In Situ Observation of Liquid Solder Alloys and Solid Substrate Reactions Using High-Voltage Transmission Electron Microscopy
Abstract
:1. Introduction
2. Materials and Methods
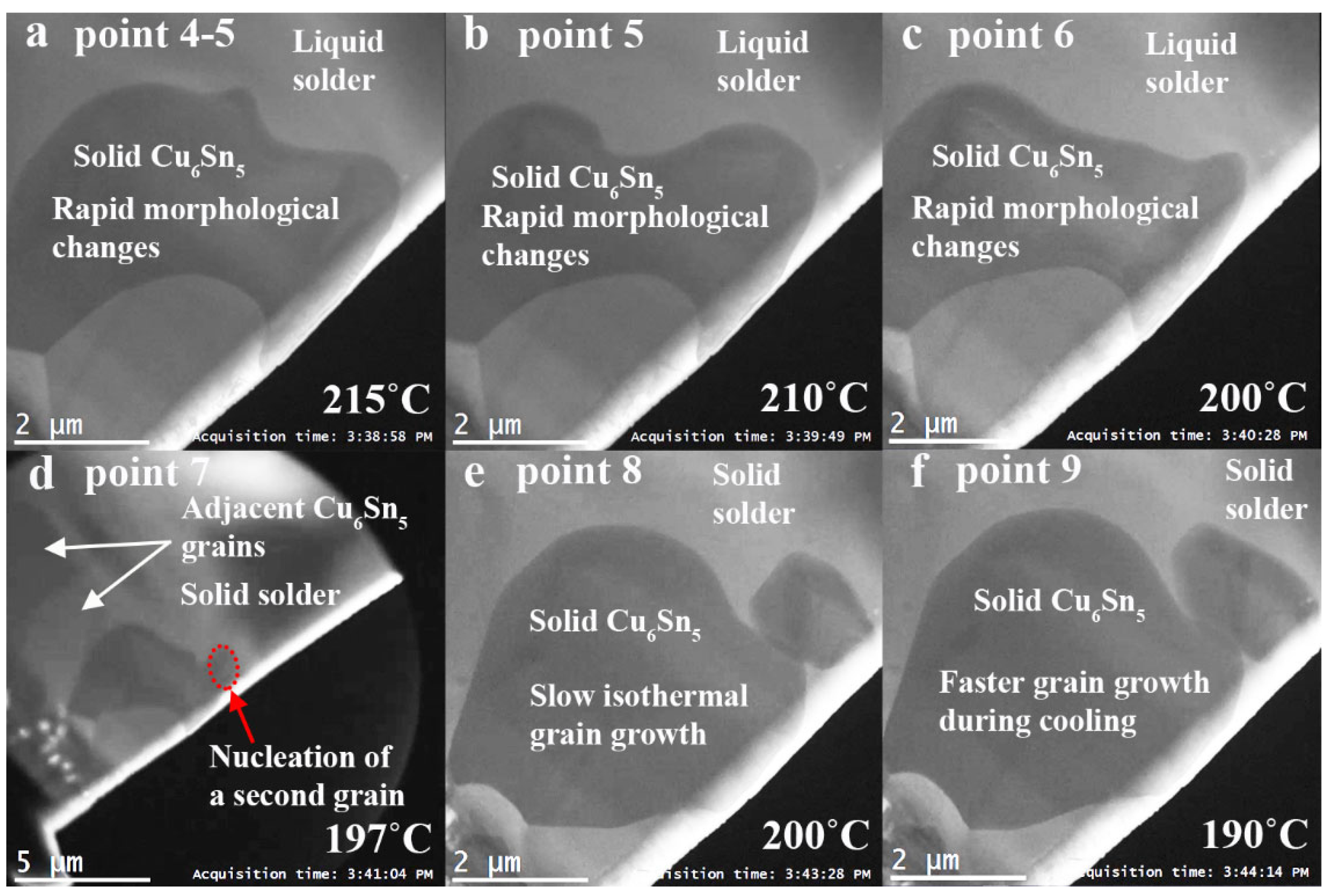
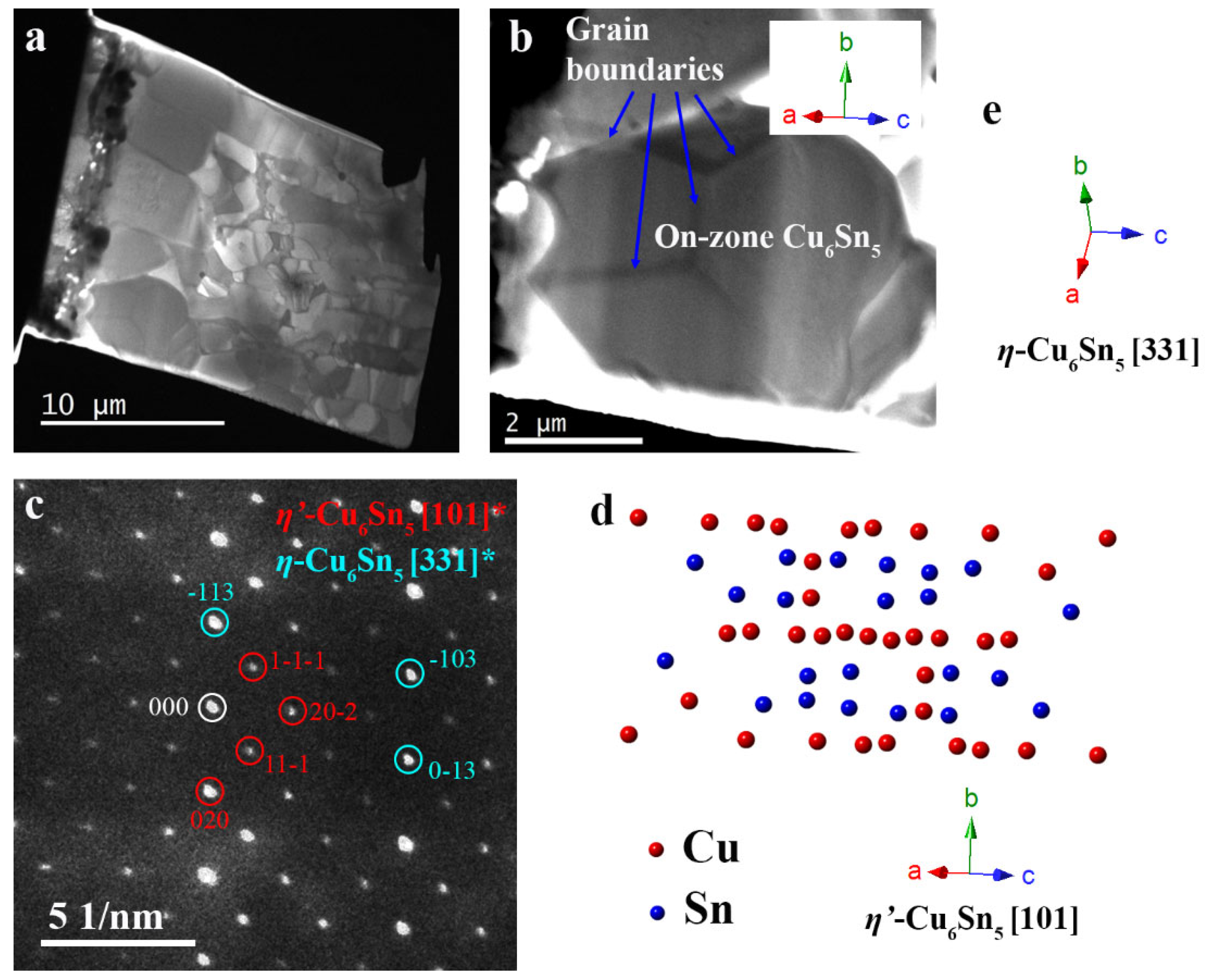
3. Results and Discussion
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Cheng, S.; Huang, C.-M.; Pecht, M. A review of lead-free solders for electronics applications. Microelectron. Reliab. 2017, 75, 77–95. [Google Scholar] [CrossRef]
- Mayappan, R.; Ghani, N.A.A.; Yahya, I. The effect of Ni addition on Cu-Sn intermetallic growth rate values in the SAC solder. AIP Conf. Proc. 2018, 2031, 020016. [Google Scholar] [CrossRef]
- Ng, W.C.W.; Sweatman, K.; Akaiwa, T.; Nishimura, T.; Sato, M.; Gourlay, C.; Belyakov, S. Dissolution in service of the copper substrate of solder joints. In Proceedings of the 2016 IEEE 18th Electronics Packaging Technology Conference (EPTC), Singapore, 30 November–3 December 2016. [Google Scholar]
- Somidin, F.; Maeno, H.; Mohd Salleh, M.A.A.; Tran, X.Q.; McDonald, S.D.; Matsumura, S.; Nogita, K. Characterising the polymorphic phase transformation at a localised point on a Cu6Sn5 grain. Mater. Charact. 2018, 138, 113–119. [Google Scholar] [CrossRef]
- Somidin, F.; Maeno, H.; Tran, X.Q.; McDonald, S.D.; Mohd Salleh, M.A.A.; Matsumura, S.; Nogita, K. Imaging the Polymorphic Transformation in a Single Cu6Sn5 Grain in a Solder Joint. Materials 2018, 11, 2229. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Somidin, F.; Maeno, H.; Toriyama, T.; McDonald, S.D.; Yang, W.; Matsumura, S.; Nogita, K. Direct observation of the Ni stabilising effect in interfacial (Cu,Ni)6Sn5 intermetallic compounds. Materialia 2020, 9, 100530. [Google Scholar] [CrossRef]
- Mohd Salleh, M.A.A.; McDonald, S.D.; Yasuda, H.; Sugiyama, A.; Nogita, K. Rapid Cu6Sn5 growth at liquid Sn/solid Cu interfaces. Scr. Mater. 2015, 100, 17–20. [Google Scholar] [CrossRef]
- Mohd Salleh, M.A.A.; Gourlay, C.M.; Xian, J.W.; Belyakov, S.A.; Yasuda, H.; McDonald, S.D.; Nogita, K. In situ imaging of microstructure formation in electronic interconnections. Sci. Rep. 2017, 7, 40010. [Google Scholar] [CrossRef] [Green Version]
- Lord, R.A.; Umantsev, A. Early stages of soldering reactions. J. Appl. Phys. 2005, 98, 063525. [Google Scholar] [CrossRef] [Green Version]
- Pan, C.-C.; Yu, C.-H.; Lin, K.-L. The amorphous origin and the nucleation of intermetallic compounds formed at the interface during the soldering of Sn–3.0Ag–0.5Cu on a Cu substrate. Appl. Phys. Lett. 2008, 93, 061912. [Google Scholar] [CrossRef]
- Gong, J.; Liu, C.; Conway, P.P.; Silberschmidt, V.V. Evolution of CuSn intermetallics between molten SnAgCu solder and Cu substrate. Acta Mater. 2008, 56, 4291–4297. [Google Scholar] [CrossRef]
- Howe, J.M.; Saka, H. In Situ Transmission Electron Microscopy Studies of the Solid–Liquid Interface. MRS Bull. 2011, 29, 951–957. [Google Scholar] [CrossRef]
- Matsuki, H.; Ibuka, H.; Saka, H. TEM observation of interfaces in a solder joint in a semiconductor device. Sci. Technol. Adv. Mater. 2002, 3, 261–270. [Google Scholar] [CrossRef]
- Saka, H.; Sasaki, K.; Tsukimoto, S.; Arai, S. In situ Observation of Solid–liquid Interfaces by Transmission Electron Microscopy. J. Mater. Res. 2005, 20, 1629–1640. [Google Scholar] [CrossRef]
- Torazawa, N.; Arai, S.; Takase, Y.; Sasaki, K.; Saka, H. Transmission Electron Microscopy of Interfaces in Joints between Pb-Free Solders and Electroless Ni-P. Mater. Trans. 2003, 44, 1438–1447. [Google Scholar] [CrossRef]
- Lee, J.G.; Mori, H. In-situobservation of alloy phase formation in nanometre-sized particles in the Sn–Bi system. Philos. Mag. 2004, 84, 2675–2686. [Google Scholar] [CrossRef]
- Lee, J.G.; Mori, H.; Yasuda, H. In situobservation of a fluid amorphous phase formed in isolated nanometer-sized particles in the Sn-Bi system. Phys. Rev. B 2002, 66, 012105. [Google Scholar] [CrossRef]
- Allen, G.L.; Bayles, R.A.; Gile, W.W.; Jesser, W.A. Small particle melting of pure metals. Thin Solid Film. 1986, 144, 297–308. [Google Scholar] [CrossRef]
- Lai, S.L.; Guo, J.Y.; Petrova, V.V.; Ramanath, G.; Allen, L.H. Size-Dependent Melting Properties of Small Tin Particles: Nanocalorimetric Measurements. Phys. Rev. Lett. 1996, 77, 99–102. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tan, X.F.; Yang, W.; Aso, K.; Matsumura, S.; McDonald, S.D.; Nogita, K. Evidence of Copper Separation in Lithiated Cu6Sn5 Lithium-Ion Battery Anodes. ACS Appl. Energy Mater. 2019, 3, 141–145. [Google Scholar] [CrossRef] [Green Version]
- Sadamatsu, S.; Tanaka, M.; Higashida, K.; Matsumura, S. Transmission electron microscopy of bulk specimens over 10microm in thickness. Ultramicroscopy 2016, 162, 10–16. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Omoto, K.; Tsuno, K.; Ohsaki, M.; Matsumura, S.; Tomokiyo, Y. Omega filter installed in the 1MV microscope of Kyushu University. Micron 2008, 39, 666–675. [Google Scholar] [CrossRef]
- Zhang, X.; Hu, X.; Jiang, X.; Li, Y. Effect of Ni addition to the Cu substrate on the interfacial reaction and IMC growth with Sn3.0Ag0.5Cu solder. Appl. Phys. A 2018, 124, 315. [Google Scholar] [CrossRef]
- Li, S.; Liu, Y.; Zhang, H.; Cai, H.; Sun, F.; Zhang, G. Microstructure and hardness of SAC305 and SAC305-0.3Ni solder on Cu, high temperature treated Cu, and graphene-coated Cu substrates. Results Phys. 2018, 11, 617–622. [Google Scholar] [CrossRef]
- Zeng, G.; McDonald, S.D.; Read, J.J.; Gu, Q.; Nogita, K. Kinetics of the polymorphic phase transformation of Cu6Sn5. Acta Mater. 2014, 69, 135–148. [Google Scholar] [CrossRef]
- Passerone, A.; Ricci, E.; Sangiorgi, R. Influence of oxygen contamination on the surface tension of liquid tin. J. Mater. Sci. 1990, 25, 4266–4272. [Google Scholar] [CrossRef]
- Lide, D.R. CRC Handbook of Chemistry and Physics, 87th ed Editor-in-Chief: David R. Lide (National Institute of Standards and Technology). CRC Press/Taylor and Francis Group: Boca Raton, FL. 2006. 2608 pp. $139.95. ISBN 0-8493-0487-3. J. Am. Chem. Soc. 2006, 129, 724. [Google Scholar] [CrossRef]
- Jeong, J.; Lee, S.; Jeon, J.-B.; Kim, S. Excessively High Vapor Pressure of Al-based Amorphous Alloys. Metals 2015, 5, 1878–1886. [Google Scholar] [CrossRef] [Green Version]
- Xian, J.W.; Belyakov, S.A.; Ivier, M.; Nogita, K.; Yasuda, H.; Gourlay, C.M. Cu6Sn5 crystal growth mechanisms during solidification of electronic interconnections. Acta Mater. 2017, 126, 540–551. [Google Scholar] [CrossRef]
- Nakano, K.; Narumi, T.; Morishita, K.; Yasuda, H. Characterization of Growing Dendrites in CrMnFeCoNi High-Entropy Alloy by Time-Resolved and In-Situ Tomography. Mater. Trans. 2020, 61, 596–604. [Google Scholar] [CrossRef] [Green Version]
- Kim, H.K.; Tu, K.N. Kinetic analysis of the soldering reaction between eutectic SnPb alloy and Cu accompanied by ripening. Phys. Rev. B Condens. Matter. 1996, 53, 16027–16034. [Google Scholar] [CrossRef]
- Gusak, A.M.; Tu, K.N.; Chen, C. Extremely rapid grain growth in scallop-type Cu6Sn5 during solid–liquid interdiffusion reactions in micro-bump solder joints. Scr. Mater. 2020, 179, 45–48. [Google Scholar] [CrossRef]
- Kim, H.K.; Liou, H.K.; Tu, K.N. Three-dimensional morphology of a very rough interface formed in the soldering reaction between eutectic SnPb and Cu. Appl. Phys. Lett. 1995, 66, 2337–2339. [Google Scholar] [CrossRef]



Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Tan, X.F.; Somidin, F.; McDonald, S.D.; Bermingham, M.J.; Maeno, H.; Matsumura, S.; Nogita, K. In Situ Observation of Liquid Solder Alloys and Solid Substrate Reactions Using High-Voltage Transmission Electron Microscopy. Materials 2022, 15, 510. https://doi.org/10.3390/ma15020510
Tan XF, Somidin F, McDonald SD, Bermingham MJ, Maeno H, Matsumura S, Nogita K. In Situ Observation of Liquid Solder Alloys and Solid Substrate Reactions Using High-Voltage Transmission Electron Microscopy. Materials. 2022; 15(2):510. https://doi.org/10.3390/ma15020510
Chicago/Turabian StyleTan, Xin F., Flora Somidin, Stuart D. McDonald, Michael J. Bermingham, Hiroshi Maeno, Syo Matsumura, and Kazuhiro Nogita. 2022. "In Situ Observation of Liquid Solder Alloys and Solid Substrate Reactions Using High-Voltage Transmission Electron Microscopy" Materials 15, no. 2: 510. https://doi.org/10.3390/ma15020510







