Real Time SPR Assessment of the Structural Changes of Adaptive Dynamic Constitutional Frameworks as a New Route for Sensing
Abstract
:1. Introduction
1.1. Dynamers and Their Analytical Potential
1.2. SPR in Sensing, in Particular for Conformational Changes
1.3. Aim of the Study
1.4. Justification of the Selection of the Model
2. Materials and Methods
2.1. Materials
2.2. Gold NPs
2.3. Au-DCFs Synthesis
2.4. Sensor Chip Functionalization
2.5. Surface Plasmon Resonance Measurements
2.6. Complementary Characterization (AFM)
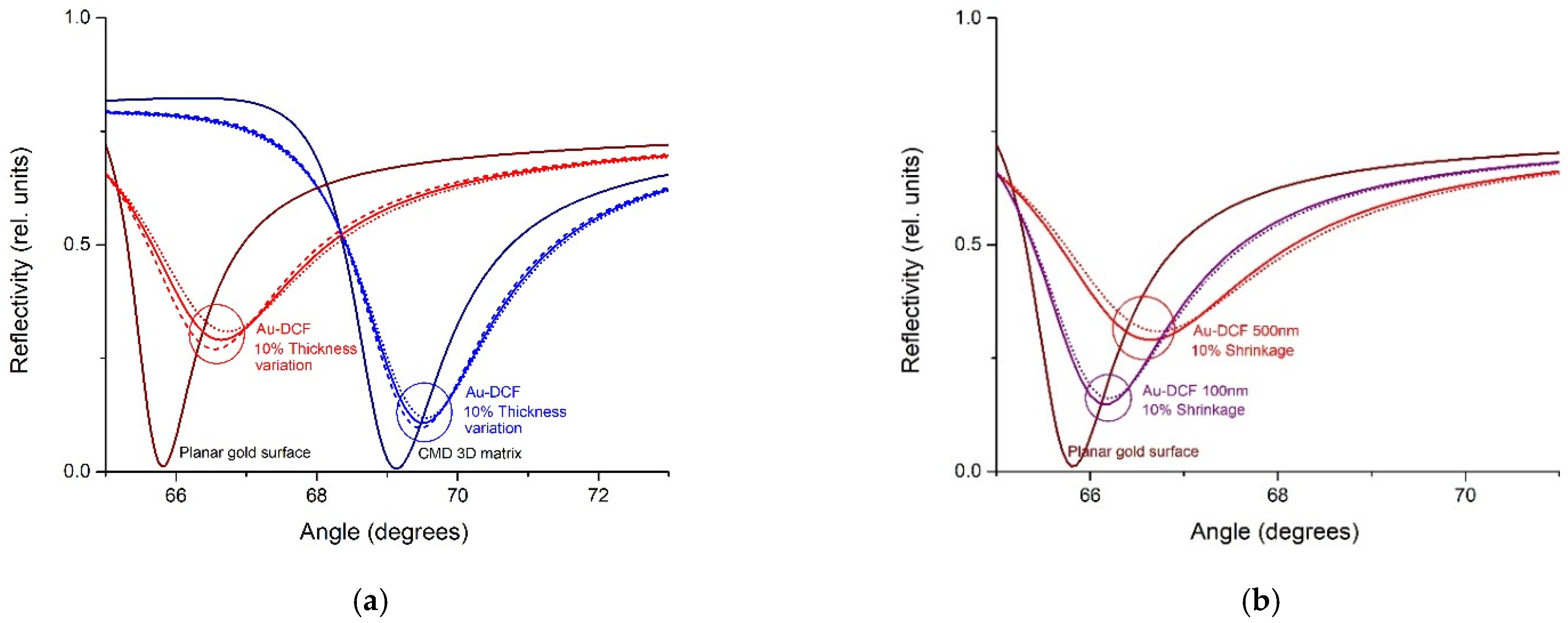
2.7. Theoretical Framework
3. Results and Discussion
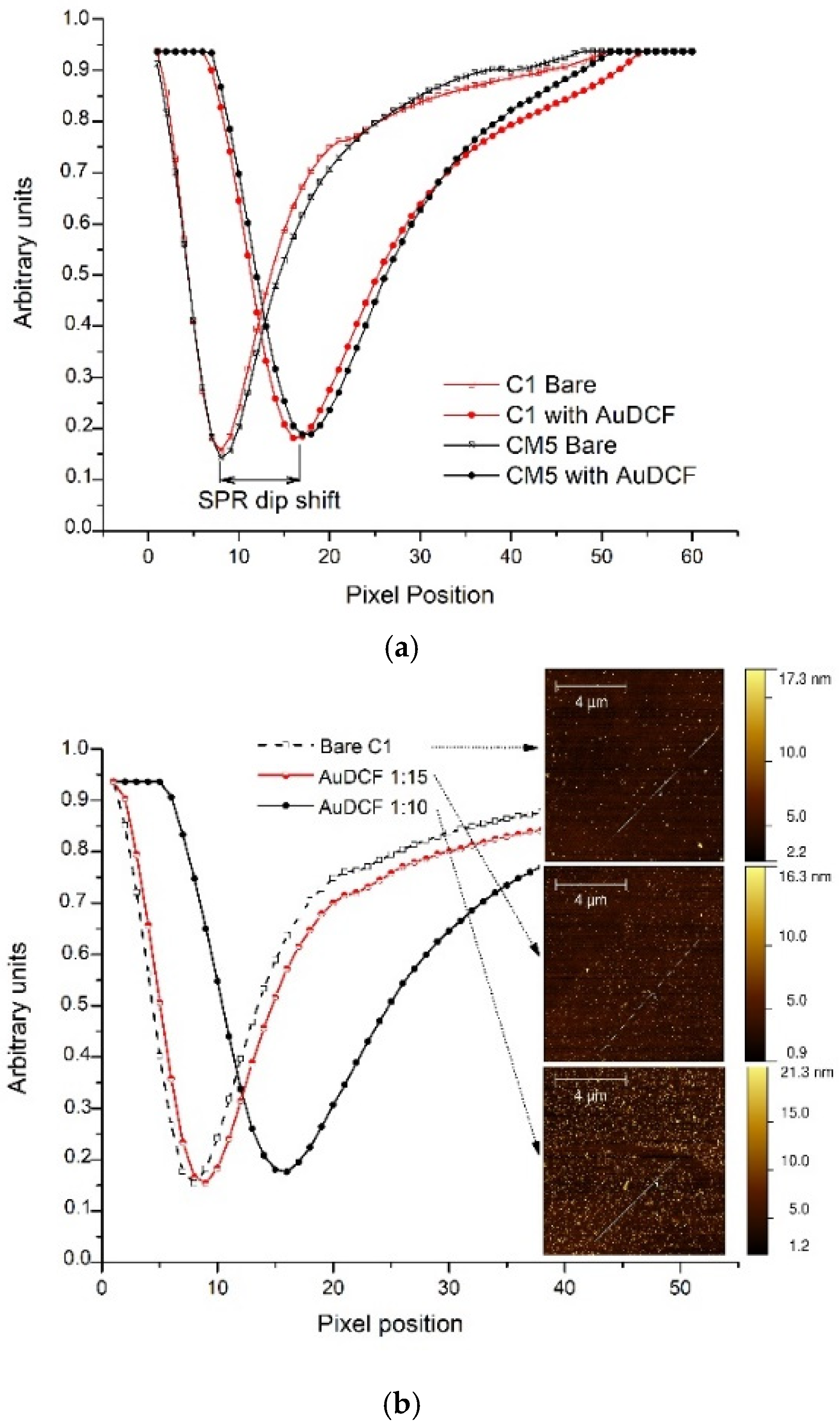
3.1. Characterization of Au-DCFs
3.2. SPR Measurement of Au-DCF
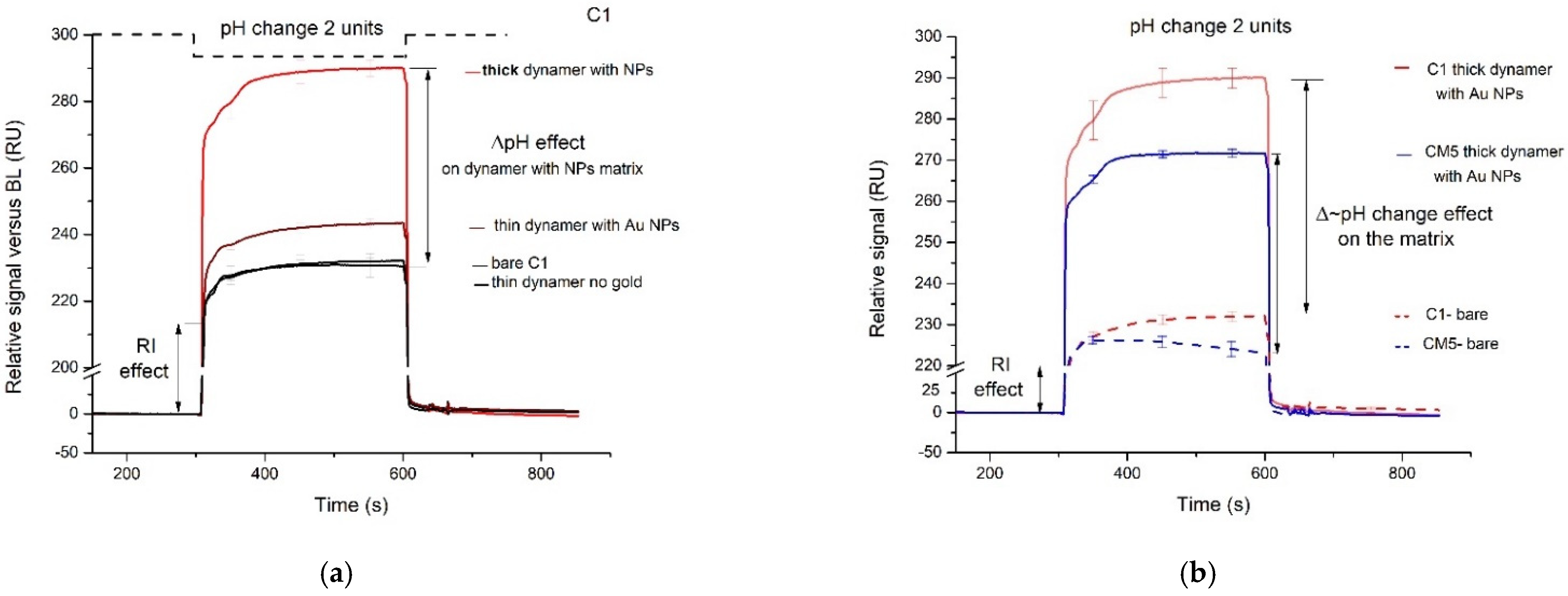
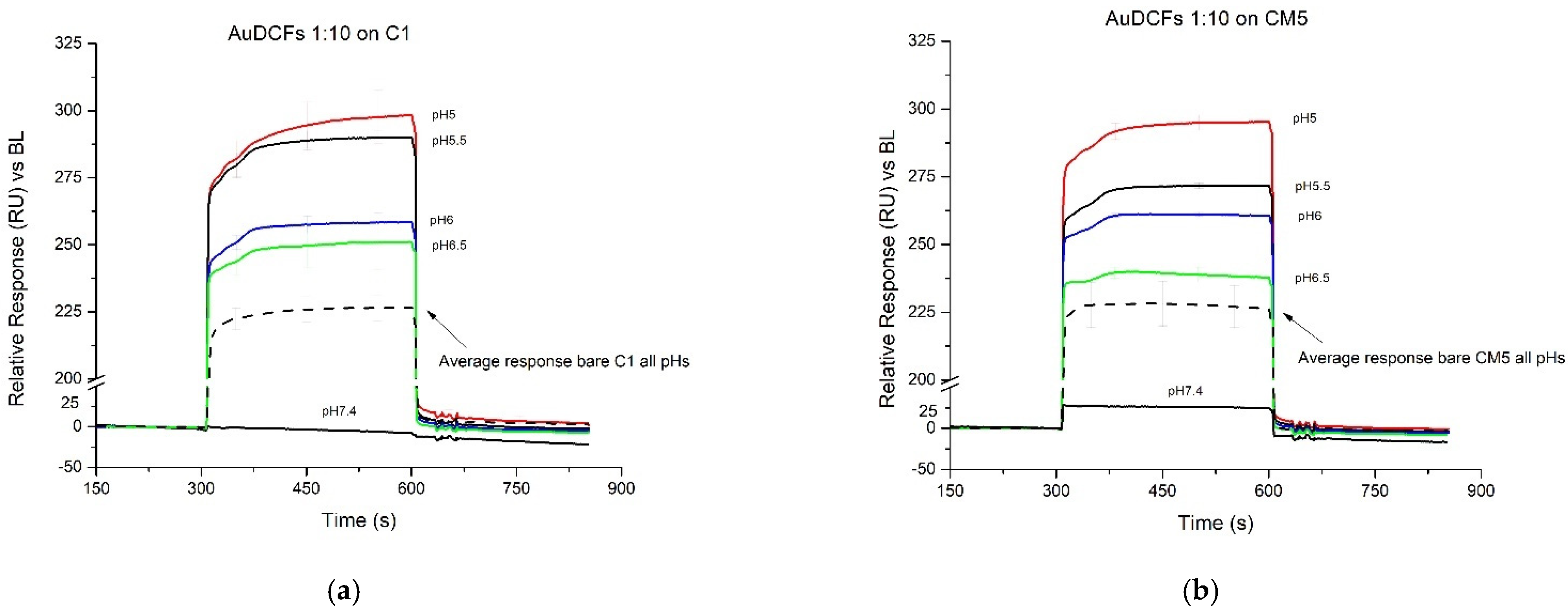
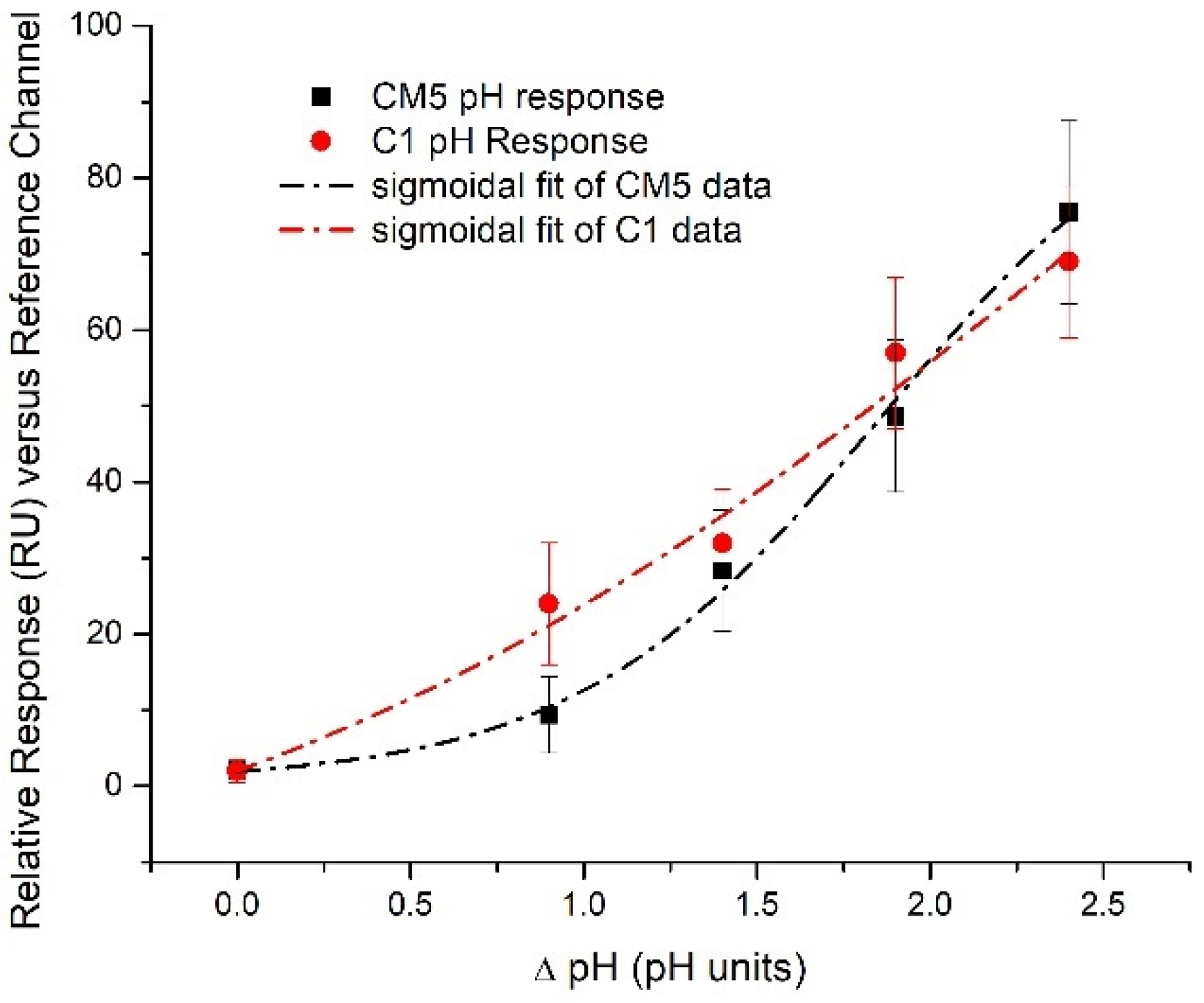
3.3. Conformational Changes of the Au-DCFs Due to pH Changes Revealed by SPR Measurements
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Skene, W.G.; Lehn, J.M.P. Dynamers: Polyacylhydrazone reversible covalent polymers, component exchange, and constitutional diversity. Proc. Natl. Acad. Sci. USA 2004, 101, 8270–8275. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Roy, N.; Bruchmann, B.; Lehn, J.M. DYNAMERS: Dynamic polymers as self-healing materials. Chem. Soc. Rev. 2015, 44, 3786–3807. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Su, D.; Zhang, Y.; Ulrich, S.; Barboiu, M. Constitutional Dynamic Inhibition/Activation of Carbonic Anhydrases. Chempluschem 2021, 86, 1499. [Google Scholar] [CrossRef] [PubMed]
- Su, D.; Coste, M.; Diaconu, A.; Barboiu, M.; Ulrich, S. Cationic dynamic covalent polymers for gene transfection. J. Mater. Chem. B 2020, 8, 9385–9403. [Google Scholar] [CrossRef]
- Zhang, Y.; Wang, Q.; Wang, Z.; Zhang, D.; Gu, J.; Ye, K.; Su, D.; Zhang, Y.; Chen, J.; Barboiu, M. Strong, self-healing gelatin hydrogels cross-linked by double dynamic covalent chemistry. Chempluschem 2021, 86, 1524–1529. [Google Scholar] [CrossRef] [PubMed]
- Herizchi, R.; Abbasi, E.; Milani, M.; Akbarzadeh, A. Current methods for synthesis of gold nanoparticles. Artif. Cells Nanomed. Biotechnol. 2016, 44, 596–602. [Google Scholar] [CrossRef]
- Seok, J.S.; Ju, H. Plasmonic optical biosensors for detecting c-reactive protein: A review. Micromachines 2020, 11, 895. [Google Scholar] [CrossRef]
- Tu, M.H.; Sun, T.; Grattan, K.T.V. Optimization of gold-nanoparticle-based optical fibre surface plasmon resonance (SPR)-based sensors. Sens. Actuators B Chem. 2012, 164, 43–53. [Google Scholar] [CrossRef]
- Nowak, P.; Saggiomo, V.; Salehian, F.; Colomb-Delsuc, M.; Han, Y.; Otto, S. Localized template-driven functionalization of nanoparticles by dynamic combinatorial chemistry. Angew. Chemie-Int. Ed. 2015, 54, 4192–4197. [Google Scholar] [CrossRef]
- Maiti, S.; Prins, L.J. Dynamic combinatorial chemistry on a monolayer protected gold nanoparticle. Chem. Commun. 2015, 51, 5714–5716. [Google Scholar] [CrossRef]
- Rozkiewicz, D.I.; Ravoo, B.J.; Reinhoudt, D.N. Reversible covalent patterning of self-assembled monolayers on gold and silicon oxide surfaces. Langmuir 2005, 21, 6337–6343. [Google Scholar] [CrossRef]
- Yu, R.; Zhang, Y.; Barboiu, M.; Maumus, M.; Noël, D.; Jorgensen, C.; Li, S. Biobased pH-responsive and self-healing hydrogels prepared from O-carboxymethyl chitosan and a 3-dimensional dynamer as cartilage engineering scaffold. Carbohydr. Polym. 2020, 244, 116471. [Google Scholar] [CrossRef]
- Tuoriniemi, J.; Gorton, L.; Ludwig, R.; Safina, G. Determination of the Distance between the Cytochrome and Dehydrogenase Domains of Immobilized Cellobiose Dehydrogenase by Using Surface Plasmon Resonance with a Center of Mass Based Model. Anal. Chem. 2020, 92, 2620–2627. [Google Scholar] [CrossRef]
- Dejeu, J.; Bonnet, H.; Coche-Guérente, L.; Defrancq, E.; Spinelli, N.; van der Heyden, A. Negative SPR signals during low molecular weight analyte recognition. Anal. Chem. 2021, 93, 4134–4140. [Google Scholar] [CrossRef]
- Miyazaki, C.M.; Shimizu, F.M.; Mejía-Salazar, J.R.; Oliveira, O.N.; Ferreira, M. Surface plasmon resonance biosensor for enzymatic detection of small analytes. Nanotechnology 2017, 28, 145501. [Google Scholar] [CrossRef] [PubMed]
- Gestwicki, J.E.; Hsieh, H.V.; Pitner, J.B. Using receptor conformational change to detect low molecular weight analytes by surface plasmon resonance. Anal. Chem. 2001, 73, 5732–5737. [Google Scholar] [CrossRef] [PubMed]
- Dell’Orco, D.; Koch, K.W. Fingerprints of Calcium-Binding Protein Conformational Dynamics Monitored by Surface Plasmon Resonance. ACS Chem. Biol. 2016, 11, 2390–2397. [Google Scholar] [CrossRef] [PubMed]
- Paynter, S.; Russell, D.A. Surface plasmon resonance measurement of pH-induced responses of immobilized biomolecules: Conformational change or electrostatic interaction effects? Anal. Biochem. 2002, 309, 85–95. [Google Scholar] [CrossRef]
- Wang, F.; Wang, J.; Liu, X.; Dong, S. Nanoparticle-amplified surface plasmon resonance study of protein conformational change at interface. Talanta 2008, 77, 628–634. [Google Scholar] [CrossRef]
- De Fernández, C.; Manera, M.G.; Spadavecchia, J.; Maggioni, G.; Quaranta, A.; Mattei, G.; Bazzan, M.; Cattaruzza, E.; Bonafini, M.; Negro, E.; et al. Study of the gas optical sensing properties of Au-polyimide nanocomposite films prepared by ion implantation. Sens. Actuators B Chem. 2005, 111–112, 225–229. [Google Scholar] [CrossRef]
- Ruff, Y.; Buhler, E.; Candau, S.J.; Kesselman, E.; Talmon, Y.; Lehn, J.M. Glycodynamers: Dynamic polymers bearing oligosaccharides residues—Generation, structure, physicochemical, component exchange, and lectin binding properties. J. Am. Chem. Soc. 2010, 132, 2573–2584. [Google Scholar] [CrossRef]
- Lehn, J.M. Dynamers: From supramolecular polymers to adaptive dynamic polymers. Adv. Polym. Sci. 2013, 261, 155–172. [Google Scholar] [CrossRef]
- Howe, R.C.T.; Smalley, A.P.; Guttenplan, A.P.M.; Doggett, M.W.R.; Eddleston, M.D.; Tanb, J.C.; Lloyd, G.O. A family of simple benzene 1,3,5-tricarboxamide (BTA) aromatic carboxylic acid hydrogels. Chem. Commun. 2013, 49, 4268–4270. [Google Scholar] [CrossRef]
- Zhang, Y.; Feng, W.X.; Legrand, Y.M.; Supuran, C.T.; Su, C.Y.; Barboiu, M. Dynameric host frameworks for the activation of lipase through H-bond and interfacial encapsulation. Chem. Commun. 2016, 52, 13768–13770. [Google Scholar] [CrossRef]
- Turkevich, J.; Stevenson, P.C.; Hillier, J. A study of the nucleation and growth processes in the synthesis of colloidal gold. Discuss. Faraday Soc. 1951, 11, 55–75. [Google Scholar] [CrossRef]
- Olaru, A.; Gheorghiu, M.; David, S.; Wohland, T.; Gheorghiu, E. Assessment of the multiphase interaction Between a membrane disrupting peptide and a lipid membrane. J. Phys. Chem. B 2009, 113, 14369–14380. [Google Scholar] [CrossRef]
- Saftics, A.; Prósz, G.A.; Türk, B.; Peter, B.; Kurunczi, S.; Horvath, R. In situ viscoelastic properties and chain conformations of heavily hydrated carboxymethyl dextran layers: A comparative study using OWLS and QCM-I chips coated with waveguide material. Sci. Rep. 2018, 8, 11840. [Google Scholar] [CrossRef]
- Daniel, M.-C.; Astruc, D. Gold Nanoparticles: Assembly, Supramolecular Chemistry, Quantum-Size-Related Properties, and Applications Toward Biology, Catalysis, and Nanotechnology. ChemInform 2004, 35, 239–346. [Google Scholar] [CrossRef]
- Sandu, T.; Vrinceanu, D.; Gheorghiu, E. Surface Plasmon Resonances of Clustered Nanoparticles. Plasmonics 2011, 6, 407–412. [Google Scholar] [CrossRef] [Green Version]
- Sharma, S.; Patel, P.B.; Patel, R.S.; Vora, J.J. Density and comparative refractive index study on mixing properties of binary liquid mixtures of eucalyptol with hydrocarbons at 303.15, 308.15 and 313.15 K. E-J. Chem. 2007, 4, 343–349. [Google Scholar] [CrossRef] [Green Version]



Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
David, S.; Gheorghiu, M.; Daakour, S.; Munteanu, R.-E.; Polonschii, C.; Gáspár, S.; Barboiu, M.; Gheorghiu, E. Real Time SPR Assessment of the Structural Changes of Adaptive Dynamic Constitutional Frameworks as a New Route for Sensing. Materials 2022, 15, 483. https://doi.org/10.3390/ma15020483
David S, Gheorghiu M, Daakour S, Munteanu R-E, Polonschii C, Gáspár S, Barboiu M, Gheorghiu E. Real Time SPR Assessment of the Structural Changes of Adaptive Dynamic Constitutional Frameworks as a New Route for Sensing. Materials. 2022; 15(2):483. https://doi.org/10.3390/ma15020483
Chicago/Turabian StyleDavid, Sorin, Mihaela Gheorghiu, Sanaa Daakour, Raluca-Elena Munteanu, Cristina Polonschii, Szilveszter Gáspár, Mihail Barboiu, and Eugen Gheorghiu. 2022. "Real Time SPR Assessment of the Structural Changes of Adaptive Dynamic Constitutional Frameworks as a New Route for Sensing" Materials 15, no. 2: 483. https://doi.org/10.3390/ma15020483







