Nanoplatforms for Irinotecan Delivery Based on Mesoporous Silica Modified with a Natural Polysaccharide
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Methods
2.2.1. Ulvan Extract Preparation and Characterization
2.2.2. Synthesis of Mesoporous Silica-Type Supports
2.2.3. Drug-Loaded Samples Preparation
2.2.4. Irinotecan Release Experiments
2.2.5. Materials Characterization
2.2.6. Biological Evaluations of Ulvan Extract and Irinotecan-Loaded Samples
3. Results and Discussion
3.1. Characterization of Ulvan, Silica-Ulvan Nanoplatforms and Irinotecan-Loaded Samples
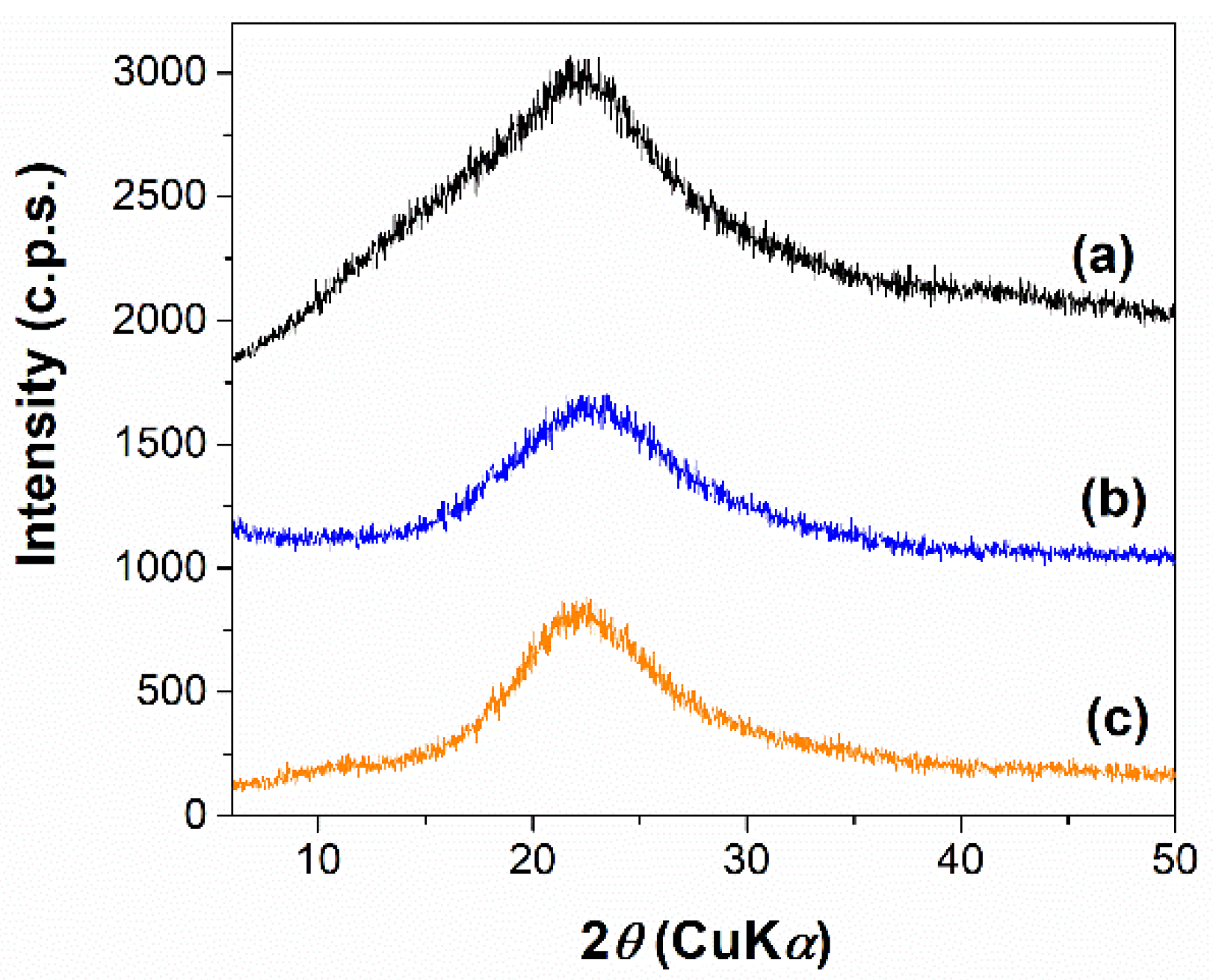
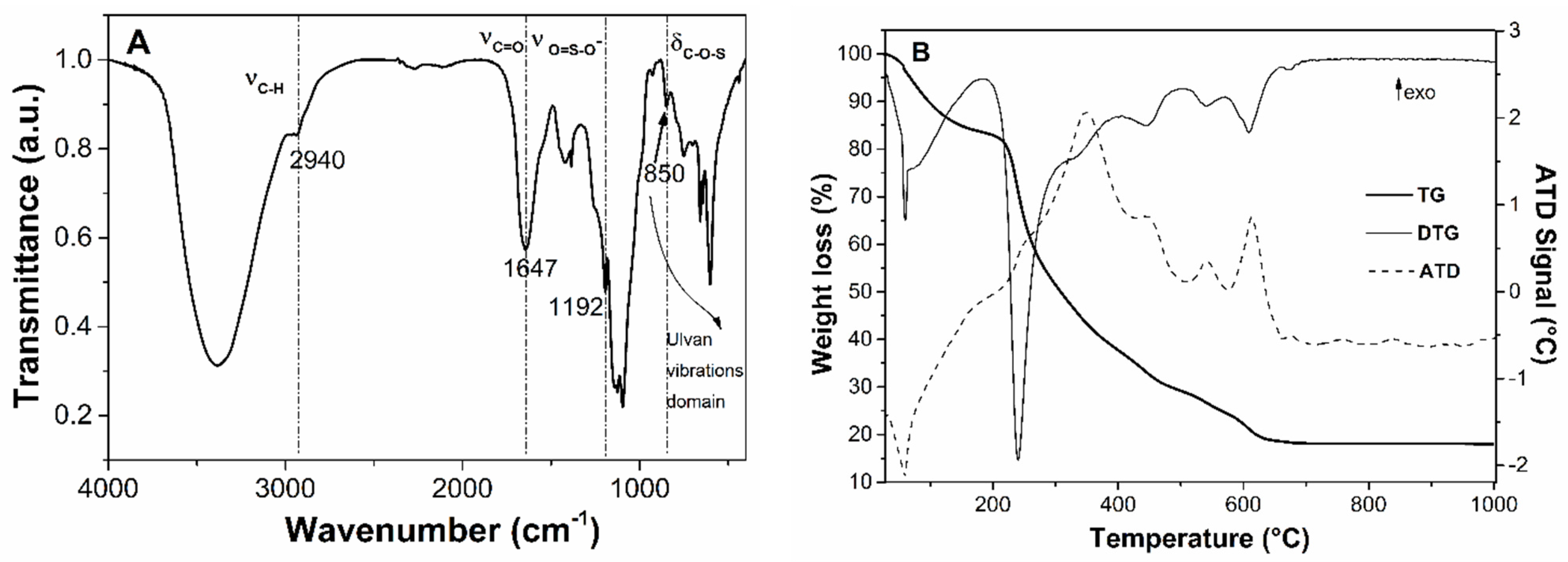
3.1.1. Characterization of Pretreatment Fractions and Ulvan
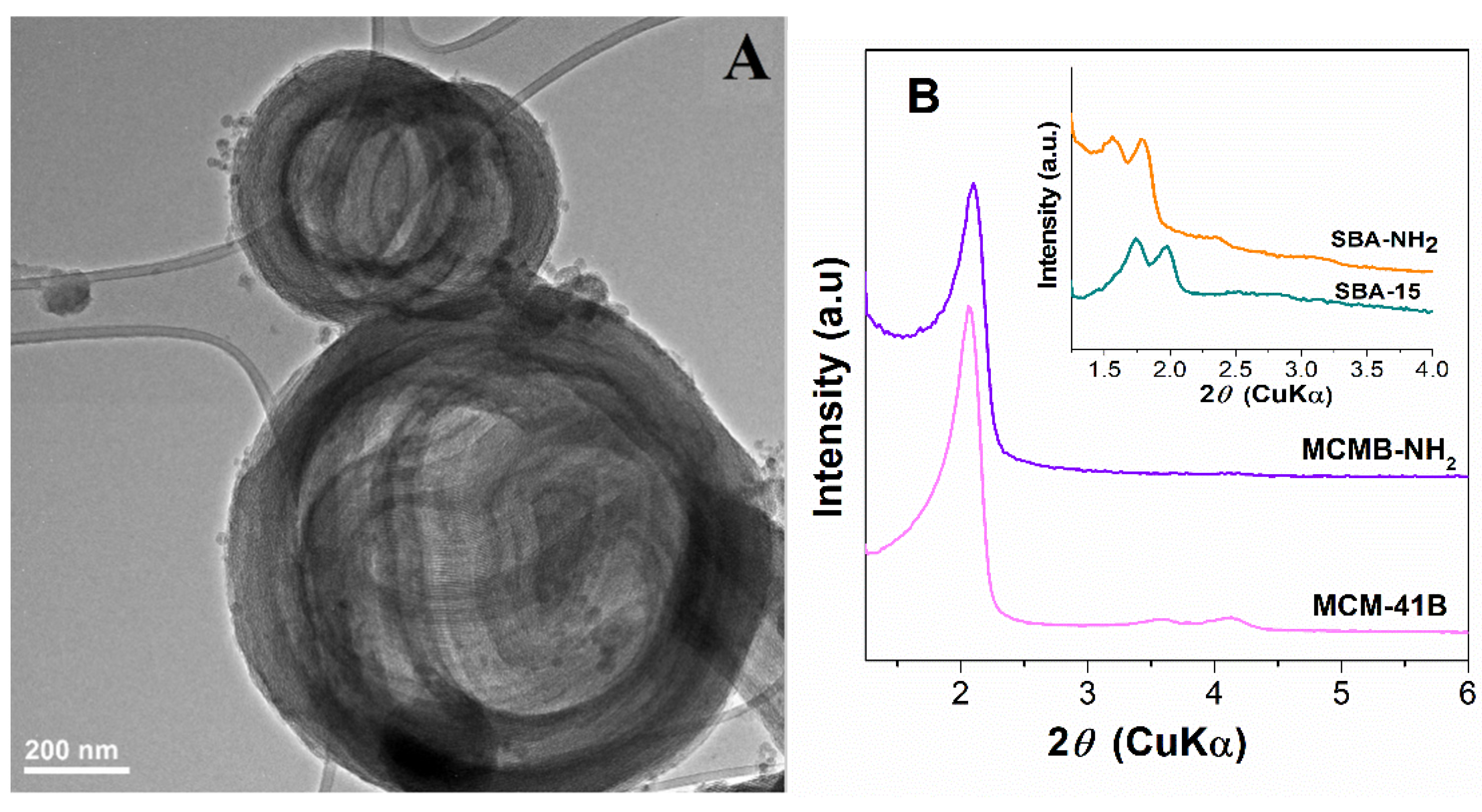
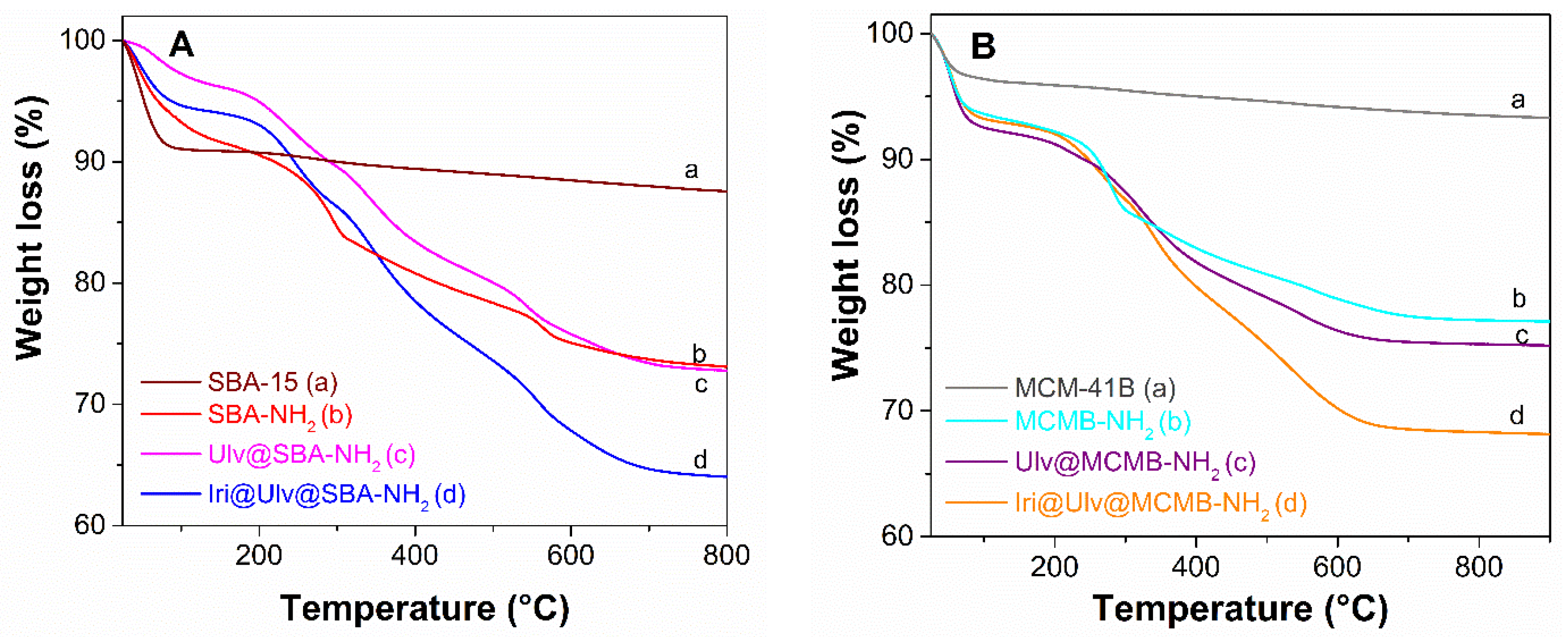
3.1.2. Characterization of Silica-Ulvan Nanoplatforms and Irinotecan-Loaded Samples
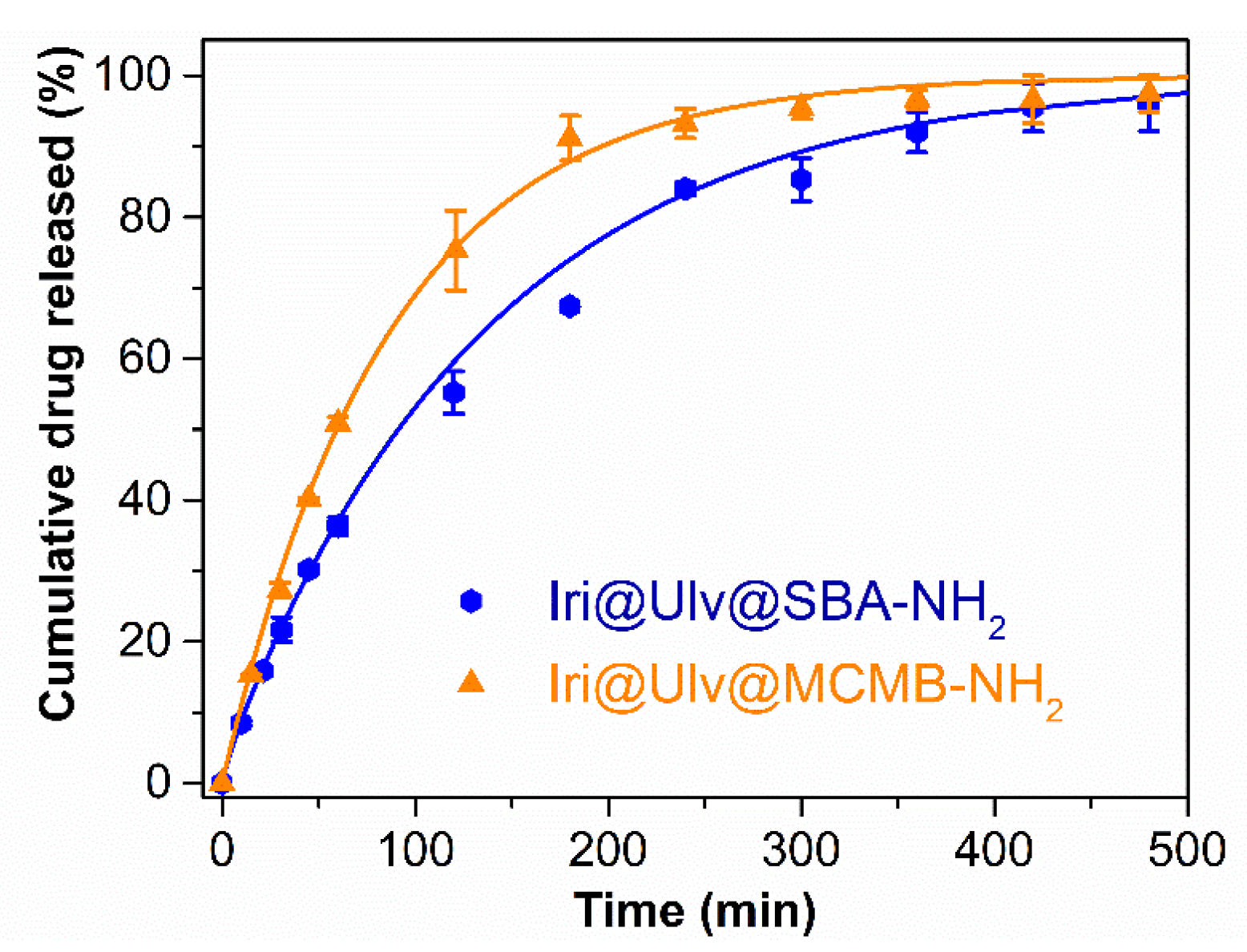
3.2. In Vitro Release Experiments of Irinotecan from Silica-Ulvan Nanoplatforms
3.3. Biological Evaluation of Ulvan, Ulvan-Silica Nanoplatforms and Irinotecan-Loaded Samples
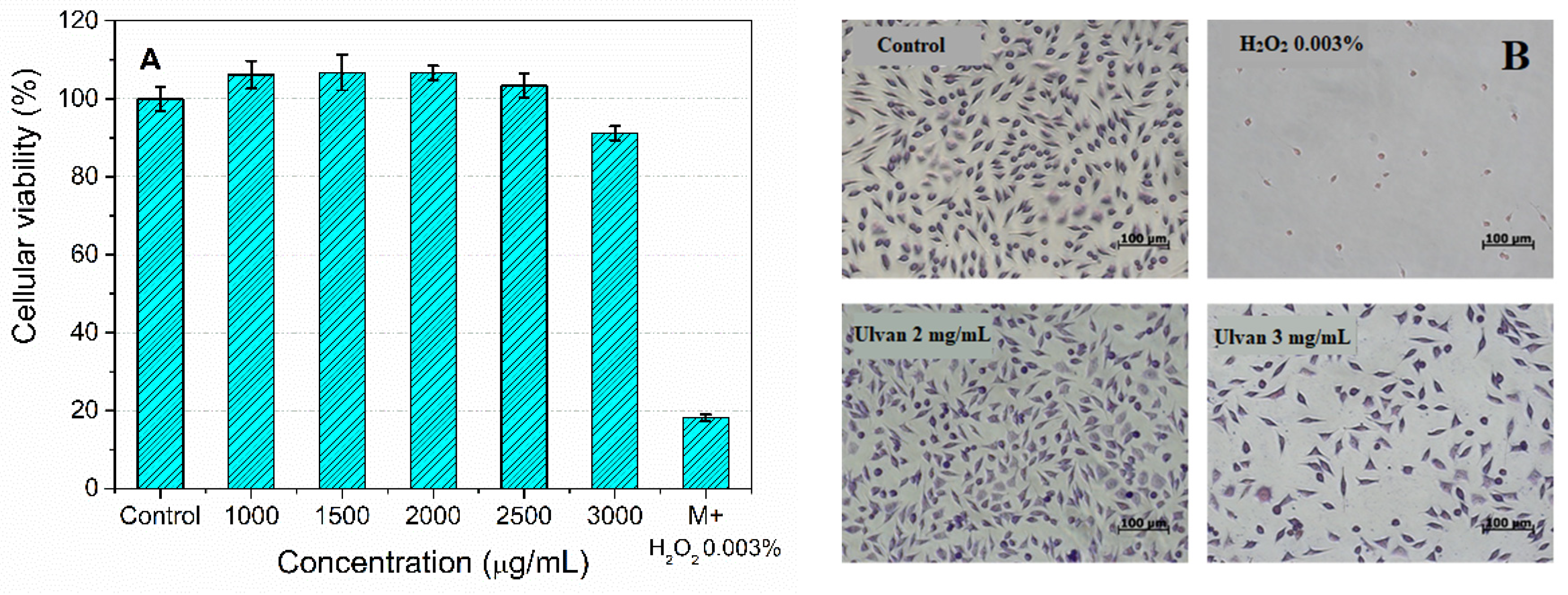
3.3.1. Ulvan Biocompatibility Assessment
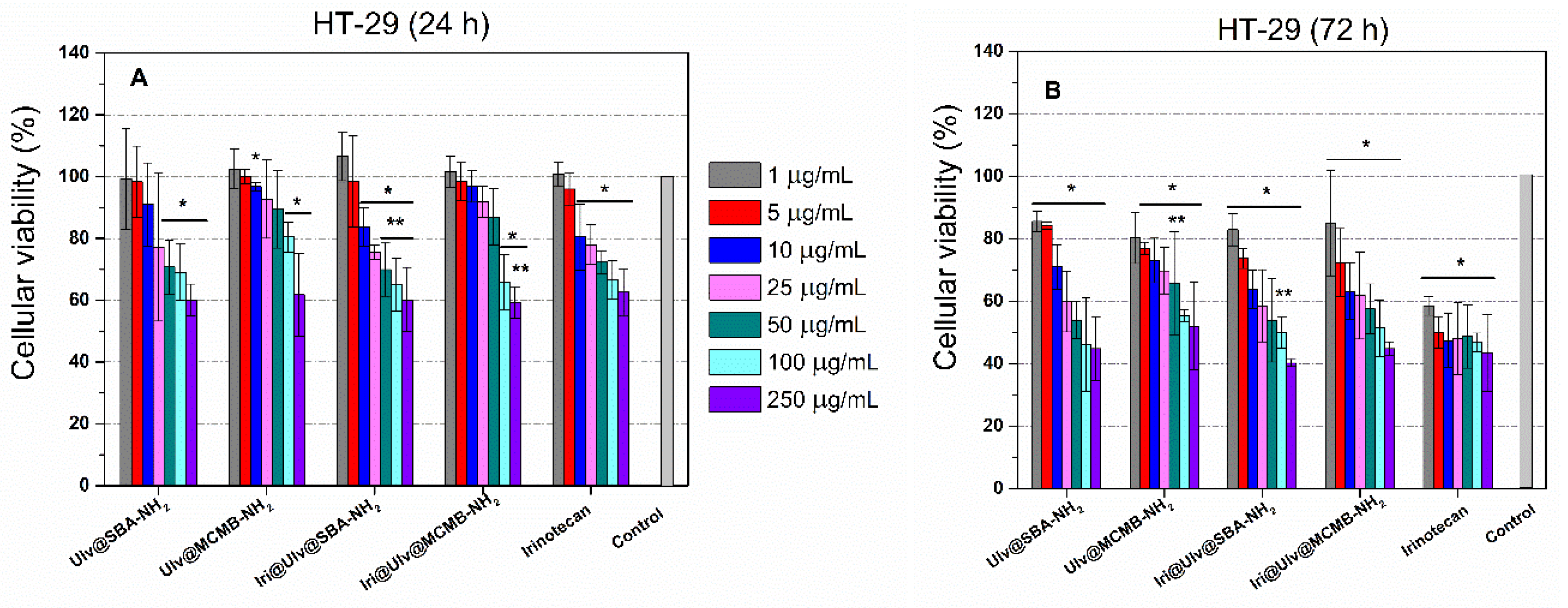
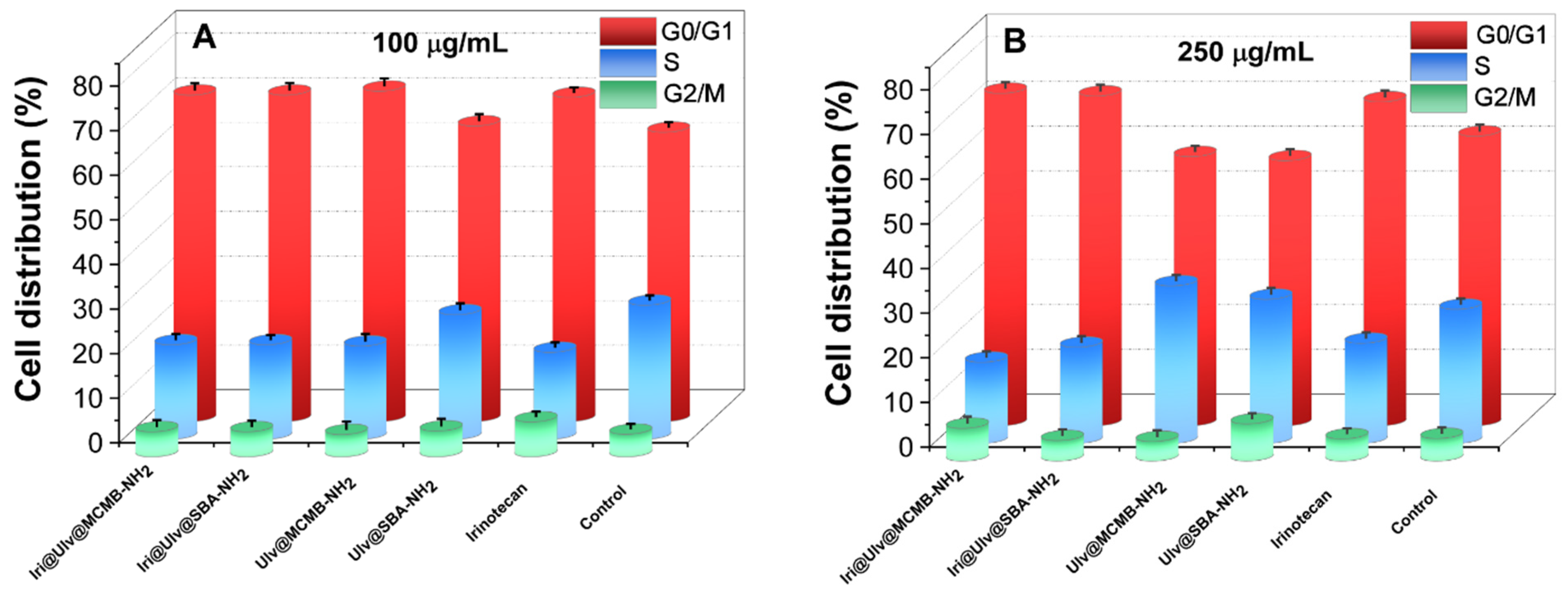
3.3.2. Cytotoxicity Evaluation of Irinotecan-Loaded Nanoplatforms
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Ginghină, O.; Hudit, A.; Zaharia, C.; Tsatsakis, A.; Mezhuev, Y.; Costache, M.; Gălăteanu, B. Current Landscape in Organic Nanosized Materials Advances for Improved Management of Colorectal Cancer Patients. Materials 2021, 14, 2440. [Google Scholar] [CrossRef] [PubMed]
- Raveendran, S.; Yasuhiko Yoshida, Y.; Maekawa, T.; Kumar, D.S. Pharmaceutically versatile sulfated polysaccharide based bionano platforms. Nanomed. Nanotechnol. Biol. Med. 2013, 9, 605–626. [Google Scholar] [CrossRef] [PubMed]
- Cunha, L.; Grenha, A. Sulfated Seaweed Polysaccharides as Multifunctional Materials in Drug Delivery Applications. Marine Drugs 2016, 14, 42. [Google Scholar] [CrossRef] [Green Version]
- Medina, S.H.; Tekumalla, T.; Chevliakov, M.V.; Shewach, D.S.; Ensminger, W.D.; El-Sayedad, M.E.H. N-acetylgalactosamine-functionalized dendrimers as hepatic cancer cell-targeted carriers. Biomaterials 2011, 32, 4118–4129. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Luo, Y.; Ziebell, M.R.; Prestwich, G.D. A Hyaluronic Acid−Taxol Antitumor Bioconjugate Targeted to Cancer Cells. Biomacromolecules 2000, 1, 208–218. [Google Scholar] [CrossRef]
- Liu, Z.; Ye, L.; Xi, J.; Wang, J.; Feng, Z. Cyclodextrin polymers: Structure, synthesis, and use as drug carriers. Prog. Polym. Sci. 2021, 118, 101408. [Google Scholar] [CrossRef]
- Iliescu, R.I.; Andronescu, E.; Ghițulia, C.D.; Voicu, G.; Ficai, A.; Holteiu, M. Montmorillonite–alginate nanocomposite as a drug delivery system—Incorporation and in vitro release of irinotecan. Int. J. Pharm. 2014, 463, 184–192. [Google Scholar] [CrossRef] [PubMed]
- Soe, Z.C.; Thapa, R.K.; Ou, W.; Gautam, M.; Nguyen, H.T.; Jin, S.G.; Ku, S.K.; Oh, K.T.; Choi, H.-G.; Yong, C.S.; et al. Folate receptor-mediated celastrol and irinotecan combination delivery using liposomes for effective chemotherapy. Colloids Surfaces. B. Biointerfaces 2018, 170, 718–728. [Google Scholar] [CrossRef]
- Wang, T.; He, W.; Du, Y.; Wang, J.; Li, X. Redox-sensitive irinotecan liposomes with active ultra-high loading and enhanced intracellular drug release. Colloids Surfaces B Biointerfaces 2021, 206, 111967. [Google Scholar] [CrossRef] [PubMed]
- Wu, D.; Zhu, L.; Li, Y.; Wang, H.; Xu, S.; Zhang, X.; Wu, R.; Yang, G. Superparamagnetic chitosan nanocomplexes for colorectal tumor-targeted delivery of irinotecan. Int. J. Pharm. 2020, 584, 119394. [Google Scholar] [CrossRef]
- Nastase, S.; Bajenaru, L.; Constantin, D.; Savopol, T.; Matei, C.; Berger, D. Mesostructured silica matrix for irinotecan delivery systems. Open Chem. 2014, 12, 813–820. [Google Scholar] [CrossRef]
- Hudson, S.P.; Padera, R.F.; Langer, R.; Kohane, D.S. The biocompatibility of mesoporous silicates. Biomaterials 2008, 29, 4045–4055. [Google Scholar] [CrossRef] [Green Version]
- Bowditch, A.; Waters, K.; Gale, H.; Rice, P.; Scott, E.A.M.; Canham, L.T.; Reeves, C.L.; Loni, A.; Cox, T.I. In-Vivo Assessment of Tissue Compatibility and Calcification of Bulk and Porous Silicon. MRS Online Proc. Libr. 1998, 536, 149–154. [Google Scholar] [CrossRef]
- Vallet-Regí, M.; Balas, F.; Arcos, D. Mesoporous Materials for Drug Delivery. Angew. Chem. Int. Ed. 2007, 46, 7548–7558. [Google Scholar] [CrossRef]
- Ambrogi, V.; Perioli, L.; Pagano, C.; Marmottini, F.; Moretti, M.; Mizzi, F.; Rossi, C. Econazole nitrate-loaded MCM-41 for an antifungal topical powder formulation. J. Pharm. Sci. 2010, 99, 4738–4745. [Google Scholar] [CrossRef]
- Berger, D.; Nastase, S.; Mitran, R.A.; Petrescu, M.; Vasile, E.; Matei, C.; Negreanu-Pirjol, T. Mesostructured silica and aluminosilicate carriers for oxytetracycline delivery systems. Int. J. Pharm. 2016, 510, 524–531. [Google Scholar] [CrossRef]
- Nastase, S.; Bajenaru, L.; Constantin, D.; Savopol, T.; Matei, C.; Berger, D. Ordered mesoporous silica and aluminosilicate-type matrix for amikacin delivery systems. Micropor. Mesopor. Mater. 2013, 182, 32–39. [Google Scholar] [CrossRef]
- Brezoiu, A.-M.; Deaconu, M.; Matei, C.; Berger, D. Heteroatom modified MCM-41-silica carriers for Lomefloxacin delivery systems. Micropor. Mesopor. Mater. 2019, 275, 214–222. [Google Scholar] [CrossRef]
- Nairi, V.; Medda, L.; Monduzzi, M.; Salis, A. Adsorption and release of ampicillin antibiotic from ordered mesoporous silica. J. Coll. Interface Sci. 2017, 497, 217–225. [Google Scholar] [CrossRef]
- Deaconu, M.; Nicu, I.; Tincu, R.; Brezoiu, A.M.; Mitran, R.A.; Vasile, E.; Matei, C.; Berger, D. Tailored doxycycline delivery from MCM-41-type silica carriers. Chem. Pap. 2018, 72, 1869–1880. [Google Scholar] [CrossRef]
- Deaconu, M.; Brezoiu, A.-M.; Mitran, R.-A.; Nicu, I.; Manolescu, B.; Matei, C.; Berger, D. Exploiting the zwitterionic properties of lomefloxacin to tailor its delivery from functionalized MCM-41 silica. Micropor. Mesopor. Mater. 2020, 305, 110323. [Google Scholar] [CrossRef]
- Ugazio, E.; Gastaldi, L.; Brunella, V.; Scalarone, D.; Jadhav, S.A.; Oliaro-Bosso, S.; Zonari, D.; Berlier, G.; Miletto, I.; Sapino, S. Thermoresponsive mesoporous silica nanoparticles as a carrier for skin delivery of quercetin. Int. J. Pharm. 2016, 511, 446–454. [Google Scholar] [CrossRef]
- Park, S.-Y.; Pendleton, P. Mesoporous silica SBA-15 for natural antimicrobial delivery. Powder Technol. 2012, 223, 77–82. [Google Scholar] [CrossRef]
- Brezoiu, A.M.; Lincu, D.; Deaconu, M.; Matei, C.; Berger, D. Enhanced stability of polyphenolic extracts from grape pomace achieved by embedding into mesoporous silica-type matrices. UPB Sci. Bull. Series B: Chem. Mater. Sci. 2020, 82, 3–20. [Google Scholar]
- Brezoiu, A.-M.; Bajenaru, L.; Berger, D.; Mitran, R.-A.; Deaconu, M.; Lincu, D.; Stoica Guzun, A.; Matei, C.; Moisescu, M.G.; Negreanu-Pirjol, T. Effect of Nanoconfinement of Polyphenolic Extract from Grape Pomace into Functionalized Mesoporous Silica on Its Biocompatibility and Radical Scavenging Activity. Antioxidants 2020, 9, 696. [Google Scholar] [CrossRef]
- Buda, V.; Brezoiu, A.-M.; Berger, D.; Zinuca Pavel, I.; Muntean, D.; Minda, D.; Dehelean, C.A.; Soica, C.; Diaconeasa, Z.; Folescu, R.; et al. Biological Evaluation of Black Chokeberry Extract Free and Embedded in Two Mesoporous Silica-Type Matrices. Pharmaceutics 2020, 12, 838. [Google Scholar] [CrossRef]
- Brezoiu, A.-M.; Prundeanu, M.; Berger, D.; Deaconu, M.; Matei, C.; Oprea, O.; Vasile, E.; Negreanu-Pirjol, T.; Munteanu, D.; Danciu, C. Properties of Salvia officinalis L. and Thymus serpyllum L. Extracts Free and Embedded into Mesopores of Silica and Titania Nanomaterials. Nanomaterials 2020, 10, 820. [Google Scholar] [CrossRef]
- Maria, G.; Berger, D.; Nastase, S.; Luta, G. Kinetic studies on the irinotecan release based on structural properties of functionalized mesoporous-silica supports. Micropor. Mesopor. Mater. 2012, 149, 25–35. [Google Scholar] [CrossRef]
- Kumar, B.; Kulanthaivel, S.; Mondal, A.; Mishra, S.; Banerjee, B.; Bhaumik, A.; Banerjee, I.; Giri, S. Mesoporous silica nanoparticle based enzyme responsive system for colon specific drug delivery through guar gum capping. Colloids Surf. B Biointerfaces 2017, 150, 352–361. [Google Scholar] [CrossRef]
- Juère, E.; Kleitz, F. On the nanopore confinement of therapeutic drugs into mesoporous silica materials and its implications. Micropor. Mesopor. Mater. 2018, 270, 109–119. [Google Scholar] [CrossRef]
- Mužík, J.; Lizoňová, D.; Zadražil, A.; Štěpánek, F. Drug amorphisation by fluid bed hot-melt impregnation of mesoporous silica carriers. Chem. Eng. J. 2020, 392, 123754. [Google Scholar] [CrossRef]
- Zhou, S.; Zhong, Q.; Wang, Y.; Hu, P.; Zhong, W.; Huang, C.B.; Fu, J. Chemically engineered mesoporous silica nanoparticles-based intelligent delivery systems for theranostic applications in multiple cancerous/non-cancerous diseases. Coord. Chem. Rev. 2022, 452, 214309. [Google Scholar] [CrossRef]
- Kankala, R.K.; Han, Y.H.; Xia, H.Y.; Wang, S.B.; Chen, A.Z. Nanoarchitectured prototypes of mesoporous silica nanoparticles for innovative biomedical applications. J. Nanobiotechnol. 2022, 20, 126. [Google Scholar] [CrossRef]
- Liu, Z.; Ran, Y.; Xi, J.; Wang, J. Polymeric hybrid aerogels and their biomedical applications. Soft Matter 2020, 16, 9160–9175. [Google Scholar] [CrossRef]
- Brezoiu, A.-M.; Matei, C.; Deaconu, M.; Stanciuc, A.M.; Trifan, A.; Gaspar-Pintiliescu, A.; Berger, D. Polyphenols extract from grape pomace. Characterization and valorisation through encapsulation into mesoporous silica-type matrices. Food Chem. Toxicol. 2019, 133, 110787. [Google Scholar] [CrossRef]
- Miclea, L.C.; Mihailescu, M.; Tarba, N.; Brezoiu, A.M.; Sandu, A.M.; Mitran, R.A.; Berger, D.; Matei, C.; Moisescu, M.G.; Savopol, T. Evaluation of intracellular distribution of folate functionalized silica nanoparticles using fluorescence and hyperspectral enhanced dark field microscopy. Nanoscale 2022. [Google Scholar] [CrossRef]
- Ioniţă, S.; Lincu, D.; Mitran, R.A.; Ziko, L.; Sedky, N.K.; Deaconu, M.; Brezoiu, A.-M.; Matei, C.; Berger, D. Resveratrol encapsulation and release from pristine and functionalized mesoporous silica carriers. Pharmaceutics 2022, 14, 203. [Google Scholar] [CrossRef]
- Suzuki, T.M.; Nakamura, T.; Fukumoto, K.; Yamamoto, M.; Akimoto, Y.; Yano, K. Direct synthesis of amino-functionalized monodispersed mesoporous silica spheres and their catalytic activity for nitroaldol condensation. J. Molec. Catal. A Chemical 2008, 280, 224–232. [Google Scholar] [CrossRef]
- Charnay, C.; Bégu, S.; Tourné-Péteilh, C.; Nicole, L.; Lerner, D.A.; Devoisselle, J.M. Inclusion of ibuprofen in mesoporous templated silica: Drug loading and release property. Eur. J. Pharm. Biopharm. 2016, 57, 533–540. [Google Scholar] [CrossRef]
- PBS Buffer pH 7.6-1x conc. Available online: https://www.jenabioscience.com/molecular-biology/buffers-and-reagents/bu-115-1-pbs-buffer-ph-7-6-1x-conc (accessed on 10 May 2022).
- Adb El-Baky, H.; El Baz, F.K.; El Baroty, G.S. Evaluation of Marine Alga Ulva lactuca L. as A Source of Natural Preservative Ingredient. Am. Eurasian J. Agric. Environ. Sci. 2008, 3, 434–444. [Google Scholar]
- El Boukhari, M.E.M.; Barakate, M.; Choumani, N.; Bouhia, Y.; Lyamloul, K. Ulva lactuca Extract and Fractions as Seed Priming Agents Mitigate Salinity Stress in Tomato Seedlings. Plants 2021, 10, 1104. [Google Scholar] [CrossRef] [PubMed]
- Kidgell, J.T.; Magnusson, M.; de Nys, R.; Glasson, C.R.K. Ulvan: A systematic review of extraction, composition and function. Algal Res. 2019, 39, 101422. [Google Scholar] [CrossRef]
- Andrade Figueira, T.; Ribeiro da Silva, A.J.; Enrich-Prast, A.; Yoneshigue-Valentin, Y.; Peruzzi de Oliveira, V. Structural Characterization of Ulvan Polysaccharide from Cultivated and Collected Ulva fasciata (Chlorophyta). Adv. Biosci. Biotechnol. 2020, 11, 206–216. [Google Scholar] [CrossRef]
- Alves, A.; Caridade, S.G.; Mano, J.F.; Sousa, R.A.; Reis, R.L. Extraction and physico-chemical characterization of a versatile biodegradable polysaccharide obtained from green algae. Carbohydr. Res. 2010, 345, 2194–2200. [Google Scholar] [CrossRef]
- Masood, S.A.; Maheen, S.; Khan, H.U.; Zafar, M.N.; Shafqat, S.S.; Mujtaba, M.A.; Khalifa, A.S. In vitro/in vivo evaluation of statistically engineered alginate scaffold reinforced with dual drugs loaded silica nanoparticles for enhanced fungal therapeutics. Alex. Eng. J. 2022, 61, 4041–4056. [Google Scholar] [CrossRef]
- Mitran, R.A.; Matei, C.; Berger, D. Correlation of Mesoporous Silica Structural and Morphological Features with Theoretical Three-Parameter Model for Drug Release Kinetics. J. Phys. Chem. C 2016, 120, 29202–29209. [Google Scholar] [CrossRef]
- Alves, A.; Sousa, R.A.; Reis, R.L. In vitro Cytotoxicity Assessment of Ulvan, a Polysaccharide Extracted from Green Algae. Phytother. Res. 2013, 27, 1143–1148. [Google Scholar] [CrossRef]


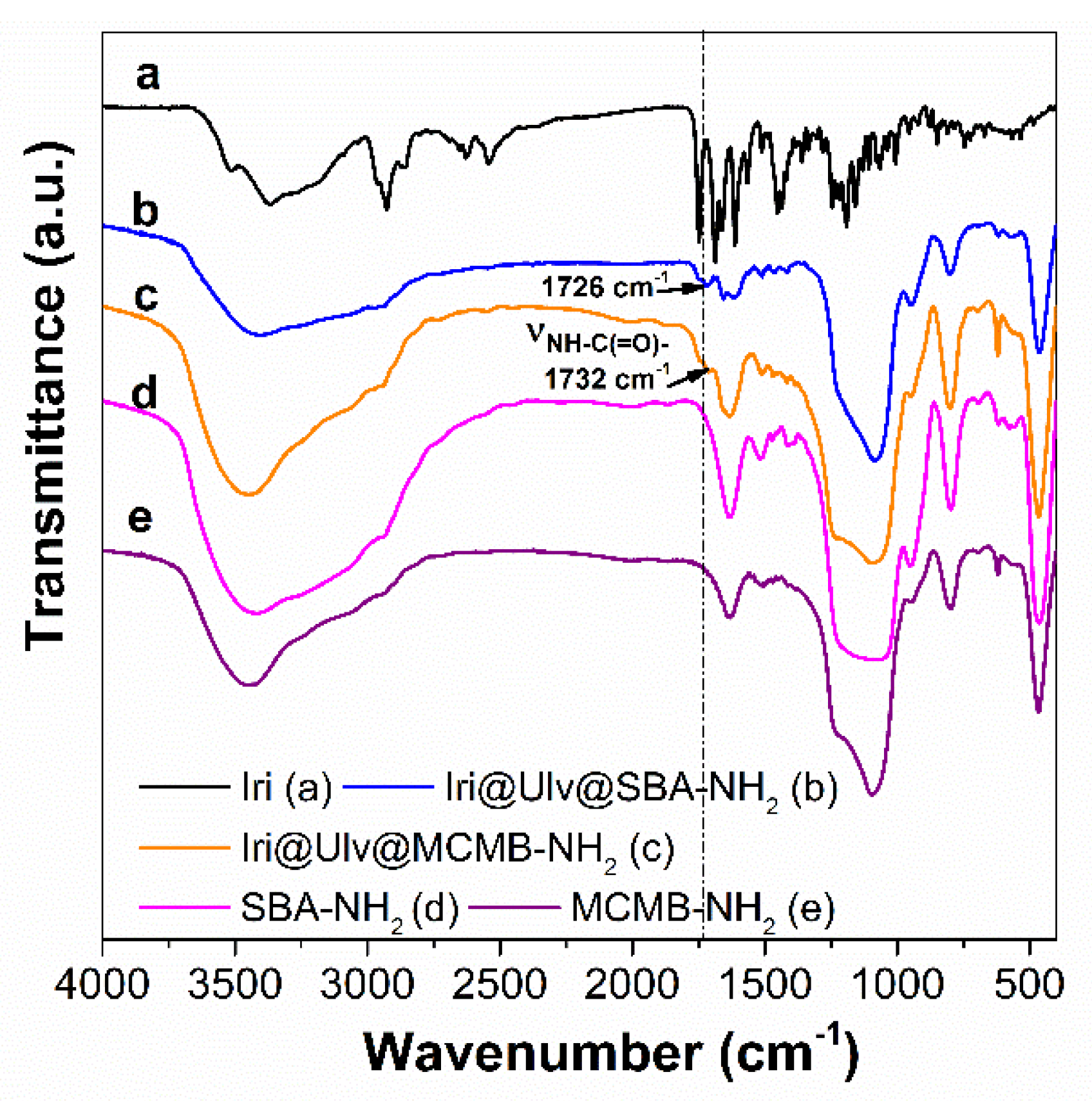
| Solvent | CHa (mgCHa/gextract) | CHb (mgCHb/gextract) | CHT (mgCHt/gextract) | TPC (mg GAE/gextract) |
|---|---|---|---|---|
| Methanol | 17.1 ± 0.1 | 25.7 ± 0.2 | 42.8 ± 0.2 | 30.4 ± 0.4 |
| Acetone | 12.3 ± 0.2 | 2.9 ± 0.0 | 15.2 ± 0.2 | - |
| Support-Type | Support | Iri@support | |
|---|---|---|---|
| nSiO2/nOG | Ulv (%w/w) | Irinotecan (%w/w) | |
| Ulv@MCMB-NH2 | 6.25 | 1.5 | 8.3 |
| Ulv@SBA-NH2 | 5.06 | 6.0 | 8.6 |
| Irinotecan Loaded Ulvan-Silica Nanoplatforms | Three-Parameter Model | Maximum Amount of Drug Released (%) | ||||
|---|---|---|---|---|---|---|
| ΔG (1021 J) | kd (min−1) | koff (min−1) | kon (min−1) | R2 | ||
| Iri@Ulv@SBA-NH2 | −3.36 | 0.032 | 0.015 | 0.034 | 0.9978 | 96.1 ± 3.9 |
| Iri@Ulv@MCMB-NH2 | 1.87 | 0.019 | 1.501 | 0.969 | 0.9976 | 97.4 ± 2.6 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Brezoiu, A.-M.; Prelipcean, A.-M.; Lincu, D.; Deaconu, M.; Vasile, E.; Tatia, R.; Seciu-Grama, A.-M.; Matei, C.; Berger, D. Nanoplatforms for Irinotecan Delivery Based on Mesoporous Silica Modified with a Natural Polysaccharide. Materials 2022, 15, 7003. https://doi.org/10.3390/ma15197003
Brezoiu A-M, Prelipcean A-M, Lincu D, Deaconu M, Vasile E, Tatia R, Seciu-Grama A-M, Matei C, Berger D. Nanoplatforms for Irinotecan Delivery Based on Mesoporous Silica Modified with a Natural Polysaccharide. Materials. 2022; 15(19):7003. https://doi.org/10.3390/ma15197003
Chicago/Turabian StyleBrezoiu, Ana-Maria, Ana-Maria Prelipcean, Daniel Lincu, Mihaela Deaconu, Eugeniu Vasile, Rodica Tatia, Ana-Maria Seciu-Grama, Cristian Matei, and Daniela Berger. 2022. "Nanoplatforms for Irinotecan Delivery Based on Mesoporous Silica Modified with a Natural Polysaccharide" Materials 15, no. 19: 7003. https://doi.org/10.3390/ma15197003
APA StyleBrezoiu, A. -M., Prelipcean, A. -M., Lincu, D., Deaconu, M., Vasile, E., Tatia, R., Seciu-Grama, A. -M., Matei, C., & Berger, D. (2022). Nanoplatforms for Irinotecan Delivery Based on Mesoporous Silica Modified with a Natural Polysaccharide. Materials, 15(19), 7003. https://doi.org/10.3390/ma15197003










