A Water-Stable Zn-MOF Used as Multiresponsive Luminescent Probe for Sensing Fe3+/Cu2+, Trinitrophenol and Colchicine in Aqueous Medium
Abstract
:1. Introduction
2. Physical Measurements
3. Synthesis of the Compound
| Compound | Zn-MOF |
|---|---|
| Molecular formula | C24H18N4O16Zn4 |
| Formula weight | 879.82 |
| Crystal system | Monoclinic |
| Space group | C 2/c |
| a, Å | 26.7137(7) |
| Β, Å | 11.1860(3) |
| c, Å | 19.7493(5) |
| α | 90 |
| β | 132.56 |
| γ | 90 |
| V, Å3 | 4347.1(2) |
| Z | 4 |
| Dcalc, g/cm3 | 1.260 |
| F(000) | 1632.0 |
| GoF | 1.057 |
| R1, wR2 [I > 2σ(I)] a,b | R1 = 0.036, wR2 = 0.1029 |
| R1, wR2 (all data) | R1 = 0.0435 wR2 = 0.1059 |
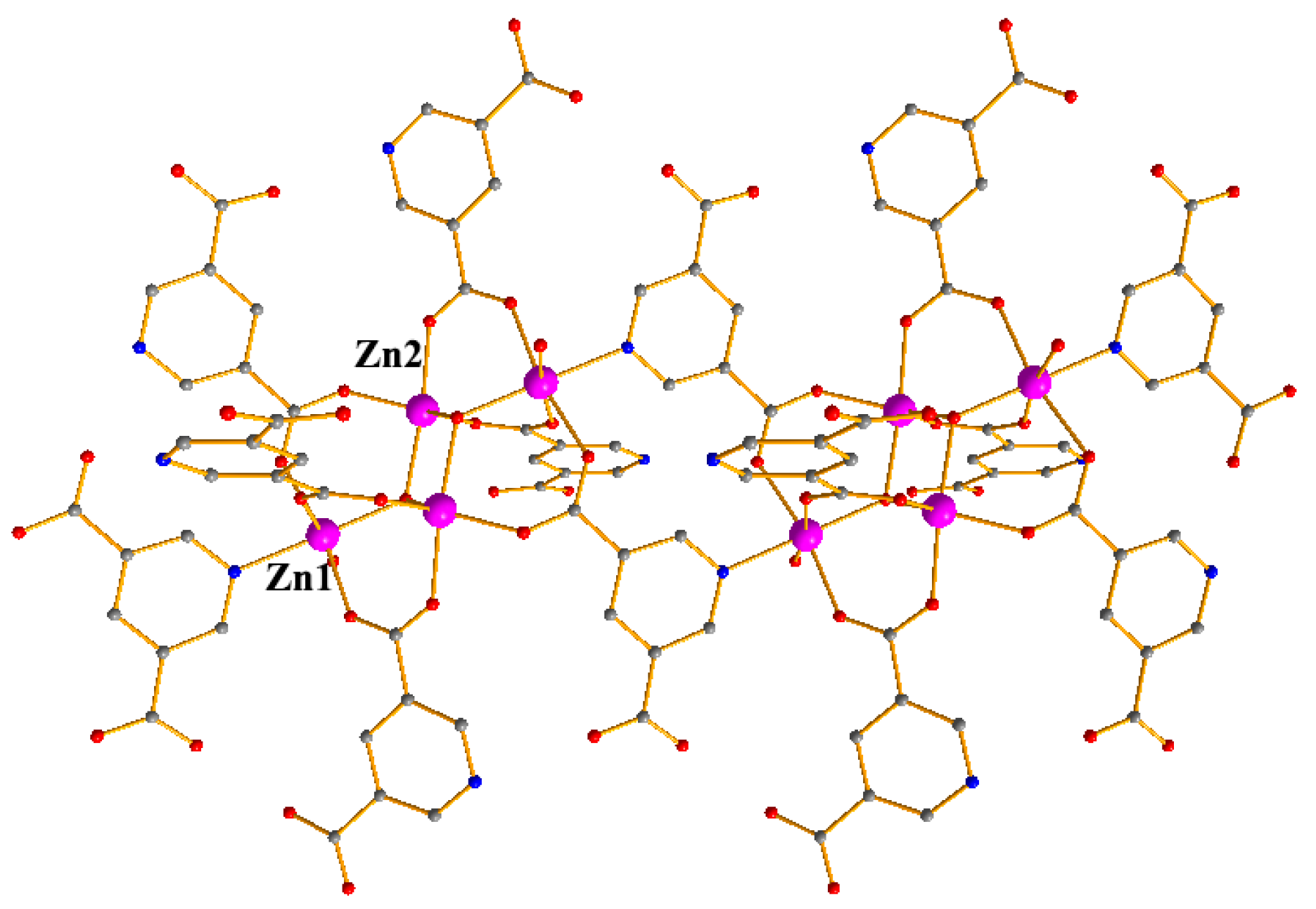
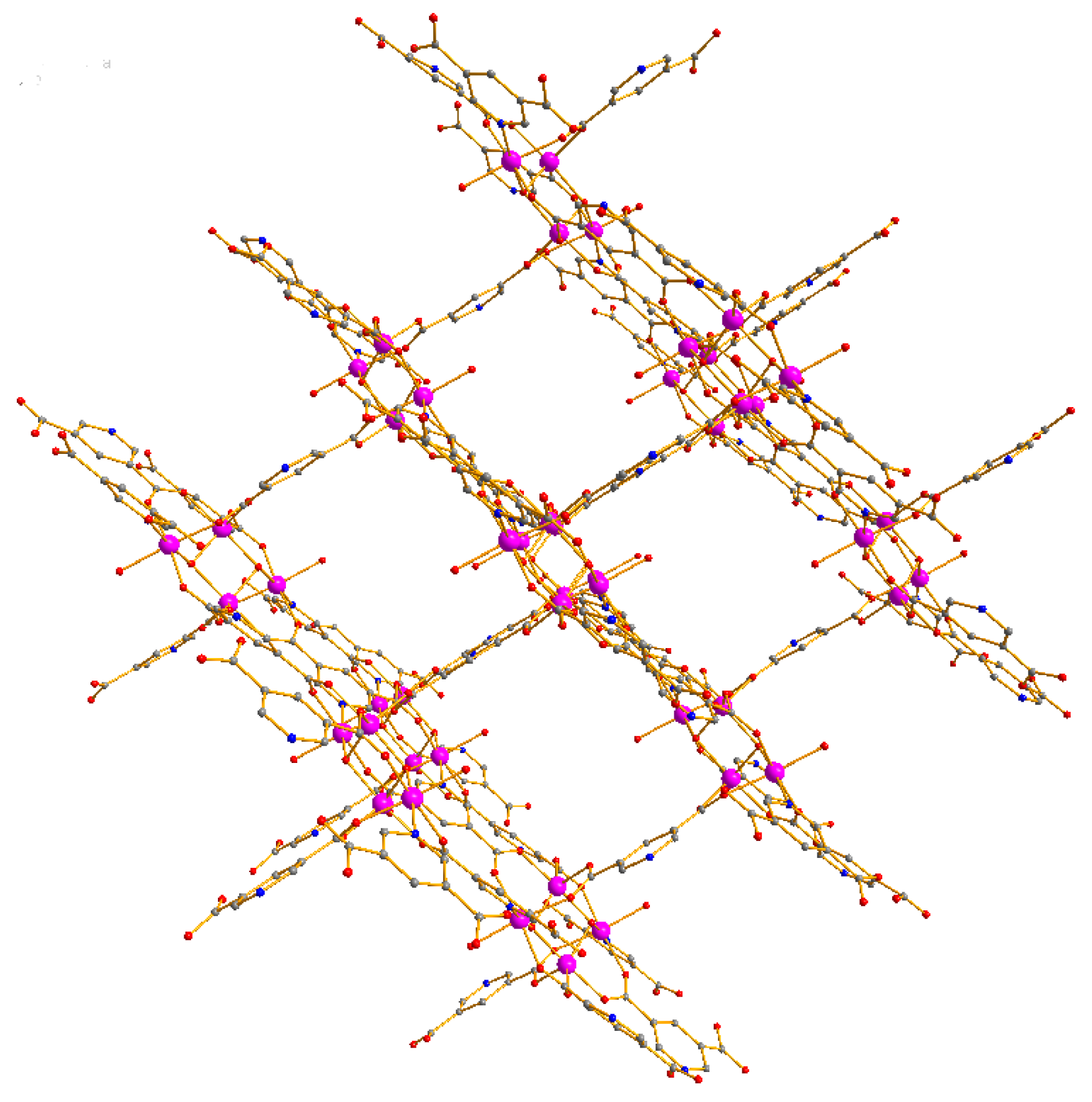
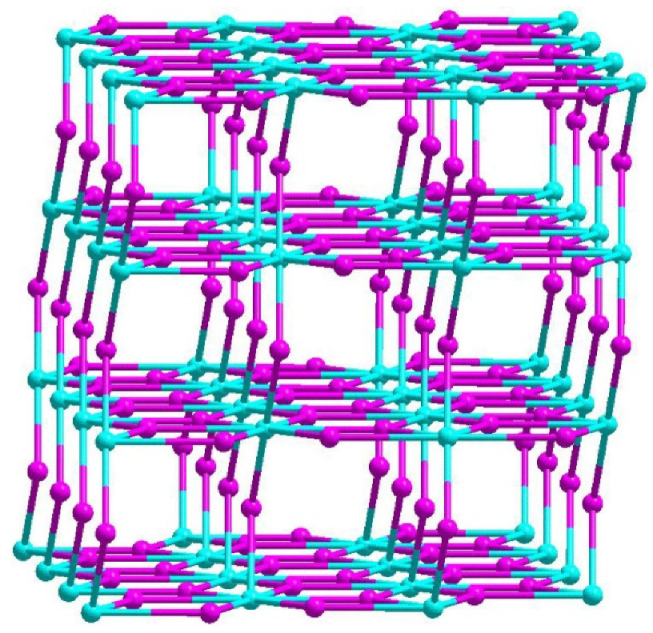
4. Crystal Structure of the Compound
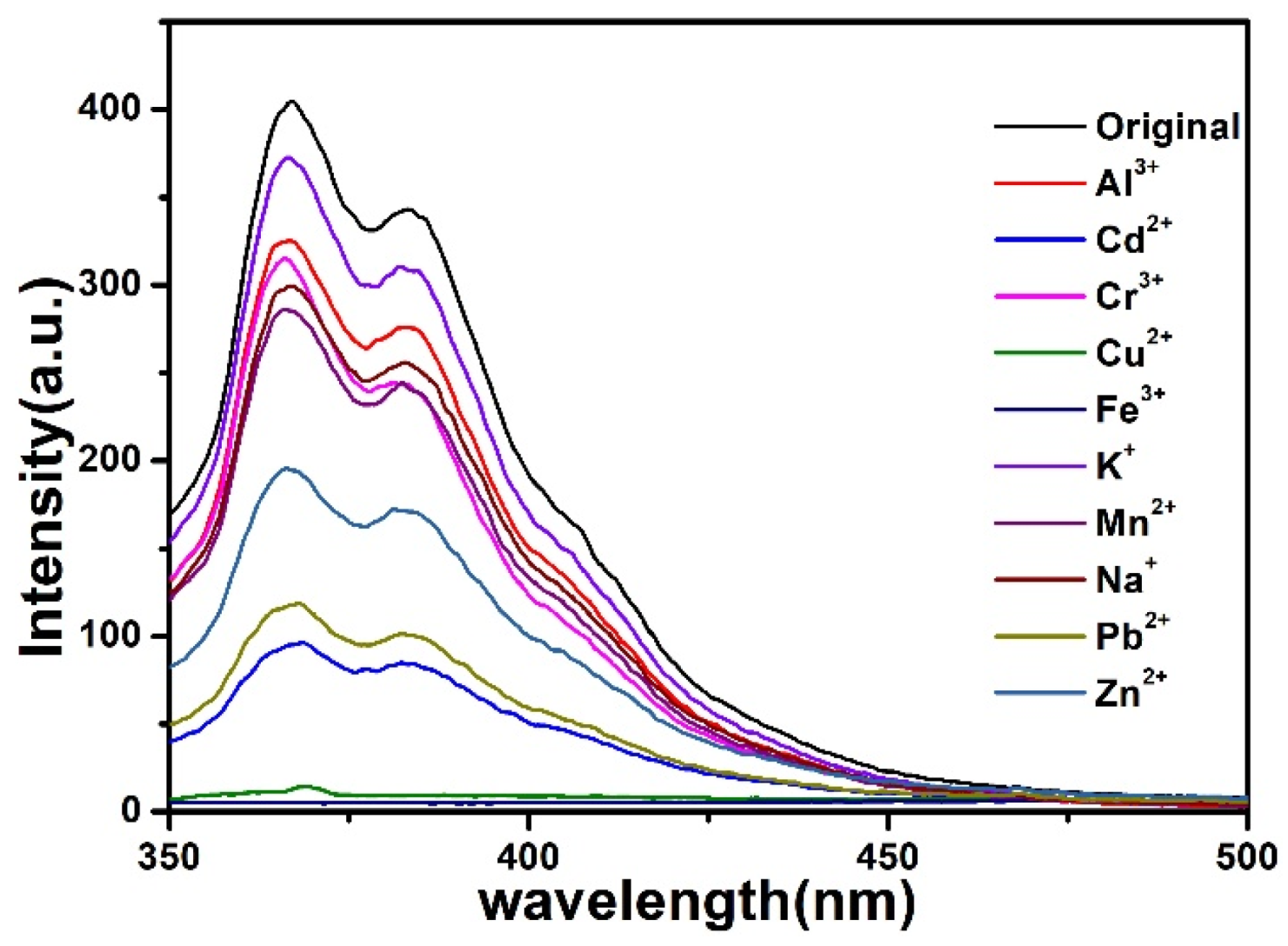
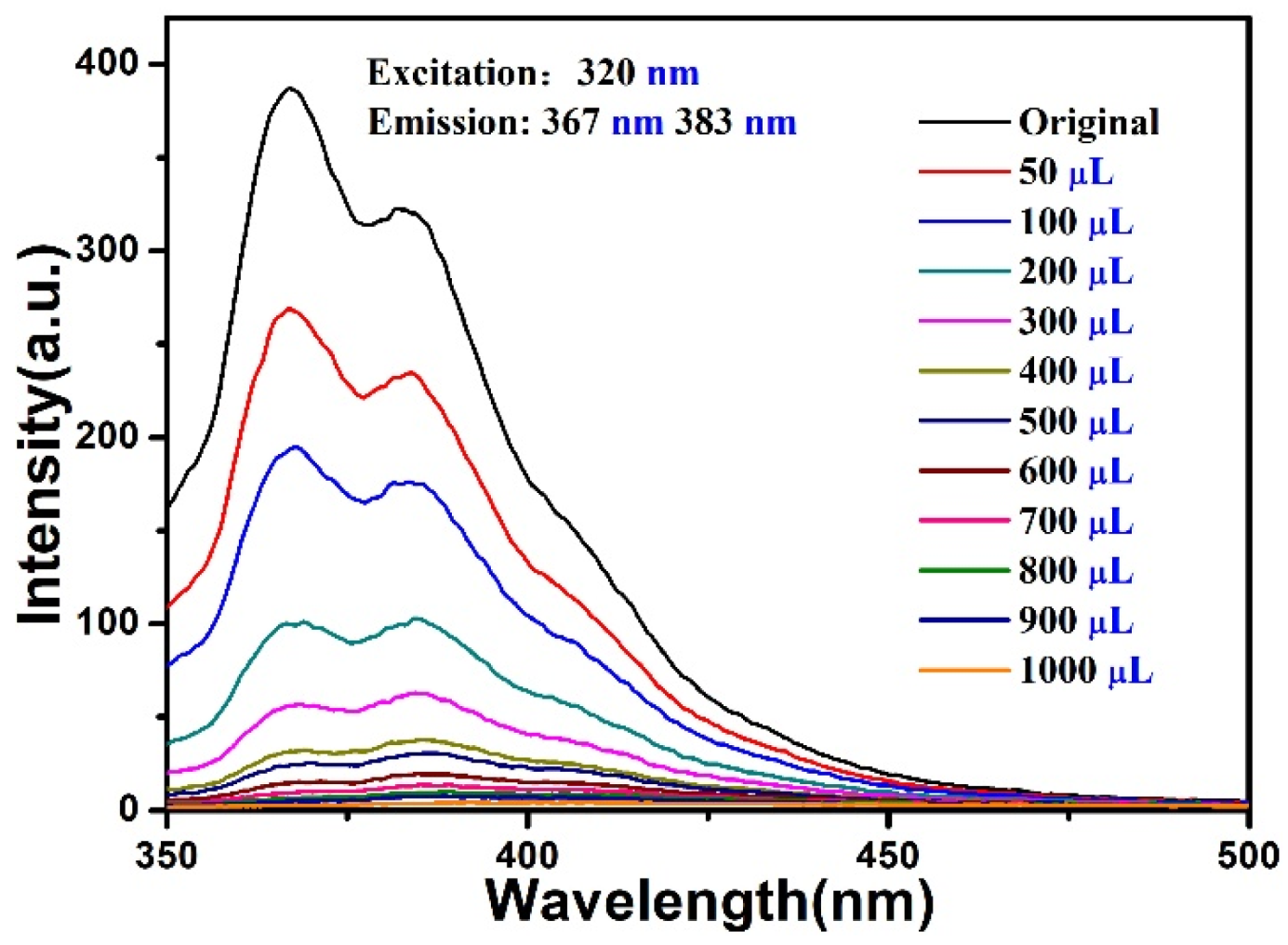
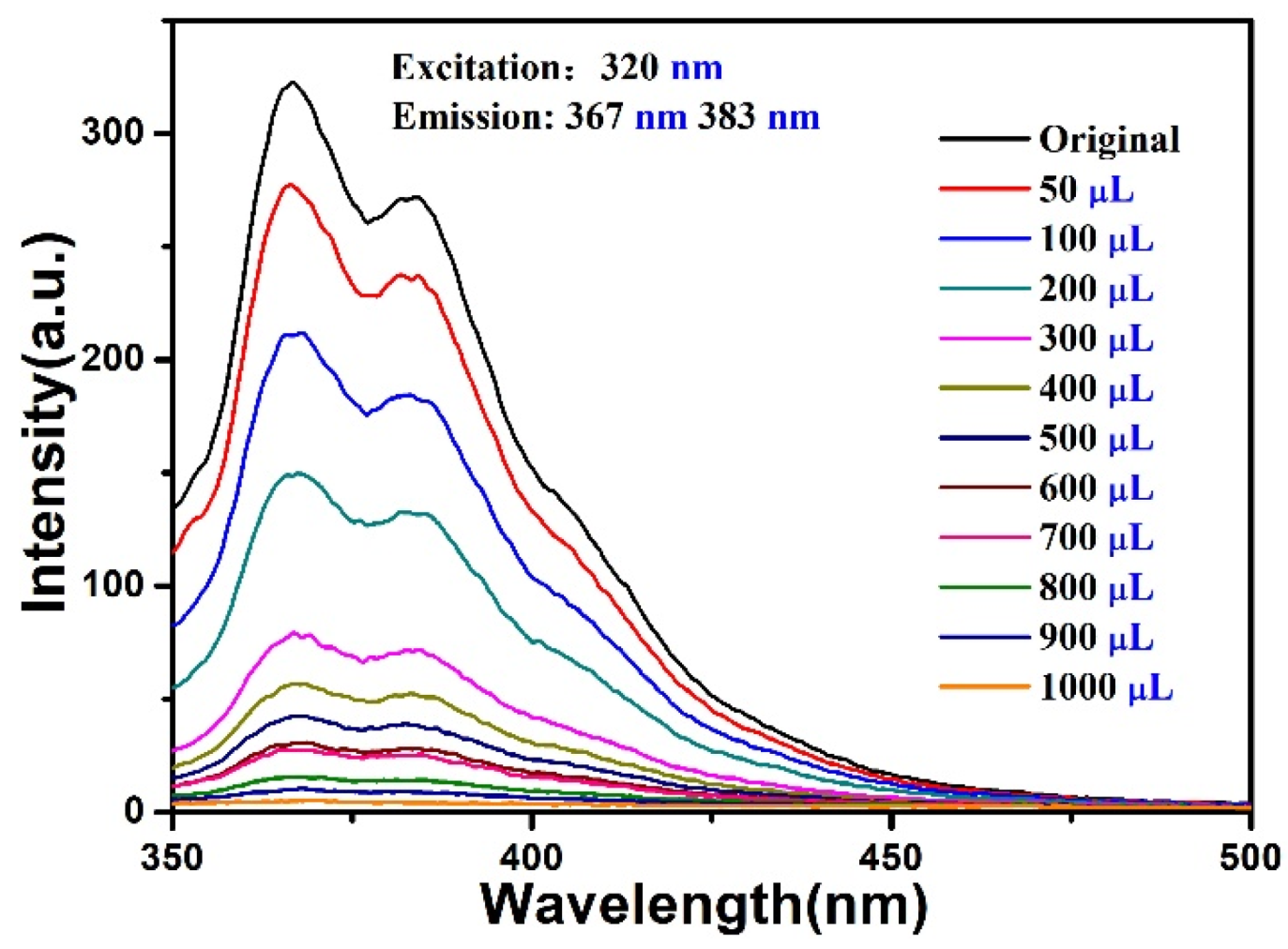
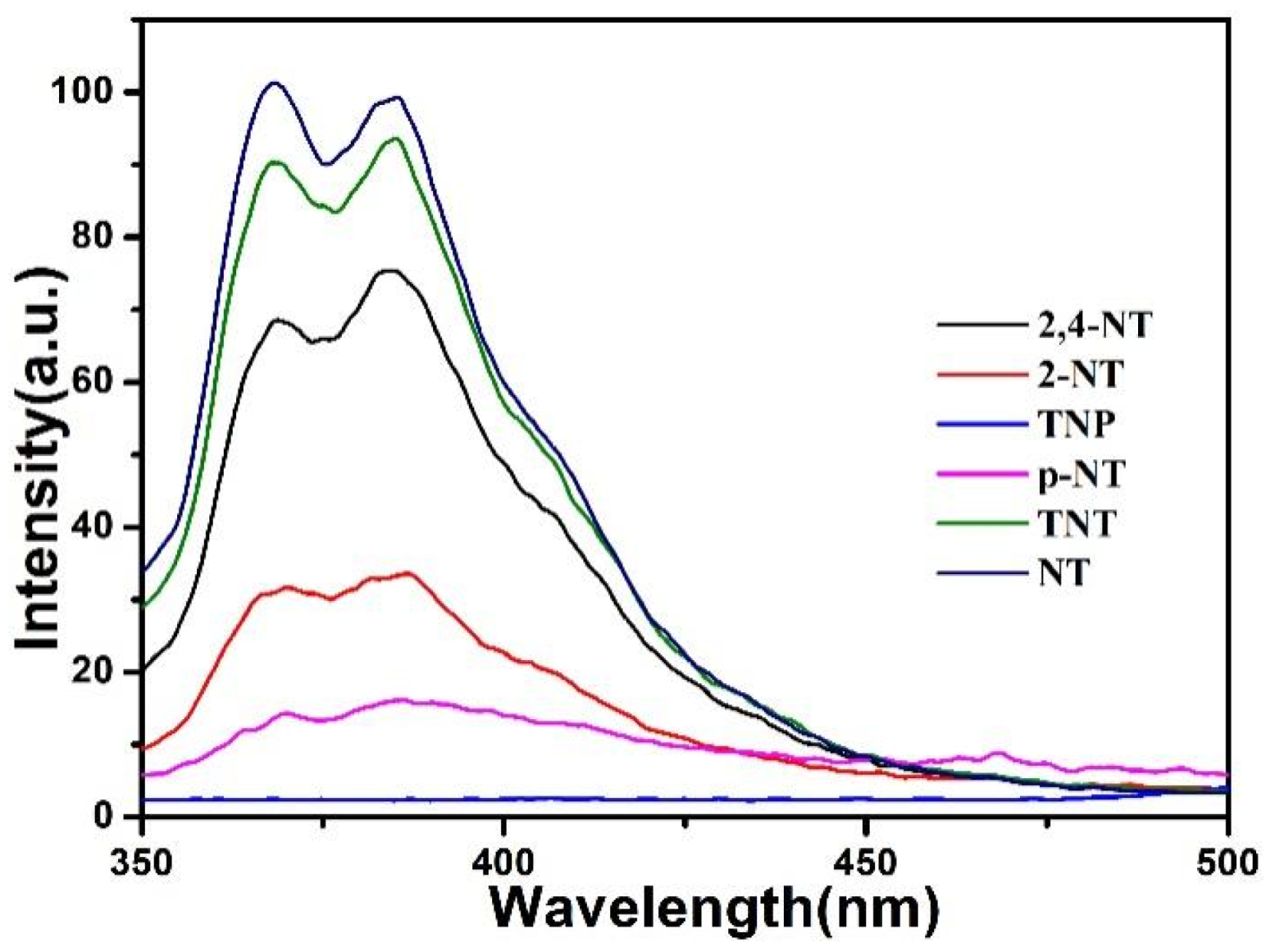
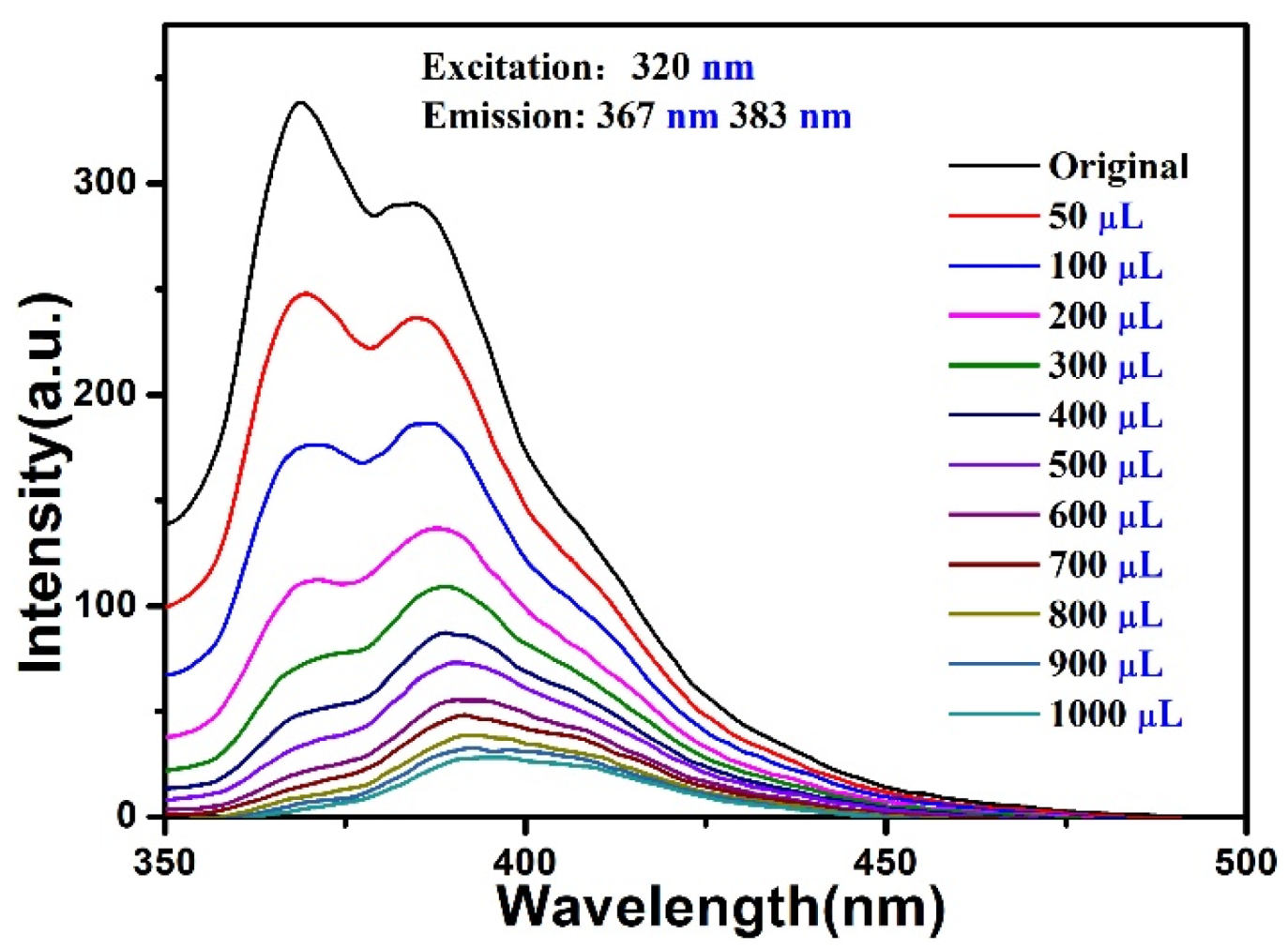
5. Luminescent Emission
6. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Conflicts of Interest
References
- Nagajyoti, P.C.; Lee, K.D.; Sreekanth, T.V.M. Heavy metals, occurrence and toxicity for plants: A review. Environ. Chem. Lett. 2010, 8, 199–216. [Google Scholar] [CrossRef]
- Ahmad Malik, L.; Bashir, A.; Qureashi, A.; Pandith, A.H. Detection and removal of heavy metal ions: A review. Environ. Chem. Lett. 2019, 17, 1495–1521. [Google Scholar] [CrossRef]
- Uddin, M.J.; Ampiaw, R.E.; Lee, W. Adsorptive removal of dyes from wastewater using a metal-organic framework: A review. Chemosphere 2021, 284, 131314. [Google Scholar] [CrossRef]
- Hyman, L.M.; Franz, K.J. Probing oxidative stress: Small molecule fluorescent sensors of metal ions, reactive oxygen species and thiols. Coor. Chem. Rev. 2012, 256, 2333–2356. [Google Scholar]
- Carter, K.P.; Young, A.M.; Palmer, A.E. Fluorescent Sensors for Measuring Metal Ions in Living Systems. Chem. Rev. 2014, 114, 4564–4601. [Google Scholar] [CrossRef] [PubMed]
- Zhong, W.S.; Ren, T.; Zhao, L.J. Determination of Pb (Lead), Cd (Cadmium), Cr (Chromium), Cu (Copper), and Ni (Nickel) in Chinese tea with high resolution continuum source graphite furnace atomic absorption spectrometry. J. Food. Drug. Anal. 2016, 24, 46–55. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wang, Y.; Wang, K.; Liu, W.; Shen, G.; Yu, R. A new fluorescence optical-fiber sensor for Colchicine. Anal. Sci. 1997, 13, 447–451. [Google Scholar] [CrossRef] [Green Version]
- Jiang, X.L.; Hou, S.L.; Jiao, Z.H.; Zhao, B. Luminescent Detection of Colchicine by a Unique Indium-Organic Framework in Water with High Sensitivity. Anal. Chem. 2019, 91, 9754–9759. [Google Scholar] [CrossRef] [PubMed]
- Wollin, M.; Dieter, H.H. Toxicological Guidelines for Monocyclic Nitro-, Amino- and Aminonitroaromatics, Nitramines, and Nitrate Esters in Drinking Water. Arch. Environ. Contam. Toxicol. 2005, 49, 18–26. [Google Scholar] [CrossRef] [PubMed]
- Germain, M.E.; Knapp, M.J. Optical explosives detection: From color changes to fluorescence turn-on. Chem. Soc. Rev. 2009, 38, 2543–2555. [Google Scholar] [CrossRef] [PubMed]
- Najarro, M.; Morris, M.E.D.; Staymates, M.E.; Fletcher, R.; Gillen, G. Optimized thermal desorption for improved sensitivity in trace explosives detection by ion mobility spectrometry. Analyst 2012, 137, 2614–2622. [Google Scholar] [CrossRef] [PubMed]
- Pavlačka, M.; Bajerová, P.; Kortánková, K.; Bláha, J.; Zástěra, M.; Mázl, R.; Ventura, K. Analysis of explosives using differential mobility spectrometry. Int. J. Ion. Mobil. Spectrom. 2016, 19, 31–39. [Google Scholar] [CrossRef]
- Chen, L.; Ma, N.; Park, Y.; Jin, S.; Hwang, H.; Jiang, D.; Jung, Y.M. Highly sensitive determination of iron (III) ion based on phenanthroline probe: Surface-enhanced Raman spectroscopy methods. Spectrochim. Acta Part. A 2018, 197, 43–46. [Google Scholar] [CrossRef] [PubMed]
- Séby, F.; Charles, S.; Gagean, M.; Garraud, H.; Donard, O.F.X. Chromium speciation by hyphenation of high-performance liquid chromatography to inductively coupled plasma-mass spectrometry-study of the influence of interfering ions. J. Anal. Spectrom. 2003, 18, 1386–1390. [Google Scholar] [CrossRef]
- Goswami, S.; Aich, K.; Das, A.K.; Manna, A.; Das, S. A naphthalimide-quinoline based probe for selective, fluorescence ratiometric sensing of trivalent ions. RSC Adv. 2013, 3, 2412–2416. [Google Scholar] [CrossRef]
- Zhang, L.; Kang, Z.; Xina, X.; Sun, D. Metal-organic frameworks based luminescent materials for nitroaromatics sensing. Cryst. Eng. Comm. 2016, 18, 193–206. [Google Scholar] [CrossRef]
- Chen, W.; Fan, R.; Fan, J.; Liu, H.; Sun, T.; Wang, P.; Yang, Y. Lanthanide Coordination Polymer-Based Composite Films for Selective and Highly Sensitive Detection of Cr2O72– in Aqueous Media. Inorg. Chem. 2019, 58, 15118–15125. [Google Scholar] [CrossRef]
- Ma, L.; Abney, C.; Lin, W. Enantioselective catalysis with homochiral metal-organic frameworks. Chem. Soc. Rev. 2009, 38, 1248–1256. [Google Scholar] [CrossRef]
- Xuan, W.; Zhu, C.; Liu, Y.; Cui, Y. Mesoporous metal-organic framework materials. Chem. Soc. Rev. 2012, 41, 1677–1695. [Google Scholar] [CrossRef]
- Wang, H.; Lustig, W.P.; Li, J. Sensing and capture of toxic and hazardous gases and vapors by metal-organic frameworks. Chem. Soc. Rev. 2018, 47, 4729–4756. [Google Scholar] [CrossRef]
- Ding, M.; Flaig, R.W.; Jiang, H.L.; Yaghi, O.M. Carbon capture and conversion using metal-organic frameworks and MOF-based materials. Chem. Soc. Rev. 2019, 48, 2783–2828. [Google Scholar] [CrossRef] [PubMed]
- Wang, P.L.; Xie, L.H.; Joseph, E.A.; Li, J.R.; Su, X.O.; Zhou, H.C. Metal-Organic Framework for Food Safety. Chem. Rev. 2019, 119, 10638–10690. [Google Scholar] [CrossRef] [PubMed]
- Zhao, Y.; Zeng, H.; Zhu, X.; Lu, W.; Li, D. Metal-organic frameworks as photoluminescent biosensing platforms: Mechanisms and applications. Chem. Soc. Rev. 2021, 50, 4484–4513. [Google Scholar] [CrossRef] [PubMed]
- Cheng, W.; Tang, X.; Zhang, Y.; Wu, D.; Yang, W. Applications of metal-organic framework (MOF)-based sensors for food safety: Enhancing mechanisms and recent advances. Trends. Food. Sci. Tech. 2021, 112, 268–282. [Google Scholar] [CrossRef]
- Lan, A.; Li, K.; Wu, H.; Olson, D.; Emge, T.; Ki, W.; Hong, M.; Li, J. A Luminescent Microporous Metal-Organic Framework for the Fast and Reversible Detection of High Explosives. Angew. Chem. Int. Ed. 2009, 48, 2334–2338. [Google Scholar] [CrossRef]
- Zhao, J.; Wang, Y.-N.; Dong, W.-W.; Wu, Y.-P.; Li, D.; Zhang, Q.C. A Robust Luminescent Tb(III)-MOF with Lewis Basic Pyridyl Sites for the Highly Sensitive Detection of Metal Ions and Small Molecules. Inorg. Chem. 2016, 55, 3265–3271. [Google Scholar] [CrossRef]
- Wang, C.; Liu, X.; Demir, N.K.; Chen, J.P.; Li, K. Applications of water stable metal-organic frameworks. Chem. Soc. Rev. 2016, 45, 5107–5134. [Google Scholar] [CrossRef]
- Ma, D.; Li, B.; Zhou, X.; Zhou, Q.; Liu, K.; Zeng, G.; Li, G.; Shi, Z.; Feng, S. A dual functional MOF as a luminescent sensor for quantitatively detecting the concentration of nitrobenzene and temperature. Chem. Comm. 2013, 49, 8964–8966. [Google Scholar] [CrossRef]
- Masoomi, M.Y.; Beheshti, S.; Morsali, A. Mechanosynthesis of new azine-functionalized Zn(II) metal-organic frameworks for improved catalytic performance. J. Mater. Chem. A 2014, 2, 16863–16866. [Google Scholar] [CrossRef]
- Liu, Z.-Q.; Zhao, Y.; Deng, Y.; Zhang, X.-D.; Kang, Y.-S.; Lu, Q.-Y.; Sun, W.-Y. Selectively sensing and adsorption properties of nickel(II) and cadmium(II) architectures with rigid 1H-imidazol-4-yl containing ligands and 1,3,5-tri(4-carboxyphenyl)benzene. Sens. Actuators B 2017, 250, 179–188. [Google Scholar] [CrossRef]
- Zhang, X.; Luo, X.; Zhang, N.; Wu, J.; Huang, Y.Q. A highly selective and sensitive Zn(II) coordination polymer luminescent sensor for Al3+ and NACs in the aqueous phase. Inorg. Chem. Front. 2017, 4, 1888–1894. [Google Scholar] [CrossRef]
- Gao, E.; Liu, D.; Xing, J.; Feng, Y.; Su, J.; Liu, J.; Zhao, H.; Wang, N.; Jia, Z.; Zhang, X.; et al. A Recyclable bi-functional Luminescent Zinc (II) metal-organic framework as highly selective and sensitive sensing probe for nitroaromatic explosives and Fe3+ ions. Appl. Organomet. Chem. 2019, 33, 9. [Google Scholar] [CrossRef]
- Li, X.; Tang, J.; Liu, H.; Gao, K.; Meng, P.X.; Wu, J.; Hou, H. A high Sensitive and Recyclable Ln-MOF Luminescent Sensor for Efficient Detection of Fe3+, CrVI Anions with Carbazolyltetracarboxylate Ligand. Chem. Asian J. 2019, 14, 3721–3727. [Google Scholar] [CrossRef] [PubMed]
- Du, Y.; Yang, H.; Liu, R.; Shao, C.; Yang, L. A multi-responsive chemosensor for highly sensitive and selective detection of Fe3+, Cu2+, Cr2O72− and nitrobenzene based on a luminescent lanthanide metal*organic framework. Dalton Trans. 2020, 49, 13003–13016. [Google Scholar] [CrossRef] [PubMed]
- Joarder, B.; Desai, A.V.; Samanta, P.; Mukherjee, S.; Ghosh, S.K. Selective and Sensitive Aqueous-Phase Detection of 2,4,6-Trinitrophenol (TNP) by an Amine-Functionalized Metal-Organic Framework. Chem. Eur. J. 2015, 21, 965–969. [Google Scholar] [CrossRef] [PubMed]
- Wu, K.; Hu, J.; Shi, S.; Li, J.; Cheng, X. A thermal stable pincer-MOF with high selective and sensitive nitro explosive TNP, metal ion Fe3+ and pH sensing in aqueous solution. Dye. Pigment. 2020, 173, 107993. [Google Scholar] [CrossRef]
- Senthilkumar, S.; Goswami, R.; Smith, V.J.; Bajaj, H.C.; Neogi, S. Pore Wall-Functionalized Luminescent Cd(II) Framework for Selective CO2 Adsorption, Highly Specific 2,4,6-Trinitrophenol Detection, and Colorimetric Sensing of Cu2+ Ions. ACS Sustain. Chem. Eng. 2018, 6, 10295–10306. [Google Scholar] [CrossRef]
- Li, J.X.; Guan, Q.L.; Wang, Y.; You, Z.X.; Xing, Y.H.; Bai, F.Y.; Sun, L.X. A lanthanide-organic crystalline framework material encapsulating 1,3,6,8-tetrakis(p-benzoic acid)pyrene: Selective sensing of Fe3+, Cr2O72− and colchicine and white-light emission. New J. Chem. 2020, 44, 1446–1454. [Google Scholar] [CrossRef]
- Chen, C.; Wang, X.; Li, L.; Huang, Y.; Gao, R. Highly selective sensing of Fe3+ by an anionic metal-organic framework containing uncoordinated nitrogen and carboxylate oxygen sites. Dalton Trans. 2018, 47, 3452–3458. [Google Scholar] [CrossRef]


Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhou, X.; Liu, L.; Niu, Y.; Song, M.; Feng, Y.; Lu, J.; Tai, X. A Water-Stable Zn-MOF Used as Multiresponsive Luminescent Probe for Sensing Fe3+/Cu2+, Trinitrophenol and Colchicine in Aqueous Medium. Materials 2022, 15, 7006. https://doi.org/10.3390/ma15197006
Zhou X, Liu L, Niu Y, Song M, Feng Y, Lu J, Tai X. A Water-Stable Zn-MOF Used as Multiresponsive Luminescent Probe for Sensing Fe3+/Cu2+, Trinitrophenol and Colchicine in Aqueous Medium. Materials. 2022; 15(19):7006. https://doi.org/10.3390/ma15197006
Chicago/Turabian StyleZhou, Xiaojing, Lili Liu, Yue Niu, Mingjun Song, Yimin Feng, Jitao Lu, and Xishi Tai. 2022. "A Water-Stable Zn-MOF Used as Multiresponsive Luminescent Probe for Sensing Fe3+/Cu2+, Trinitrophenol and Colchicine in Aqueous Medium" Materials 15, no. 19: 7006. https://doi.org/10.3390/ma15197006
APA StyleZhou, X., Liu, L., Niu, Y., Song, M., Feng, Y., Lu, J., & Tai, X. (2022). A Water-Stable Zn-MOF Used as Multiresponsive Luminescent Probe for Sensing Fe3+/Cu2+, Trinitrophenol and Colchicine in Aqueous Medium. Materials, 15(19), 7006. https://doi.org/10.3390/ma15197006







