Physical Modeling of the Impeller Construction Impact on the Aluminum Refining Process
Abstract
1. Introduction
2. Research Study and the Object
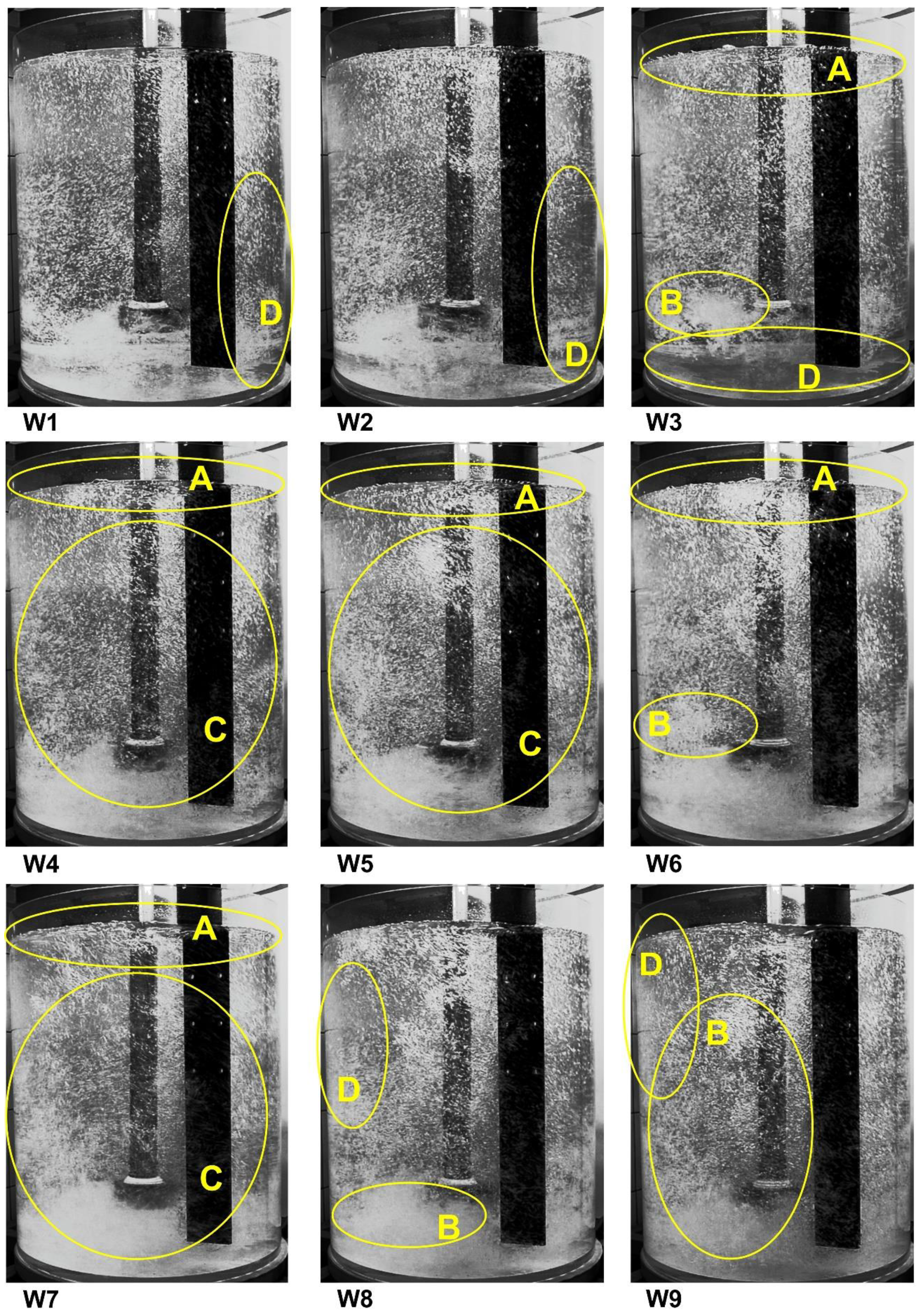
3. Results and Discussion
- A—single gas bubbles formed on the surface of the modeling liquid,
- B—excessive formation of gas chains and swirls,
- C—uniform distribution of gas bubbles in the entire volume of the tank,
- D—dead zones without gas bubbles, no dispersion.
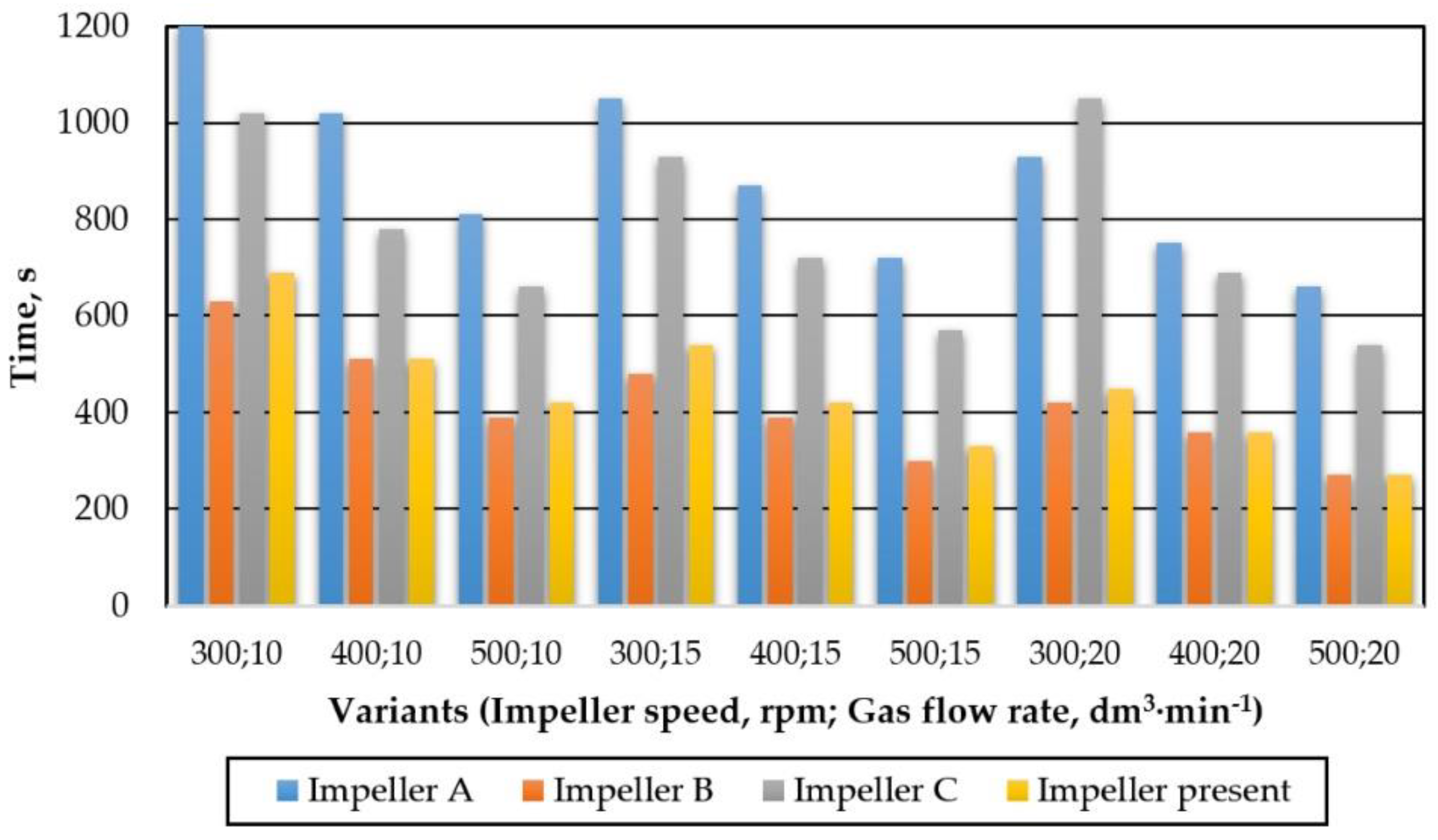
4. Comparison of Results for a New Impeller with Other Industrial Applications
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Schmitz, C. Handbook of Aluminium Recycling; Vulkan-Verlag GmbH: Essen, Germany, 2006. [Google Scholar]
- Zhang, L.; Lv, X.; Torgeson, A.T.; Long, M. Removal of impurity elements from molten aluminum: A review. Miner. Process. Extr. Metall. Rev. 2011, 32, 150–228. [Google Scholar] [CrossRef]
- Taylor, M.B. Molten metal fluxing/treatment: How best achieve the desired quality requirements. Aluminum 2003, 79, 44–50. [Google Scholar]
- Sigworth, G.K. A scientific basis for degassing aluminum. AFS Trans. 1987, 95, 73–78. [Google Scholar]
- Siemensen, C.J.; Berg, G. A survey of inclussions in aluminium. Aluminum 1980, 56, 335–340. [Google Scholar]
- Aarflot, A. New melt cleaning technology. In Light Metals; TMS: Warrendale, PA, USA, 1991; pp. 1133–1137. [Google Scholar]
- Chateau, J.M. Latest trends in molten metal in-line treatment. Alum. Times 2003, 04/05, 34–35. [Google Scholar]
- Diaz, M.C.; Komarov, S.V.; Sano, M. Bubble behaviour and absorption rate in gas injection through rotary lances. ISIJ Int. 1997, 37, 1–8. [Google Scholar] [CrossRef]
- Johansen, S.; Graadahl, S.; Tetlie, P.; Rasch, B.; Myrbostad, E. Can rotor-based refining units be developed and optimized based on water model experiments? In Light Metals; TMS: Warrendale, PA, USA, 1998; pp. 805–810. [Google Scholar]
- Saternus, M.; Merder, T. Numerical and physical modelling of aluminium refining process conducted in uro-200 reactor. Solid State Phenom. 2012, 191, 3–12. [Google Scholar] [CrossRef]
- Tovio, D.O.; Mugica, G.W.; González, A.C.; Cuyás, J.C. Formation and size of bubbles in degassing system of aluminum. AFS Trans. 2000, 108, 457–462. [Google Scholar]
- Hernández-Hernández, M.; Camacho-Martínez, J.L.; González-Rivera, C.; Ramírez-Argáez, M.A. Impeller design assisted by physical modeling and pilot plant trials. J. Mat. Process. Technol. 2016, 236, 1–8. [Google Scholar] [CrossRef]
- Michalek, K.; Socha, L.; Gryc, K.; Tkadleckova, M.; Saternus, M.; Pieprzyca, J.; Merder, T. Modelling of technological parameters of aluminium melt refining in the ladle by blowing of inert gas through the rotating impeller. Arch. Metall. Mater. 2018, 63, 987–992. [Google Scholar]
- Mi, G.; Liu, X.; Wang, K.; Qi, S.; Wang, H.W.; Niu, J.T. Analyses of the influencing factors of rotating impeller degassing process and water simulation experiment. J. Mater. Sci. Forum 2008, 575–578, 1258–1265. [Google Scholar] [CrossRef]
- Ramos Gomez, E.; Zenit, R.; González Rivera, C.; Trápaga, G.; Ramírez-Argáez, M.A. Mathematical modeling of fluid flow in a water physical model of an aluminum degassing ladle equipped with an impeller-injector. Metall. Mater. Trans. B 2013, 44B, 423–435. [Google Scholar] [CrossRef]
- Panic, B. 3D Model studies on the effect of bed and powder type upon radial static pressure and powder distribution in metallurgical shaft furnaces. Arch. Metall. Mater. 2017, 62, 1449–1452. [Google Scholar] [CrossRef][Green Version]
- Michalek, K.; Gryc, K.; Moravka, J. Physical modelling of bath homogenization in argon stirred ladle. Metalurgija 2009, 48, 215–218. [Google Scholar]
- Mazdumar, D.; Guthrie, R.I.L. The physical and mathematical modelling of gas stirred ladle systems. ISIJ Int. 1995, 35, 1–20. [Google Scholar]
- Mishra, S.K.; Jha, P.K.; Sharma, S.C.; Ajamani, S.K. Effect of blockage of outlet nozzle on fluid flow and heat transfer in continuously cast multistrand billet caster tundish. Can. Metall. Q. 2012, 51, 170–183. [Google Scholar] [CrossRef]
- Wan, B.; Chen, W.; Mao, M.; Fu, Z.; Zhu, D. Numerical simulation of a stirring purifying technology for aluminum melt. J. Mater. Process. Tech. 2018, 251, 330–342. [Google Scholar] [CrossRef]
- Fornalczyk, A.; Golak, S.; Przyłucki, R. Investigation of the influence of supply parameters on the velocity of molten metal in a metallurgical reactor used for platinum recovery. Arch. Civ. Mech. Eng. 2015, 15, 171–178. [Google Scholar] [CrossRef]
- Fornalczyk, A.; Golak, S.; Saternus, M. Model of infiltration of spent automotive catalysts by molten metal in process of platinum metals recovery. Math. Probl. Eng. 2013, 2013, 461085. [Google Scholar] [CrossRef]
- Michalek, K. The Use of Physical Modeling and Numerical Optimization for Metallurgical Processes; Publishing of the VSB: Ostrawa, Czech Republic, 2001. [Google Scholar]
- Müller, L. Application of Dimensional Analysis in Model Research; Publishing of the PWN: Warsawa, Poland, 1983. [Google Scholar]
- Pieprzyca, J.; Kudliński, Z. Mathematical and Physical Modeling of Phenomena in Technological Processes; Publishing of the WIMiM: Katowice, Poland, 2006. [Google Scholar]
- Saternus, M. Physical Modelling of Phenomena Occurring During Refining Process of Fe and Al Solutions by Means of Inert Gases; Publishing of the Silesian University of Technology: Gliwice, Poland, 2020. [Google Scholar]
- Camacho-Martínez, J.L.; Ramírez-Argáez, M.A.; Zenit-Camacho, R.; Juárez-Hernández, A.; Berceinas-Sanchez, J.O.; Trápaga-Martánez, G. Physical modelling of an aluminium degassing operation with rotating impellers—A comparative hydrodynamic analysis. Mater. Manuf. Processes 2010, 2, 581–591. [Google Scholar] [CrossRef]
- Saternus, M.; Merder, T. Influence of processing parameters on the gas dispersion level in the process of argon blowing through aluminium. Acta Metall. Slovaca 2014, 20, 181–188. [Google Scholar] [CrossRef][Green Version]
- Chen, J.; Zhao, J. Bubble distribution in a melt treatment water model. In Light Metals; TMS: Warrendale, PA, USA, 1995; pp. 1227–1231. [Google Scholar]
- Camacho-Martínez, J.L.; Ramírez-Argáez, M.A.; Juárez-Hernández, A.; González-Rivera, C.; Trápaga-Martánez, G. Novel degasification design for aluminum using an impeller degasification water physical model. Mater. Manuf. Processes 2012, 27, 556–560. [Google Scholar] [CrossRef]
- Saternus, M.; Botor, J. Physical model of aluminium refining process in URC-7000. Metalurgija 2009, 48, 175–179. [Google Scholar]
- Mancilla, E.; Cruz-Mendez, W.; Garduno, I.E.; Gonzalez-Rivera, C.; Ramirez-Argaez, M.A.; Ascanio, G. Comparison of the hydrodynamic performance of rotor-injector devices in a water physical model of an aluminum degassing ladle. Chem. Eng. Res. Des. 2017, 118, 158–169. [Google Scholar] [CrossRef]
- Saternus, M.; Merder, T.; Pieprzyca, J. The influence of impeller geometry on the gas bubbles dispersion in uro-200 reactor—RTD curves. Arch. Metall. Mater. 2015, 60, 2887–2893. [Google Scholar] [CrossRef]
- Saternus, M.; Szudy, R.; Róg, M.; Pieprzyca, J.; Merder, T. Research considering the influence of the construction of rotary impeller on the hydrodynamics of the aluminium batch refining process. Rudy Met. Nieżelazne 2017, 62, 9–15. [Google Scholar]
- Saternus, M.; Merder, T. Physical modelling of aluminum refining process conducted in batch reactor with rotary impeller. Metals 2018, 8, 726. [Google Scholar] [CrossRef]
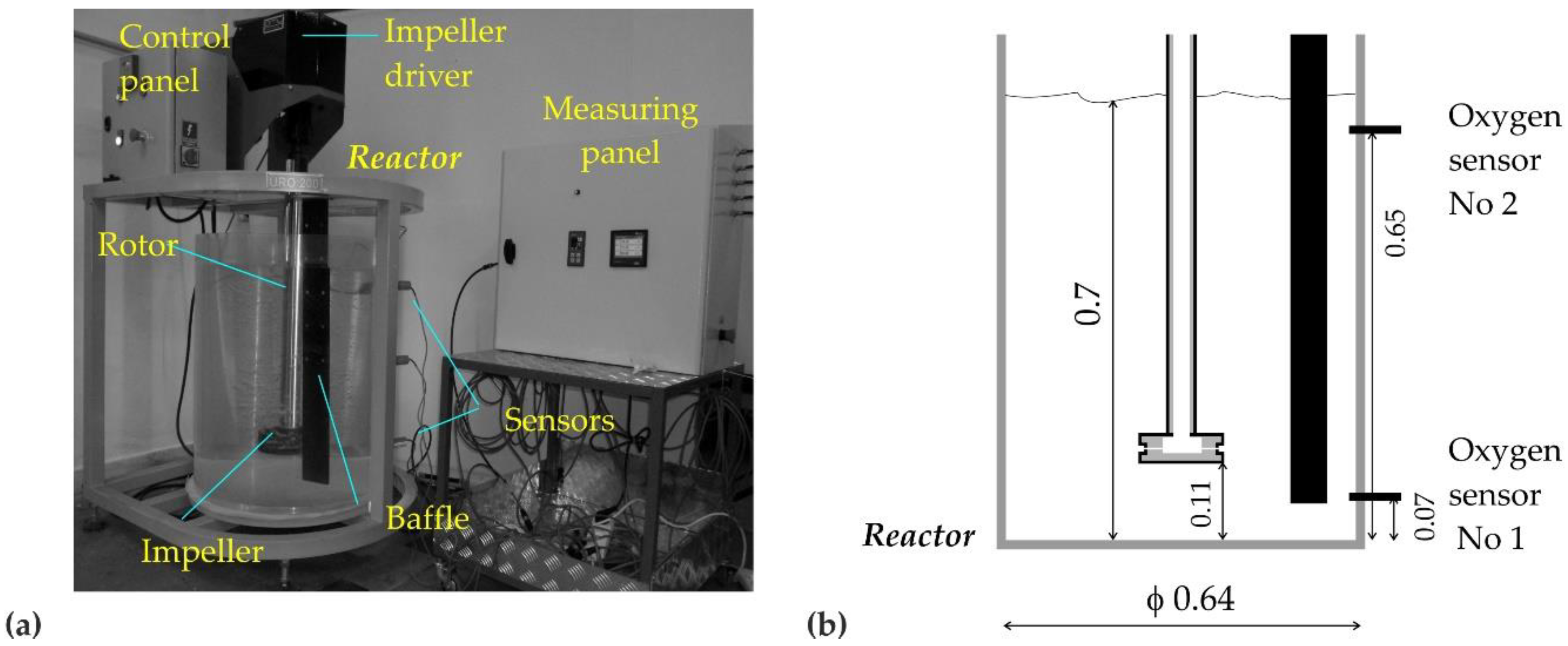
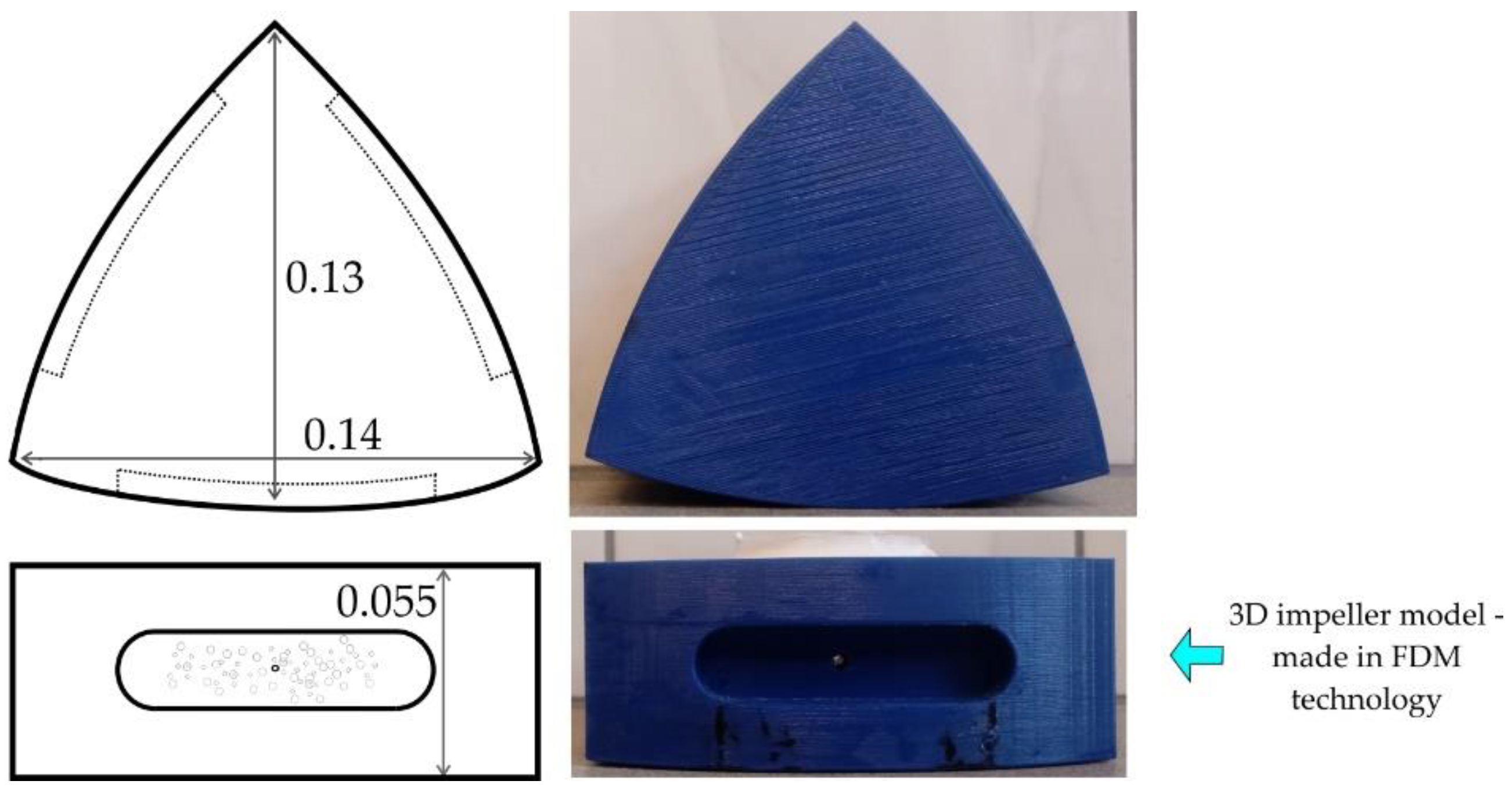
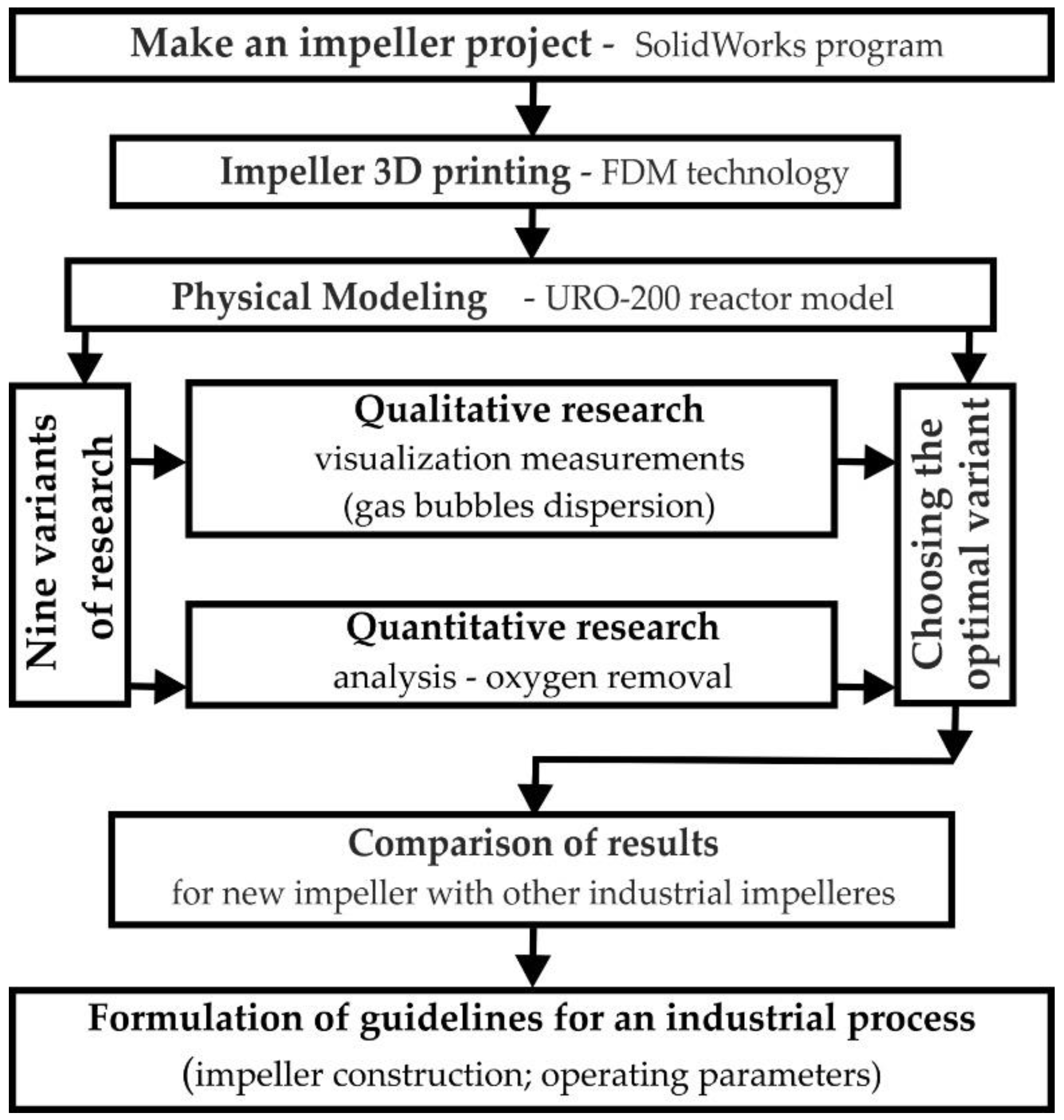
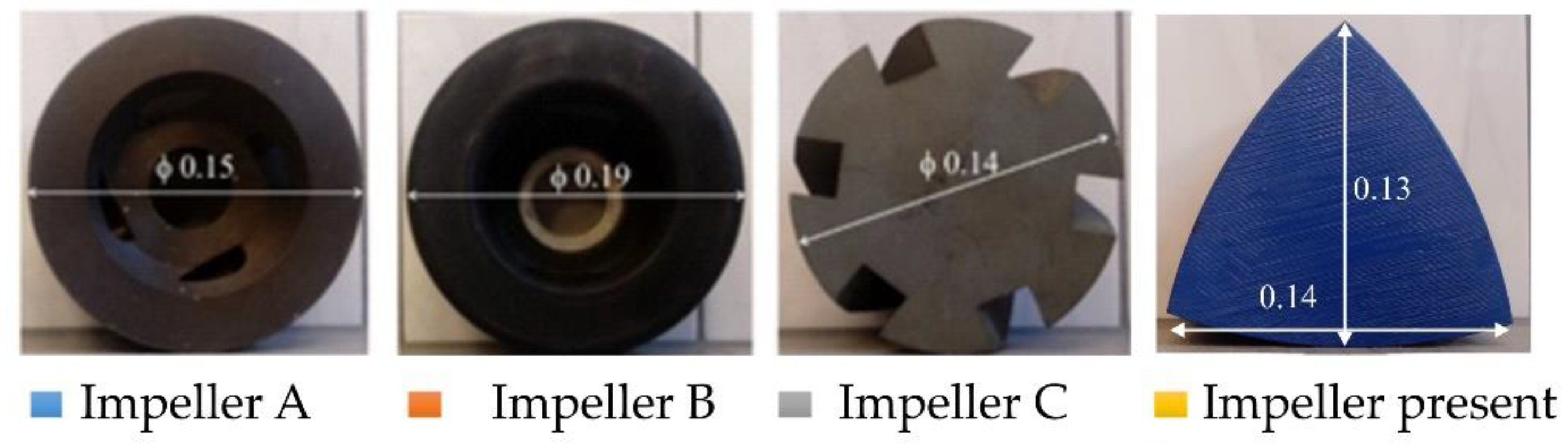

| Parameter | Unit | Value | |
|---|---|---|---|
| Volume of the model | m3 | 0.23 | |
| Characteristic dimension of the impeller | m | 0.14 | |
| Liquids | water | aluminum | |
| Temperature | K | 293 | 973 |
| Kinematic viscosity | m2·s−1 | 1.005 · 10−6 | 1.000 · 10−6 |
| Surface tension | N·m−1 | 0.072 | 0.868 |
| Density | kg·m−3 | 1000 | 2700 |
| Reynolds number | - | 49,000 | 132,300 |
| Weber number | - | 238.19 | 53.35 |
| Froude number | - | 0.089 | 0.089 |
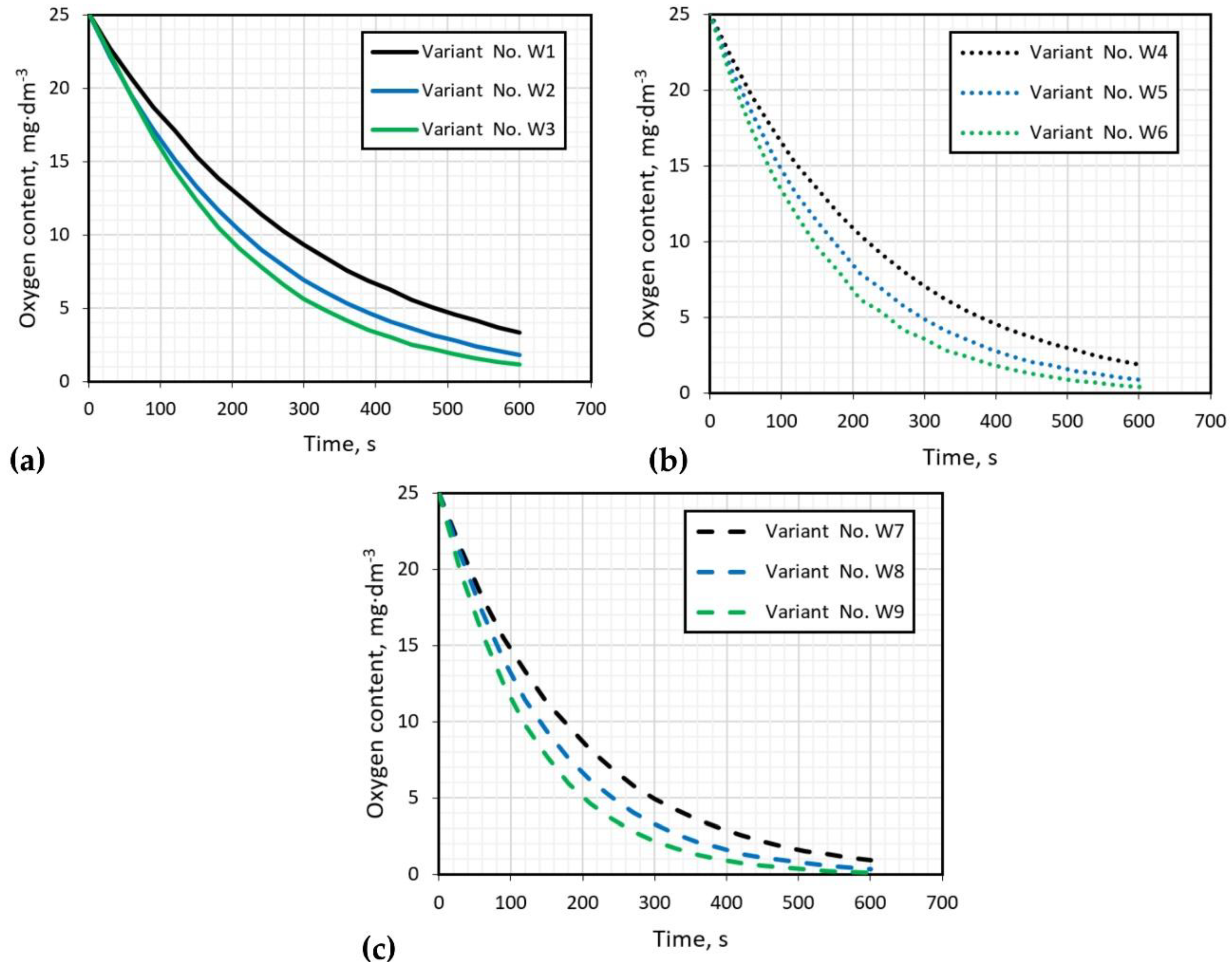
| No | Impeller Speed | Gas Flow Rate | No | Impeller Speed | Gas Flow Rate | No | Impeller Speed | Gas Flow Rate |
|---|---|---|---|---|---|---|---|---|
| rpm | dm3·min−1 | rpm | dm3·min−1 | rpm | dm3·min−1 | |||
| W1 | 300 | 10 | W4 | 400 | 10 | W7 | 500 | 10 |
| W2 | 15 | W5 | 15 | W8 | 15 | |||
| W3 | 20 | W6 | 20 | W9 | 20 |
| Gas Flow Rate, dm3·min−1 | Type of Dispersion/Impeller Speed, rpm | ||
|---|---|---|---|
| 300 | 400 | 500 | |
| 10 | intimate | uniform | uniform |
| 15 | intimate | uniform | excessive |
| 20 | intimate | excessive | excessive |
| Variant | Time, s | Variant | Time, s | Variants | Time, s |
|---|---|---|---|---|---|
| W1 | 690 | W4 | 510 | W7 | 420 |
| W2 | 540 | W5 | 420 | W8 | 330 |
| W3 | 450 | W6 | 360 | W9 | 270 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Saternus, M.; Merder, T. Physical Modeling of the Impeller Construction Impact on the Aluminum Refining Process. Materials 2022, 15, 575. https://doi.org/10.3390/ma15020575
Saternus M, Merder T. Physical Modeling of the Impeller Construction Impact on the Aluminum Refining Process. Materials. 2022; 15(2):575. https://doi.org/10.3390/ma15020575
Chicago/Turabian StyleSaternus, Mariola, and Tomasz Merder. 2022. "Physical Modeling of the Impeller Construction Impact on the Aluminum Refining Process" Materials 15, no. 2: 575. https://doi.org/10.3390/ma15020575
APA StyleSaternus, M., & Merder, T. (2022). Physical Modeling of the Impeller Construction Impact on the Aluminum Refining Process. Materials, 15(2), 575. https://doi.org/10.3390/ma15020575







