of Biomaterials Used in Hernia Surgery and Chronic Mesh—Infection. Mater. Plast. 2015, 52, 484–486. [Google Scholar]
- Cirstoiu, M.; Cirstoiu, C.; Antoniac, I.; Munteanu, O. Levonorgestrel-Releasing Intrauterine Systems: Device Design, Biomaterials, Mechanism of Action and Surgical Technique. Mater. Plast. 2015, 52, 258–262. [Google Scholar]
- Râpă, M.; Stefan, L.M.; Zaharescu, T.; Seciu, A.-M.; Țurcanu, A.A.; Matei, E.; Predescu, A.M.; Antoniac, I.; Predescu, C. Development of Bionanocomposites Based on PLA, Collagen and AgNPs and Characterization of Their Stability and In Vitro Biocompatibility. Appl. Sci. 2020, 10, 2265. [Google Scholar] [CrossRef]
- Antoniac, I.V.; Ionescu, R.D.; Burcea, M.; Balta, F. IOL’s Opacification: A Complex Analysis Based on the Clinical Aspects, Biomaterials Used and Surface Characterization of Explanted IOL’s. Mater. Plast. 2015, 52, 109–112. [Google Scholar]
- National Pressure Ulcer Advisory Panel (U.S.); Haesler, E.; European Pressure Ulcer Advisory Panel; Pan Pacific Pressure Injury Alliance. Prevention and Treatment of Pressure Ulcers; Quick Reference Guide; Cambridge Media: Osborne Park, Australia, 2014; ISBN 9780957934368. [Google Scholar]
- da Silva, L.P.; Reis, R.L.; Correlo, V.M.; Marques, A.P. Hydrogel-Based Strategies to Advance Therapies for Chronic Skin Wounds. Annu. Rev. Biomed. Eng. 2019, 21, 145–169. [Google Scholar] [CrossRef] [Green Version]
- Wallenstein, S.; Brem, H. Statistical Analysis of Wound-Healing Rates for Pressure Ulcers. Am. J. Surg. 2004, 188, 73–78. [Google Scholar] [CrossRef]
- Afzali Borojeny, L.; Albatineh, A.N.; Hasanpour Dehkordi, A.; Ghanei Gheshlagh, R. The Incidence of Pressure Ulcers and Its Associations in Different Wards of the Hospital: A Systematic Review and Meta-Analysis. Int. J. Prev. Med. 2020, 11, 171. [Google Scholar] [CrossRef] [PubMed]
- Dorner, B.; Mary, L.; Posthauer, E.; Thomas, D. The Role of Nutrition in Pressure Ulcer Prevention and Treatment: National Pressure Ulcer Advisory Panel White Paper. Adv. Ski. Wound Care 2009, 22, 212–221. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Schindler, C.A.; Mikhailov, T.A.; Kuhn, E.M.; Christopher, J.; Conway, P.; Ridling, D.; Scott, A.M.; Simpson, V.S. Protecting Fragile Skin: Nursing Interventions to Decrease Development of Pressure Ulcers in Pediatric Intensive Care. Am. J. Crit. Care 2011, 20, 26–35. [Google Scholar] [CrossRef] [PubMed]
- Smith, D.M.; Snow, D.E.; Rees, E.; Zischkau, A.M.; Delton Hanson, J.; Wolcott, R.D.; Sun, Y.; White, J.; Kumar, S.; Dowd, S.E. Evaluation of the Bacterial Diversity of Pressure Ulcers Using BTEFAP Pyrosequencing. BMC Med. Genom. 2010, 3, 41. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kottner, J.; Dassen, T. Pressure Ulcer Risk Assessment in Critical Care: Interrater Reliability and Validity Studies of the Braden and Waterlow Scales and Subjective Ratings in Two Intensive Care Units. Int. J. Nurs. Stud. 2010, 47, 671–677. [Google Scholar] [CrossRef] [PubMed]
- Anghel, A.; Taranu, G.; Seclaman, E.; Rata, A.; Tamas, L.; Moldovan, H.; Ursoniu, S.; Samoila, C.; Ionac, M.; Popa-Wagner, A. Safety of Vascular Endothelial and Hepatocyte Growth Factor Gene Therapy in Patients with Critical Limb Ischemia. Curr. Neurovasc. Res. 2011, 8, 183–189. [Google Scholar] [CrossRef] [PubMed]
- Moldovan, H.; Popescu, D.; Buliga, T.; Filip, A.; Antoniac, I.; Gheorghiţӑ, D.; Molnar, A. Gastric Adenocarcinoma Associated with Acute Endocarditis of the Aortic Valve and Coronary Artery Disease in a 61-Year-Old Male with Multiple Comorbidities—Combined Surgical Management—Case Report. Medicina 2019, 55, 242. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Available online: https://www.fortunebusinessinsights.com/ (accessed on 20 May 2022).
- Moura, L.I.F.; Dias, A.M.A.; Carvalho, E.; de Sousa, H.C. Recent Advances on the Development of Wound Dressings for Diabetic Foot Ulcer Treatment—A Review. Acta Biomater. 2013, 9, 7093–7114. [Google Scholar] [CrossRef] [PubMed]
- Rather, A.H.; Wani, T.U.; Khan, R.S.; Pant, B.; Park, M.; Sheikh, F.A. Prospects of Polymeric Nanofibers Loaded with Essential Oils for Biomedical and Food-packaging Applications. Int. J. Mol. Sci. 2021, 22, 4017. [Google Scholar] [CrossRef]
- Fan, C.; Xu, Q.; Hao, R.; Wang, C.; Que, Y.; Chen, Y.; Yang, C.; Chang, J. Multi-Functional Wound Dressings Based on Silicate Bioactive Materials. Biomaterials 2022, 287, 121652. [Google Scholar] [CrossRef]
- Naseri, E.; Ahmadi, A. A Review on Wound Dressings: Antimicrobial Agents, Biomaterials, Fabrication Techniques, and Stimuli-Responsive Drug Release. Eur. Polym. J. 2022, 173, 111293. [Google Scholar] [CrossRef]
- Peng, W.; Li, D.; Dai, K.; Wang, Y.; Song, P.; Li, H.; Tang, P.; Zhang, Z.; Li, Z.; Zhou, Y.; et al. Recent Progress of Collagen, Chitosan, Alginate and Other Hydrogels in Skin Repair and Wound Dressing Applications. Int. J. Biol. Macromol. 2022, 208, 400–408. [Google Scholar] [CrossRef]
- Caridade, S.G.; Monge, C.; Gilde, F.; Boudou, T.; Mano, J.F.; Picart, C. Free-Standing Polyelectrolyte Membranes Made of Chitosan and Alginate. Biomacromolecules 2013, 14, 1653–1660. [Google Scholar] [CrossRef]
- Wang, X.; Chang, J.; Wu, C. Bioactive Inorganic/Organic Nanocomposites for Wound Healing. Appl. Mater. Today 2018, 11, 308–319. [Google Scholar] [CrossRef]
- El Fawal, G.; Hong, H.; Mo, X.; Wang, H. Fabrication of Scaffold Based on Gelatin and Polycaprolactone (PCL) for Wound Dressing Application. J. Drug Deliv. Sci. Technol. 2021, 63, 102501. [Google Scholar] [CrossRef]
- Fan, T.; Daniels, R. Preparation and Characterization of Electrospun Polylactic Acid (PLA) Fiber Loaded with Birch Bark Triterpene Extract for Wound Dressing. AAPS PharmSciTech 2021, 22, 205. [Google Scholar] [CrossRef] [PubMed]
- Bardania, H.; Mahmoudi, R.; Bagheri, H.; Salehpour, Z.; Fouani, M.H.; Darabian, B.; Khoramrooz, S.S.; Mousavizadeh, A.; Kowsari, M.; Moosavifard, S.E.; et al. Facile Preparation of a Novel Biogenic Silver-Loaded Nanofilm with Intrinsic Anti-Bacterial and Oxidant Scavenging Activities for Wound Healing. Sci. Rep. 2020, 10, 6129. [Google Scholar] [CrossRef] [Green Version]
- Powers, J.G.; Higham, C.; Broussard, K.; Phillips, T.J. Wound Healing and Treating Wounds. J. Am. Acad. Dermatol. 2016, 74, 607–625. [Google Scholar] [CrossRef]
- Amalraj, A.; Haponiuk, J.T.; Thomas, S.; Gopi, S. Preparation, Characterization and Antimicrobial Activity of Polyvinyl Alcohol/Gum Arabic/Chitosan Composite Films Incorporated with Black Pepper Essential Oil and Ginger Essential Oil. Int. J. Biol. Macromol. 2020, 151, 366–375. [Google Scholar] [CrossRef]
- Sarbu, I.; Vassu, T.; Chifiriuc, M.C.; Bucur, M.; Stoica, I.; Stefana, P.; Rusu, E.; Moldovan, H.; Pelinescu, D. Assessment the Activity of Some Enzymes and Antibiotic Substances Sensitivity on Pathogenic Bacteria Species. Rev. Chim. 2018, 68, 3015–3021. [Google Scholar] [CrossRef]
- Prisada, R.M. Perspectives to describe surface properties of raw pharmaceutical materials. A fractal approach on the wetting of powders. Farmacia 2020, 68, 354–361. [Google Scholar] [CrossRef]
- Vivcharenko, V.; Przekora, A. Modifications of Wound Dressings with Bioactive Agents to Achieve Improved Pro-Healing Properties. Appl. Sci. 2021, 11, 4114. [Google Scholar] [CrossRef]
- Farkas, L. Active Principles of Plants of Traditional Medicine as Models of New Drugs. J. Ethnopharmacol. 1980, 2, 145–148. [Google Scholar] [CrossRef]
- Ahmad Ganaie, H. Review of the Active Principles of Medicinal and Aromatic Plants and Their Disease Fighting Properties. In Medicinal and Aromatic Plants; Elsevier: Amsterdam, The Netherlands, 2021; pp. 1–36. [Google Scholar]
- Arraiza, M.P.; Guerrero, C.C.; Guillén, S.C.; Sarmiento, M.Á. Active Principles in MAPs European Hub on New Challenges in the Field of Essential Oils (EOHUB) View Project MEST; MSc Program in Ecological Ans Sustainable Tourism View Project; Bentham Science Publishers: Sharjah, United Arab Emirates, 2016. [Google Scholar]
- Active Plant Ingredients Used for Medicinal Purposes. Available online: https://www.fs.usda.gov/wildflowers/ethnobotany/medicinal/ingredients.shtml (accessed on 20 May 2022).
- Harrigan, G.G.; Glenn, K.C.; Ridley, W.P. Assessing the Natural Variability in Crop Composition. Regul. Toxicol. Pharmacol. 2010, 58, S13–S20. [Google Scholar] [CrossRef]
- Capasso, F.; Gaginella, T.S.; Grandolini, G.; Angelo, A.I. Active Principles. In Phytotherapy: A Quick Reference to Herbal Medicine; Springer: Berlin/Heidelberg, Germany, 2003; pp. 31–32. [Google Scholar]
- Nair, A.; Mallya, R.; Suvarna, V.; Khan, T.A.; Momin, M.; Omri, A. Nanoparticles—Attractive Carriers of Antimicrobial Essential Oils. Antibiotics 2022, 11, 108. [Google Scholar] [CrossRef] [PubMed]
- Guan, M.; Hao, L.; Chen, L.; Gao, F.; Qiu, S.; Zhou, H.; Chen, H.; Zhou, X. Facile Mechanical-Induced Functionalization of Hexagonal Boron Nitride and Its Application as Vehicles for Antibacterial Essential Oil. ACS Sustain. Chem. Eng. 2020, 8, 15120–15133. [Google Scholar] [CrossRef]
- Munhuweyi, K.; Caleb, O.J.; van Reenen, A.J.; Opara, U.L. Physical and Antifungal Properties of β-Cyclodextrin Microcapsules and Nanofibre Films Containing Cinnamon and Oregano Essential Oils. LWT—Food Sci. Technol. 2018, 87, 413–422. [Google Scholar] [CrossRef]
- Martins, I.M.; Barreiro, M.F.; Coelho, M.; Rodrigues, A.E. Microencapsulation of Essential Oils with Biodegradable Polymeric Carriers for Cosmetic Applications. Chem. Eng. J. 2014, 245, 191–200. [Google Scholar] [CrossRef]
- Hajifathaliha, F.; Mahboubi, A.; Nematollahi, L.; Mohit, E.; Bolourchian, N. Comparison of Different Cationic Polymers Efficacy in Fabrication of Alginate Multilayer Microcapsules. Asian J. Pharm. Sci. 2020, 15, 95–103. [Google Scholar] [CrossRef] [PubMed]
- Rodríguez, J.; Martín, M.J.; Ruiz, M.A.; Clares, B. Current Encapsulation Strategies for Bioactive Oils: From Alimentary to Pharmaceutical Perspectives. Food Res. Int. 2016, 83, 41–59. [Google Scholar] [CrossRef]
- Husain, M.S.B.; Gupta, A.; Alashwal, B.Y.; Sharma, S. Synthesis of PVA/PVP Based Hydrogel for Biomedical Applications: A Review. Energy Sources Part A Recovery Util. Environ. Eff. 2018, 40, 2388–2393. [Google Scholar] [CrossRef]
- Huang, M.; Hou, Y.; Li, Y.; Wang, D.; Zhang, L. High Performances of Dual Network PVA Hydrogel Modified by PVP Using Borax as the Structure-Forming Accelerator. Des. Monomers. Polym. 2017, 20, 505–513. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Li, P.; Jiang, S.; Yu, Y.; Yang, J.; Yang, Z. Biomaterial Characteristics and Application of Silicone Rubber and PVA Hydrogels Mimicked in Organ Groups for Prostate Brachytherapy. J. Mech. Behav. Biomed. Mater. 2015, 49, 220–234. [Google Scholar] [CrossRef]
- Ma, Y.; Bai, T.; Wang, F. The Physical and Chemical Properties of the Polyvinylalcohol/Polyvinylpyrrolidone/Hydroxyapatite Composite Hydrogel. Mater. Sci. Eng. C 2016, 59, 948–957. [Google Scholar] [CrossRef]
- Huang, X.; Zuo, Y.; Li, J.D.; Li, Y.B. Study on Crystallisation of Nano-Hydroxyapatite/Polyvinyl Alcohol Composite Hydrogel. Mater. Res. Innov. 2009, 13, 98–102. [Google Scholar] [CrossRef]
- Peers, S.; Montembault, A.; Ladavière, C. Chitosan Hydrogels for Sustained Drug Delivery. J. Control. Release 2020, 326, 150–163. [Google Scholar] [CrossRef]
- Kong, M.; Chen, X.G.; Xing, K.; Park, H.J. Antimicrobial Properties of Chitosan and Mode of Action: A State of the Art Review. Int. J. Food Microbiol. 2010, 144, 51–63. [Google Scholar] [CrossRef] [PubMed]
- Zhou, H.Y.; Jiang, L.J.; Cao, P.P.; Li, J.B.; Chen, X.G. Glycerophosphate-Based Chitosan Thermosensitive Hydrogels and Their Biomedical Applications. Carbohydr. Polym. 2015, 117, 524–536. [Google Scholar] [CrossRef] [PubMed]
- Palazzolo, G.; Broguiere, N.; Cenciarelli, O.; Dermutz, H.; Zenobi-Wong, M. Ultrasoft Alginate Hydrogels Support Long-Term Three-Dimensional Functional Neuronal Networks. Tissue Eng. Part A 2015, 21, 2177–2185. [Google Scholar] [CrossRef] [PubMed]
- Zhao, L.; Weir, M.D.; Xu, H.H.K. An Injectable Calcium Phosphate-Alginate Hydrogel-Umbilical Cord Mesenchymal Stem Cell Paste for Bone Tissue Engineering. Biomaterials 2010, 31, 6502–6510. [Google Scholar] [CrossRef] [Green Version]
- Agulhon, P.; Markova, V.; Robitzer, M.; Quignard, F.; Mineva, T. Structure of Alginate Gels: Interaction of Diuronate Units with Divalent Cations from Density Functional Calculations. Biomacromolecules 2012, 13, 1899–1907. [Google Scholar] [CrossRef]
- Wang, K.; Nune, K.C.; Misra, R.D.K. The Functional Response of Alginate-Gelatin-Nanocrystalline Cellulose Injectable Hydrogels toward Delivery of Cells and Bioactive Molecules. Acta Biomater. 2016, 36, 143–151. [Google Scholar] [CrossRef]
- Liu, J.; Tian, B.; Liu, Y.; Wan, J.B. Cyclodextrin-Containing Hydrogels: A Review of Preparation Method, Drug Delivery, and Degradation Behavior. Int. J. Mol. Sci. 2021, 22, 13516. [Google Scholar] [CrossRef]
- Kiti, K.; Suwantong, O. Bilayer Wound Dressing Based on Sodium Alginate Incorporated with Curcumin-β-Cyclodextrin Inclusion Complex/Chitosan Hydrogel. Int. J. Biol. Macromol. 2020, 164, 4113–4124. [Google Scholar] [CrossRef]
- Moradi, S.; Barati, A.; Tonelli, A.E.; Hamedi, H. Chitosan-Based Hydrogels Loading with Thyme Oil Cyclodextrin Inclusion Compounds: From Preparation to Characterization. Eur. Polym. J. 2020, 122, 109303. [Google Scholar] [CrossRef]
- Ramírez Barragán, C.A.; Macías Balleza, E.R.; García-Uriostegui, L.; Andrade Ortega, J.A.; Toríz, G.; Delgado, E. Rheological Characterization of New Thermosensitive Hydrogels Formed by Chitosan, Glycerophosphate, and Phosphorylated β-Cyclodextrin. Carbohydr. Polym. 2018, 201, 471–481. [Google Scholar] [CrossRef]
- Giri, T.K.; Ghosh, B. Plant and Algal Hydrogels for Drug Delivery and Regenerative Medicine, 1st ed.; Giri, T.K., Ghosh, B., Eds.; Woodhead Publishing Series in Biomaterials; Woodhead Publishing: Cambridge, UK, 2021. [Google Scholar]
- Viguier, A.; Boyer, C.; Chassenieux, C.; Benyahia, L.; Guicheux, J.; Weiss, P.; Rethore, G.; Nicolai, T. Interpenetrated Si-HPMC/Alginate Hydrogels as a Potential Scaffold for Human Tissue Regeneration. J. Mater. Sci. Mater. Med. 2016, 27, 99. [Google Scholar] [CrossRef]
- Perez-robles, S.; Carotenuto, C.; Minale, M. HPMC Hydrogel Formation Mechanisms Unveiled by the Evaluation of the Activation Energy. Polymers 2022, 14, 635. [Google Scholar] [CrossRef]
- Joshi, S.C. Sol-Gel Behavior of Hydroxypropyl Methylcellulose (HPMC) in Ionic Media Including Drug Release. Materials 2011, 4, 1861–1905. [Google Scholar] [CrossRef] [Green Version]
- Caballero Aguilar, L.; Stoddart, P.R.; McArthur, S.L.; Moulton, S.E. Polycaprolactone Porous Template Facilitates Modulated Release of Molecules from Alginate Hydrogels. React Funct. Polym. 2018, 133, 29–36. [Google Scholar] [CrossRef]
- Patel, P.N.; Gobin, A.S.; West, J.L.; Patrick, C.W. Poly(Ethylene Glycol) Hydrogel System Supports Preadipocyte Viability, Adhesion, and Proliferation. Tissue Eng. 2005, 11, 1498–1505. [Google Scholar] [CrossRef]
- Hernandez, I.; Kumar, A.; Joddar, B. A Bioactive Hydrogel and 3d Printed Polycaprolactone System for Bone Tissue Engineering. Gels 2017, 3, 26. [Google Scholar] [CrossRef] [Green Version]
- Russo, E.; Villa, C. Poloxamer Hydrogels for Biomedical Applications. Pharmaceutics 2019, 11, 671. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gioffredi, E.; Boffito, M.; Calzone, S.; Giannitelli, S.M.; Rainer, A.; Trombetta, M.; Mozetic, P.; Chiono, V. Pluronic F127 Hydrogel Characterization and Biofabrication in Cellularized Constructs for Tissue Engineering Applications. Procedia CIRP 2016, 49, 125–132. [Google Scholar] [CrossRef] [Green Version]
- Li, J.; Stachowski, M.; Zhang, Z. Application of Responsive Polymers in Implantable Medical Devices and Biosensors. In Switchable and Responsive Surfaces and Materials for Biomedical Applications; Elsevier Inc.: Amsterdam, The Netherlands, 2015; pp. 259–298. ISBN 9780857097170. [Google Scholar]
- Pitto-Barry, A.; Barry, N.P.E. Pluronic® Block-Copolymers in Medicine: From Chemical and Biological Versatility to Rationalisation and Clinical Advances. Polym. Chem. 2014, 5, 3291–3297. [Google Scholar] [CrossRef] [Green Version]
- Bunker, A. Poly(Ethylene Glycol) in Drug Delivery, Why Does It Work, and Can We Do Better? All Atom Molecular Dynamics Simulation Provides Some Answers. Phys. Procedia 2012, 34, 24–33. [Google Scholar] [CrossRef] [Green Version]
- Hardman, D.; George Thuruthel, T.; Iida, F. Self-Healing Ionic Gelatin/Glycerol Hydrogels for Strain Sensing Applications. NPG Asia Mater. 2022, 14, 11. [Google Scholar] [CrossRef]
- Chin, S.F.; Romainor, A.N.B.; Pang, S.C.; Lihan, S. Antimicrobial Starch-Citrate Hydrogel for Potential Applications as Drug Delivery Carriers. J. Drug Deliv. Sci. Technol. 2019, 54, 101239. [Google Scholar] [CrossRef]
- Arora, D.; Sharma, N.; Sharma, V.; Abrol, V.; Shankar, R.; Jaglan, S. An Update on Polysaccharide-Based Nanomaterials for Antimicrobial Applications. Appl. Microbiol. Biotechnol. 2016, 100, 2603–2615. [Google Scholar] [CrossRef]
- Kanca, Y.; Milner, P.; Dini, D.; Amis, A.A. Tribological Properties of PVA/PVP Blend Hydrogels against Articular Cartilage. J. Mech. Behav. Biomed. Mater. 2018, 78, 36–45. [Google Scholar] [CrossRef] [Green Version]
- Ara, M.; Alcântara, T.S.; Giannini, D.R.; Brant, A.J.C.; Riella, H.G.; Lugão, A.B. Effect of Different Plasticizers on Poly(^-Vinyl-2-Pyrrolidone) Hydrogels Cross-Linked by Radiation. In Proceedings of the INAC 2011: International Nuclear Atlantic Conference Nuclear Energy, Belo Horizonte, Brasil, 24–28 October 2011; ISBN 978-85-99141-04-5. [Google Scholar]
- Ajji, Z.; Othman, I.; Rosiak, J.M. Production of Hydrogel Wound Dressings Using Gamma Radiation. Nucl. Instrum. Methods Phys Res. B 2005, 229, 375–380. [Google Scholar] [CrossRef]
- Laurienzo, P. Marine Polysaccharides in Pharmaceutical Applications: An Overview. Mar. Drugs 2010, 8, 2435–2465. [Google Scholar] [CrossRef] [Green Version]
- Coviello, T.; Matricardi, P.; Marianecci, C.; Alhaique, F. Polysaccharide Hydrogels for Modified Release Formulations. J. Control. Release 2007, 119, 5–24. [Google Scholar] [CrossRef]
- Lamim, R.; de Freitas, R.A.; Rudek, E.I.; Wilhelm, H.M.; Cavalcanti, O.A.; Bresolin, T.M.B. Films of Chitosan and N-Carboxymethyl-Chitosan. Part II: Effect of Plasticizers on Their Physiochemical Properties. Polym. Int. 2006, 55, 970–977. [Google Scholar] [CrossRef]
- Gal, A.; Nussinovitch, A. Plasticizers in the Manufacture of Novel Skin-Bioadhesive Patches. Int. J. Pharm. 2009, 370, 103–109. [Google Scholar] [CrossRef] [PubMed]
- Gallardo, V.; Muñoz, M.; Ruíz, M.A. Formulations of Hydrogels and Lipogels with Vitamin E. J. Cosmet. Dermatol. 2005, 4, 187–192. [Google Scholar] [CrossRef] [PubMed]
- Perchyonok, V.T. Protective Effect of Conventional Antioxidant (β-Carotene, Resveratrol and Vitamin E) in Chitosan-Containing Hydrogels Against Oxidative Stress and Reversal of DNA Double Stranded Breaks Induced by Common Dental Composites: In-Vitro Model. Open Nanosci. J. 2013, 7, 1–7. [Google Scholar] [CrossRef] [Green Version]
- Acton, Q.A. Polyethylene Glycols—Advances in Research and Application; Acton, Q.A., Ed.; ScholarlyEditions: Atlanta, GA, USA, 2012. [Google Scholar]
- Banerjee, I.; Sarkar, P.; Kim, D.; Deng, W.-P.; Kumar, N.; Kaustav Majumder, D. Biopolymer-Based Formulations Biomedical and Food Applications; Elsevier: Amsterdam, The Netherlands, 2020. [Google Scholar]
- 9 Health Benefits of Fennel + Risks & Side Effects. Available online: https://supplements.selfdecode.com/blog/fennel-benefits/ (accessed on 8 June 2022).
- Peppermint Oil Uses and Benefits to Try Now for Beauty, Health and Home. Available online: https://www.today.com/style/peppermint-oil-uses-benefits-beauty-health-home-cleanliness-t145583 (accessed on 8 June 2022).
- National Library of Medicine, National Institutes of Health. Available online: https://pubchem.ncbi.nlm.nih.gov/compound/fennel-oil (accessed on 16 June 2022).
- National Library of Medicine, National Institutes of Health. Available online: https://pubchem.ncbi.nlm.nih.gov/compound/peppermint-oil (accessed on 16 June 2022).
- 9 Amazing Benefits of Pine Essential Oil. Available online: https://www.organicfacts.net/health-benefits/essential-oils/pine-essential-oil.html (accessed on 8 June 2022).
- Thyme Oil Uses and Benefits. Available online: https://www.doterra.com/US/en/blog/spotlight-thyme-oil (accessed on 8 June 2022).
- Zeng, W.C.; Zhang, Z.; Gao, H.; Jia, L.R.; He, Q. Chemical Composition, Antioxidant, and Antimicrobial Activities of Essential Oil from Pine Needle (Cedrus Deodara). J. Food Sci. 2012, 77, C824–C829. [Google Scholar] [CrossRef]
- National Library of Medicine, National Institutes of Health. Available online: https://pubchem.ncbi.nlm.nih.gov/compound/thyme-oil (accessed on 16 June 2022).
- Liu, B.; Zhang, J.; Guo, H. Research Progress of Polyvinyl Alcohol Water-Resistant Film Materials. Membranes 2022, 12, 347. [Google Scholar] [CrossRef]
- Bahadoran, M.; Shamloo, A.; Nokoorani, Y.D. Development of a Polyvinyl Alcohol/Sodium Alginate Hydrogel-Based Scaffold Incorporating BFGF-Encapsulated Microspheres for Accelerated Wound Healing. Sci. Rep. 2020, 10, 7342. [Google Scholar] [CrossRef]
- Hwang, M.R.; Kim, J.O.; Lee, J.H.; Kim, Y.i.; Kim, J.H.; Chang, S.W.; Jin, S.G.; Kim, J.A.; Lyoo, W.S.; Han, S.S.; et al. Gentamicin-Loaded Wound Dressing with Polyvinyl Alcohol/Dextran Hydrogel: Gel Characterization and in Vivo Healing Evaluation. AAPS PharmSciTech 2010, 11, 1092–1103. [Google Scholar] [CrossRef] [Green Version]
- Chen, Q.; Wang, W.; Chen, G.; Li, X.; Wang, Y.; Xiong, J.; Wei, L. Preparation and Characterization of Polyvinyl Alcohol-Chitosan/Cerium Hydrogel with Significant Antibacterial Activity. Starch/Staerke 2021, 73, 2000253. [Google Scholar] [CrossRef]
- Shen, X.L.; Wu, J.M.; Chen, Y.; Zhao, G. Antimicrobial and Physical Properties of Sweet Potato Starch Films Incorporated with Potassium Sorbate or Chitosan. Food Hydrocoll. 2010, 24, 285–290. [Google Scholar] [CrossRef]
- Limpan, N. Properties of Biodegradable Film Based on Fish Myofibrillar Protein and Poly(Vinyl Alcohol) Blend. Ph.D. Thesis, Prince of Songkla University, Songkhla, Thailand, 2009. [Google Scholar]
- Su, J.F.; Huang, Z.; Zhao, Y.H.; Yuan, X.Y.; Wang, X.Y.; Li, M. Moisture Sorption and Water Vapor Permeability of Soy Protein Isolate/Poly(Vinyl Alcohol)/Glycerol Blend Films. Ind. Crops Prod. 2010, 31, 266–276. [Google Scholar] [CrossRef]
- EN 14885; Chemical Disinfectants and Antiseptics-Application of European Standards for Chemical Disinfectants and Antiseptics. British Standard Institution: London, UK, 2015.
- Balouiri, M.; Sadiki, M.; Ibnsouda, S.K. Methods for in Vitro Evaluating Antimicrobial Activity: A Review. J. Pharm. Anal. 2016, 6, 71–79. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mueller Hinton Agar (MHA)—Composition, Principle, Uses and Preparation. Available online: https://microbiologyinfo.com/Mueller-Hinton-Agar-Mha-Composition-Principle-Uses-and-Preparation/ (accessed on 26 May 2022).
- Mueller Hinton Agar (MHA): Composition, Preparation, Uses. Available online: https://Microbeonline.Com/Mueller-Hinton-Agar (accessed on 1 August 2022).
- Mueller Hinton Agar.Principle.Preparation.Procedure. Available online: https://microbiologie-clinique.com/mueller-hinton-agar.html (accessed on 19 May 2022).
- Sabouraud Dextrose Agar (SDA)—Composition, Principle, Uses, Preparation and Colony Morphology. Available online: https://Microbiologyinfo.Com/Sabouraud-Dextrose-Agar-Sda-Composition-Principle-Uses-Preparation-and-Colony-Morphology (accessed on 23 May 2022).
- Available online: https://Clsi.Org/ (accessed on 17 June 2022).
- Implementation of the CLSI Method for Direct Disk Diffusion Testing From Positive Blood Cultures. Available online: https://Clsi.Org/about/Blog/Implementation-of-the-Clsi-Method-for-Direct-Disk-Diffusion-Testing-from-Positive-Blood-Cultures/ (accessed on 17 June 2022).
- Singh Arora, D.; Jeet Kaur, G.; Kaur, H. Antibacterial Activity of Tea and Coffee: Their Extracts and Preparations. Int. J. Food Prop. 2009, 12, 286–294. [Google Scholar] [CrossRef]
- Heilos, D.; Rodríguez-Carrasco, Y.; Englinger, B.; Timelthaler, G.; van Schoonhoven, S.; Sulyok, M.; Boecker, S.; Süssmuth, R.; Heffeter, P.; Lemmens-Gruber, R.; et al. The Natural Fungal Metabolite Beauvericin Exerts Anticancer Activity In Vivo: A Pre-Clinical Pilot Study. Toxins 2017, 9, 258. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mahajan, S.D.; Law, W.-C.; Aalinkeel, R.; Reynolds, J.; Nair, B.B.; Yong, K.-T.; Roy, I.; Prasad, P.N.; Schwartz, S.A. Nanoparticle-Mediated Targeted Delivery of Antiretrovirals to the Brain. In Methods in Enzymology; Academic Press: Cambridge, MA, USA, 2012; pp. 41–60. [Google Scholar]
- Dinç, C.Ö.; Kibarer, G.; Güner, A. Solubility Profiles of Poly(Ethylene Glycol)/Solvent Systems. II. Comparison of Thermodynamic Parameters from Viscosity Measurements. J. Appl. Polym. Sci. 2010, 117, 1100–1119. [Google Scholar] [CrossRef]
- Knop, K.; Hoogenboom, R.; Fischer, D.; Schubert, U.S. Poly(Ethylene Glycol) in Drug Delivery: Pros and Cons as Well as Potential Alternatives. Angew. Chem.-Int. Ed. 2010, 49, 6288–6308. [Google Scholar] [CrossRef] [PubMed]
- Abd El-Kader, M.F.H.; Elabbasy, M.T.; Ahmed, M.K.; Menazea, A.A. Structural, Morphological Features, and Antibacterial Behavior of PVA/PVP Polymeric Blends Doped with Silver Nanoparticles via Pulsed Laser Ablation. J. Mater. Res. Technol. 2021, 13, 291–300. [Google Scholar] [CrossRef]
- Mallakpour, S.; Mansourzadeh, S. Sonochemical Synthesis of PVA/PVP Blend Nanocomposite Containing Modified CuO Nanoparticles with Vitamin B1 and Their Antibacterial Activity against Staphylococcus Aureus and Escherichia Coli. Ultrason. Sonochem. 2018, 43, 91–100. [Google Scholar] [CrossRef]
- Caporalini, S.; Cinelli, P.; Cristallini, C.; Seggiani, M.; Barbani, N. Engineered Matrices Based on Gellan Gum and Biocompatible Synthetic Polymers for the Release of Molecules with Antioxidant Activity. Chem. Eng. Trans. 2022, 93, 181–186. [Google Scholar] [CrossRef]
- Pushp, P.; Bhaskar, R.; Kelkar, S.; Sharma, N.; Pathak, D.; Gupta, M.K. Plasticized Poly(Vinylalcohol) and Poly(Vinylpyrrolidone) Based Patches with Tunable Mechanical Properties for Cardiac Tissue Engineering Applications. Biotechnol. Bioeng. 2021, 118, 2312–2325. [Google Scholar] [CrossRef]
- Qureshi, D.; Sahoo, A.; Mohanty, B.; Anis, A.; Kulikouskaya, V.; Hileuskaya, K.; Agabekov, V.; Sarkar, P.; Ray, S.S.; Maji, S.; et al. Fabrication and Characterization of Poly (Vinyl Alcohol) and Chitosan Oligosaccharide-Based Blend Films. Gels 2021, 7, 55. [Google Scholar] [CrossRef] [PubMed]
- Hu, Y.; Wei, J.; Liang, Y.; Zhang, H.; Zhang, X.; Shen, W.; Wang, H. Zeolitic Imidazolate Framework/Graphene Oxide Hybrid Nanosheets as Seeds for the Growth of Ultrathin Molecular Sieving Membranes. Angew. Chem. 2016, 128, 2088–2092. [Google Scholar] [CrossRef]
- Guo, L.; Sato, H.; Hashimoto, T.; Ozaki, Y. FTIR Study on Hydrogen-Bonding Interactions in Biodegradable Polymer Blends of Poly(3-Hydroxybutyrate) and Poly(4-Vinylphenol). Macromolecules 2010, 43, 3897–3902. [Google Scholar] [CrossRef]
- Negim, E.; Rakhmetullayeva, R.; Yeligbayeva, G.; Urkimbaeva, P.; Primzharova, S.; Kaldybekov, D.; Khatib, J.; Mun, G.W.C. Improving Biodegradability of Polyvinyl Alcohol/Starch Blend Films for Packaging Applications. Int. J. Basic Appl. Sci. 2014, 3, 263. [Google Scholar] [CrossRef] [Green Version]
- Jaipakdee, N.; Pongjanyakul, T.; Limpongsa, E. Preparation and Characterization of Poly (Vinyl Alcohol)-Poly (Vinyl Pyrrolidone) Mucoadhesive Buccal Patches for Delivery of Lidocaine HCL. Int. J. Appl. Pharm. 2018, 10, 115–123. [Google Scholar] [CrossRef]
- Abdollahi, S.; Raoufi, Z. Gelatin/Persian Gum/Bacterial Nanocellulose Composite Films Containing Frankincense Essential Oil and Teucrium Polium Extract as a Novel and Bactericidal Wound Dressing. J. Drug Deliv. Sci. Technol. 2022, 72, 103423. [Google Scholar] [CrossRef]
- Jaiswal, L.; Shankar, S.; Rhim, J.W. Carrageenan-Based Functional Hydrogel Film Reinforced with Sulfur Nanoparticles and Grapefruit Seed Extract for Wound Healing Application. Carbohydr. Polym. 2019, 224, 115191. [Google Scholar] [CrossRef]
- Amirian, J.; Zeng, Y.; Shekh, M.I.; Sharma, G.; Stadler, F.J.; Song, J.; Du, B.; Zhu, Y. In-Situ Crosslinked Hydrogel Based on Amidated Pectin/Oxidized Chitosan as Potential Wound Dressing for Skin Repairing. Carbohydr. Polym. 2021, 251, 117005. [Google Scholar] [CrossRef]
- Muchová, M.; Münster, L.; Capáková, Z.; Mikulcová, V.; Kuřitka, I.; Vícha, J. Design of Dialdehyde Cellulose Crosslinked Poly(Vinyl Alcohol) Hydrogels for Transdermal Drug Delivery and Wound Dressings. Mater. Sci. Eng. C 2020, 116, 111242. [Google Scholar] [CrossRef]
- Hashemi Doulabi, A.; Mirzadeh, H.; Imani, M.; Samadi, N. Chitosan/Polyethylene Glycol Fumarate Blend Film: Physical and Antibacterial Properties. Carbohydr. Polym. 2013, 92, 48–56. [Google Scholar] [CrossRef]
- Koosehgol, S.; Ebrahimian-Hosseinabadi, M.; Alizadeh, M.; Zamanian, A. Preparation and Characterization of in Situ Chitosan/Polyethylene Glycol Fumarate/Thymol Hydrogel as an Effective Wound Dressing. Mater. Sci. Eng. C 2017, 79, 66–75. [Google Scholar] [CrossRef]
- Bajpai, S.K.; Daheriya, P.; Ahuja, S.; Gupta, K. Water Absorption and Antimicrobial Behavior of Physically Cross Linked Poly (Vinyl Alcohol)/Carrageenan Films Loaded with Minocycline. Des. Monomers Polym. 2016, 19, 630–642. [Google Scholar] [CrossRef] [Green Version]
- Pan, H.; Fan, D.; Cao, W.; Zhu, C.; Duan, Z.; Fu, R.; Li, X.; Ma, X. Preparation and Characterization of Breathable Hemostatic Hydrogel Dressings and Determination of Their Effects on Full-Thickness Defects. Polymers 2017, 9, 727. [Google Scholar] [CrossRef] [PubMed]








| Essential Oil | Property | Application |
|---|---|---|
| Eucalyptus (Eucalyptus globulus) | Antibacterial, antispasmodic, and antiviral | Effective in combating Staphylococccus aureus infections. |
| Frankincense (Boswellia carteri, Frereana & Sacra) | Anti-inflammatory | Boswellic acid can improve the immunesystem. Beneficial effects in topical applications used to heal pain and inflammation. |
| Lavender (Lavandula angustifolia) | Calming properties | Accelerating the healing time for burns, cuts, stings, and other wounds; decreasing oxidative stress. |
| Peppermint (Mentha piperita) | Antibacterial | Antibiotic-resistant. |
| Thyme (Thymus sp.) | Antibacterial | Treatment of cutaneous lesions, acts against Staphylococcus aureus and Klebsiella pneumoniae. |
| Pine (Pinus sylvestris) | Antibacterial | Treatment of infections with Staphylococcus aureus, Escherichia coli, and Candida albicans. |
| Fennel (Foeniculum vulgare) | Antibacterial | Treatment of infections with Salmonella enteritidis and Salmonella typhimurium. |
| Property | Fennel Essential Oil | Peppermint Essential Oil | Pine Essential Oil | Thyme Essential Oil |
|---|---|---|---|---|
| Relative density [g/cm3] | 0.961–0.975 | 0.900–0.916 | 0.855–0.875 | 0.915–0.935 |
| Refraction index | 1.528–1.539 | 1.457–1.467 | 1.465–1.480 | 1.490–1.505 |
| Optical rotation, [°] | 10.0–24.0 | −30–−10 | −9.0–30.0 | −21.0–+15.0 |
| Residue on evaporation, [%] | max 1.5 | max 1.5 | max 1.5 | max 1.5 |
| Solubility in: | hexane | hexane | hexane | hexane |
| Antioxidant activity (mg equivalent to Fe2SO4 × 7H2O/g for sample) | 6.09 | 6.07 | 7.09 | 6.79 |
| Peppermint Essential Oil | Thyme Essential Oil | Pine Essential Oil | Fennel Essential Oil | ||||
|---|---|---|---|---|---|---|---|
| Comp. | RT | Comp. | RT | Comp. | RT | Comp. | RT |
| β-Pinene | 7.035 | α-Pinene | 5.21 | α-Pinene | 4.83 | α-Pinene | 7.02 |
| Sabinene | 7.909 | α-Phellandrene | 5.42 | Camphene | 6.16 | Camphene | 8.75 |
| d-Limonene | 12.657 | α-Myrcene | 12.84 | β-Pinene | 7.94 | β-Pinene | 10.68 |
| Eucalyptol | 12.922 | 4-Carene | 13.23 | 3-Carene | 10.63 | β-Phellandrene | 11.55 |
| o-Cymene | 16.462 | Cineole | 14.42 | β-Myrcene | 12.04 | γ-Phellandrene | 13.96 |
| Isomenthone | 23.829 | 3-Carene | 16.28 | d-Limonene | 13.40 | Myrcene | 14.28 |
| d-Menthone | 24.754 | o-Cimene | 17.26 | β-Phellandrene | 13.74 | d-Limonene | 15.94 |
| Menthol, acetate | 27.353 | Camphor | 24.70 | Terpinolene | 16.98 | Cineol | 16.40 |
| Isopulegol | 27.533 | Linalool | 25.75 | Cariofilene | 26.22 | α-Phellandrene | 18.85 |
| Caryophyllene | 28.063 | Caryophilene | 26.90 | Germacrene | 28.94 | γ-Terpinene | 20.42 |
| β-Terpineol | 28.502 | Terpinene-4-ol | 27.04 | α-Eudesmene | 29.15 | o-Cimene | 21.12 |
| Pulegone | 29.614 | Carvacol methyl ether | 27.16 | α-Murolene | 29.33 | Fenchone | 28.26 |
| Levomenthol | 29.778 | Bomeol | 29.34 | Elixene | 29.49 | Camphor | 35.11 |
| Terpineol | 31.410 | Isoledene | 30.70 | Cadinene | 30.04 | Estragol | 44.73 |
| Piperitone | 31.930 | Tymol | 39.32 | Cadinol | 39.54 | Anethole | 53.57 |
| Carvacrol | 39.84 | Benzaldehyde | 62.80 | ||||
| Sample/ Component | E1 [%] | E2 [%] | E3 [%] | E4 [%] | E5 [%] | E6 [%] | E7 [%] | E8 [%] |
|---|---|---|---|---|---|---|---|---|
| PVA | 25 | 23 | 23 | 23 | 23 | 46.5 | 46.5 | |
| PVP | 15 | 10 | 10 | 10 | 10 | 16.5 | 18 | 18 |
| Sodium alginate | - | - | 1 g in 100 mL water | |||||
| Tween 80 | 0.02 | |||||||
| CaCl2 | 30 g in 100 mL water | |||||||
| CMC * | 2 | 2 | 2 | 2 | 2 | 2 | 2 | 2 |
| Fennel essential oil | 12 | |||||||
| Peppermint essential oil | 12 | 12 | ||||||
| Pine essential oil | 12 | 12 | ||||||
| Thyme essential oil | 12 | 12 | ||||||
| Vitamin A | 3 | 3 | 3 | 3 | 3 | 1.5 | 1.5 | 1.5 |
| Vitamin E | 2 | 2 | 2 | 2 | 2 | 2 | 2 | 2 |
| Deionized water | 50 | 50 | 50 | 50 | 50 | 14.5 | 14.5 | 14.5 |
| Glycerol | 2.5 | 2.5 | 2.5 | 2.5 | 2.5 | 3 | 3 | 3 |
| Polyethylene glycol | 8 | |||||||
| Glutaraldehyde | 0.5 | 0.5 | 0.5 | 0.5 | 0.5 | 0.5 | 0.5 | 0.5 |
| Zn stearate | 0.6 | 0.6 | 0.6 | 0.6 | 0.6 | 0.6 | 0.6 | 0.6 |
| Essential Oil Encapsulated | Wall Thickness [µm] | Diameter [µm] |
|---|---|---|
| Pine essential oil | 89.40 ± 0.96 | 1523.31 ± 1.23 |
| Thyme essential oil | 66.05 ± 0.33 | 1142.49 ± 1.03 |
| Peppermint essential oil | 74.08 ± 0.89 | 1233.24 ± 0.74 |
| E0_1 | E0_2 | ||
|---|---|---|---|
| Wavenumber [cm−1] | Corresponding Band | Wavenumber [cm−1] | Corresponding Band |
| 3256.22 | Stretching OH from PVA | 3273.57 | Stretching OH from PVA |
| 2926.45 | Asymmetric stretching of CH2 from PVA | 2925.48 | Asymmetric stretching of CH2 from PVA |
| 1740.44 | Symmetric stretching of C=O from PVP | 1742.37 | Symmetric stretching of C=O from PVP |
| 1428.03 | Symmetric deformation of CH from PVA | 1428.99 | Symmetric deformation of CH from PVA |
| 1319.07 | Stretching asymmetric due to CH-OH from ethanol | ||
| 1289.18 | Deformation of CH from PVA | 1286.29 | Deformation of CH from PVP |
| 1234.22 | Stretching secondary C=O from GTA | ||
| 1084.76 | Stretching of C-N from PVP | 1085.73 | Stretching of C-N from PVP |
| 921.807 | Deformation C-H from PVP | 923.736 | Deformation C-H from PVP |
| 835.026 | Stretching C-C from PVA | 845.633 | Stretching C-C from PVA |
| Sample | δ × 103, (m) | SD | WS, (%) | WVTR, (gm−2 day−1) | WVP × 1010, (g m−1 s−1 Pa−1) |
|---|---|---|---|---|---|
| E0_2 | 0.167 ± 0.003 | 7.019 ± 0.389 | 43.35 ± 0.73 | 624.9 ± 21.5 | 5.276 ± 0.034 |
| E1 | 0.238 ± 0.003 | 4.847 ± 0.518 | 59.27 ± 0.68 | 578.5 ± 10.4 | 6.816 ± 0.123 |
| E2 | 0.307 ± 0.030 | 6.472 ± 0.325 | 59.34 ± 0.61 | 493.3 ± 7.4 | 7.496 ± 0.112 |
| E3 | 0.247 ± 0.003 | 5.775 ± 0.624 | 59.69 ± 0.97 | 575.9 ± 14.4 | 7.041 ± 0.176 |
| E4 | 0.237 ± 0.003 | 6.048 ± 0.831 | 66.02 ± 1.05 | 521.2 ± 5.1 | 6.115 ± 0.596 |
| E5 | 0.297 ± 0.004 | 4.654 ± 0.254 | 76.97 ± 1.03 | 604.9 ± 11.5 | 8.894 ± 0.170 |
| E6 | 0.360 ± 0.044 | 3.735 ± 0.365 | 58.75 ± 0.94 | 612.9 ± 16.5 | 10.925 ± 0.296 |
| E7 | 0.188 ± 0.012 | 3.206 ± 0.158 | 48.64 ± 0.65 | 606.8 ± 24.2 | 5.647 ± 0.225 |
| E8 | 0.184 ± 0.027 | 9.631 ± 0.832 | 57.01 ± 1.32 | 134.7 ± 3.1 | 1.227 ± 0.028 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Gheorghita, D.; Grosu, E.; Robu, A.; Ditu, L.M.; Deleanu, I.M.; Gradisteanu Pircalabioru, G.; Raiciu, A.-D.; Bita, A.-I.; Antoniac, A.; Antoniac, V.I. Essential Oils as Antimicrobial Active Substances in Wound Dressings. Materials 2022, 15, 6923. https://doi.org/10.3390/ma15196923
Gheorghita D, Grosu E, Robu A, Ditu LM, Deleanu IM, Gradisteanu Pircalabioru G, Raiciu A-D, Bita A-I, Antoniac A, Antoniac VI. Essential Oils as Antimicrobial Active Substances in Wound Dressings. Materials. 2022; 15(19):6923. https://doi.org/10.3390/ma15196923
Chicago/Turabian StyleGheorghita, Daniela, Elena Grosu, Alina Robu, Lia Mara Ditu, Iuliana Mihaela Deleanu, Gratiela Gradisteanu Pircalabioru, Anca-Daniela Raiciu, Ana-Iulia Bita, Aurora Antoniac, and Vasile Iulian Antoniac. 2022. "Essential Oils as Antimicrobial Active Substances in Wound Dressings" Materials 15, no. 19: 6923. https://doi.org/10.3390/ma15196923
APA StyleGheorghita, D., Grosu, E., Robu, A., Ditu, L. M., Deleanu, I. M., Gradisteanu Pircalabioru, G., Raiciu, A.-D., Bita, A.-I., Antoniac, A., & Antoniac, V. I. (2022). Essential Oils as Antimicrobial Active Substances in Wound Dressings. Materials, 15(19), 6923. https://doi.org/10.3390/ma15196923












