Impact of Torrefaction on Fuel Properties of Aspiration Cleaning Residues
Abstract
:1. Introduction
2. Materials and Methods
2.1. Samples and Preparation
2.2. Preparation of Samples for Torrefaction
2.3. Sample Analysis
3. Results and Discussion
3.1. Elemental Analysis and Calorific Value of Waste from Maize
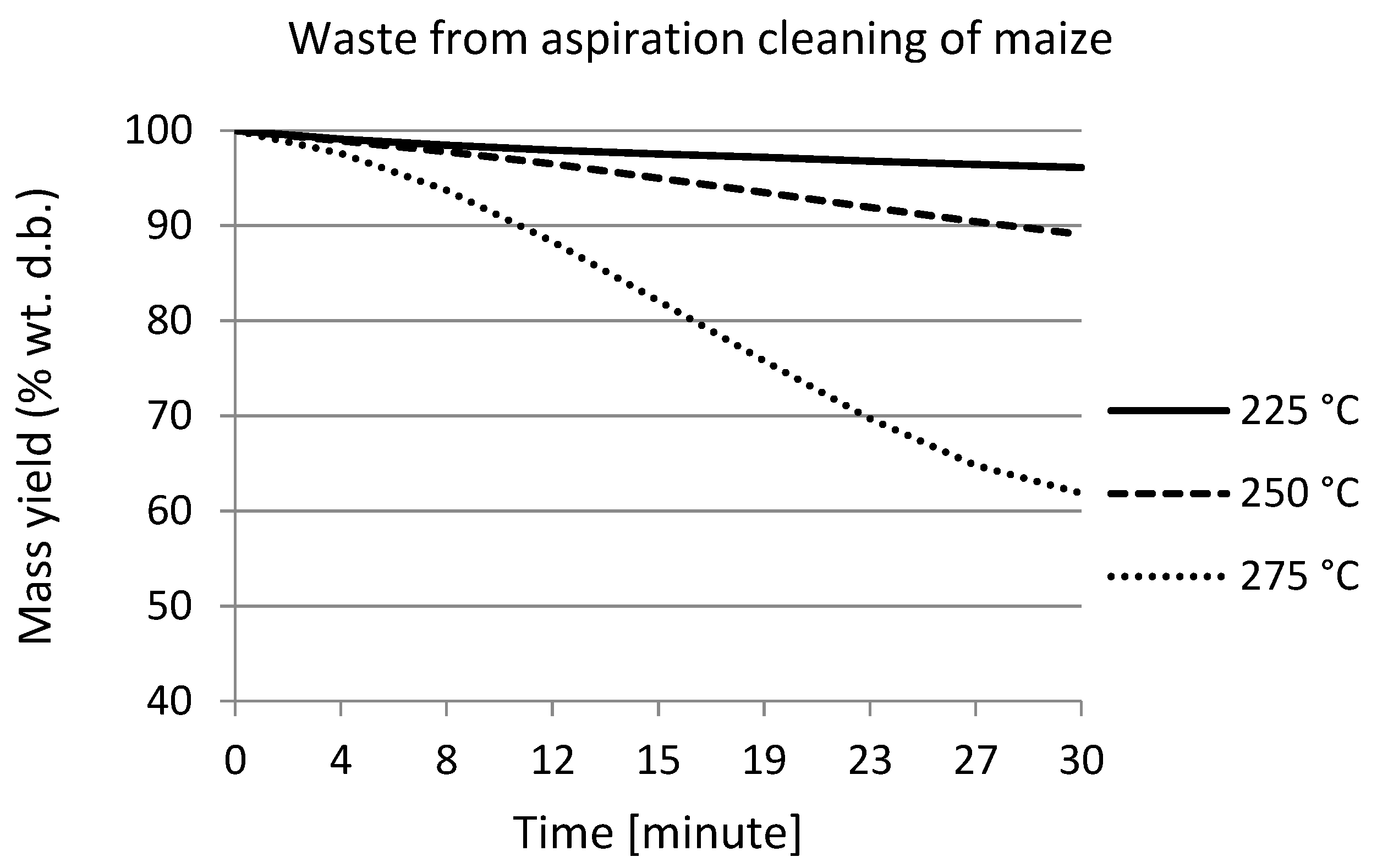
3.2. Weight Loss of the Waste from Maize during Torrefaction
3.3. Stoichiometric Combustion Analysis of Treated Wastes from Maize
3.4. Elemental Analysis and Calorific Values of Wheat Waste Samples
3.5. Weight Loss of the Waste from Aspiration Cleaning of Wheat during Torrefaction
3.6. Stoichiometric Combustion of Sample Waste from Aspiration Cleaning of Grains
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Chen, W.-H.; Lu, K.-M.; Tsai, C.-M. An experimental analysis on property and structure variations of agricultural wastes undergoing torrefaction. Appl. Energy 2012, 100, 318–325. [Google Scholar] [CrossRef]
- Ong, H.C.; Yu, K.L.; Chen, W.-H.; Pillejera, M.K.; Bi, X.; Tran, K.-Q.; Pétrissans, A.; Pétrissans, M. Variation of lignocellulosic biomass structure from torrefaction: A critical review. Renew. Sustain. Energy Rev. 2021, 152, 111698. [Google Scholar] [CrossRef]
- Sheldon, R.A. Green and sustainable manufacture of chemicals from biomass: State of the art. Green Chem. 2014, 16, 950–963. [Google Scholar] [CrossRef]
- Kärkäs, M.D.; Matsuura, B.S.; Monos, T.M.; Magallanes, G.; Stephenson, C.R.J. Transition-metal catalyzed valorization of lignin: The key to a sustainable carbon-neutral future. Org. Biomol. Chem. 2016, 14, 1853–1914. [Google Scholar] [CrossRef] [Green Version]
- Bradna, J.; Malaťák, J. Flue gases thermal emission concentration during waste biomass combustion in small combustion device with manual fuel supply. Res. Agric. Eng. 2016, 62, 1–8. [Google Scholar] [CrossRef] [Green Version]
- Aniszewska, M.; Gendek, A.; Zychowicz, W. Analysis of Selected Physical Properties of Conifer Cones with Relevance to Energy Production Efficiency. Forests 2018, 9, 405. [Google Scholar] [CrossRef] [Green Version]
- Akhtar, A.; Krepl, V.; Ivanova, T. A Combined Overview of Combustion, Pyrolysis, and Gasification of Biomass. Energy Fuels 2018, 32, 7294–7318. [Google Scholar] [CrossRef]
- Nurek, T.; Gendek, A.; Roman, K. Forest residues as a renewable source of energy: Elemental composition and physical properties. BioResources 2019, 14, 6–20. [Google Scholar] [CrossRef]
- Kučerová, V.; Lagaňa, R.; Hýrošová, T. Changes in chemical and optical properties of silver fir (Abies alba L.) wood due to thermal treatment. J. Wood Sci. 2019, 65, 21. [Google Scholar] [CrossRef] [Green Version]
- Kučerová, V.; Výbohová, E.; Hönig, V.; Čabalová, I. Chemical changes within solids during liquid hot water pretreatment of wood. BioResources 2020, 15, 38–48. [Google Scholar] [CrossRef]
- Deng, J.; Wang, G.; Kuang, J.; Zhang, Y.; Luo, Y. Pretreatment of agricultural residues for co-gasification via torrefaction. J. Anal. Appl. Pyrolysis 2009, 86, 331–337. [Google Scholar] [CrossRef]
- Tuck, C.O.; Pérez, E.; Horváth, I.T.; Sheldon, R.A.; Poliakoff, M. Valorization of Biomass: Deriving More Value from Waste. Science 2012, 337, 695–699. [Google Scholar] [CrossRef]
- Ijaz, N.; Dai, F.; Meng, L.; ur Rehman, Z.; Zhang, H. Integrating lignosulphonate and hydrated lime for the amelioration of expansive soil: A sustainable waste solution. J. Clean. Prod. 2020, 254, 119985. [Google Scholar] [CrossRef]
- Ijaz, N.; Dai, F.; ur Rehman, Z. Paper and wood industry waste as a sustainable solution for environmental vulnerabilities of expansive soil: A novel approach. J. Environ. Manag. 2020, 262, 110285. [Google Scholar] [CrossRef] [PubMed]
- McKendry, P. Energy production from biomass (part 2): Conversion technologies. Bioresour. Technol. 2002, 83, 47–54. [Google Scholar] [CrossRef]
- Huang, Y.F.; Chen, W.R.; Chiueh, P.T.; Kuan, W.H.; Lo, S.L. Microwave torrefaction of rice straw and pennisetum. Bioresour. Technol. 2012, 123, 1–7. [Google Scholar] [CrossRef]
- Kažimírová, V.; Kubík, Ľ.; Mihina, Š. Evaluation of Properties of Pellets Made of Swine Manure. Acta Technol. Agric. 2020, 23, 137–143. [Google Scholar] [CrossRef]
- Souček, J.; Jasinskas, A. Assessment of the Use of Potatoes as a Binder in Flax Heating Pellets. Sustainability 2020, 12, 10481. [Google Scholar] [CrossRef]
- Mudryk, K.; Hutsol, T.; Wrobel, M.; Jewiarz, M.; Dziedzic, B. Determination of friction coefficients of fast-growing tree biomass. Eng. Rural. Dev. 2019, 1568–1573. [Google Scholar] [CrossRef]
- Cahyanti, M.N.; Doddapaneni, T.R.K.C.; Kikas, T. Biomass torrefaction: An overview on process parameters, economic and environmental aspects and recent advancements. Bioresour. Technol. 2020, 301, 122737. [Google Scholar] [CrossRef]
- Ohliger, A.; Förster, M.; Kneer, R. Torrefaction of beechwood: A parametric study including heat of reaction and grindability. Fuel 2013, 104, 607–613. [Google Scholar] [CrossRef]
- Bridgeman, T.G.; Jones, J.M.; Shield, I.; Williams, P.T. Torrefaction of reed canary grass, wheat straw and willow to enhance solid fuel qualities and combustion properties. Fuel 2008, 87, 844–856. [Google Scholar] [CrossRef]
- Tamelová, B.; Malaťák, J.; Velebil, J.; Gendek, A.; Aniszewska, M. Energy Utilization of Torrefied Residue from Wine Production. Materials 2021, 14, 1610. [Google Scholar] [CrossRef] [PubMed]
- Simonic, M.; Goricanec, D.; Urbancl, D. Impact of torrefaction on biomass properties depending on temperature and operation time. Sci. Total Environ. 2020, 740, 140086. [Google Scholar] [CrossRef] [PubMed]
- Pulka, J.; Manczarski, P.; Koziel, J.A.; Białowiec, A. Torrefaction of Sewage Sludge: Kinetics and Fuel Properties of Biochars. Energies 2019, 12, 565. [Google Scholar] [CrossRef] [Green Version]
- Bach, Q.V.; Tran, K.Q. Dry and Wet Torrefaction of Woody Biomass-A Comparative Study on Combustion Kinetics. Energy Procedia 2015, 75, 150–155. [Google Scholar] [CrossRef] [Green Version]
- Bach, Q.-V.; Skreiberg, Ø. Upgrading biomass fuels via wet torrefaction: A review and comparison with dry torrefaction. Renew. Sustain. Energy Rev. 2016, 54, 665–677. [Google Scholar] [CrossRef]
- Novák, V.; Křížová, K.; Šařec, P. Biochar dosage impact on physical soil properties and crop status. Agron. Res. 2020, 18, 2501–2511. [Google Scholar] [CrossRef]
- Tamelová, B.; Malaťák, J.; Velebil, J. Energy valorisation of citrus peel waste by torrefaction treatment. Agron. Res. 2018, 16, 276–285. [Google Scholar] [CrossRef]
- Chen, W.-H.; Kuo, P.-C. Torrefaction and co-torrefaction characterization of hemicellulose, cellulose and lignin as well as torrefaction of some basic constituents in biomass. Energy 2011, 36, 803–811. [Google Scholar] [CrossRef]
- Parmar, A.; Nema, P.K.; Agarwal, T. Biochar production from agro-food industry residues: A sustainable approach for soil and environmental management. Curr. Sci. 2014, 107, 1673–1682, ISSN 0011-3891. [Google Scholar]
- Palanivelu, K.; Ramachandran, A.; Raghavan, V. Biochar from biomass waste as a renewable carbon material for climate change mitigation in reducing greenhouse gas emissions—A review. Biomass Convers. Biorefinery 2021, 11, 2247–2267. [Google Scholar] [CrossRef] [Green Version]
- Yahya, M.A.; Al-Qodah, Z.; Ngah, C.W.Z. Agricultural bio-waste materials as potential sustainable precursors used for activated carbon production: A review. Renew. Sustain. Energy Rev. 2015, 46, 218–235. [Google Scholar] [CrossRef]
- Rousset, P.; Petithuguenin, T.; Rodrigues, T.; Azevedo, A.-C. The fluidization behaviour of torrefied biomass in a cold model. Fuel 2012, 102, 256–263. [Google Scholar] [CrossRef]
- Malaták, J.; Velebil, J.; Bradna, J.; Gendek, A.; Tamelová, B. Evaluation of Co and Nox Emissions in Real-Life Operating Conditions of Herbaceous Biomass Briquettes Combustion. Acta Technol. Agric. 2020, 23, 53–59. [Google Scholar] [CrossRef]
- Tumuluru, J.S.; Sokhansanj, S.; Hess, J.R.; Wright, C.T.; Boardman, R.D. A review on biomass torrefaction process and product properties for energy applications. Ind. Biotechnol. 2011, 7, 384–401. [Google Scholar] [CrossRef] [Green Version]
- Li, H.; Liu, X.; Legros, R.; Bi, X.T.; Lim, C.J.; Sokhansanj, S. Pelletization of torrefied sawdust and properties of torrefied pellets. Appl. Energy 2012, 93, 680–685. [Google Scholar] [CrossRef]
- Tsalidis, G.A.; Joshi, Y.; Korevaar, G.; de Jong, W. Life cycle assessment of direct co-firing of torrefied and/or pelletised woody biomass with coal in The Netherlands. J. Clean. Prod. 2014, 81, 168–177. [Google Scholar] [CrossRef]
- Dyjakon, A.; Sobol, Ł.; Krotowski, M.; Mudryk, K.; Kawa, K. The impact of particles comminution on mechanical durability of wheat straw briquettes. Energies 2020, 13, 6186. [Google Scholar] [CrossRef]
- Wrobel, M.; Mudryk, K.; Jewiarz, M.; Knapczyk, A. Impact of raw material properties and agglomeration pressure on selected parmeters of granulates obtained from willow and black locust biomass. Eng. Rural Dev. 2018, 17, 1933–1938. [Google Scholar] [CrossRef]
- Bai, X.; Wang, G.; Gong, C.; Yu, Y.; Liu, W.; Wang, D. Co-pelletizing characteristics of torrefied wheat straw with peanut shell. Bioresour. Technol. 2017, 233, 373–381. [Google Scholar] [CrossRef]
- Liu, Z.; Zhang, F.; Liu, H.; Ba, F.; Yan, S.; Hu, J. Pyrolysis/gasification of pine sawdust biomass briquettes under carbon dioxide atmosphere: Study on carbon dioxide reduction (utilization) and biochar briquettes physicochemical properties. Bioresour. Technol. 2018, 249, 983–991. [Google Scholar] [CrossRef]
- Stelte, W.; Nielsen, N.P.K.; Hansen, H.O.; Dahl, J.; Shang, L.; Sanadi, A.R. Reprint of: Pelletizing properties of torrefied wheat straw. Biomass Bioenergy 2013, 53, 105–112. [Google Scholar] [CrossRef]
- Safar, M.; Lin, B.J.; Chen, W.H.; Langauer, D.; Chang, J.S.; Raclavska, H.; Pétrissans, A.; Rousset, P.; Pétrissans, M. Catalytic effects of potassium on biomass pyrolysis, combustion and torrefaction. Appl. Energy 2019, 235, 346–355. [Google Scholar] [CrossRef]
- Woolf, D.; Amonette, J.E.; Street-Perrott, F.A.; Lehmann, J.; Joseph, S. Sustainable biochar to mitigate global climate change. Nat. Commun. 2010, 1, 56. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Prins, M.J.; Ptasinski, K.J.; Janssen, F.J.J.G. Torrefaction of wood: Part 1. Weight loss kinetics. J. Anal. Appl. Pyrolysis 2006, 77, 28–34. [Google Scholar] [CrossRef]
- Pelaez-Samaniego, M.R.; Yadama, V.; Garcia-Perez, M.; Lowell, E.; McDonald, A.G. Effect of temperature during wood torrefaction on the formation of lignin liquid intermediates. J. Anal. Appl. Pyrolysis 2014, 109, 222–233. [Google Scholar] [CrossRef]
- Poudel, J.; Karki, S.; Oh, S.C. Valorization of waste wood as a solid fuel by torrefaction. Energies 2018, 11, 1641. [Google Scholar] [CrossRef] [Green Version]
- Odhady Sklizně—Operativní Zpráva—k 15. 9. 2021|ČSÚ. Available online: https://www.czso.cz/csu/czso/odhady-sklizne-operativni-zprava-k-15-9-2021 (accessed on 30 August 2022).
- Unpinit, T.; Poblarp, T.; Sailoon, N.; Wongwicha, P.; Thabuot, M. Fuel Properties of Bio-Pellets Produced from Selected Materials under Various Compacting Pressure. Energy Procedia 2015, 79, 657–662. [Google Scholar] [CrossRef] [Green Version]
- Mcfall, K.L.; Fowler, M.E. Overview of Wheat Classification and Trade. In Wheat Science and Trade; Wiley Online Library: Hoboken, NJ, USA, 2009; pp. 437–454. [Google Scholar] [CrossRef]
- Wang, X.; Wu, J.; Chen, Y.; Pattiya, A.; Yang, H.; Chen, H. Comparative study of wet and dry torrefaction of corn stalk and the effect on biomass pyrolysis polygeneration. Bioresour. Technol. 2018, 258, 88–97. [Google Scholar] [CrossRef]
- Zheng, A.; Zhao, Z.; Chang, S.; Huang, Z.; Zhao, K.; Wei, G.; He, F.; Li, H. Comparison of the effect of wet and dry torrefaction on chemical structure and pyrolysis behavior of corncobs. Bioresour. Technol. 2015, 176, 15–22. [Google Scholar] [CrossRef] [PubMed]
- Medic, D.; Darr, M.; Shah, A.; Potter, B.; Zimmerman, J. Effects of torrefaction process parameters on biomass feedstock upgrading. Fuel 2012, 91, 147–154. [Google Scholar] [CrossRef]
- Chen, D.; Cen, K.; Cao, X.; Li, Y.; Zhang, Y.; Ma, H. Restudy on torrefaction of corn stalk from the point of view of deoxygenation and decarbonization. J. Anal. Appl. Pyrolysis 2018, 135, 85–93. [Google Scholar] [CrossRef]
- Chaloupková, V.; Ivanova, T.; Hutla, P.; Špunarová, M. Ash Melting Behavior of Rice Straw and Calcium Additives. Agriculture 2021, 11, 1282. [Google Scholar] [CrossRef]
- Satpathy, S.K.; Tabil, L.G.; Meda, V.; Naik, S.N.; Prasad, R. Torrefaction of wheat and barley straw after microwave heating. Fuel 2014, 124, 269–278. [Google Scholar] [CrossRef]
- Cheng, X.; Huang, Z.; Wang, Z.; Ma, C.; Chen, S. A novel on-site wheat straw pretreatment method: Enclosed torrefaction. Bioresour. Technol. 2019, 281, 48–55. [Google Scholar] [CrossRef] [PubMed]
- Jeníček, L.; Neškudla, M.; Malaťák, J.; Velebil, J.; Passian, L. Spruce and Barley Elemental and Stochiometric Analysis Affected by the Impact of Pellet Production and Torrefaction. Acta Technol. Agric. 2021, 24, 166–172. [Google Scholar] [CrossRef]

| Temp. | C | H | N | S | O | Ash | GCV 1 | NCV 2 |
|---|---|---|---|---|---|---|---|---|
| °C | % wt. | % wt. | % wt. | % wt. | % wt. | % wt. | MJ·kg−1 | MJ·kg−1 |
| Maize waste | 47.20 ± 0.10 | 6.25 ± 0.08 | 1.31 ± 0.12 | 0.11 ± 0.01 | 43.13 | 2.01 ± 0.05 | 18.88 ± 0.10 | 17.51 |
| MT-30-225 | 48.36 ± 0.08 | 6.20 ± 0.07 | 1.38 ± 0.10 | 0.09 ± 0.01 | 41.93 | 2.04 ± 0.02 | 19.22 ± 0.09 | 17.87 |
| MT-30-250 | 51.34 ± 0.09 | 6.00 ± 0.07 | 1.54 ± 0.02 | 0.08 ± 0.01 | 38.72 | 2.32 ± 0.09 | 20.33 ± 0.09 | 19.02 |
| MT-30-275 | 63.03 ± 0.02 | 5.21 ± 0.08 | 1.98 ± 0.06 | 0.09 ± 0.01 | 26.31 | 3.38 ± 0.05 | 24.69 ± 0.10 | 23.56 |
| Dry Basis | T-30-225 | T-30-250 | T-30-275 | |||
|---|---|---|---|---|---|---|
| L min | Stoichiometric volume of air for complete combustion | (m3·kg−1) | 4.41 | 4.54 | 4.86 | 6.10 |
| vssp min | Stoichiometric volume of dry flue gas | (m3·kg−1) | 4.33 | 4.45 | 4.76 | 5.95 |
| vCO2 | Stoichiometric volume of CO2 | (m3·kg−1) | 0.88 | 0.90 | 0.95 | 1.17 |
| vH2O | Stoichiometric volume of H2O | (m3·kg−1) | 0.87 | 0.87 | 0.86 | 0.82 |
| vN2 | Stoichiometric volume of N2 | (m3·kg−1) | 3.45 | 3.55 | 3.80 | 4.78 |
| CO2max | Concentration of carbon dioxide in dry flue gas after stoichiometric combustion | (% vol.) | 20.22 | 20.15 | 20.02 | 19.66 |
| Samples | Heat Output (kW) | ||||
|---|---|---|---|---|---|
| 20 | 50 | 100 | 300 | ||
| Maize waste | Mass flow rate of fuel (kg h−1) | 4.57 | 11.42 | 22.84 | 68.53 |
| MT-30-225 | 4.48 | 11.19 | 21.38 | 67.15 | |
| MT-30-250 | 4.21 | 10.52 | 21.03 | 63.09 | |
| MT-30-275 | 3.40 | 8.49 | 16.98 | 50.93 | |
| Temp. | C | H | N | S | O | Ash | GCV 1 | NCV 2 |
|---|---|---|---|---|---|---|---|---|
| °C | % wt. | % wt. | % wt. | % wt. | % wt. | % wt. | MJ·kg−1 | MJ·kg−1 |
| Wheat waste | 41.75 ± 0.09 | 5.20 ± 0.09 | 1.65 ± 0.12 | 0.14 ± 0.01 | 34.43 | 16.83 ± 0.10 | 16.72 ± 0.10 | 15.59 |
| WT-30-225 | 43.43 ± 0.10 | 5.12 ± 0.04 | 1.71 ± 0.10 | 0.13 ± 0.01 | 31.18 | 18.43 ± 0.08 | 17.16 ± 0.11 | 16.04 |
| WT-30-250 | 45.79 ± 0.07 | 4.73 ± 0.05 | 1.93 ± 0.07 | 0.13 ± 0.01 | 26.25 | 21.17 ± 0.10 | 18.38 ± 0.09 | 17.35 |
| WT-30-275 | 47.56 ± 0.02 | 4.42 ± 0.08 | 2.12 ± 0.01 | 0.14 ± 0.01 | 21.62 | 24.14 ± 0.09 | 19.09 ± 0.09 | 18.12 |
| Dry Basis | T-30-225 | T-30-250 | T-30-275 | |||
|---|---|---|---|---|---|---|
| L min | Stoichiometric volume of air for complete combustion | (m3·kg−1) | 3.94 | 4.17 | 4.45 | 4.68 |
| vssp min | Stoichiometric volume of dry flue gas | (m3·kg−1) | 3.86 | 4.08 | 4.34 | 4.55 |
| vCO2 | Stoichiometric volume of CO2 | (m3·kg−1) | 0.78 | 0.81 | 0.85 | 0.88 |
| vH2O | Stoichiometric volume of H2O | (m3·kg−1) | 0.74 | 0.74 | 0.70 | 0.68 |
| vN2 | Stoichiometric volume of N2 | (m3·kg−1) | 3.09 | 3.27 | 3.49 | 3.67 |
| CO2max | Concentration of carbon dioxide in dry flue gas after stoichiometric combustion | (% vol.) | 20.04 | 19.75 | 19.59 | 19.39 |
| Samples | Heat Output (kW) | ||||
|---|---|---|---|---|---|
| 20 | 50 | 100 | 300 | ||
| Wheat waste | Mass flow rate of fuel (kg h−1) | 4.78 | 11.96 | 23.92 | 71.77 |
| WT-30-225 | 4.66 | 11.66 | 23.31 | 69.93 | |
| WT-30-250 | 4.35 | 10.88 | 21.76 | 65.29 | |
| WT-30-275 | 4.19 | 10.48 | 20.95 | 62.86 | |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Tamelová, B.; Malaťák, J.; Velebil, J.; Gendek, A.; Aniszewska, M. Impact of Torrefaction on Fuel Properties of Aspiration Cleaning Residues. Materials 2022, 15, 6949. https://doi.org/10.3390/ma15196949
Tamelová B, Malaťák J, Velebil J, Gendek A, Aniszewska M. Impact of Torrefaction on Fuel Properties of Aspiration Cleaning Residues. Materials. 2022; 15(19):6949. https://doi.org/10.3390/ma15196949
Chicago/Turabian StyleTamelová, Barbora, Jan Malaťák, Jan Velebil, Arkadiusz Gendek, and Monika Aniszewska. 2022. "Impact of Torrefaction on Fuel Properties of Aspiration Cleaning Residues" Materials 15, no. 19: 6949. https://doi.org/10.3390/ma15196949
APA StyleTamelová, B., Malaťák, J., Velebil, J., Gendek, A., & Aniszewska, M. (2022). Impact of Torrefaction on Fuel Properties of Aspiration Cleaning Residues. Materials, 15(19), 6949. https://doi.org/10.3390/ma15196949






