Design and Manufacturing of Si-Based Non-Oxide Cellular Ceramic Structures through Indirect 3D Printing
Abstract
:1. Introduction
2. Materials and Methods
2.1. Reagents and Materials
2.2. Preparation of Preceramic Polymer Mixtures
2.3. 3D Printing of Honeycomb Structures
2.4. Dip-Coating of PLA Molds with Preceramic Polymer Solutions
2.5. Polymer-to-Ceramic Conversion
2.6. Characterization
3. Results
3.1. Characterization of Preceramic Polymers
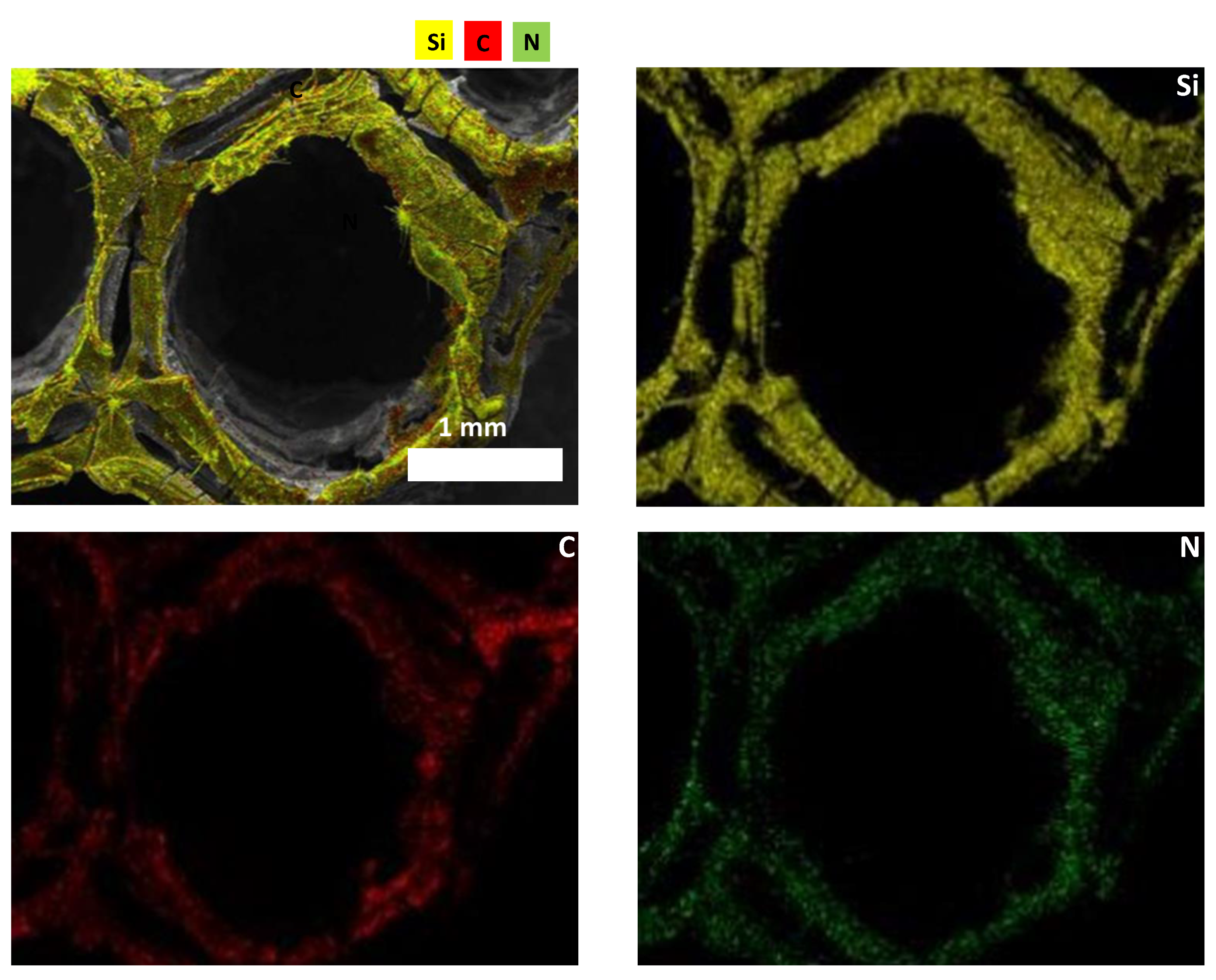
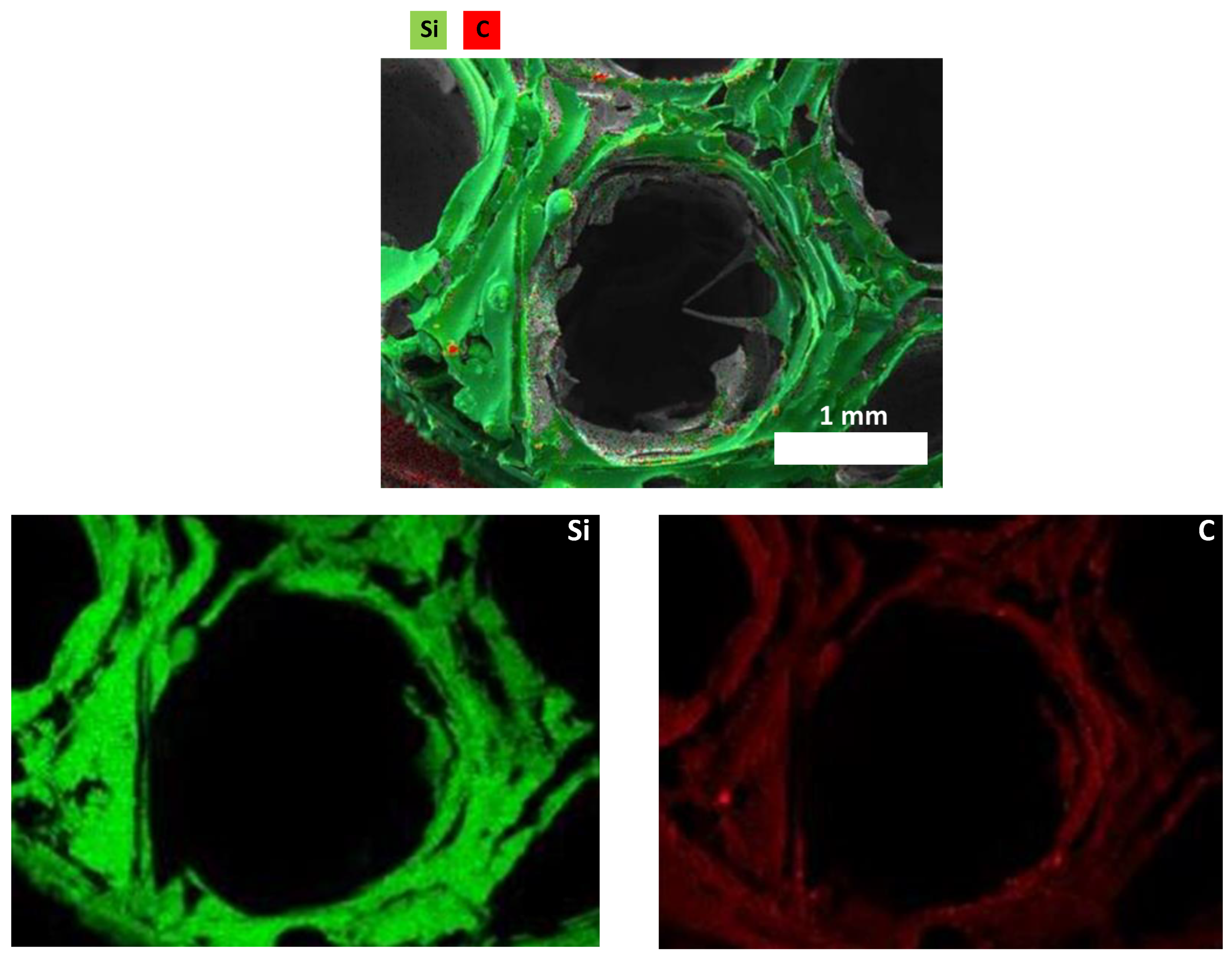
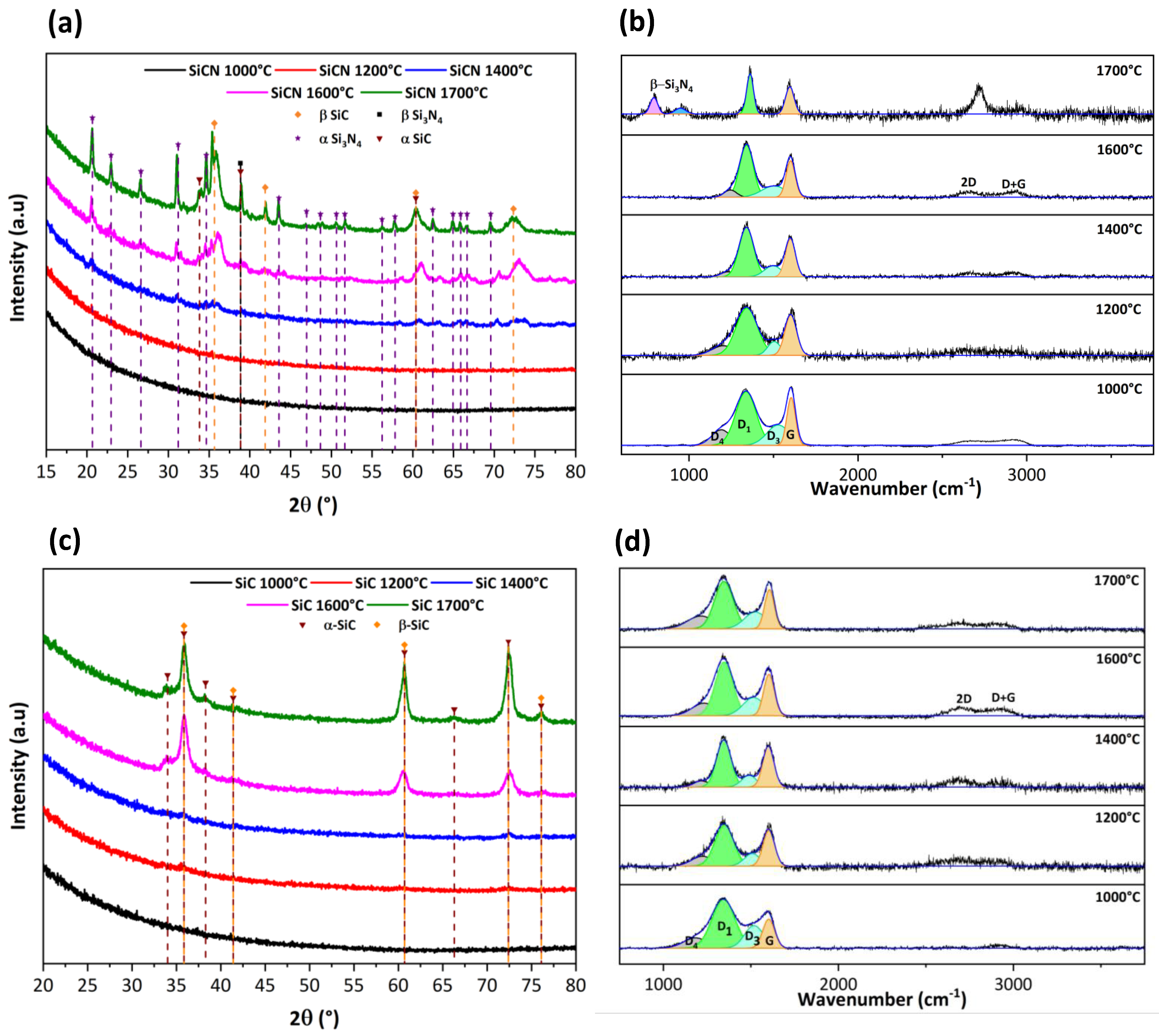
3.2. Characterization of SiCN and SiC Ceramics
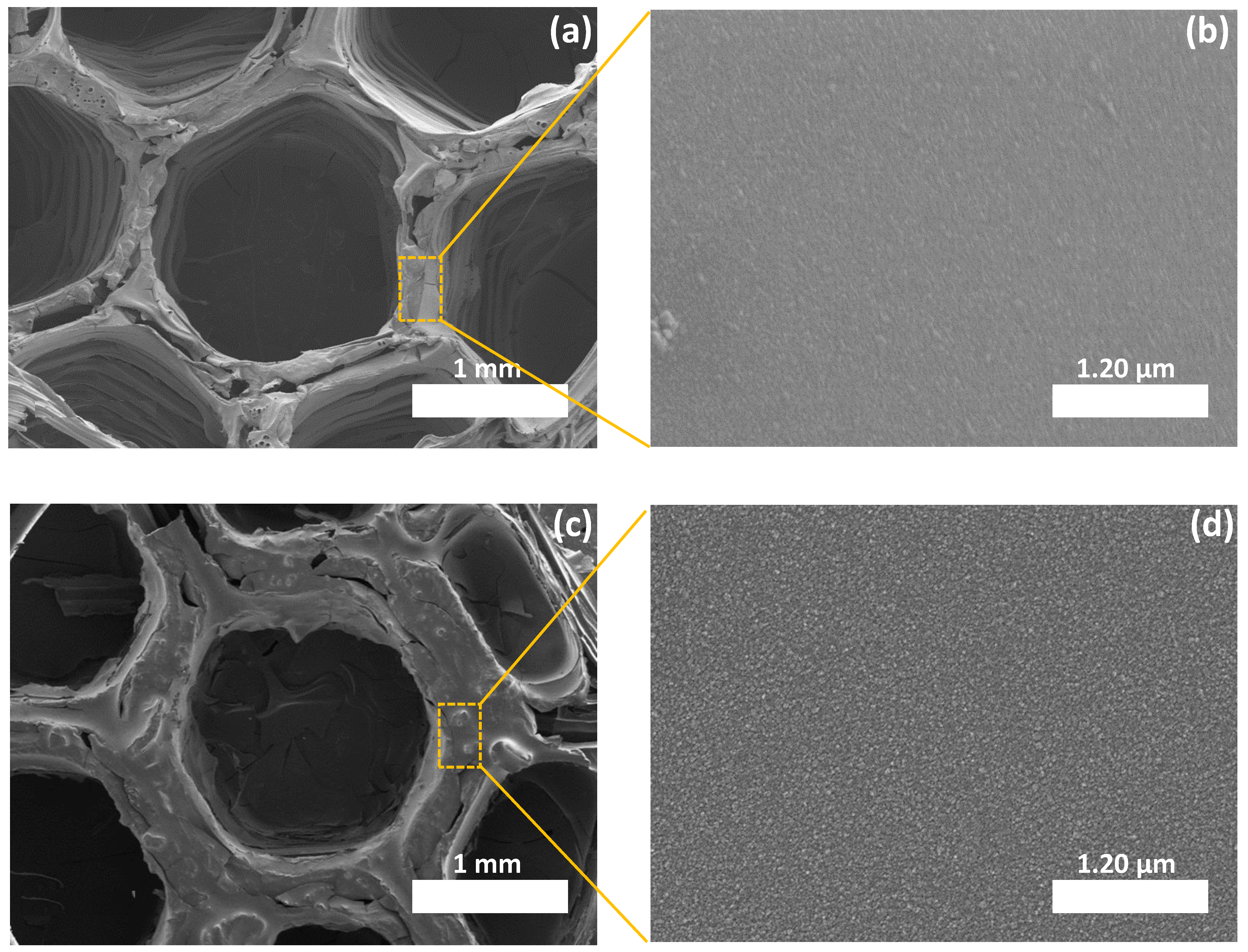
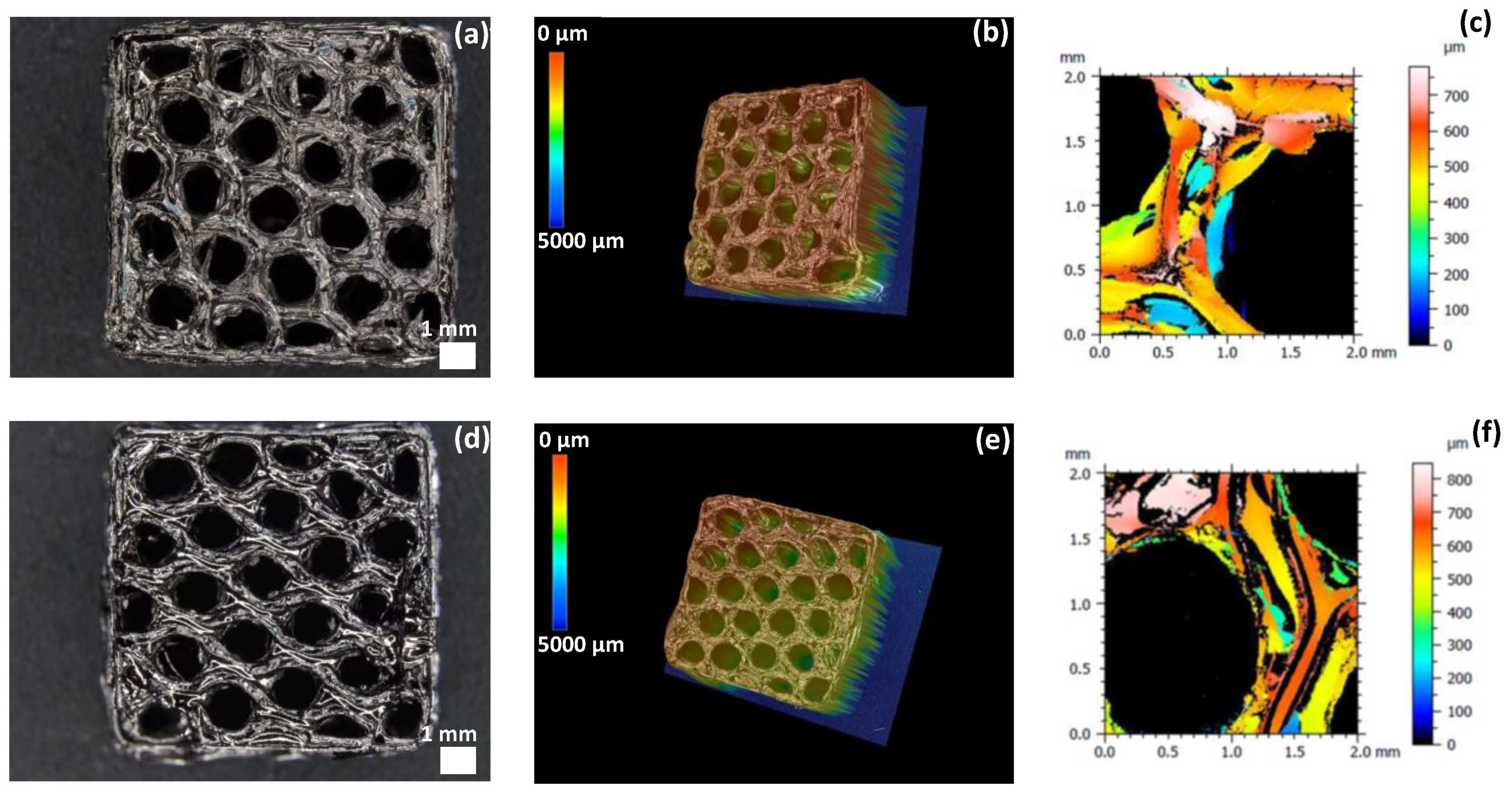
3.2.1. Morphological Properties
3.2.2. Microstructural Evolution
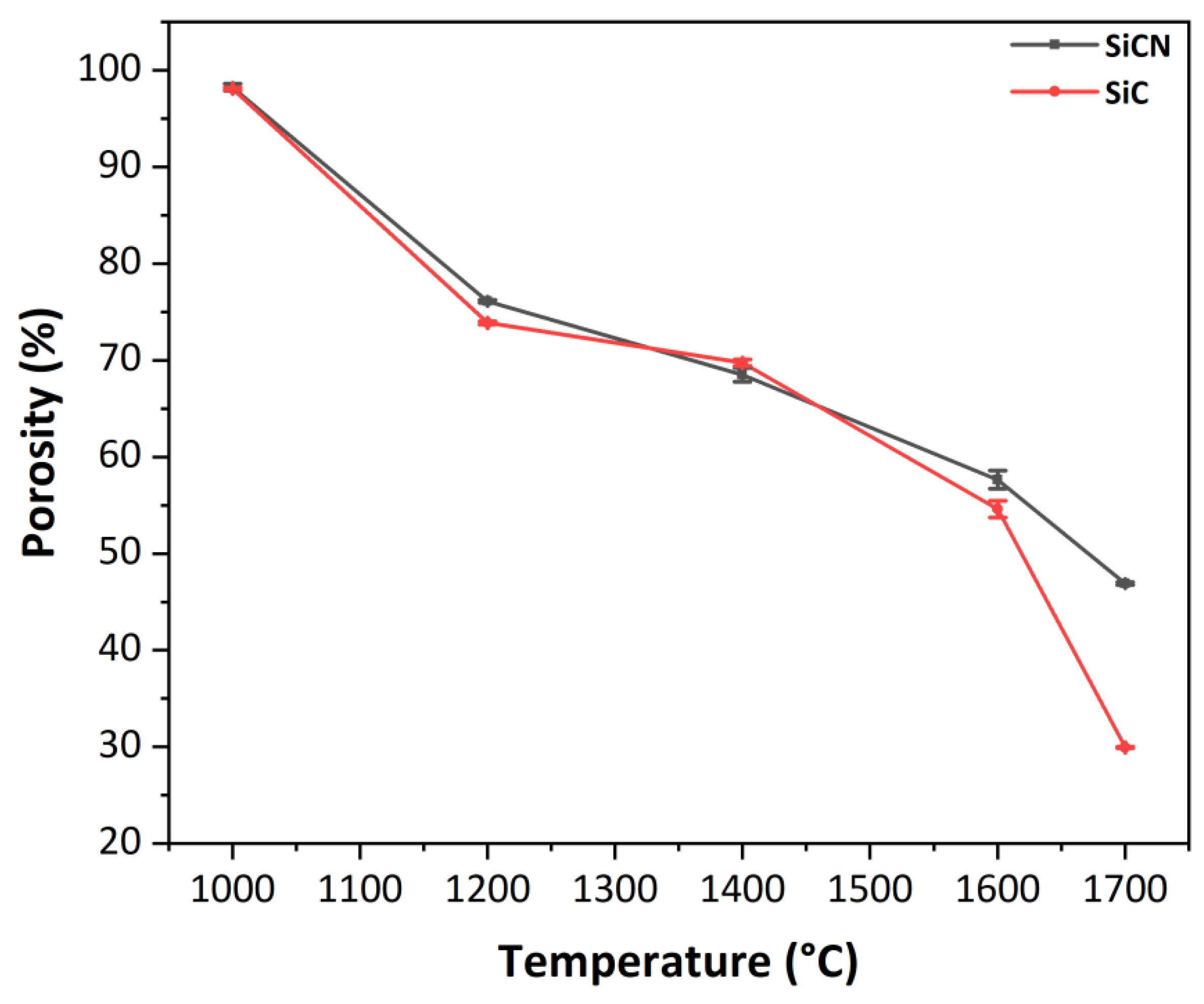
3.2.3. Evolution of the Porosity with the Temperature
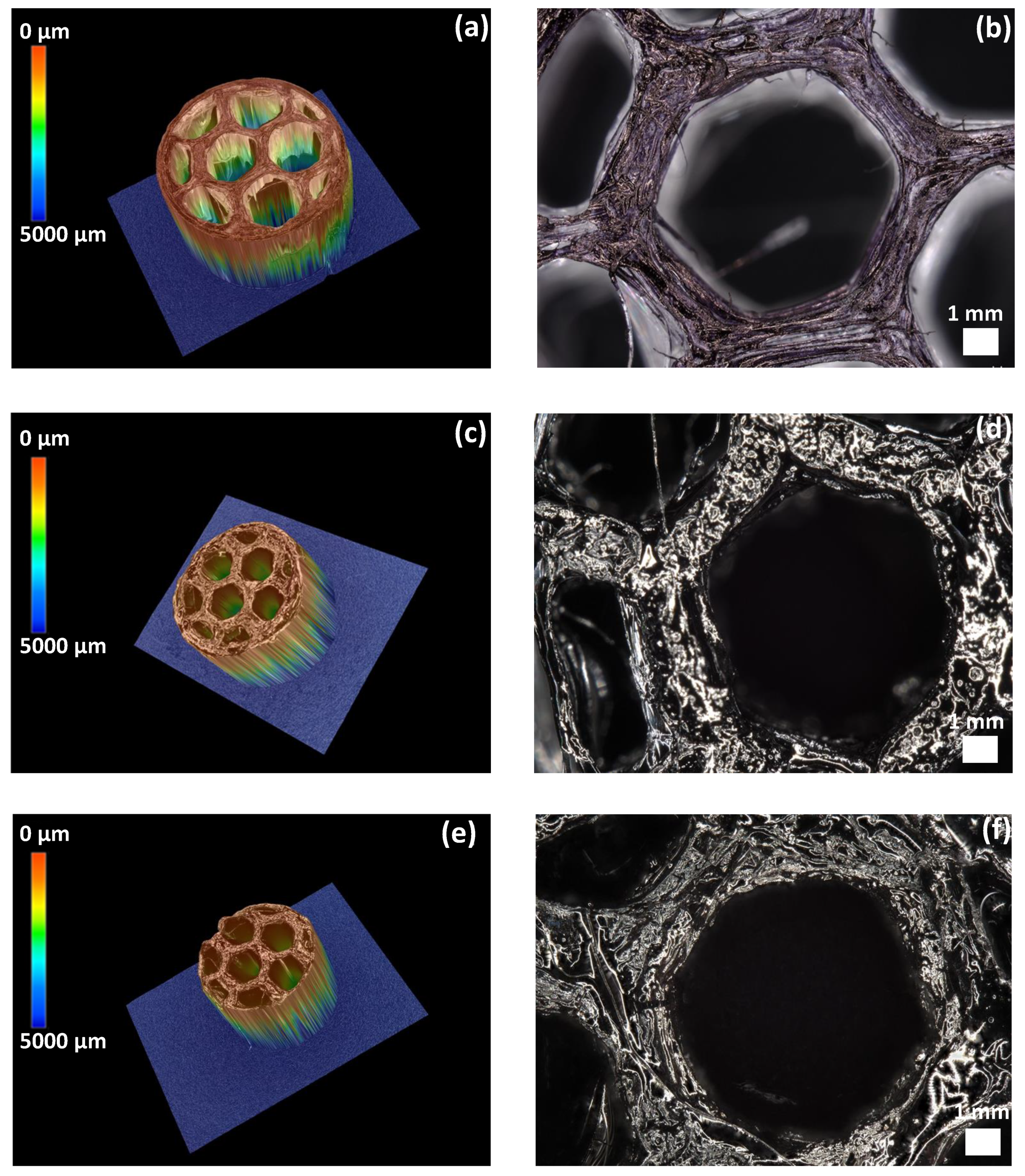
3.2.4. Roughness of SiCN and SiC 3D Ceramics
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Wang, M.; Xie, C.; He, R.; Ding, G.; Zhang, K.; Wang, G.; Fang, D. Polymer-Derived Silicon Nitride Ceramics by Digital Light Processing Based Additive Manufacturing. J. Am. Ceram. Soc. 2019, 102, 5117–5126. [Google Scholar] [CrossRef]
- Schmidt, J.; Altun, A.A.; Schwentenwein, M.; Colombo, P. Complex Mullite Structures Fabricated via Digital Light Processing of a Preceramic Polysiloxane with Active Alumina Fillers. J. Eur. Ceram. Soc. 2019, 39, 1336–1343. [Google Scholar] [CrossRef]
- Lee, J.-Y.; Tan, W.S.; An, J.; Chua, C.K.; Tang, C.Y.; Fane, A.G.; Chong, T.H. The Potential to Enhance Membrane Module Design with 3D Printing Technology. J. Membr. Sci. 2016, 499, 480–490. [Google Scholar] [CrossRef]
- Shahrubudin, N.; Lee, T.C.; Ramlan, R. An Overview on 3D Printing Technology: Technological, Materials, and Applications. Procedia Manuf. 2019, 35, 1286–1296. [Google Scholar] [CrossRef]
- Kamran, M.; Saxena, A. A Comprehensive Study on 3D Printing Technology. MIT Int. J. Mech. Eng. 2016, 6, 63–69. [Google Scholar]
- Cumpston, B.H.; Ananthavel, S.P.; Barlow, S.; Dyer, D.L.; Ehrlich, J.E.; Erskine, L.L.; Heikal, A.A.; Kuebler, S.M.; Lee, I.-Y.S.; McCord-Maughon, D. Two-Photon Polymerization Initiators for Three-Dimensional Optical Data Storage and Microfabrication. Nature 1999, 398, 51–54. [Google Scholar] [CrossRef]
- Zhou, S.; Mei, H.; Chang, P.; Lu, M.; Cheng, L. Molecule Editable 3D Printed Polymer-Derived Ceramics. Coord. Chem. Rev. 2020, 422, 213486. [Google Scholar] [CrossRef]
- Zeng, X.; Li, E.; Xia, G.; Xie, N.; Shen, Z.-Y.; Moskovits, M.; Yu, R. Silica-Based Ceramics toward Electromagnetic Microwave Absorption. J. Eur. Ceram. Soc. 2021, 41, 7381–7403. [Google Scholar] [CrossRef]
- Mei, H.; Zhao, X.; Zhou, S.; Han, D.; Xiao, S.; Cheng, L. 3D-Printed Oblique Honeycomb Al2O3/SiCw Structure for Electromagnetic Wave Absorption. Chem. Eng. J. 2019, 372, 940–945. [Google Scholar] [CrossRef]
- Zocca, A.; Colombo, P.; Gomes, C.M.; Günster, J. Additive Manufacturing of Ceramics: Issues, Potentialities, and Opportunities. J. Am. Ceram. Soc. 2015, 98, 1983–2001. [Google Scholar] [CrossRef]
- Eckel, Z.C.; Zhou, C.; Martin, J.H.; Jacobsen, A.J.; Carter, W.B.; Schaedler, T.A. Additive Manufacturing of Polymer-Derived Ceramics. Science 2016, 351, 58–62. [Google Scholar] [CrossRef] [Green Version]
- Colombo, P.; Mera, G.; Riedel, R.; Soraru, G.D. Polymer-Derived Ceramics: 40 Years of Research and Innovation in Advanced Ceramics. J. Am. Ceram. Soc. 2010, 93, 1805–1837. [Google Scholar] [CrossRef]
- Wang, P.; Liu, F.; Wang, H.; Li, H.; Gou, Y. A Review of Third Generation SiC Fibers and SiCf/SiC Composites. J. Mater. Sci. Technol. 2019, 35, 2743–2750. [Google Scholar] [CrossRef]
- Schmidt, M.; Durif, C.; Acosta, E.D.; Salameh, C.; Plaisantin, H.; Miele, P.; Backov, R.; Machado, R.; Gervais, C.; Alauzun, J.G.; et al. Molecular-Level Processing of Si-(B)-C Materials with Tailored Nano/Microstructures. Chem. Eur. J. 2017, 23, 17103–17117. [Google Scholar] [CrossRef]
- Herrmann, M.; Adloff, L.; Matthey, B.; Gestrich, T. Oxidation Behaviour of Silicon Carbide Bonded Diamond Materials. Open Ceram. 2020, 2, 100017. [Google Scholar] [CrossRef]
- Hundley, J.M.; Eckel, Z.C.; Schueller, E.; Cante, K.; Biesboer, S.M.; Yahata, B.D.; Schaedler, T.A. Geometric Characterization of Additively Manufactured Polymer Derived Ceramics. Addit. Manuf. 2017, 18, 95–102. [Google Scholar] [CrossRef]
- Mondschein, R.J.; Kanitkar, A.; Williams, C.B.; Verbridge, S.S.; Long, T.E. Polymer Structure-Property Requirements for Stereolithographic 3D Printing of Soft Tissue Engineering Scaffolds. Biomaterials 2017, 140, 170–188. [Google Scholar] [CrossRef]
- Salameh, C.; Bernard, S.; Gervais, C.; Babonneau, F.; Bruma, A.; Malo, S.; Miele, P. Chemistry of a Series of Aluminum-Modified Polysilazanes: Synthesis, Pyrolysis Behaviour and Microstructural Evolution. J. Eur. Ceram. Soc. 2019, 39, 183–194. [Google Scholar] [CrossRef]
- Lewis, J.A. Direct Ink Writing of 3D Functional Materials. Adv. Funct. Mater. 2006, 16, 2193–2204. [Google Scholar] [CrossRef]
- Stansbury, J.W.; Idacavage, M.J. 3D Printing with Polymers: Challenges among Expanding Options and Opportunities. Dent. Mater. 2016, 32, 54–64. [Google Scholar] [CrossRef]
- Colombo, P.; Schmidt, J.; Franchin, G.; Zocca, A.; Günster, J. Additive Manufacturing Techniques for Fabricating Complex Ceramic Components from Preceramic Polymers. Am. Ceram. Soc. Bull. 2017, 96, 16–23. [Google Scholar]
- Gorjan, L.; Tonello, R.; Sebastian, T.; Colombo, P.; Clemens, F. Fused Deposition Modeling of Mullite Structures from a Preceramic Polymer and γ-Alumina. J. Eur. Ceram. Soc. 2019, 39, 2463–2471. [Google Scholar] [CrossRef]
- Zhao, L.; Wang, X.; Xiong, H.; Zhou, K.; Zhang, D. Optimized Preceramic Polymer for 3D Structured Ceramics via Fused Deposition Modeling. J. Eur. Ceram. Soc. 2021, 41, 5066–5074. [Google Scholar] [CrossRef]
- Kulkarni, A.; Sorarù, G.D.; Pearce, J.M. Polymer-Derived SiOC Replica of Material Extrusion-Based 3-D Printed Plastics. Addit. Manuf. 2020, 32, 100988. [Google Scholar] [CrossRef] [Green Version]
- Zhao, H.; Chen, L.; Luan, X.; Zhang, X.; Yun, J.; Xu, T. Synthesis, Pyrolysis of a Novel Liquid SiBCN Ceramic Precursor and Its Application in Ceramic Matrix Composites. J. Eur. Ceram. Soc. 2017, 37, 1321–1329. [Google Scholar] [CrossRef]
- Lu, B.; Zhang, Y. Oxidation Behavior of SiC–SiBCN Ceramics. Ceram. Int. 2015, 41, 1023–1030. [Google Scholar] [CrossRef]
- Zhang, Z.; Zeng, F.; Han, J.; Luo, Y.; Xu, C. Synthesis and Characterization of a New Liquid Polymer Precursor for Si–B–C–N Ceramics. J. Mater. Sci. 2011, 46, 5940–5947. [Google Scholar] [CrossRef]
- Ji, X.; Shao, C.; Wang, H.; Cheng, J.; Li, H. Curing Green Fibres Infusible by Electron Beam Irradiation for the Preparation of SiBNC Ceramic Fibres. Ceram. Int. 2017, 43, 11218–11224. [Google Scholar] [CrossRef]
- Pham, T.A.; Kim, D.-P.; Lim, T.-W.; Park, S.-H.; Yang, D.-Y.; Lee, K.-S. Three-Dimensional SiCN Ceramic Microstructures via Nano-Stereolithography of Inorganic Polymer Photoresists. Adv. Funct. Mater. 2006, 16, 1235–1241. [Google Scholar] [CrossRef]
- Zanchetta, E.; Cattaldo, M.; Franchin, G.; Schwentenwein, M.; Homa, J.; Brusatin, G.; Colombo, P. Stereolithography of SiOC Ceramic Microcomponents. Adv. Mater. 2016, 28, 370–376. [Google Scholar] [CrossRef]
- Li, S.; Zhang, Y.; Zhao, T.; Han, W.; Duan, W.; Wang, L.; Dou, R.; Wang, G. Additive Manufacturing of SiBCN/Si 3 N 4 w Composites from Preceramic Polymers by Digital Light Processing. RSC Adv. 2020, 10, 5681–5689. [Google Scholar] [CrossRef] [Green Version]
- Chen, J.; Wang, Y.; Pei, X.; Bao, C.; Huang, Z.; He, L.; Huang, Q. Preparation and Stereolithography of SiC Ceramic Precursor with High Photosensitivity and Ceramic Yield. Ceram. Int. 2020, 46, 13066–13072. [Google Scholar] [CrossRef]
- De Hazan, Y.; Penner, D. SiC and SiOC Ceramic Articles Produced by Stereolithography of Acrylate Modified Polycarbosilane Systems. J. Eur. Ceram. Soc. 2017, 37, 5205–5212. [Google Scholar] [CrossRef]
- Xiao, J.; Jia, Y.; Liu, D.; Cheng, H. Three-Dimensional Printing of SiCN Ceramic Matrix Composites from Preceramic Polysilazane by Digital Light Processing. Ceram. Int. 2020, 46, 25802–25807. [Google Scholar] [CrossRef]
- Xiao, J.; Liu, D.; Cheng, H.; Jia, Y.; Zhou, S.; Zu, M. Carbon Nanotubes as Light Absorbers in Digital Light Processing Three-Dimensional Printing of SiCN Ceramics from Preceramic Polysilazane. Ceram. Int. 2020, 46, 19393–19400. [Google Scholar] [CrossRef]
- Li, S.; Duan, W.; Zhao, T.; Han, W.; Wang, L.; Dou, R.; Wang, G. The Fabrication of SiBCN Ceramic Components from Preceramic Polymers by Digital Light Processing (DLP) 3D Printing Technology. J. Eur. Ceram. Soc. 2018, 38, 4597–4603. [Google Scholar] [CrossRef]
- Zhou, Y.-G.; Su, B.; Turng, L. Deposition-Induced Effects of Isotactic Polypropylene and Polycarbonate Composites during Fused Deposition Modeling. Rapid Prototyp. J. 2017, 23, 869–880. [Google Scholar] [CrossRef]
- Hanniet, Q.; Boussmen, M.; Barés, J.; Huon, V.; Iatsunskyi, I.; Coy, E.; Bechelany, M.; Gervais, C.; Voiry, D.; Miele, P.; et al. Investigation of Polymer-Derived Si–(B)–C–N Ceramic/Reduced Graphene Oxide Composite Systems as Active Catalysts towards the Hydrogen Evolution Reaction. Sci. Rep. 2020, 10, 22003. [Google Scholar] [CrossRef]
- Viard, A.; Fonblanc, D.; Schmidt, M.; Lale, A.; Salameh, C.; Soleilhavoup, A.; Wynn, M.; Champagne, P.; Cerneaux, S.; Babonneau, F. Molecular Chemistry and Engineering of Boron-Modified Polyorganosilazanes as New Processable and Functional SiBCN Precursors. Chem. Eur. J. 2017, 23, 9076–9090. [Google Scholar] [CrossRef] [PubMed]
- Lücke, J.; Hacker, J.; Suttor, D.; Ziegler, G. Synthesis and Characterization of Silazane-Based Polymers as Precursors for Ceramic Matrix Composites. Appl. Organomet. Chem. 1997, 11, 181–194. [Google Scholar] [CrossRef]
- Yu, Z.; Yang, L.; Min, H.; Zhang, P.; Liu, A.; Riedel, R. High-Ceramic-Yield Precursor to SiC-Based Ceramic: A Hyperbranched Polytitaniumcarbosilane Bearing Self-Catalyzing Units. J. Eur. Ceram. Soc. 2015, 35, 851–858. [Google Scholar] [CrossRef]
- Flores, O.; Schmalz, T.; Krenkel, W.; Heymann, L.; Motz, G. Selective Cross-Linking of Oligosilazanes to Tailored Meltable Polysilazanes for the Processing of Ceramic SiCN Fibres. J. Mater. Chem. A 2013, 1, 15406–15415. [Google Scholar] [CrossRef]
- Viard, A.; Gottardo, L.; Lopez-Ferber, D.; Soleilhavoup, A.; Salameh, C.; Samal, S.; Gueguen, Y.; Rouxel, T.; Motz, G.; Babonneau, F.; et al. Molecular Design of Melt-Spinnable Co-Polymers as Si–B–C–N Fiber Precursors. Dalton Trans. 2017, 46, 13510–13523. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.-L.; Kroke, E.; Riedel, R.; Fasel, C.; Gervais, C.; Babonneau, F. Thermal Cross-Linking and Pyrolytic Conversion of Poly (Ureamethylvinyl) Silazanes to Silicon-Based Ceramics. Appl. Organomet. Chem. 2001, 15, 820–832. [Google Scholar] [CrossRef]
- Yu, Z.; Yang, L.; Zhan, J.; Zhou, C.; Min, H.; Zheng, Q.; Xia, H. Preparation, Cross-Linking and Ceramization of AHPCS/Cp2ZrCl2 Hybrid Precursors for SiC/ZrC/C Composites. J. Eur. Ceram. Soc. 2012, 32, 1291–1298. [Google Scholar] [CrossRef]
- Jana, P.; Santoliquido, O.; Ortona, A.; Colombo, P.; Sorarù, G.D. Polymer-Derived SiCN Cellular Structures from Replica of 3D Printed Lattices. J. Am. Ceram. Soc. 2018, 101, 2732–2738. [Google Scholar] [CrossRef] [Green Version]
- Soraru, G.D.; Babonneau, F.; Mackenzie, J.D. Structural Evolutions from Polycarbosilane to SiC Ceramic. J. Mater. Sci. 1990, 25, 3886–3893. [Google Scholar] [CrossRef]
- Yan, M.; Song, W.; Zhao-Hui, C. Raman Spectroscopy Studies of the High-Temperature Evolution of the Free Carbon Phase in Polycarbosilane Derived SiC Ceramics. Ceram. Int. 2010, 36, 2455–2459. [Google Scholar] [CrossRef]
- Matthews, S.; Edirisinghe, M.J.; Folkes, M.J. Effect of Pre-Pyrolysis Heat Treatment on the Preparation of Silicon Carbide from a Polycarbosilane Precursor. Ceram. Int. 1999, 25, 49–60. [Google Scholar] [CrossRef]
- Sadezky, A.; Muckenhuber, H.; Grothe, H.; Niessner, R.; Pöschl, U. Raman Microspectroscopy of Soot and Related Carbonaceous Materials: Spectral Analysis and Structural Information. Carbon 2005, 43, 1731–1742. [Google Scholar] [CrossRef]
- Urbonaite, S.; Hälldahl, L.; Svensson, G. Raman Spectroscopy Studies of Carbide Derived Carbons. Carbon 2008, 46, 1942–1947. [Google Scholar] [CrossRef]
- Yeon, S.-H.; Reddington, P.; Gogotsi, Y.; Fischer, J.E.; Vakifahmetoglu, C.; Colombo, P. Carbide-Derived-Carbons with Hierarchical Porosity from a Preceramic Polymer. Carbon 2010, 48, 201–210. [Google Scholar] [CrossRef]
- Chen, Y.; Yang, X.; Cao, Y.; Gan, Z.; An, L. Quantitative Study on Structural Evolutions and Associated Energetics in Polysilazane-Derived Amorphous Silicon Carbonitride Ceramics. Acta Mater. 2014, 72, 22–31. [Google Scholar] [CrossRef]
- Eom, J.-H.; Kim, Y.-W.; Song, I.-H.; Kim, H.-D. Microstructure and Properties of Porous Silicon Carbide Ceramics Fabricated by Carbothermal Reduction and Subsequent Sintering Process. Mater. Sci. Eng. A 2007, 464, 129–134. [Google Scholar] [CrossRef]
- Chae, S.-H.; Kim, Y.-W.; Song, I.-H.; Kim, H.-D.; Narisawa, M. Porosity Control of Porous Silicon Carbide Ceramics. J. Eur. Ceram. Soc. 2009, 29, 2867–2872. [Google Scholar] [CrossRef]

| Honeycomb Cylinder Diameter | Pore Diameter | Honeycomb Cylinder Thickness | ||||
|---|---|---|---|---|---|---|
| Measure (mm) | Shrinkage (%) | Measure (mm) | Shrinkage (%) | Measure (mm) | Shrinkage (%) | |
| PLA | 10.00 | - | 2.85 | - | 6.00 | - |
| SiCN | 6.35 ± 0.12 | 36.5 | 1.75 ± 0.01 | 38.6 | 4.65 ± 0.13 | 22.5 |
| SiC | 6.78 ± 0.16 | 32.2 | 1.78 ± 0.01 | 37.5 | 4.85 ± 0.12 | 19.2 |
| Ceramic Composition | Si (wt%) | C (wt%) | N (wt%) | O (wt%) | Empirical Formula |
|---|---|---|---|---|---|
| SiCN | 36.71 ± 1.91 | 37.54 ± 2.67 | 23.46 ± 1.48 | 2.27 ± 0.98 | (Si1C1.02N0.63O0.06) |
| SiC | 49.97 ± 1.81 | 46.78 ± 1.91 | - | 3.23 ± 0.51 | (Si1C0.93O0.06) |
| SiCN Ceramic | SiCN Ceramic | |||||
|---|---|---|---|---|---|---|
| Position (cm−1) | FWHM (cm−1) | Id/Ig | Position (cm−1) | FWHM (cm−1) | Id/Ig | |
| 1000 °C | 1601 | 90 | 1.12 | 1604 | 80 | 1.67 |
| 1200 °C | 1602 | 82 | 1.16 | 1602 | 78 | 1.19 |
| 1400 °C | 1602 | 79 | 1.30 | 1600 | 75 | 1.16 |
| 1600 °C | 1603 | 64 | 1.43 | 1601 | 71 | 1.28 |
| 1700 °C | 1605 | 60 | 1.48 | 1596 | 64 | 1.21 |
| Ceramics Roughness | SiCN | SiC |
|---|---|---|
| Sq (µm) | 109.44 ± 15.54 | 107.46 ± 27.87 |
| Ssk | −0.92 ± 0.26 | −0.55 ± 0.61 |
| Sku | 4.57 ± 0.86 | 3.62 ± 1.53 |
| Sz | 589.40 ± 58.31 | 552.40 ± 189.75 |
| Sa | 80.50 ± 10.66 | 83.22 ± 27.28 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
El Chawich, G.; El Hayek, J.; Rouessac, V.; Cot, D.; Rebière, B.; Habchi, R.; Garay, H.; Bechelany, M.; Zakhour, M.; Miele, P.; et al. Design and Manufacturing of Si-Based Non-Oxide Cellular Ceramic Structures through Indirect 3D Printing. Materials 2022, 15, 471. https://doi.org/10.3390/ma15020471
El Chawich G, El Hayek J, Rouessac V, Cot D, Rebière B, Habchi R, Garay H, Bechelany M, Zakhour M, Miele P, et al. Design and Manufacturing of Si-Based Non-Oxide Cellular Ceramic Structures through Indirect 3D Printing. Materials. 2022; 15(2):471. https://doi.org/10.3390/ma15020471
Chicago/Turabian StyleEl Chawich, Ghenwa, Joelle El Hayek, Vincent Rouessac, Didier Cot, Bertrand Rebière, Roland Habchi, Hélène Garay, Mikhael Bechelany, Mirvat Zakhour, Philippe Miele, and et al. 2022. "Design and Manufacturing of Si-Based Non-Oxide Cellular Ceramic Structures through Indirect 3D Printing" Materials 15, no. 2: 471. https://doi.org/10.3390/ma15020471
APA StyleEl Chawich, G., El Hayek, J., Rouessac, V., Cot, D., Rebière, B., Habchi, R., Garay, H., Bechelany, M., Zakhour, M., Miele, P., & Salameh, C. (2022). Design and Manufacturing of Si-Based Non-Oxide Cellular Ceramic Structures through Indirect 3D Printing. Materials, 15(2), 471. https://doi.org/10.3390/ma15020471











