Effect of pH on the In Vitro Degradation of Borosilicate Bioactive Glass and Its Modulation by Direct Current Electric Field
Abstract
1. Introduction
2. Materials and Methods
2.1. Preparation of the Glass
2.2. Immersion Solutions
2.3. Short-Term Degradation
2.4. Long-Term Degradation
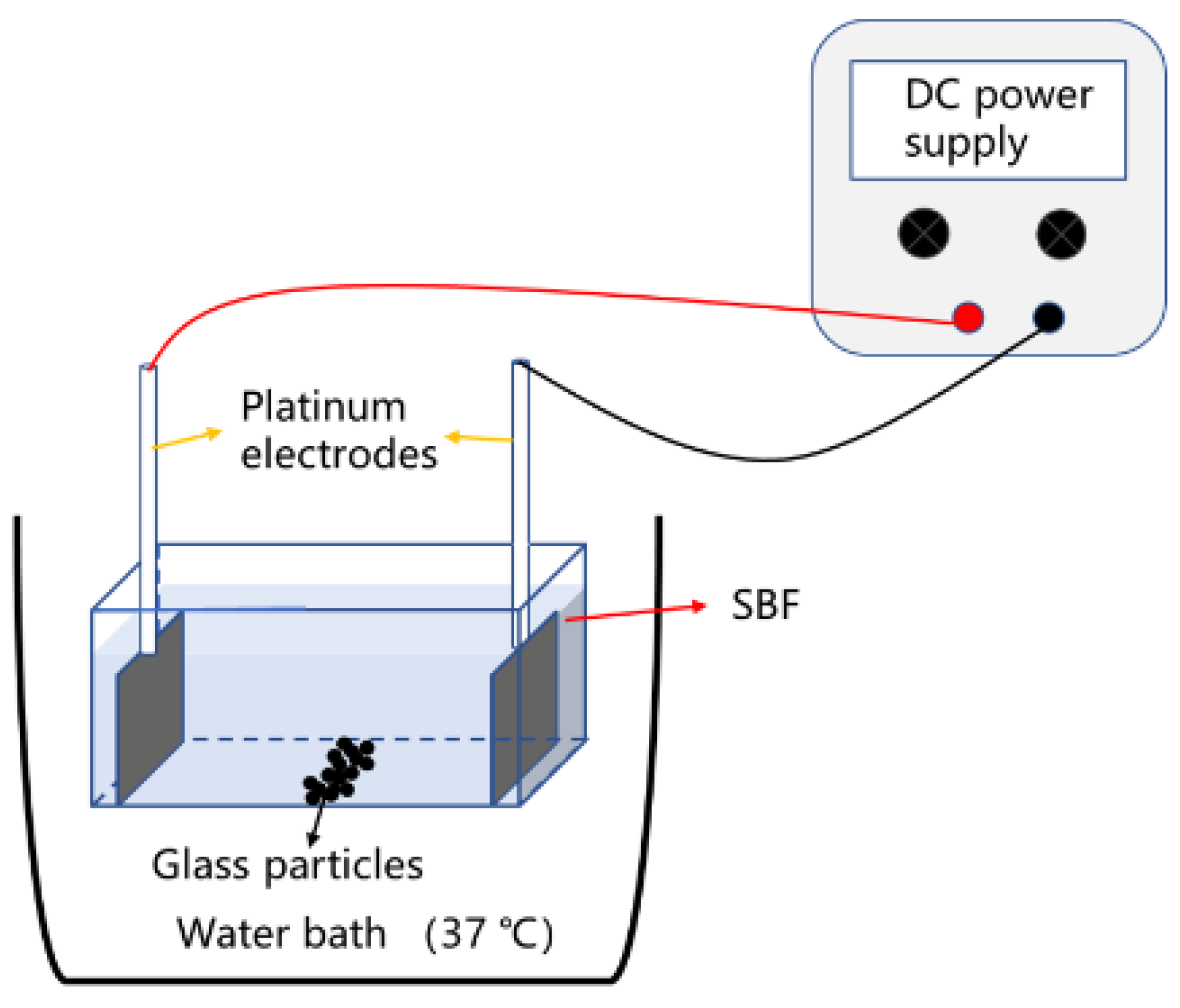
2.5. Degradation of the Glass with a DC Electric Field
3. Results and Discussion
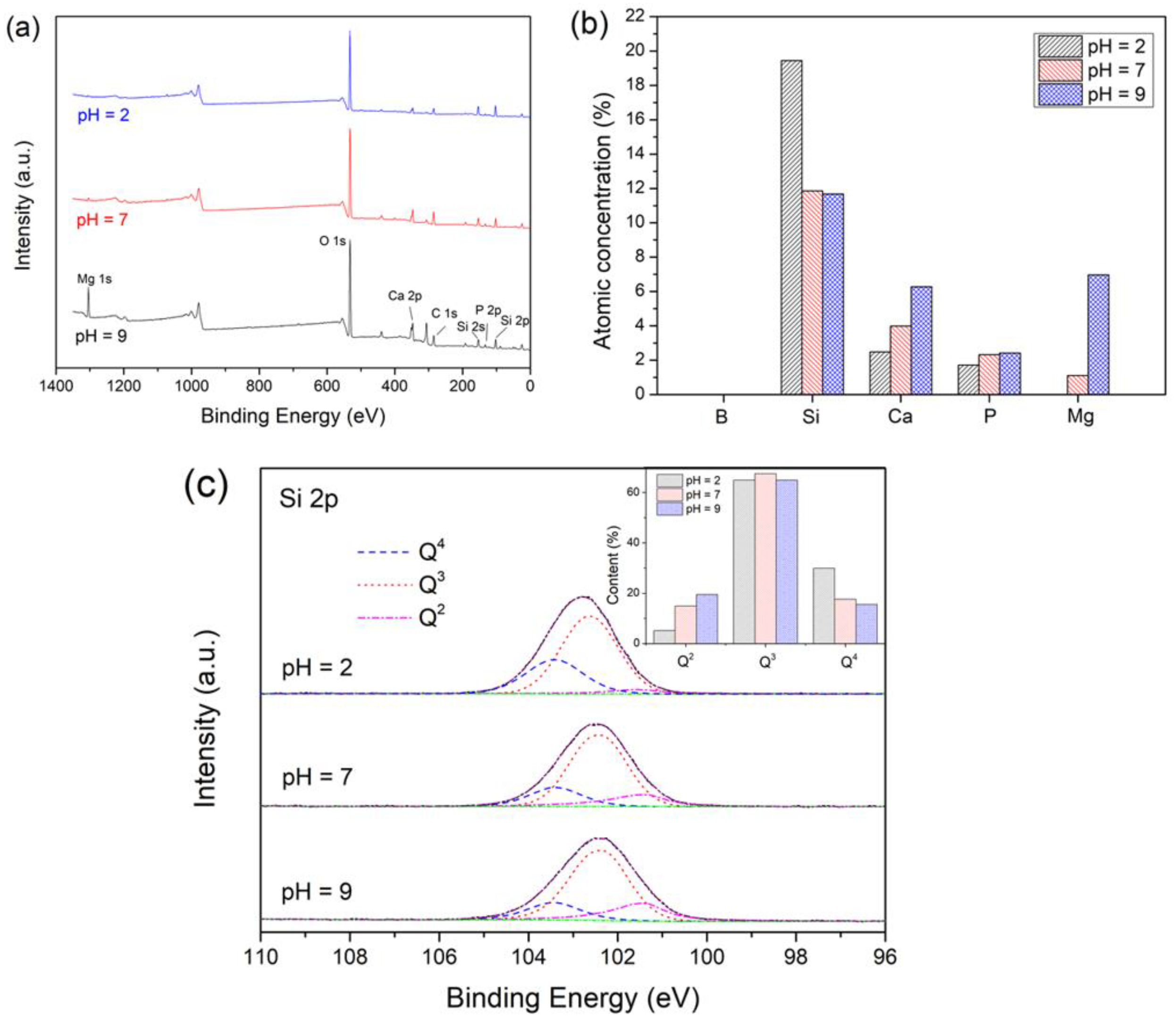
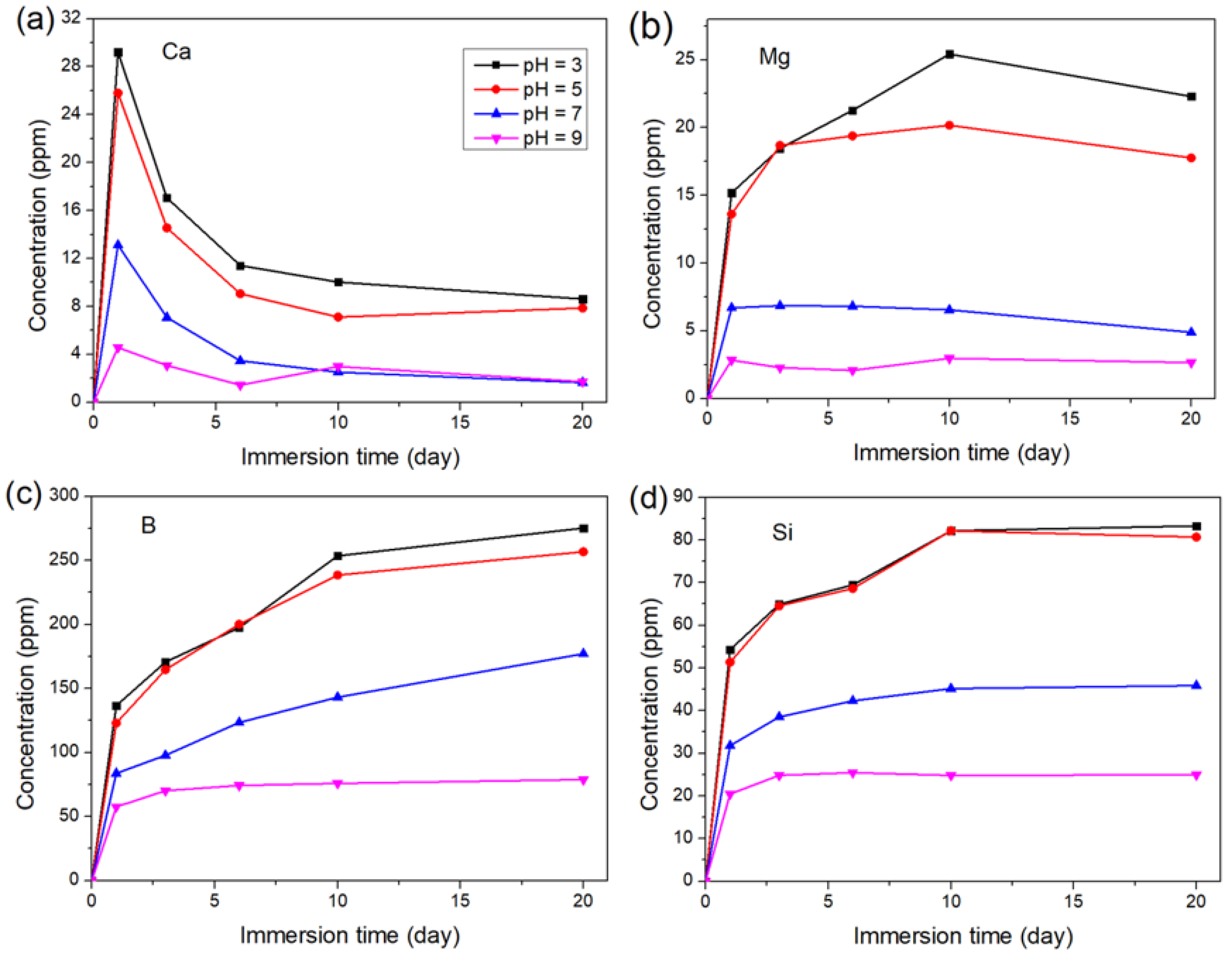
3.1. The Effect of pH on the Degradation of the Glass
3.1.1. Short-Term Degradation
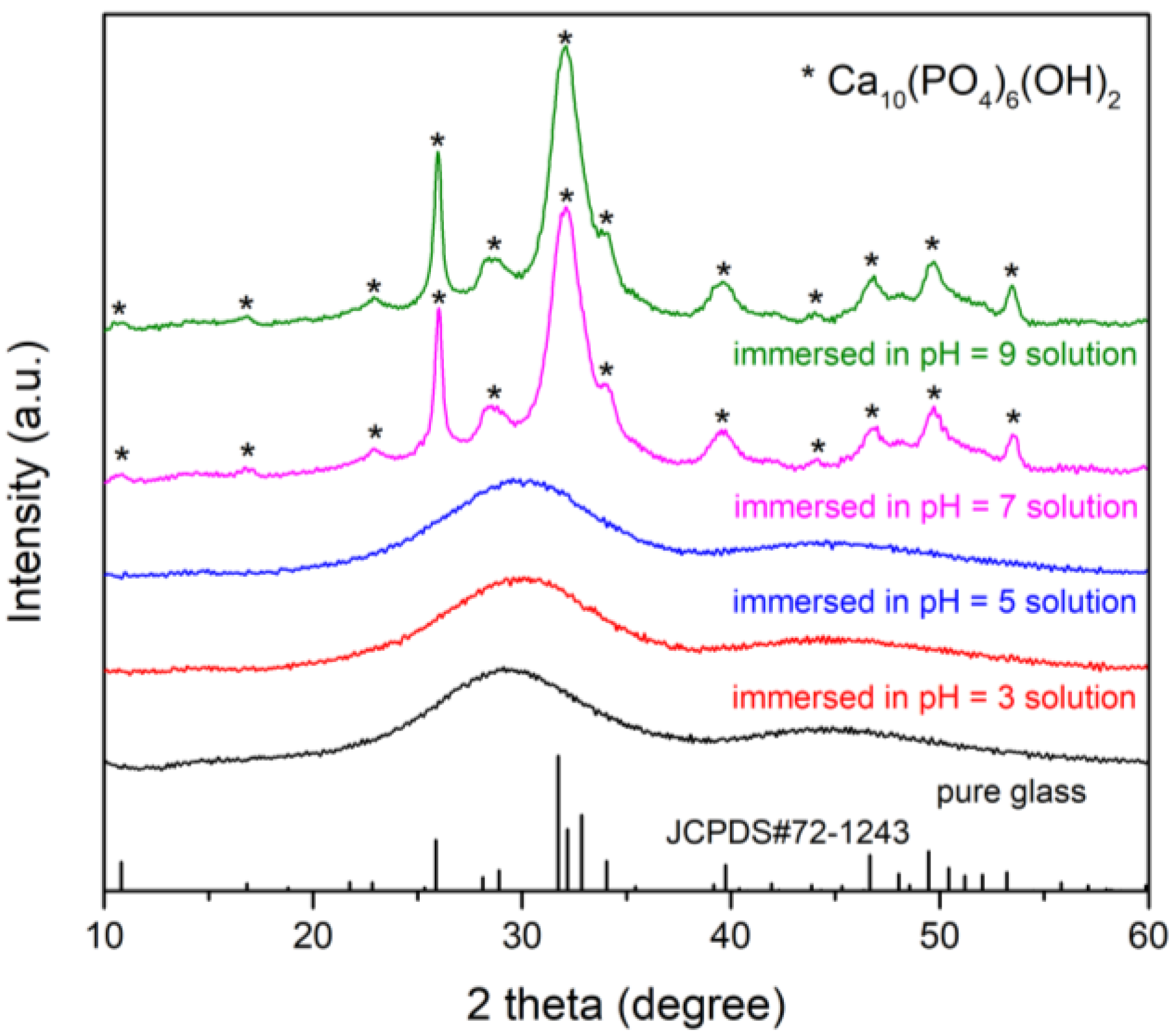
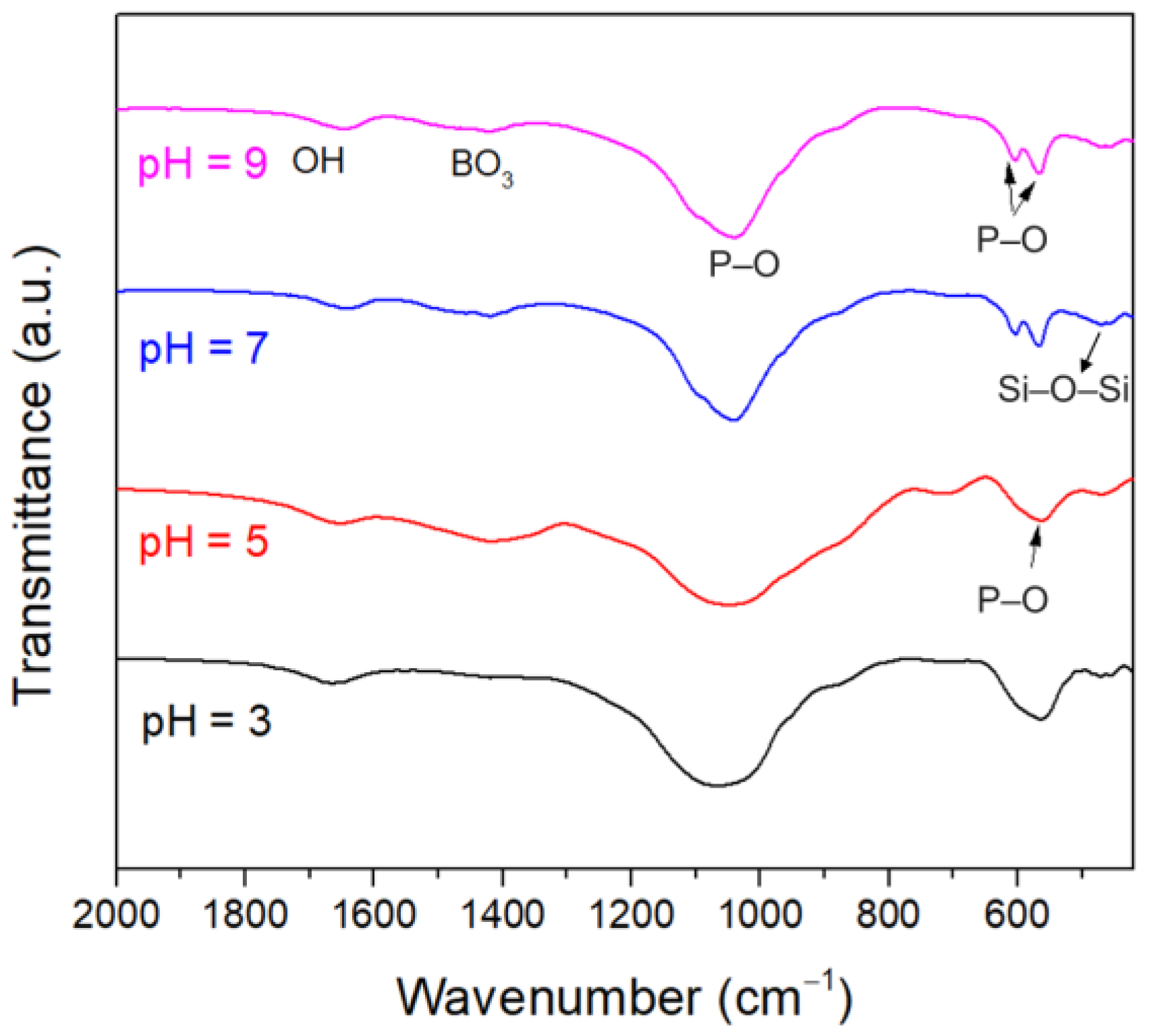
3.1.2. Long-Term Degradation
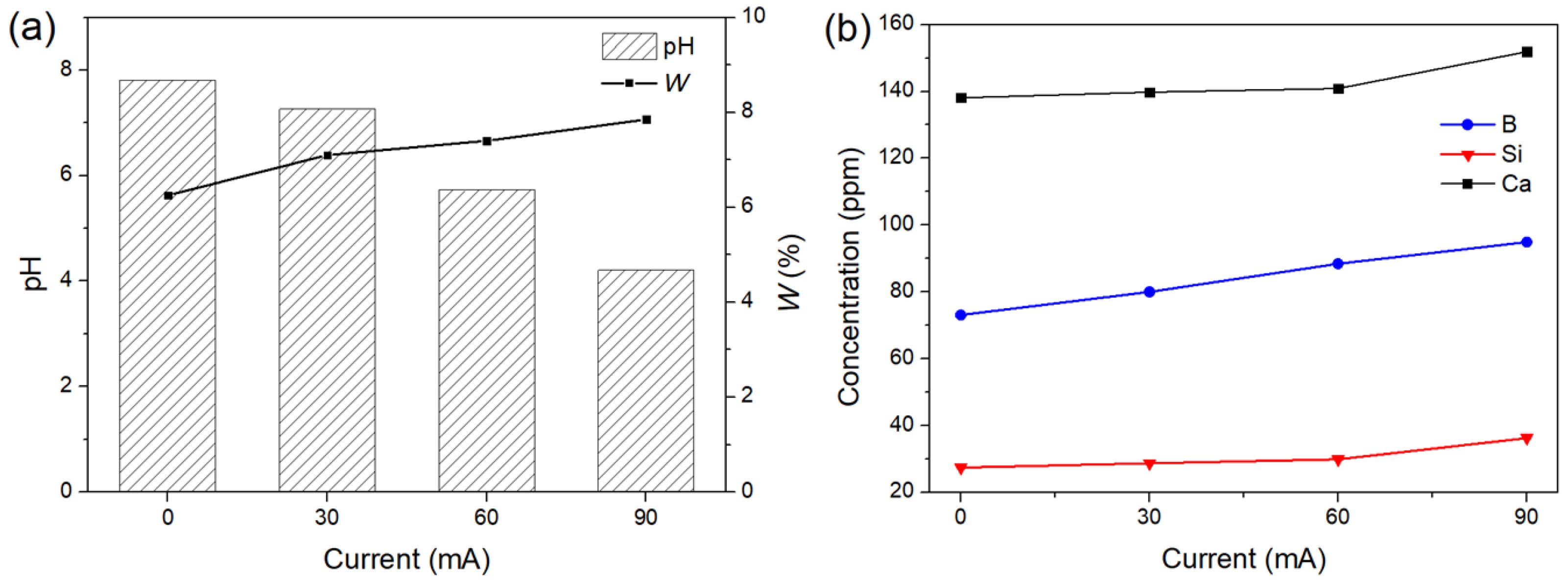
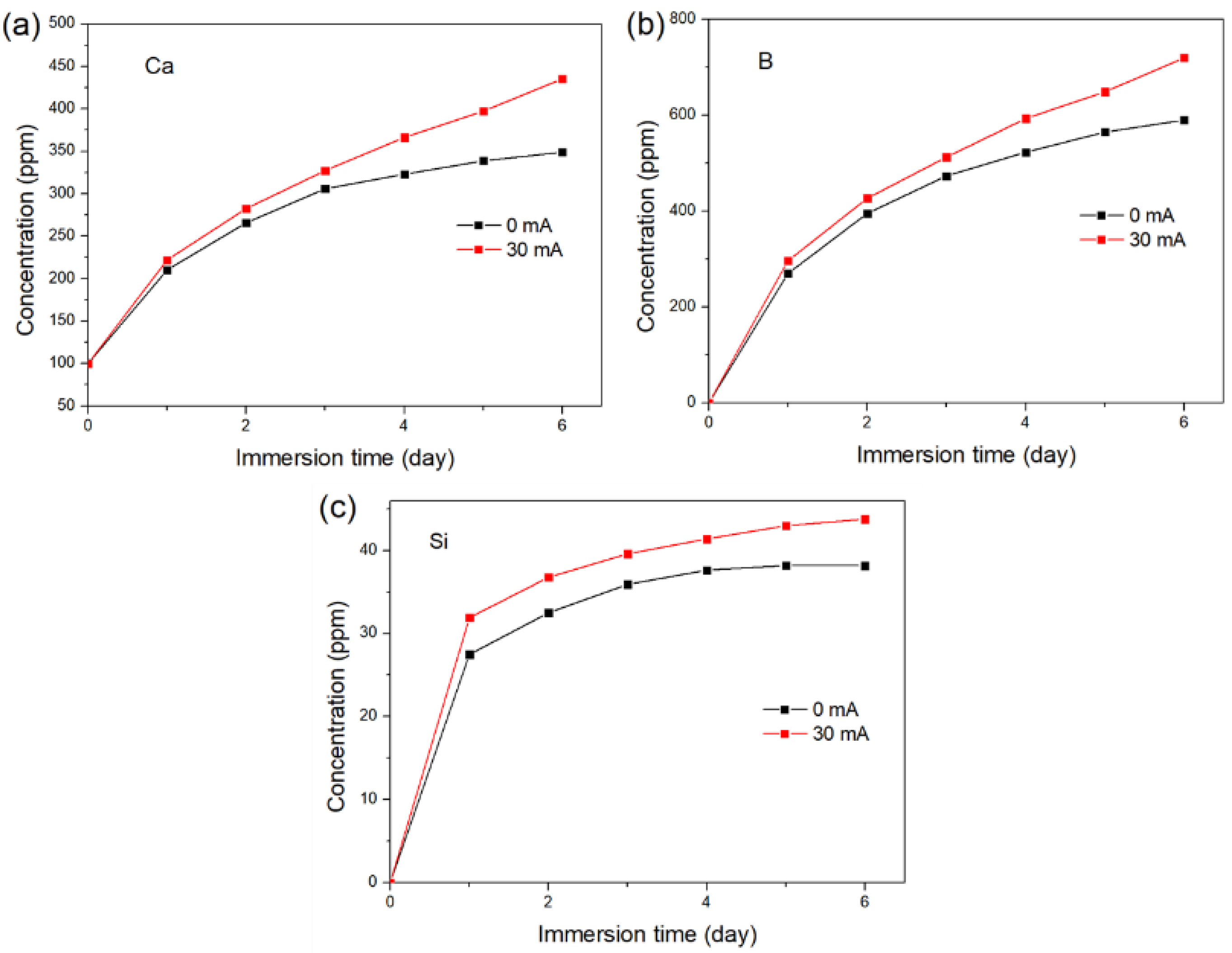
3.2. The Degradation of the Glass with a DC Electric Field
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Lindfors, N.C.; Hyvonen, P.; Nyyssonen, M.; Kirjavainen, M.; Kankare, J.; Gullichsen, E.; Salo, J. Bioactive glass S53P4 as bone graft substitute in treatment of osteomyelitis. Bone 2010, 47, 212–218. [Google Scholar] [CrossRef] [PubMed]
- Jia, W.; Lau, G.Y.; Huang, W.; Zhang, C.; Tomsia, A.P.; Fu, Q. Bioactive Glass for Large Bone Repair. Adv. Healthc. Mater. 2015, 4, 2842–2848. [Google Scholar] [CrossRef] [PubMed]
- Rahaman, M.N.; Xiao, W.; Huang, W. Review-bioactive glass implants for potential application in structural bone repair. Biomed. Glasses 2017, 3, 56–66. [Google Scholar] [CrossRef]
- Hench, L.L. The story of Bioglass. J. Mater. Sci. Mater. Med. 2006, 17, 967–978. [Google Scholar] [CrossRef] [PubMed]
- Xynos, I.D.; Edgar, A.J.; Buttery, L.D.; Hench, L.L.; Polak, J.M. Ionic products of bioactive glass dissolution increase proliferation of human osteoblasts and induce insulin-like growth factor II mRNA expression and protein synthesis. Biochem. Biophys. Res. Commun. 2000, 276, 461–465. [Google Scholar] [CrossRef]
- Xynos, I.D.; Edgar, A.J.; Buttery, L.D.; Hench, L.L.; Polak, J.M. Gene-expression profiling of human osteoblasts following treatment with the ionic products of Bioglass 45S5 dissolution. J. Biomed. Mater. Res. 2001, 55, 151–157. [Google Scholar] [CrossRef]
- Day, R.M.; Boccaccini, A.R.; Shurey, S.; Roether, J.A.; Forbes, A.; Hench, L.L.; Gabe, S.M. Assessment of polyglycolic acid mesh and bioactive glass for soft-tissue engineering scaffolds. Biomaterials 2004, 25, 5857–5866. [Google Scholar] [CrossRef] [PubMed]
- Day, R.M. Bioactive glass stimulates the secretion of angiogenic growth factors and angiogenesis in vitro. Tissue Eng. 2005, 11, 768–777. [Google Scholar] [CrossRef]
- Hench, L.L. Genetic design of bioactive glass. J. Eur. Ceram. Soc. 2009, 29, 1257–1265. [Google Scholar] [CrossRef]
- Filgueiras, M.R.; Latorre, G.; Hench, L.L. Solution Effects on the Surface-Reactions of a Bioactive Glass. J. Biomed. Mater. Res. 1993, 27, 445–453. [Google Scholar] [CrossRef] [PubMed]
- Yao, A.; Wang, D.; Huang, W.; Fu, Q.; Rahaman, M.N.; Day, D.E. In Vitro Bioactive Characteristics of Borate-Based Glasses with Controllable Degradation Behavior. J. Am. Ceram. Soc. 2007, 90, 303–306. [Google Scholar] [CrossRef]
- Schumacher, M.; Habibovic, P.; van Rijt, S. Mesoporous bioactive glass composition effects on degradation and bioactivity. Bioact. Mater. 2021, 6, 1921–1931. [Google Scholar] [CrossRef] [PubMed]
- Schepers, E.J.G.; Ducheyne, P. Bioactive glass particles of narrow size range for the treatment of oral bone defects: A 1-24 month experiment with several materials and particle sizes and size ranges. J. Oral Rehabil. 1997, 24, 171–181. [Google Scholar] [CrossRef]
- Cerruti, M.G.; Greenspan, D.; Powers, K. An analytical model for the dissolution of different particle size samples of Bioglass in TRIS-buffered solution. Biomaterials 2005, 26, 4903–4911. [Google Scholar] [CrossRef]
- Barrioni, B.R.; de Laia, A.G.S.; Valverde, T.M.; Martins, T.M.D.; Caliari, M.V.; de Sa, M.A.; de Goes, A.M.; Pereira, M.D. Evaluation of in vitro and in vivo biocompatibility and structure of cobalt releasing sol-gel bioactive glass. Ceram. Int. 2018, 44, 20337–20347. [Google Scholar] [CrossRef]
- Luz, G.M.; Mano, J.F. Preparation and characterization of bioactive glass nanoparticles prepared by sol-gel for biomedical applications. Nanotechnology 2011, 22, 494014. [Google Scholar] [CrossRef] [PubMed]
- Rezaei, Y.; Moztarzadeh, F.; Shahabi, S.; Tahriri, M. Synthesis, Characterization, and In Vitro Bioactivity of Sol-Gel-Derived SiO2–CaO–P2O5–MgO-SrO Bioactive Glass. Synth. React. Inorg. Met.-Org. Chem. 2013, 44, 692–701. [Google Scholar] [CrossRef]
- Yılmaz, D.; Aktaş, B.; Yalçın, Ş.; Albaşkara, M. Erbium oxide and Cerium oxide-doped borosilicate glasses as radiation shielding material. Radiat. Eff. Defects Solids 2019, 175, 458–471. [Google Scholar] [CrossRef]
- Aktas, B.; Acikgoz, A.; Yilmaz, D.; Yalcin, S.; Dogru, K.; Yorulmaz, N. The role of TeO2 insertion on the radiation shielding, structural and physical properties of borosilicate glasses. J. Nucl. Mater. 2022, 563, 153619. [Google Scholar] [CrossRef]
- Aktas, B.; Yalcin, S.; Dogru, K.; Uzunoglu, Z.; Yilmaz, D. Structural and radiation shielding properties of chromium oxide doped borosilicate glass. Radiat. Phys. Chem. 2019, 156, 144–149. [Google Scholar] [CrossRef]
- Zhang, M.; Yao, A.; Lin, J.; Shi, P.; Sun, G.; Jin, C. Photothermally active borosilicate-based composite bone cement for near-infrared light controlled mineralisation. Mater. Technol. 2022, 37, 1243–1250. [Google Scholar] [CrossRef]
- Höhn, S.; Zheng, K.; Romeis, S.; Brehl, M.; Peukert, W.; Ligny, D.; Virtanen, S.; Boccaccini, A.R. Effects of Medium pH and Preconditioning Treatment on Protein Adsorption on 45S5 Bioactive Glass Surfaces. Adv. Mater. Interfaces 2020, 7, 2000420. [Google Scholar] [CrossRef]
- Li, P.J.; Zhang, F.P. The Electrochemistry of a Glass-Surface and Its Application to Bioactive Glass in Solution. J. Non-Cryst. Solids 1990, 119, 112–118. [Google Scholar] [CrossRef]
- Cerruti, M.; Greenspan, D.; Powers, K. Effect of pH and ionic strength on the reactivity of Bioglass 45S5. Biomaterials 2005, 26, 1665–1674. [Google Scholar] [CrossRef] [PubMed]
- Shah, F.A.; Brauer, D.S.; Wilson, R.M.; Hill, R.G.; Hing, K.A. Influence of cell culture medium composition on in vitro dissolution behavior of a fluoride-containing bioactive glass. J. Biomed. Mater. Res. A 2014, 102, 647–654. [Google Scholar] [CrossRef]
- Huang, W.; Yang, J.; Feng, Q.; Shu, Y.; Liu, C.; Zeng, S.; Guan, H.; Ge, L.; Pathak, J.L.; Zeng, S. Mesoporous Bioactive Glass Nanoparticles Promote Odontogenesis and Neutralize Pathophysiological Acidic pH. Front. Mater. 2020, 7, 241. [Google Scholar] [CrossRef]
- Bingel, L.; Groh, D.; Karpukhina, N.; Brauer, D.S. Influence of dissolution medium pH on ion release and apatite formation of Bioglass® 45S5. Mater. Lett. 2015, 143, 279–282. [Google Scholar] [CrossRef]
- Arango-Ospina, M.; Hupa, L.; Boccaccini, A.R. Bioactivity and dissolution behavior of boron-containing bioactive glasses under static and dynamic conditions in different media. Biomed. Glasses 2019, 5, 124–139. [Google Scholar] [CrossRef]
- Tiktinsky, R.; Chen, L.; Narayan, P. Electrotherapy: Yesterday, today and tomorrow. Haemophilia 2010, 16 (Suppl. 5), 126–131. [Google Scholar] [CrossRef] [PubMed]
- Bozic, K.J.; Glazer, P.A.; Zurakowski, D.; Simon, B.J.; Lipson, S.J.; Hayes, W.C. In vivo evaluation of coralline hydroxyapatite and direct current electrical stimulation in lumbar spinal fusion. Spine 1999, 24, 2127–2133. [Google Scholar] [CrossRef] [PubMed]
- Brighton, C.T. Treatment of nonunion of the tibia with constant direct current (1980 Fitts Lecture, A.A.S.T.). J. Trauma 1981, 21, 189–195. [Google Scholar] [CrossRef] [PubMed]
- Karamitros, A.E.; Kalentzos, V.N.; Soucacos, P.N. Electric stimulation and hyperbaric oxygen therapy in the treatment of nonunions. Injury 2006, 37 (Suppl. 1), S63–S73. [Google Scholar] [CrossRef] [PubMed]
- Black, J.; Baranowski, T.J.; Brighton, C.T. Electrochemical Aspects of Dc Stimulation of Osteogenesis. Bioelectrochem. Bioenerg. 1984, 12, 323–327. [Google Scholar] [CrossRef]
- Zhu, Z.; Zhang, M.; Zhang, X.; Yao, A.; Lin, J.; Wang, D. In Vitro Mineralization Property of Borosilicate Bioactive Glass under DC Electric Field. J. Inorg. Mater. 2021, 36, 1006–1012. [Google Scholar]
- Fu, H.; Fu, Q.; Zhou, N.; Huang, W.; Rahaman, M.N.; Wang, D.; Liu, X. In vitro evaluation of borate-based bioactive glass scaffolds prepared by a polymer foam replication method. Mater. Sci. Eng. C 2009, 29, 2275–2281. [Google Scholar] [CrossRef]
- Deng, Z.; Lin, B.; Jiang, Z.; Huang, W.; Li, J.; Zeng, X.; Wang, H.; Wang, D.; Zhang, Y. Hypoxia-Mimicking Cobalt-Doped Borosilicate Bioactive Glass Scaffolds with Enhanced Angiogenic and Osteogenic Capacity for Bone Regeneration. Int. J. Biol. Sci. 2019, 15, 1113–1124. [Google Scholar] [CrossRef] [PubMed]
- Kokubo, T.; Takadama, H. How useful is SBF in predicting in vivo bone bioactivity? Biomaterials 2006, 27, 2907–2915. [Google Scholar] [CrossRef]
- Barrere, F.; van Blitterswijk, C.A.; de Groot, K.; Layrolle, P. Influence of ionic strength and carbonate on the Ca-P coating formation from SBF×5 solution. Biomaterials 2002, 23, 1921–1930. [Google Scholar] [CrossRef]
- Bellucci, D.; Bolelli, G.; Cannillo, V.; Cattini, A.; Sola, A. In situ Raman spectroscopy investigation of bioactive glass reactivity: Simulated body fluid solution vs. TRIS-buffered solution. Mater. Charact. 2011, 62, 1021–1028. [Google Scholar] [CrossRef]
- Serra, J.; Gonzalez, P.; Liste, S.; Chiussi, S.; Leon, B.; Perez-Amor, M.; Ylanen, H.O.; Hupa, M. Influence of the non-bridging oxygen groups on the bioactivity of silicate glasses. J. Mater. Sci. Mater. Med. 2002, 13, 1221–1225. [Google Scholar] [CrossRef]
- Scholze, H. Glass: Nature, Structure, and Properties, 1st ed.; Springer: New York, NY, USA, 1991; pp. 342–346. [Google Scholar]
- Bunker, B.C. Molecular Mechanisms for Corrosion of Silica and Silicate Glasses. J. Non-Cryst. Solids 1994, 179, 300–308. [Google Scholar] [CrossRef]
- Bunker, B.C.; Arnold, G.W.; Day, D.E.; Bray, P.J. The effect of molecular structure on borosilicate glass leaching. J. Non-Cryst. Solids 1986, 87, 226–253. [Google Scholar] [CrossRef]
- Stone-Weiss, N.; Smith, N.J.; Youngman, R.E.; Pierce, E.M.; Goel, A. Dissolution kinetics of a sodium borosilicate glass in Tris buffer solutions: Impact of Tris concentration and acid (HCl/HNO3) identity. Phys. Chem. Chem. Phys. 2021, 23, 16165–16179. [Google Scholar] [CrossRef] [PubMed]
- Hamilton, J.P.; Brantley, S.L.; Pantano, C.G.; Criscenti, L.J.; Kubicki, J.D. Dissolution of nepheline, jadeite and albite glasses: Toward better models for aluminosilicate dissolution. Geochim. Cosmochim. Acta 2001, 65, 3683–3702. [Google Scholar] [CrossRef]
- Yao, A.H.; Lin, J.; Duan, X.; Huang, W.H.; Rahaman, M.N. Formation mechanism of multilayered structure on surface of bioactive borosilicate glass. Chinese J. Inorg. Chem. 2008, 24, 1132–1136. [Google Scholar]
- Sawyer, R.; Nesbitt, H.W.; Secco, R.A. High resolution X-ray Photoelectron Spectroscopy (XPS) study of K2O–SiO2 glasses: Evidence for three types of O and at least two types of Si. J. Non-Cryst. Solids 2012, 358, 290–302. [Google Scholar] [CrossRef]
- Dziadek, M.; Zagrajczuk, B.; Jelen, P.; Olejniczak, Z.; Cholewa-Kowalska, K. Structural variations of bioactive glasses obtained by different synthesis routes. Ceram. Int. 2016, 42, 14700–14709. [Google Scholar] [CrossRef]
- Okada, K.; Kameshima, Y.; Yasumori, A. Chemical Shifts of Silicon X-ray Photoelectron Spectra by Polymerization Structures of Silicates. J. Am. Ceram. Soc. 2005, 81, 1970–1972. [Google Scholar] [CrossRef]
- Stone-Weiss, N.; Bradtmuller, H.; Eckert, H.; Goel, A. Composition-Structure-Solubility Relationships in Borosilicate Glasses: Toward a Rational Design of Bioactive Glasses with Controlled Dissolution Behavior. ACS Appl. Mater. Interfaces 2021, 13, 31495–31513. [Google Scholar] [CrossRef]
- Wan, J.; Cheng, J.; Lu, P. The coordination state of B and Al of borosilicate glass by IR spectra. J. Wuhan Univ. Technol. Mater. Sci. Ed. 2008, 23, 419–421. [Google Scholar] [CrossRef]
- Aktas, B.; Yalcin, S.; Albaskara, M.; Aytar, E.; Ceyhan, G.; Turhan, Z.Ş. Effect of Er2O3 on structural, mechanical, and optical properties of Al2O3-Na2O-B2O3-SiO2 glass. J. Non-Cryst. Solids 2022, 584, 121516. [Google Scholar] [CrossRef]
- Pan, H.B.; Zhao, X.L.; Zhang, X.; Zhang, K.B.; Li, L.C.; Li, Z.Y.; Lam, W.M.; Lu, W.W.; Wang, D.P.; Huang, W.H.; et al. Strontium borate glass: Potential biomaterial for bone regeneration. J. R. Soc. Interface 2010, 7, 1025–1031. [Google Scholar] [CrossRef] [PubMed]
- Ferraris, S.; Nommeots-Nomm, A.; Spriano, S.; Vernè, E.; Massera, J. Surface reactivity and silanization ability of borosilicate and Mg-Sr-based bioactive glasses. Appl. Surf. Sci. 2019, 475, 43–55. [Google Scholar] [CrossRef]
- Aguiar, H.; Serra, J.; González, P.; León, B. Structural study of sol–gel silicate glasses by IR and Raman spectroscopies. J. Non-Cryst. Solids 2009, 355, 475–480. [Google Scholar] [CrossRef]
- Mariappan, C.R.; Ranga, N. Influence of silver on the structure, dielectric and antibacterial effect of silver doped bioglass-ceramic nanoparticles. Ceram. Int. 2017, 43, 2196–2201. [Google Scholar] [CrossRef]
- Kim, C.Y.; Clark, A.E.; Hench, L.L. Early stages of calcium-phosphate layer formation in bioglasses. J. Non-Cryst. Solids 1989, 113, 195–202. [Google Scholar] [CrossRef]
- Liu, X.; Rahaman, M.N.; Day, D.E. Conversion of melt-derived microfibrous borate (13-93B3) and silicate (45S5) bioactive glass in a simulated body fluid. J. Mater. Sci. Mater. Med. 2013, 24, 583–595. [Google Scholar] [CrossRef]
- Vallet-Regí, M.; Rámila, A. New Bioactive Glass and Changes in Porosity during the Growth of a Carbonate Hydroxyapatite Layer on Glass Surfaces. Chem. Mater. 2000, 12, 961–965. [Google Scholar] [CrossRef]
- Andersson, O.H.; Kangasniemi, I. Calcium phosphate formation at the surface of bioactive glass in vitro. J. Biomed. Mater. Res. 1991, 25, 1019–1030. [Google Scholar] [CrossRef]
- Liu, X.; Rahaman, M.N.; Day, D.E.; Bose, S. In Vitro Degradation and Conversion of Melt-Derived Microfibrous Borate (13-93B3) Bioactive Glass Doped with Metal Ions. J. Am. Ceram. Soc. 2014, 97, 3501–3509. [Google Scholar] [CrossRef]
- Fu, Q.; Rahaman, M.N.; Day, D.E. Accelerated Conversion of Silicate Bioactive Glass (13-93) to Hydroxyapatite in Aqueous Phosphate Solution Containing Polyanions. J. Am. Ceram. Soc. 2009, 92, 2870–2876. [Google Scholar] [CrossRef]
- Ciraldo, F.E.; Boccardi, E.; Melli, V.; Westhauser, F.; Boccaccini, A.R. Tackling bioactive glass excessive in vitro bioreactivity: Preconditioning approaches for cell culture tests. Acta Biomater. 2018, 75, 3–10. [Google Scholar] [CrossRef] [PubMed]
- Miklavčič, D.; Serša, G.; Kryžanowski, M.; Novakovič, S.; Bobanović, F.; Golouh, R.; Vodovnik, L. Tumor treatment by direct electric current-tumor temperature and pH, electrode material and configuration. Bioelectrochem. Bioenerg. 1993, 30, 209–220. [Google Scholar] [CrossRef]
- Li, K.-H.; Xin, Y.-L.; Gu, Y.-N.; Xu, B.-L.; Fan, D.-J.; Ni, B.-F. Effects of direct current on dog liver: Possible mechanisms for tumor electrochemical treatment. Bioelectromagnetics 1997, 18, 2–7. [Google Scholar] [CrossRef]
- Griffin, M.; Bayat, A. Electrical stimulation in bone healing: Critical analysis by evaluating levels of evidence. Eplasty 2011, 11, e34. [Google Scholar]

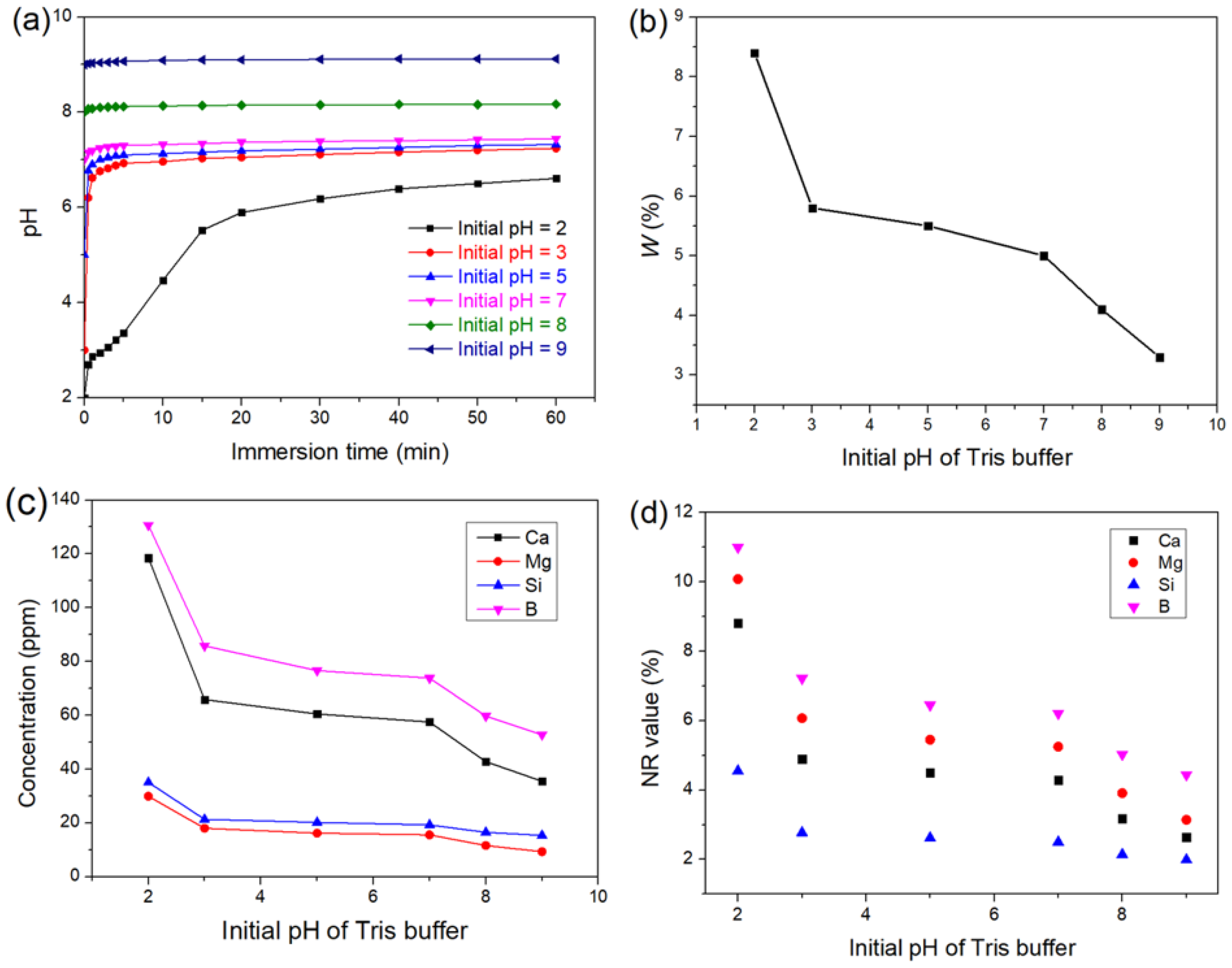




| Media | Initial pH | Ion Release (ppm) | Product | |||
|---|---|---|---|---|---|---|
| B | Si | Ca | Mg | |||
| Tris | 2 | 130.7 | 35.1 | 118.5 | 29.9 | - |
| Tris | 3 | 85.8 | 21.4 | 65.8 | 18.0 | - |
| Tris | 5 | 76.6 | 20.2 | 60.5 | 16.2 | - |
| Tris | 7 | 73.8 | 19.2 | 57.5 | 15.6 | - |
| Tris | 8 | 59.7 | 16.5 | 42.8 | 11.6 | - |
| Tris | 9 | 52.8 | 15.4 | 35.5 | 9.3 | - |
| K2HPO4 | 3 | 136.6 | 54.3 | 29.2 | 15.2 | ACP |
| K2HPO4 | 5 | 123.0 | 51.3 | 25.8 | 13.6 | ACP |
| K2HPO4 | 7 | 83.8 | 31.8 | 13.1 | 6.7 | HA |
| K2HPO4 | 9 | 57.6 | 20.5 | 4.6 | 2.8 | HA |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhang, X.; Zhang, M.; Lin, J. Effect of pH on the In Vitro Degradation of Borosilicate Bioactive Glass and Its Modulation by Direct Current Electric Field. Materials 2022, 15, 7015. https://doi.org/10.3390/ma15197015
Zhang X, Zhang M, Lin J. Effect of pH on the In Vitro Degradation of Borosilicate Bioactive Glass and Its Modulation by Direct Current Electric Field. Materials. 2022; 15(19):7015. https://doi.org/10.3390/ma15197015
Chicago/Turabian StyleZhang, Xuanyu, Minhui Zhang, and Jian Lin. 2022. "Effect of pH on the In Vitro Degradation of Borosilicate Bioactive Glass and Its Modulation by Direct Current Electric Field" Materials 15, no. 19: 7015. https://doi.org/10.3390/ma15197015
APA StyleZhang, X., Zhang, M., & Lin, J. (2022). Effect of pH on the In Vitro Degradation of Borosilicate Bioactive Glass and Its Modulation by Direct Current Electric Field. Materials, 15(19), 7015. https://doi.org/10.3390/ma15197015






