Electrical Impedance of Surface Modified Porous Titanium Implants with Femtosecond Laser
Abstract
:1. Introduction
2. Materials and Methods
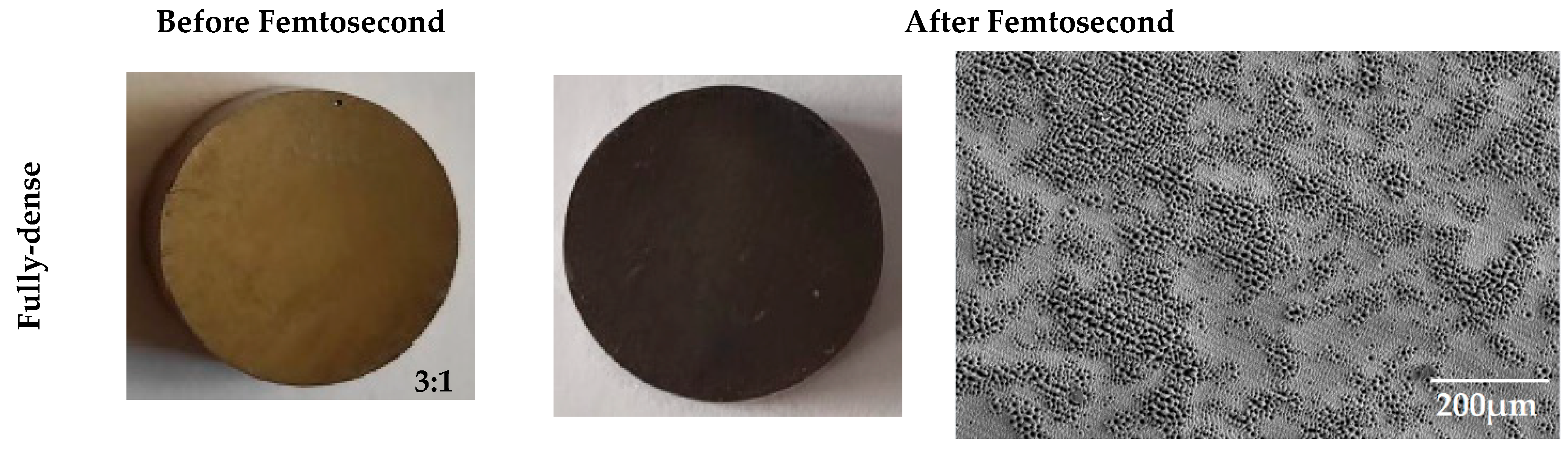
2.1. Manufacturing of Surface Modified Porous Titanium Discs Using Femtosecond Laser Surface Treatment
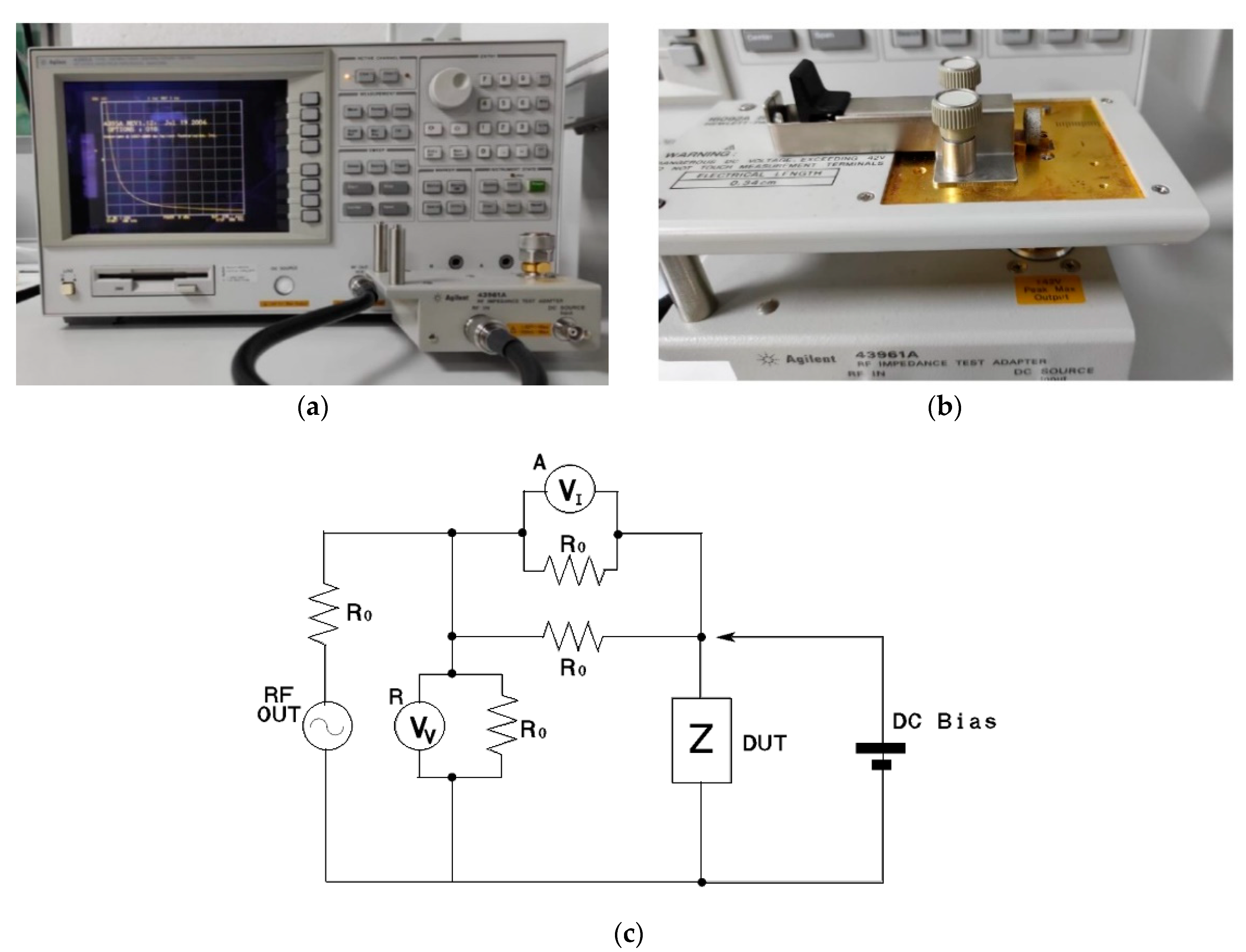
2.2. Electrical Impedance Characterization
3. Results and Discussion
3.1. Electrical Characterization of Porous Discs and Femtosecond Laser Treatment
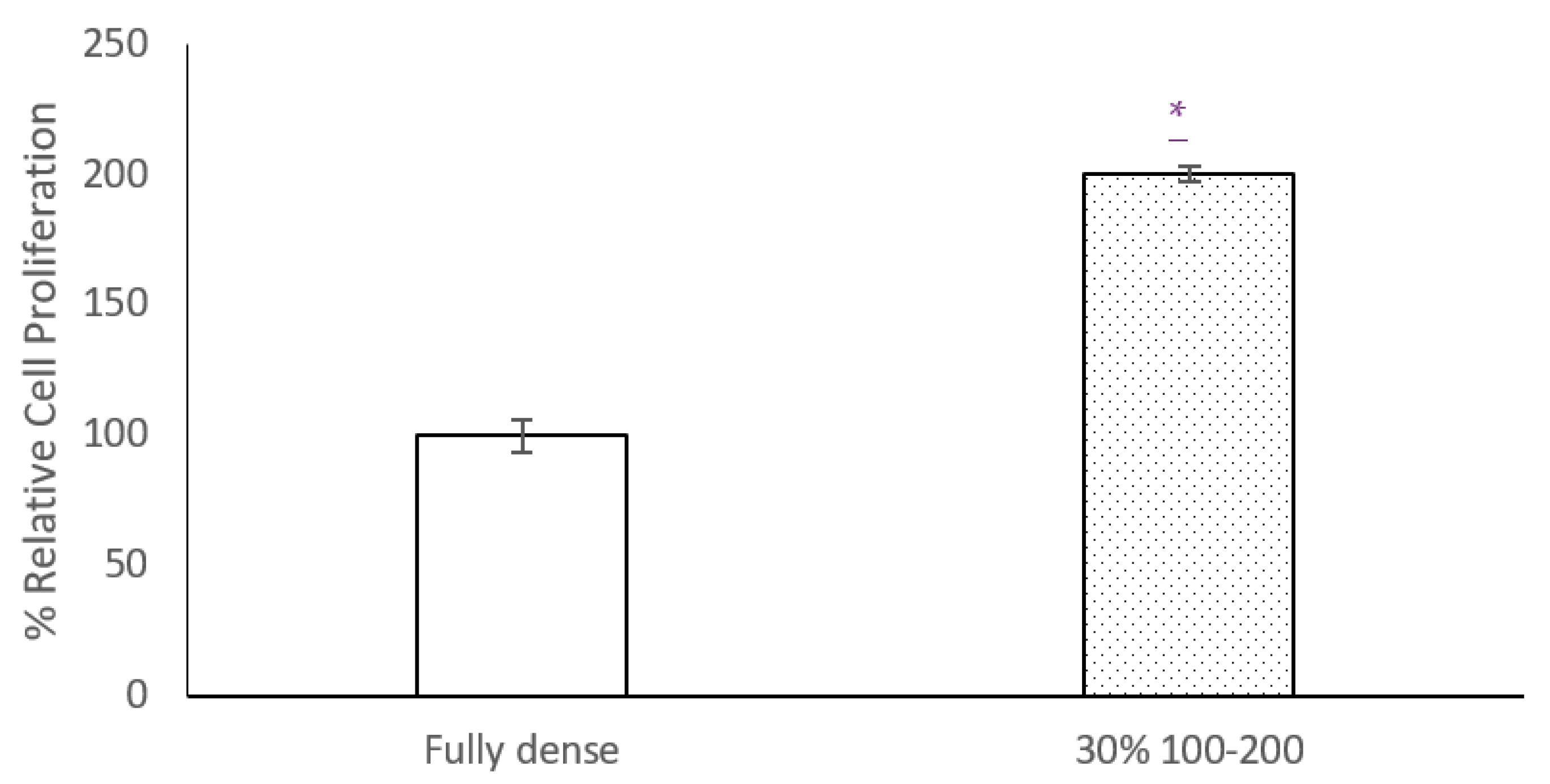
3.2. Electrical Characterization of Osteoblast Cell Cultures: Cell Proliferation and Viability Tests
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Liu, W.; Liu, S.; Wang, L. Surface Modification of Biomedical Titanium Alloy: Micromorphology, Microstructure Evolution and Biomedical Applications. Coatings 2019, 9, 249. [Google Scholar] [CrossRef] [Green Version]
- Echeverry-Rendón, M.; Galvis, O.; Giraldo, D.A.Q.; Pavón-Palacio, J.-J.; López-Lacomba, J.L.; Jimenez-Pique, E.; Anglada, M.; Robledo, S.M.; Castaño, J.G.; Echeverria, F. Osseointegration improvement by plasma electrolytic oxidation of modified titanium alloys surfaces. J. Mater. Sci. Mater. Med. 2015, 26, 72. [Google Scholar] [CrossRef] [Green Version]
- Kaur, M.; Singh, K. Review on titanium and titanium based alloys as biomaterials for orthopaedic applications. Mater. Sci. Eng. C 2019, 102, 844–862. [Google Scholar] [CrossRef]
- Khorasani, A.M.; Goldberg, M.; Doeven, E.H.; Littlefair, G. Titanium in biomedical applications—properties and fabrication: A review. J. Biomater. Tissue Eng. 2015, 5, 593–619. [Google Scholar] [CrossRef]
- Niinomi, M.; Nakai, M. Titanium-based biomaterials for preventing stress shielding between implant devices and bone. Int. J. Biomater. 2011, 2011. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Niinomi, M.; Liu, Y.; Nakai, M.; Liu, H.; Li, H. Biomedical titanium alloys with Young’s moduli close to that of cortical bone. Regen. Biomater. 2016, 3, 173–185. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Pałka, K.; Pokrowiecki, R. Porous Titanium Implants: A Review. Adv. Eng. Mater. 2018, 20, 1700648. [Google Scholar] [CrossRef]
- Naebe, M.; Shirvanimoghaddam, K. Functionally graded materials: A review of fabrication and properties. Appl. Mater. Today. 2016, 5, 223–245. [Google Scholar] [CrossRef]
- Xu, J.; Weng, X.-J.; Wang, X.; Huang, J.-Z.; Zhang, C.; Muhammad, H.; Ma, X.; Liao, Q.-D. Potential use of porous titanium-niobium alloy in orthopedic implants: Preparation and experimental study of its biocompatibility in vitro. PLoS ONE 2013, 8, e79289. [Google Scholar] [CrossRef]
- Teixeira, L.N.; Crippa, G.E.; Lefebvre, L.P.; de Oliveira, P.T.; Rosa, A.L.; Beloti, M.M. The influence of pore size on osteoblast phenotype expression in cultures grown on porous titanium. Int. J. Oral Maxillofac. Surg. 2012, 41, 1097–1101. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Taniguchi, N.; Fujibayashi, S.; Takemoto, M.; Sasaki, K.; Otsuki, B.; Nakamura, T.; Matsushita, T.; Kokubo, T.; Matsuda, S. Effect of pore size on bone ingrowth into porous titanium implants fabricated by additive manufacturing: An in vivo experiment. Mater. Sci. Eng. C 2016, 59, 690–701. [Google Scholar] [CrossRef] [Green Version]
- Civantos, A.; Domínguez, C.; Pino, R.J.; Setti, G.; Pavón, J.J.; Martínez-Campos, E.; Garcia Garcia, F.J.; Rodríguez, J.A.; Allain, J.P.; Torres, Y. Designing bioactive porous titanium interfaces to balance mechanical properties and in vitro cells behavior towards increased osseointegration. Surf. Coat. Technol. 2019, 368, 162–174. [Google Scholar] [CrossRef]
- Muñoz, S.; Pavón, J.; Rodríguez-Ortiz, J.A.; Civantos, A.; Allain, J.P.; Torres, Y. On the influence of space holder in the development of porous titanium implants: Mechanical, computational and biological evaluation. Mater. Charact. 2015, 108, 68–78. [Google Scholar] [CrossRef]
- Rodríguez, Á.; Trueba, P.; Amado, J.M.; Tobar, M.J.; Giner, M.; Amigó, V.; Torres, Y. Surface modification of porous titanium discs using femtosecond laser structuring. Metals 2020, 10, 748. [Google Scholar] [CrossRef]
- Devgan, S.; Sidhu, S.S. Evolution of surface modification trends in bone related biomaterials: A review. Mater. Chem. Phys. 2019, 233, 68–78. [Google Scholar] [CrossRef]
- Ibrahim, M.Z.; Sarhan, A.A.D.; Yusuf, F.; Hamdi, M. Biomedical materials and techniques to improve the tribological, mechanical and biomedical properties of orthopedic implants—A review article. J. Alloys Compd. 2017, 714, 636–667. [Google Scholar] [CrossRef]
- Civantos, A.; Martínez-Campos, E.; Ramos, V.; Elvira, C.; Gallardo, A.; Abarrategi, A. Titanium Coatings and Surface Modifications: Toward Clinically Useful Bioactive Implants. ACS Biomater. Sci. Eng. 2017, 3, 1245–1261. [Google Scholar] [CrossRef]
- Vorobyev, A.Y.; Chunlei, G. Femtosecond laser structuring of titanium implants. Appl. Surf. Sci. 2007, 253, 7272–7280. [Google Scholar] [CrossRef]
- Wennerberg, A.; Albrektsson, T. Effects of titanium surface topography on bone integration: A systematic review. Clin. Oral Implants Res. 2009, 20, 172–184. [Google Scholar] [CrossRef] [PubMed]
- Trueba, P.; Giner, M.; Rodríguez, Á.; Beltrán, A.M.; Amado, J.M.; Montoya-García, M.J.; Rodríguez-Albelo, L.M.; Torres, Y. Tribo-mechanical and cellular behavior of superficially modified porous titanium samples using femtosecond laser. Surf. Coat. Technol. 2021, 422, 127555. [Google Scholar] [CrossRef]
- Schweitzer, L.; Cunha, A.; Pereira, T.; Mika, K.; Rego, A.M.B.D.; Ferraria, A.M.; Kieburg, H.; Geissler, S.; Uhlmann, E.; Schoon, J. Preclinical in vitro assessment of submicron-scale laser surface texturing on Ti6Al4V. Materials 2020, 13, 5342. [Google Scholar] [CrossRef]
- Klos, A.; Sedao, X.; Itina, T.E.; Helfenstein-Didier, C.; Donnet, C.; Peyroche, S.; Vico, L.; Guignandon, A.; Dumas, V. Ultrafast laser processing of nanostructured patterns for the control of cell adhesion and migration on titanium alloy. Nanomaterials 2020, 10, 864. [Google Scholar] [CrossRef] [PubMed]
- Martínez-Calderon, M.; Martín-Palma, R.J.; Gómez-Aranzadi, M.; García-Ruiz, J.P.; Olaizola, A.M.; Manso-Silva, M. Biomimetic hierarchical micro/nano texturing of TiAlV alloys by femtosecond laser processing for the control of cell adhesion and migration. Phys. Rev. Mater. 2020, 4, 056008. [Google Scholar] [CrossRef]
- Oliveira, V.; Ausset, S.; Vilar, R. Surface micro/nanostructuring of titanium under stationary and non-stationary femtosecond laser irradiation. Appl. Surf. Sci. 2009, 255, 7556–7560. [Google Scholar] [CrossRef]
- Bonse, J.; Höhm, S.; Koter, R.; Harlet, M.; Spaltmann, D.; Pentzinen, S.; Rosenfeld, A.; Krüger, J. Tribological performance of sub-100-nm femtosecond laser-induced periodic surface structures on titanium. Appl. Surf. Sci. 2016, 374, 190–196. [Google Scholar] [CrossRef]
- Shinonaga, T.; Kinoshita, S.; Okamoto, Y.; Tsukamoto, M.; Okada, A. Formation of Periodic Nanostructures with Femtosecond Laser for Creation of New Functional Biomaterials. Procedia CIRP 2016, 42, 57–61. [Google Scholar] [CrossRef]
- Schnell, G.; Duenow, U.; Seitz, H. Effect of Laser Pulse Overlap and Scanning Line Overlap on Femtosecond Laser-Structured Ti6Al4V Surfaces. Materials 2020, 13, 969. [Google Scholar] [CrossRef] [Green Version]
- Rupp, F.; Gittens, R.A.; Scheideler, L.; Marmur, A.; Boyan, B.D.; Schwartz, Z.; Geis-Gerstorfer, J. A review on the wettability of dental implant surfaces I: Theoretical and experimental aspects. Acta Biomater. 2014, 10, 2894–2906. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Raimbault, O.; Benayoun, S.; Anselme, K.; Mauclair, C.; Bourgade, T.; Kietzig, A.-M.; Girard-Lauriault, P.-L.; Valette, S.; Donnet, C. The effects of femtosecond laser-textured Ti-6Al-4V on wettability and cell response. Mater. Sci. Eng. C. 2016, 69, 311–320. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Rui, Z.; Cheng, W.; Song, L.; Xu, Y.; Li, R.; Zhang, X. Characterization and evaluation of a femtosecond laser-induced osseointegration and an anti-inflammatory structure generated on a titanium alloy. Regen. Biomater. 2021, 8, rbab006. [Google Scholar] [CrossRef]
- Li, C.; Yang, Y.; Yang, L.; Shi, Z.; Yang, P.; Cheng, G. In vitro bioactivity and biocompatibility of bio-inspired Ti-6Al-4V alloy surfaces modified by combined laser micro/nano structuring. Molecules 2020, 25, 1494. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Dumas, V.; Guignandon, A.; Vico, L.; Mauclair, C.; Zapata, X.; Linossier, M.T.; Bouleftour, W.; Granier, J.; Peyroche, S.; Dumas, J.-C.; et al. Femtosecond laser nano/micro patterning of titanium influences mesenchymal stem cell adhesion and commitment. Biomed. Mater. 2015, 10, 055002. [Google Scholar] [CrossRef]
- Shaikh, S.; Singh, D.; Subramanian, M.; Kedia, S.; Singh, A.K.; Singh, K.; Gupta, N.; Sinha, S. Femtosecond laser induced surface modification for prevention of bacterial adhesion on 45S5 bioactive glass. J. Non. Cryst. Solids 2018, 482, 63–72. [Google Scholar] [CrossRef] [Green Version]
- Cunha, A.; Elie, A.-M.; Plawinski, L.; Serro, A.; Rego, A.M.B.D.; Almeida, A.; Urdaci, M.C.; Durrieu, M.-C.; Vilar, R. Femtosecond laser surface texturing of titanium as a method to reduce the adhesion of Staphylococcus aureus and biofilm formation. Appl. Surf. Sci. 2016, 360, 485–493. [Google Scholar] [CrossRef]
- Liang, C.; Wang, H.; Yang, J.; Cai, Y.; Hu, X.; Yang, Y.; Li, B.; Li, H.; Li, H.; Li, C.; et al. Femtosecond laser-induced micropattern and Ca/P deposition on Ti implant surface and its acceleration on early osseointegration. ACS Appl. Mater. Interfaces 2013, 5, 8179–8186. [Google Scholar] [CrossRef] [PubMed]
- Wang, C.; Hu, H.; Li, Z.; Shen, Y.; Xu, Y.; Zhang, G.; Zeng, X.; Deng, J.; Zhao, S.; Ren, T.; et al. Enhanced Osseointegration of Titanium Alloy Implants with Laser Microgrooved Surfaces and Graphene Oxide Coating . ACS Appl. Mater. Interfaces 2019, 11, 39470–39483. [Google Scholar] [CrossRef]
- Florian, C.; Wonneberger, R.; Undisz, A.; Kimer, V.S.; Wasmuth, K.; Spaltmann, D.; Krüger, J.; Bonse, J. Chemical effects during the formation of various types of femtosecond laser-generated surface structures on titanium alloy. Appl. Phys. A Mater. Sci. Process 2020, 126, 266. [Google Scholar] [CrossRef] [Green Version]
- Pan, J.; Thierry, D.; Leygraf, C. Electrochemical impedance spectroscopy study of the passive oxide film on titanium for implant application. Electrochim. Acta 1996, 41, 1143–1153. [Google Scholar] [CrossRef]
- Menini, R.; Dion, M.-J.; So, S.K.V.; Gauthier, M.; Lefebvre, L.-P. Surface and Corrosion Electrochemical Characterization of Titanium Foams for Implant Applications. J. Electrochem. Soc. 2006, 153, B13–B21. [Google Scholar] [CrossRef]
- El Daym, D.A.A.; Gheith, M.E.; Abbas, N.A.; Rashed, L.A.; El Aziz, Z.A.A. Electrochemical assessment of laser-treated titanium alloy used for dental applications at acidic pH condition (in vitro study). Dent. Res. J. 2019, 16, 304–309. [Google Scholar] [CrossRef]
- Olmo, A.; Hernández, M.; Chicardi, E.; Torres, Y. Characterization and monitoring of titanium bone implants with impedance spectroscopy. Sensors 2020, 20, 4358. [Google Scholar] [CrossRef] [PubMed]
- Chen, X.; Fu, Q.; Jin, Y.; Li, M.; Yang, R.; Cui, X.; Gong, M. In vitro studying corrosion behavior of porous titanium coating in dynamic electrolyte. Mater. Sci. Eng. C 2017, 70, 1071–1075. [Google Scholar] [CrossRef]
- Giner, M.; Olmo, A.; Hernández, M.; Trueba, P.; Chicardi, E.; Civantos, A.; Vázquez, M.Á.; Montoya-García, M.-J.; Torres, Y. Use of impedance spectroscopy for the characterization of in-vitro osteoblast cell response in porous titanium bone implants. Metals 2020, 10, 1077. [Google Scholar] [CrossRef]
- Huang, H.-H. In situ surface electrochemical characterizations of Ti and Ti-6Al-4V alloy cultured with osteoblast-like cells. Biochem. Biophys. Res. Commun. 2004, 314, 787–792. [Google Scholar] [CrossRef]
- Zhiyong, P.; Wong, C.L.; Guofeng, G.; Lin, M.N.; Chwee, T.L.; Jongyoon, H.; Krystyn, J.V.V. Bone Marrow Regeneration Promoted by Biophysically Sorted Osteoprogenitors From Mesenchymal Stromal Cells. Stemcells Transl. Med. 2015, 4, 56–65. [Google Scholar]
- Gamal, W.; Wu, H.; Underwood, I.; Jia, J.; Smith, S.; Bagnaninchi, P.O. Impedance-based cellular assays for regenerative medicine. Philos. Trans. R. Soc. B Biol. Sci. 2018, 373, 1750. [Google Scholar] [CrossRef] [PubMed] [Green Version]

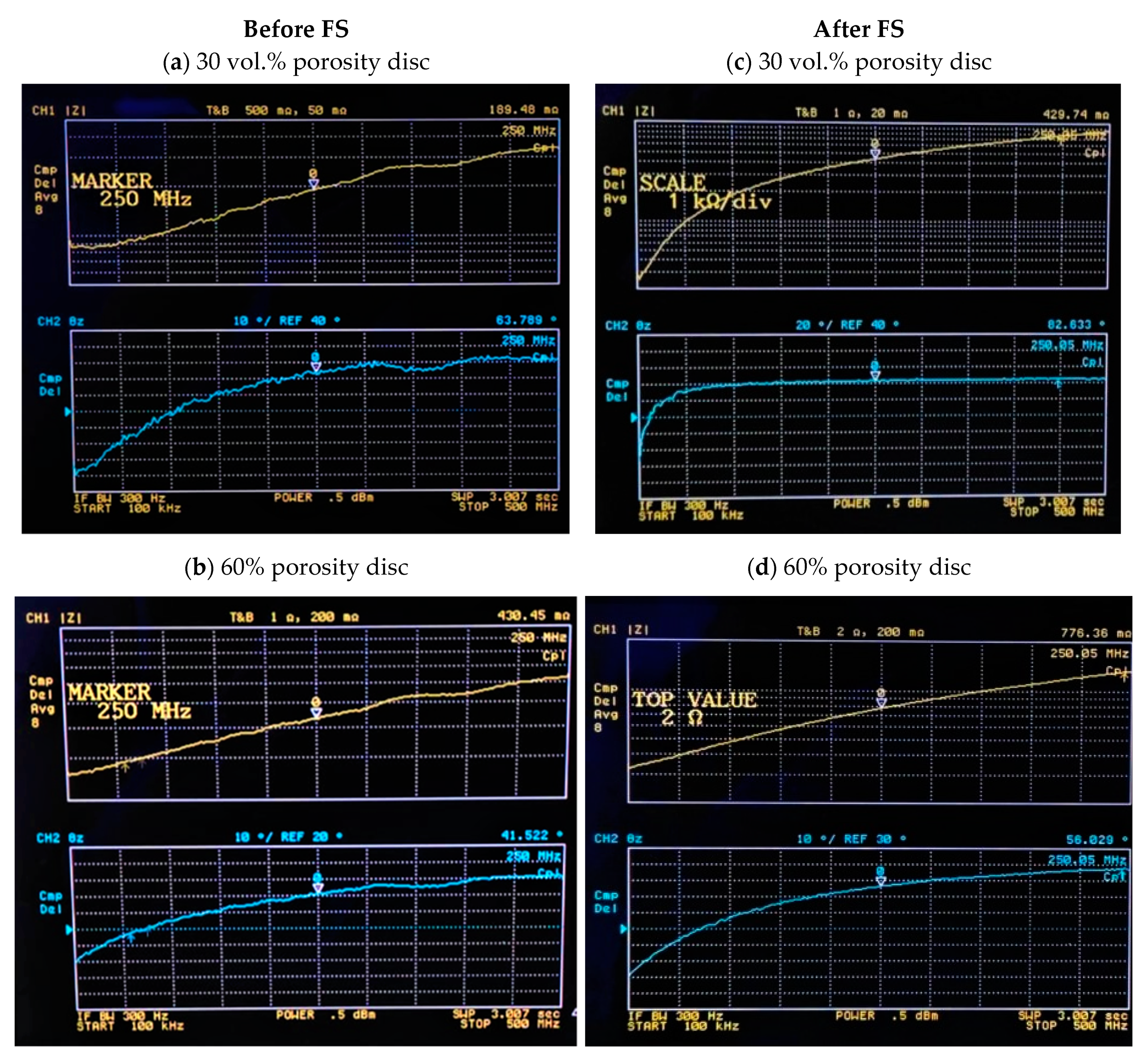
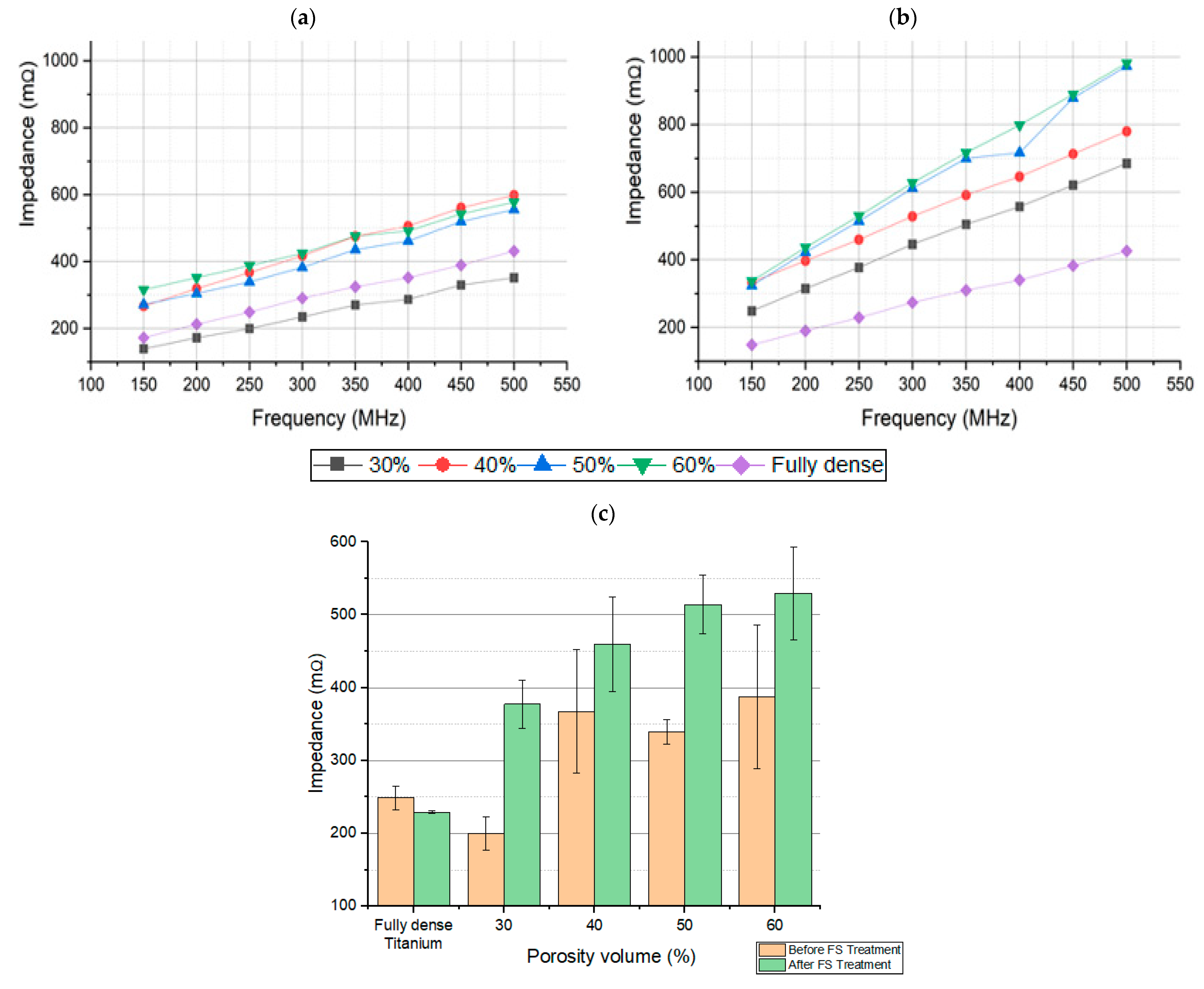


| Frequency | Fully-Dense Titanium | 30 vol.% | 40 vol.% | 50 vol.% | 60 vol.% | |||||
|---|---|---|---|---|---|---|---|---|---|---|
| |Z| (mΩ) | θ | |Z| (mΩ) | θ | |Z| (mΩ) | θ | |Z| (mΩ) | θ | |Z| (mΩ) | θ | |
| 150 MHz | 172.23 | 58.78° | 139.32 | 52.88° | 266.70 | 51.12° | 271.96 | 48.83° | 315.43 | 35.42° |
| 250 MHz | 249.02 | 66.85° | 199.57 | 65.72° | 367.48 | 56.82° | 339.57 | 58.15° | 387.5 | 44.93° |
| 500 MHz | 430.92 | 75.01° | 351.56 | 72.24° | 598.41 | 59.71° | 555.33 | 65.44° | 577.31 | 52.74° |
| Frequency | Fully-Dense Titanium | 30 vol.% | 40 vol.% | 50 vol.% | 60 vol.% | |||||
|---|---|---|---|---|---|---|---|---|---|---|
| |Z| (mΩ) | θ | |Z| (mΩ) | θ | |Z| (mΩ) | θ | |Z| (mΩ) | θ | |Z| (mΩ) | θ | |
| 150 MHz | 148.81 | 63.37° | 248.74 | 63.03° | 333.19 | 53.33° | 324.02 | 74.84° | 337.07 | 72.66° |
| 250 MHz | 229.25 | 73.21° | 377.33 | 69.02° | 459.78 | 61.16° | 514.23 | 78.37° | 529.88 | 75.58° |
| 500 MHz | 426.06 | 79.17° | 685.04 | 73.85° | 779.71 | 70.5° | 972.62 | 81.70° | 981.51 | 77.34° |
| Frequency | Fully-Dense Titanium | 30 vol.% | ||
|---|---|---|---|---|
| |Z| (mΩ) | θ | |Z| (mΩ) | Θ | |
| 150 MHz | 308.6 | 67.55° | 328.967 | 50.62° |
| 250 MHz | 472.01 | 68.19° | 457.557 | 63.71° |
| 500 MHz | 768.385 | 67.24° | 873.023 | 74.54° |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Navarro, P.; Olmo, A.; Giner, M.; Rodríguez-Albelo, M.; Rodríguez, Á.; Torres, Y. Electrical Impedance of Surface Modified Porous Titanium Implants with Femtosecond Laser. Materials 2022, 15, 461. https://doi.org/10.3390/ma15020461
Navarro P, Olmo A, Giner M, Rodríguez-Albelo M, Rodríguez Á, Torres Y. Electrical Impedance of Surface Modified Porous Titanium Implants with Femtosecond Laser. Materials. 2022; 15(2):461. https://doi.org/10.3390/ma15020461
Chicago/Turabian StyleNavarro, Paula, Alberto Olmo, Mercè Giner, Marleny Rodríguez-Albelo, Ángel Rodríguez, and Yadir Torres. 2022. "Electrical Impedance of Surface Modified Porous Titanium Implants with Femtosecond Laser" Materials 15, no. 2: 461. https://doi.org/10.3390/ma15020461
APA StyleNavarro, P., Olmo, A., Giner, M., Rodríguez-Albelo, M., Rodríguez, Á., & Torres, Y. (2022). Electrical Impedance of Surface Modified Porous Titanium Implants with Femtosecond Laser. Materials, 15(2), 461. https://doi.org/10.3390/ma15020461







