Preparation, Characterization of Granulated Sulfur Fertilizers and Their Effects on a Sandy Soils
Abstract
:1. Introduction
2. Materials and Methods
2.1. Preparation of Granular Sulfur Fertilizers
- waste sulfur (95%) + halloysite (5%);
- waste sulfur (81%) + halloysite (5%) + humic acids (14%);
- waste sulfur (50%) + halloysite (50%);
- waste sulfur (46%) + halloysite (46%) + humic acids (8%).
2.2. Establishing the Incubation Experiment
2.3. Methods of Laboratory Analyses
- 0.03 mol L−1 CH3COOH (Chempur, Piekary Śląskie, Poland); the extraction was conducted at 1:10 (m/v) ratio, the suspensions were shaken for 30 min at 30 rpm, filtrated and analyzed by inductively coupled plasma-optical emission spectrometry (ICP-OES), on an Optima 7300 DV spectrometer (Perkin-Elmer, Waltham, MA, USA);
- unbuffered, neutral 0.01 mol L−1 CaCl2 (POCH, Gliwice, Poland); the extraction was conducted at 1:10 (m/v) ratio, the suspensions were shaken for 2 h at 40 rpm, filtrated and analyzed by ICP-OES method;
- Mehlich 3 (M3) (pH 2.5) (0.2 mol L−1 CH3COOH (Chempur, Piekary Śląskie, Poland), 0.25 mol L−1 NH4NO3 (Chempur, Piekary Śląskie, Poland), 0.015 mol L−1 NH4F (Chempur, Piekary Śląskie, Poland), 0.013 mol L−1 HNO3 (Chempur, Piekary Śląskie, Poland), 0.001 mol L−1 EDTA (POCH, Gliwice, Poland)); the extraction was conducted at 1:10 (m/v) ratio, the suspensions were shaken for 5 min at 40 rpm, filtrated and analyzed by ICP-OES method.
2.4. Statistical Analysis of the Results
3. Results and Discussion
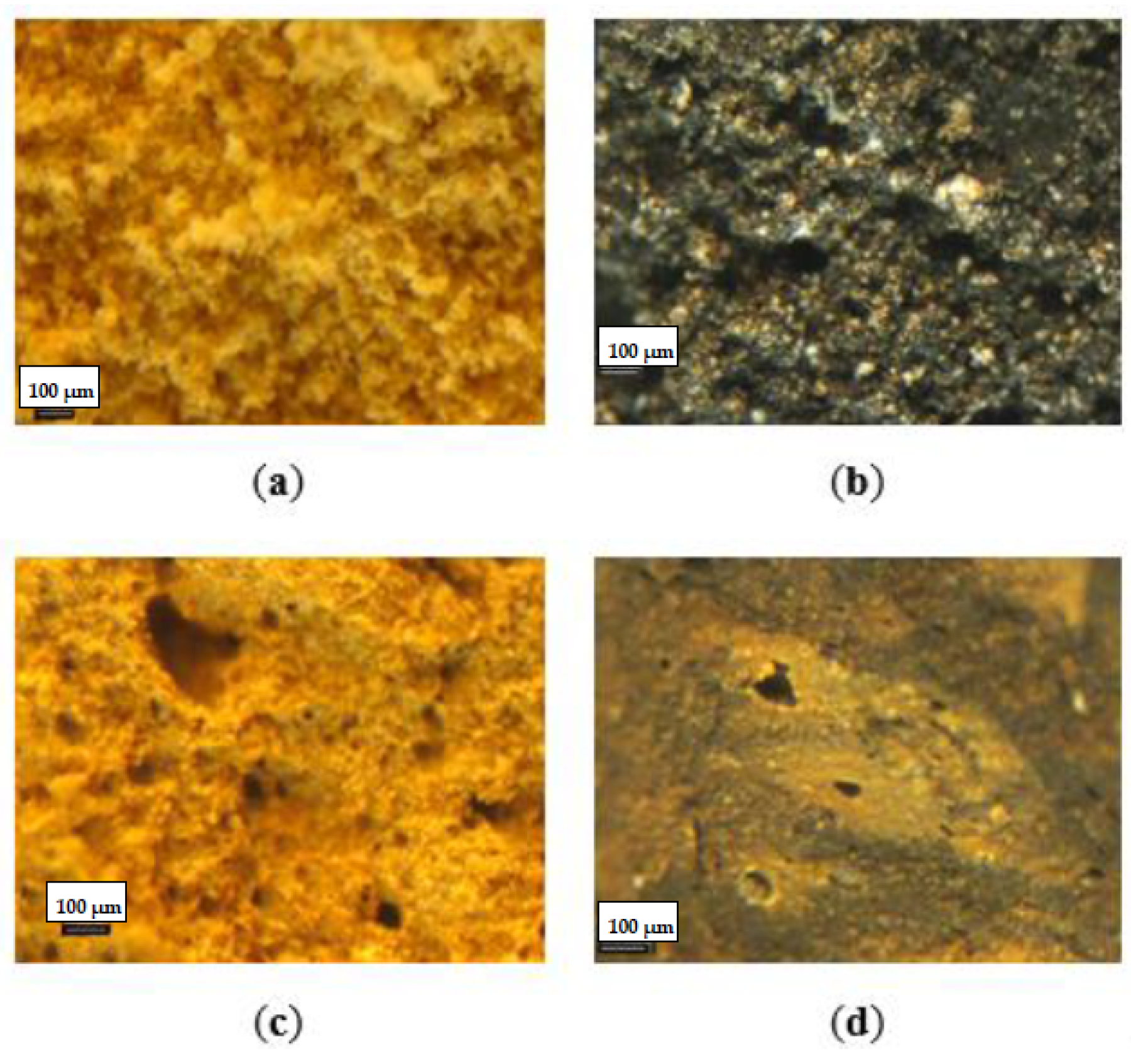
3.1. Physical Properties of Fertilizer Granules
3.2. Soil properties after Fertilizer Application
3.3. Comparison of Soil Extractants
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Fróna, D.; Szenderák, J.; Harangi-Rákos, M. The challenge of feeding the World. Sustainability 2019, 11, 5816. [Google Scholar] [CrossRef] [Green Version]
- Cakmak, I. Plant nutrition research: Priorities to meet human needs for food in sustainable ways. In Progress in Plant Nutrition: Plenary Lectures of the XIV International Plant Nutrition Colloquium; Horst, W.J., Bürkert, A., Claassen, N., Flessa, H., Frommer, W.B., Goldbach, H., Merbach, W., Olfs, H.W., Römheld, V., Sattelmacher, B., et al., Eds.; Springer: Dordrecht, The Netherlands, 2002; pp. 3–24. [Google Scholar] [CrossRef]
- Von Uexküll, H.R.; Mutert, E. Global extent, development and economic impact of acid soils. Plant Soil 1995, 17, 1–15. [Google Scholar] [CrossRef]
- Bian, M.; Zhou, M.; Sun, D.; Li, C. Molecular approaches unravel the mechanism of acid soil tolerance in plants. Crop J. 2013, 1, 91–104. [Google Scholar] [CrossRef] [Green Version]
- Zhao, F.; Hawkesford, M.; McGrath, S. Sulphur assimilation and effects on yield and quality of wheat. J. Cereal Sci. 1999, 30, 1–17. [Google Scholar] [CrossRef]
- Blake-Kalff, M.M.A.; Zhao, F.J.; Hawkesford, M.J.; Mcgrath, S.P. Using plant analysis to predict yield losses caused by sulphur deficiency. Ann. Appl. Biol. 2001, 138, 123–127. [Google Scholar] [CrossRef]
- Scherer, H.W. Sulphur in crop production—Invited paper. Eur. J. Agron. 2001, 14, 81–111. [Google Scholar] [CrossRef]
- McNeill, A.M.; Eriksen, J.; Bergstrom, L.; Smith, K.A.; Marstorp, H.; Kirchmann, H.; Nilsson, I. Nitrogen and sulphur management: Challenges for organic sources in temperate agricultural systems. Soil Use Manag. 2005, 21, 82–93. [Google Scholar] [CrossRef]
- Siebielec, G.; Smreczak, B.; Klimkowicz-Pawlas, A.; Kowalik, M.; Kaczyński, R.; Koza, P.; Ukalska-Jaruga, A.; Łysiak, M.; Wójtowicz, U.; Poręba, L.; et al. Report from the third stage of the contract implementation. In Monitoring of Chemistry of Arable Soils in Poland in 2015–2017; IUNG-PIB Puławy: Puławy, Poland, 2017; pp. 1–170. [Google Scholar]
- Wiesmeier, M.; Poeplau, C.; Sierra, C.A.; Maier, H.; Frühauf, C.; Hübner, R.; Kühnel, A.; Spörlein, P.; Geuß, U.; Hangen, E.; et al. Projected loss of soil organic carbon in temperate agricultural soils in the 21st century: Effects of climate change and carbon input trends. Sci. Rep. 2016, 6, 32525. [Google Scholar] [CrossRef] [Green Version]
- Tuttobene, R.; Avola, G.; Gresta, F.; Abbate, V. Industrial orange waste as organic fertilizer in durum wheat. Agron. Sustain. Dev. 2009, 29, 557–563. [Google Scholar] [CrossRef]
- Kizito, S.; Luo, H.; Lu, J.; Bah, H.; Dong, R.; Wu, S. Role of nutrient-enriched biochar as a soil amendment during maize growth: Exploring practical alternatives to recycle agricultural residuals and to reduce chemical fertilizer demand. Sustainability 2019, 11, 3211. [Google Scholar] [CrossRef] [Green Version]
- Szymańska, M.; Sosulski, T.; Szara, E.; Wąs, A.; Sulewski, P.; van Pruissen, G.W.P.; Cornelissen, R.L. Ammonium sulphate from a bio-refinery system as a fertilizer-agronomic and economic effectiveness on the farm scale. Energies 2019, 12, 4721. [Google Scholar] [CrossRef] [Green Version]
- Amin, A.E.E.A.Z.; Mihoub, A. Effect of sulfur-enriched biochar in combination with sulfur-oxidizing bacterium (Thiobacillus Spp.) on release and distribution of phosphorus in high calcareous p-fixing soils. J. Soil Sci. Plant Nutr. 2021, 21, 2041–2047. [Google Scholar] [CrossRef]
- Mattiello, E.M.; da Silva, R.C.; Degryse, F.; Baird, R.; Gupta, V.V.S.R.; McLaughlin, M.J. Sulfur and zinc availability from co-granulated Zn-enriched elemental sulfur fertilizers. J. Agric. Food Chem. 2017, 65, 1108–1115. [Google Scholar] [CrossRef]
- Abatzoglou, N.; Boivin, S. A review of biogas purification processes. Biofuel Bioprod. Biorefin. 2009, 3, 42–71. [Google Scholar] [CrossRef]
- Awe, O.W.; Zhao, Y.; Nzihou, A.; Minh, D.P.; Lyczko, N. A review of biogas utilisation, purification and upgrading technologies. Waste Biomass-Valoriz. 2017, 8, 267–283. [Google Scholar] [CrossRef] [Green Version]
- Ermich, S.; Pruszynska, E. Complex bio-gas cleansing treatment—BIOSULFEX device: Exploitation results. Gaz Woda Tech. Sanit. 2008, 4, 6–9. [Google Scholar]
- Kociołek-Balawejder, E.; Wilk, Ł. A review of the methods for removal of hydrogen sulfide from biogas. Przem. Chem. 2011, 90, 389–397. [Google Scholar]
- Kurczewska, J.; Grzesiak, P.; Łukaszyk, J.; Gabała, E.; Schroeder, G. High decrease in soil metal bioavailability by metal immobilization with halloysite clay. Environ. Chem. Lett. 2015, 13, 319–325. [Google Scholar] [CrossRef]
- Radziemska, M. Study of applying naturally occurring mineral sorbents of Poland (dolomite halloysite, chalcedonite) for aided phytostabilization of soil polluted with heavy metals. Catena 2018, 163, 123–129. [Google Scholar] [CrossRef]
- Liuskanto, S. The Use of Halloysite for Nutrient and Moisture Retention in Soils. Bachelor’s Thesis, Tampere University of Applied Sciences, Tampere, Finland, 2015. [Google Scholar]
- European Commission. Communication from the Commission to the European Parliament, the Council, the European Economic and Social Committee and the Committee of the Regions, a Farm to Fork Strategy for a Fair, Healthy and Environmentally-Friendly Food System. Available online: https://eur-lex.europa.eu/legal-content/EN/TXT/?uri=CELEX%3A52020DC0381 (accessed on 20 November 2021).
- Vergaro, V.; Abdullayev, E.; Lvov, Y.M.; Zeitoun, A.; Cingolani, R.; Rinaldi, R.; Leporatti, S. Cytocompatibility and uptake of halloysite clay nanotubes. Biomacromolecules 2010, 11, 820–826. [Google Scholar] [CrossRef]
- Banaś, D.; Kubala-Kukuś, A.; Braziewicz, J.; Majewska, U.; Pajeka, M.; Wudarczyk-Moćko, J.; Czech, K.; Garnuszek, M.; Słomkiewicz, P.; Szczepanik, B. Study of properties of chemically modified samples of halloysite mineral with X-ray fluorescence and X-ray powder diffraction methods. Radiat. Phys. Chem. 2013, 93, 129–134. [Google Scholar] [CrossRef]
- Massaro, M.; Noto, R.; Riela, S. Past, present and future perspectives on halloysite clay minerals. Molecules 2020, 25, 4863. [Google Scholar] [CrossRef]
- PN-EN 12048:1999. Solid Fertilizers and Liming Materials—Determination of Moisture Content—Gravimetric Method by Drying at (105 +/− 2) Degrees C; European Committee for Standarization, Management Centre: Brussels, Belgium, 2020. [Google Scholar]
- PN-EN 1235:1999. Solid Fertilizers—Test Sieving (ISO 8397:1988 Modified); European Committee for Standarization, Management Centre: Brussels, Belgium, 1995. [Google Scholar]
- ISO 3944:1992. Fertilizers—Determination of Bulk Density (Loose); International Organization for Standardization: Geneva, Switzerland, 1992. [Google Scholar]
- ISO 11265:1994/COR 1:1996. Soil Quality—Determination of the Specific Electrical Conductivity—Technical Corrigendum 1; International Organization for Standardization: Geneva, Switzerland, 1996. [Google Scholar]
- Turan, J.J.; Findura, P.J.; Djalović, I.G.; Sedlar, A.D.; Bugarin, R.M.; Janić, T.V. Influence of moisture content on the angle of repose of nitrogen fertilizers. Int. Agrophys. 2011, 25, 201–204. [Google Scholar]
- Kiiski, H.; Dittmar, H. Fertilizers, 4. Granulation. In Ullmann’s Encyclopedia of Industrial Chemistry; Wiley-VCH Verlag GmbH: Weinheim, Germany, 2016; pp. 1–32. [Google Scholar] [CrossRef]
- Diettmar, H. “Outdated Version” of Fertilizers, 4. Granulation. In Ullmann’s Encyclopedia of Industrial Chemistry; Wiley-VCH Verlag GmbH: Weinheim, Germany, 2012; Volume 14, pp. 253–272. [Google Scholar] [CrossRef]
- Falk, D. Pelleting cost centre. In Feed Manufacturing Technology III; Mcellhiney, R.R., Ed.; American Feed Manufacturers Association: Arlington, VA, USA, 1985; pp. 167–190. [Google Scholar]
- Mieldažys, R.; Jotautien, E.; Jasinskas, A. The opportunities of sustainable biomass ashes and poultry manure recycling for granulated fertilizers. Sustainability 2019, 11, 4466. [Google Scholar] [CrossRef] [Green Version]
- Gressier, J.B.; Mourgues, R.; Bodet, L.; Matthieu, J.Y.; Galland, O.; Cobbold, P. Control of pore fluid pressure on depth of emplacement of magmatic sills: An experimental approach. Tectonophysics 2010, 489, 13. [Google Scholar] [CrossRef]
- Panien, M.; Schreurs, G.; Pfiffner, O.A. Mechanical behaviour of granular materials used in analogue modelling: Insights from grain characterisation, ring-shear tests and analogue experiments. J. Struct. Geol. 2006, 28, 1710–1724. [Google Scholar] [CrossRef]
- Montanari, D.; Agostini, A.; Bonini, M.; Corti, G.; Del Ventisette, C. The use of empirical methods for testing granular materials in analogue modelling. Materials 2017, 10, 635. [Google Scholar] [CrossRef] [PubMed]
- Klinkmüller, M.; Schreurs, G.; Rosenau, M.; Kemnitz, H. Properties of granular analogue materials: A community wide survey. Tectonophysics 2016, 684, 23–38. [Google Scholar] [CrossRef] [Green Version]
- Abdulaha-Al Baquy, M.; Jiu-Yu, L.; Chen-Yang, X.; Mehmood, K.; Ren-Kou, X. Determination of critical pH and Al concentration of acidic Ultisols for wheat and canola crops. Solid Earth 2017, 8, 149–159. [Google Scholar] [CrossRef] [Green Version]
- Świercz, A.; Smorzewska, E.; Słomkiewicz, P.; Suchanek, G. Possibile use of halloysite in phytoremediation of soils contaminated with heavy metals. J. Elem. 2016, 21, 559–570. [Google Scholar] [CrossRef]
- Lesovik, V.S.; Zagorodnyuk, L.K.; Babaev, Z.K.; Dzhumaniyazov, Z.B. Analysis of the Causes of Brickwork Efflorescence in the Aral Sea Region. Glas. Ceram. 2020, 77, 277–279. [Google Scholar] [CrossRef]
- Murali, G.; Abid, S.R.; Amran, Y.M.; Abdelgader, H.S.; Fediuk, R.; Susrutha, A.; Poonguzhali, K. Impact performance of novel multi-layered prepacked aggregate fibrous composites under compression and bending. Structures 2020, 28, 1502–1515. [Google Scholar] [CrossRef]
- Belal, E.E.; El Sowfy, D.M.; Rady, M.M. Integrative soil application of humic acid and sulfur improves saline calcareous soil properties and barley plant performance. Commun. Soil Sci. Plant Anal. 2019, 50, 1919–1930. [Google Scholar] [CrossRef]
- Kunhikrishnan, A.; Thangarajan, R.; Bolan, N.S.; Xu, Y.; Mandal, S.; Gleeson, D.B.; Seshadri, B.; Zaman, M.; Barton, L.; Tang, C.; et al. Functional relationships of soil acidification, liming, and greenhouse gas flux. Adv. Agron. 2016, 139, 1–71. [Google Scholar]
- Schjoerring, J.K.; Cakmak, I.; White, P.J. Plant nutrition and soil fertility: Synergies for acquiring global green growth and sustainable development. Plant Soil 2019, 434, 1–6. [Google Scholar] [CrossRef] [Green Version]
- Fuentes-Lara, L.O.; Medrano-Macías, J.; Pérez-Labrada, F.; Rivas-Martínez, E.N.; García-Enciso, E.L.; González-Morales, S.; Juárez-Maldonado, A.; Rincón-Sánchez, F.; Benavides-Mendoza, A. From elemental sulfur to hydrogen sulfide in agricultural soils and plants. Molecules 2019, 24, 2282. [Google Scholar] [CrossRef] [Green Version]
- Zhao, C.; Degryse, F.; Gupta, V.; McLaughlin, M.J. Elemental sulfur oxidation in Australian cropping soils. Soil Sci. Soc. Am. J. 2015, 79, 89–96. [Google Scholar] [CrossRef]
- Karimizarchi, M.; Aminuddin, H.; Khanif, M.Y.; Radziah, O. Effect of elemental sulphur timing and application rates on soil P release and concentration in maize. Pertanika J. Trop. Agric. Sci. 2016, 39, 235–248. [Google Scholar] [CrossRef]
- Bobowiec, A.; Tabak, M. The effect of waste sulfur obtained during biogas desulfurization on the availability of selected trace elements in soil. Geol. Geophys. Environ. 2018, 44, 345–355. [Google Scholar] [CrossRef] [Green Version]
- Ye, R.; Wright, A.L.; McCray, J.M. Seasonal changes in nutrient availability for sulfur-amended everglades soils under sugarcane. J. Plant Nutr. 2011, 34, 2095–2113. [Google Scholar] [CrossRef]
- Shrivastava, P.; Kumar, R. Soil salinity: A serious environmental issue and plant growth promoting bacteria as one of the tools for its alleviation. Saudi J. Biol. Sci. 2015, 22, 123–131. [Google Scholar] [CrossRef] [Green Version]
- Machado, R.M.A.; Serralheiro, P. Soil salinity: Effect on vegetable crop growth. Management practices to prevent and mitigate soil salinization. Horticulturae 2017, 3, 30. [Google Scholar] [CrossRef]
- Jamil, A.; Riaz, S.; Ashraf, M.; Foolad, M.R. Gene expression profiling of plants under salt stress. Crit. Rev. Plant Sci. 2011, 30, 435–458. [Google Scholar] [CrossRef]
- Cheraghi, S.A.M. Wheat yield response and seasonal salt profile evolution under irrigation with saline waters in a semi-arid region. J. Water Land Dev. 2020, 44, 26–32. [Google Scholar] [CrossRef]
- Paul, D. Osmotic stress adaptations in rhizobacteria. J. Basic Microbiol. 2012, 53, 101–110. [Google Scholar] [CrossRef]
- Han, J.; Shi, J.; Zeng, L.; Xu, J.; Wu, L. Effects of nitrogen fertilization on the acidity and salinity of greenhouse soils. Environ. Sci. Pollut. Res. 2014, 22, 2976–2986. [Google Scholar] [CrossRef] [PubMed]
- Wei, C.; Li, F.; Yang, P.; Ren, S.; Wang, S.; Wang, Y.; Xu, Z.; Xu, Y.; Wei, R.; Zhang, Y. Effects of irrigation water salinity on soil properties, N2O emission and yield of spring maize under mulched drip irrigation. Water 2019, 11, 1548. [Google Scholar] [CrossRef] [Green Version]
- Pourbabaee, A.A.; Koohbori Dinekaboodi, S.; Seyed Hosseini, H.M.; Alikhani, H.A.; Emami, S. Potential application of selected sulfur-oxidizing bacteria and different sources of sulfur in plant growth promotion under different moisture conditions. Commun. Soil Sci. Plant Anal. 2020, 51, 735–745. [Google Scholar] [CrossRef]
- Yang, Z.H.; Stöven, K.; Haneklaus, S.; Singh, B.R.; Schnug, E. Elemental sulfur oxidation by thiobacillus spp. And aerobic heterotrophic sulfur-oxidizing bacteria. Pedosphere 2010, 20, 71–79. [Google Scholar] [CrossRef]
- El-Tarabily, K.A.; Soaud, A.A.; Saleh, M.E.; Matsumoto, S. Isolation and characterisation of sulfur-oxidising bacteria, including strains of Rhizobium, from calcareous sandy soils and their effects on nutrient uptake and growth of maize (Zea mays L.). Aust. J. Agric. Res. 2006, 57, 101–111. [Google Scholar] [CrossRef]
- Gümüş, İ.; Şeker, C. Influence of humic acid applications on modulus of rupture, aggregate stability, electrical conductivity, carbon and nitrogen content of a crusting problem soil. Solid Earth 2015, 6, 1231–1236. [Google Scholar] [CrossRef] [Green Version]
- Yılmaz, E. Changes of some soil properties by agricultural processing waste (soybean pulp) amendment. J. Food Agric. Environ. 2010, 8, 1057–1060. [Google Scholar] [CrossRef]
- Matusik, J. Kaolinite Group Minerals as Precursors of Mineral Nanotubes. Ph.D. Thesis, AGH University of Science and Technology, Krakow, Poland, 2010. [Google Scholar]
- Bailey, S.W. Halloysite—A critical assessment. In Volume II: Surface Chemistry. Structure and Mixed Layering of Clays. In Proceedings of the 9th International Clay Conference, Strasbourg, France, 9 July 2018. [Google Scholar]
- Joussein, E.; Petit, S.; Churchman, J.; Theng, B.; Righi, D.; Delvaux, B. Halloysite clay minerals—A review. Clay Miner. 2005, 40, 383–426. [Google Scholar] [CrossRef]
- Lvov, Y.M.; Shchukin, D.G.; Möhwald, H.; Price, R.R. Halloysite clay nanotubes for controlled release of protective agents. ACS Nano 2008, 2, 814–820. [Google Scholar] [CrossRef] [PubMed]
- Klikocka, H.; Cybulska, M.; Barczak, B.; Narolski, B.; Szostak, B.; Kobiałka, A.; Nowak, A.; Wójcik, E. The effect of sulphur and nitrogen fertilization on grain yield and technological quality of spring wheat. Plant Soil Environ. 2016, 62, 230–236. [Google Scholar] [CrossRef] [Green Version]
- Tabak, M.; Lepiarczyk, A.; Filipek-Mazur, B.; Bachara, P. Ammonium nitrate enriched with sulfur influences wheat yield and soil properties. Plant Soil Environ. 2019, 65, 211–217. [Google Scholar] [CrossRef] [Green Version]
- Schoenau, J.J.; Malhi, S.S.; Jez, J. Sulfur forms and cycling processes in soil and their relationship to sulfur fertility. In Sulfur: A Missing Link between Soils, Crops, and Nutritio; Jez, J., Ed.; American Society of Agronomy: Madison, WI, USA, 2008; Volume 50, pp. 1–10. [Google Scholar] [CrossRef]
- Hedge, D.M.; Murthy, I.Y.L.N. Management of secondary nutrients. Indian J. Fertil. 2005, 1, 93–100. [Google Scholar]
- Haque, M.M.; Saleque, M.A.; Shah, A.L.; Biswas, J.C.; Kim, P.J. Long-term effects of sulfur and zinc fertilization on rice productivity and nutrient efficiency in double rice cropping paddy in Bangladesh. Commun. Soil Sci. Plant Anal. 2015, 46, 2877–2887. [Google Scholar] [CrossRef]
- Degryse, F.; Ajiboye, B.; Baird, R.; da Silva, R.C.; McLaughlin, M.J. Oxidation of elemental sulfur in granular fertilizers depends on the soil-exposed surface area. Soil Sci. Soc. Am. J. 2016, 80, 294. [Google Scholar] [CrossRef]
- Singh, S.; Sarkar, D.; Bhudevi, M.; Rakesh, S.; Singh, R.K.; Kar, S.; Rakshit, A. Advanced forms of sulphur formulations for improving use efficiency in crop species. Annu. Res. Rev. Biol. 2018, 27, 1–14. [Google Scholar] [CrossRef]
- Balík, J.; Kulhánek, M.; Cerňý, J.; Száková, J.; Pavlíková, D.; Čermák, P. Differences in soil sulfur fractions due to limitation of atmospheric deposition. Plant Soil Environ. 2009, 55, 344–352. [Google Scholar] [CrossRef] [Green Version]
- Förster, S.; Welp, G.; Scherer, H.W. Sulfur specification in bulk soil as influenced by long-term application of mineral and organic fertilizers. Plant Soil Environ. 2012, 58, 316–321. [Google Scholar] [CrossRef] [Green Version]
- Janzen, H.H.; Bettany, J.R. Measurement of sulfur oxidation in soils. Soil Sci. 1987, 143, 444–452. [Google Scholar] [CrossRef]
- Janzen, H.H.; Bettany, J.R. The effect of temperature and water potential on sulfur oxidation in soils. Soil Sci. 1987, 144, 81–89. [Google Scholar] [CrossRef]
- Dick, R.P.; Deng, S. Multivariate factor analysis of sulfur oxidation and rhodanese activity in soils. Biogeochemistry 1991, 12, 87–101. [Google Scholar] [CrossRef]
- Lee, A.; Watkinson, J.H.; Lauren, D.R. Factors affecting oxidation rates of elemental sulphur in a soil under a ryegrass dominant sward. Soil Biol. Biochem. 1988, 20, 809–816. [Google Scholar] [CrossRef]
- Lettl, A.; Langkramer, O.; Lochman, V. Dynamics of oxidation of inorganic sulphur compounds in upper soil horizons of spruce forests. Folia Microbiol. 1981, 26, 24–28. [Google Scholar] [CrossRef]
- Baikhamurova, M.O.; Sainova, G.A.; Akbasova, A.D.; Anarbekova, G.D.; Ozler, M.A. The influence of the mixture of ver-micompost and sulphur-perlite-containing waste on the yield and the quality of crops. J. Water Land Dev. 2021, 49, 213–218. [Google Scholar] [CrossRef]
- Rego, T.J.; Rao, V.N.; Seeling, B.; Pardhasaradhi, G. Widespread deficiencies of sulfur, boron and zinc in Indian semiarid tropical soils on-farm crop responses. J. Plant Nutr. 2007, 30, 569–1583. [Google Scholar] [CrossRef] [Green Version]
- Nurmesniemi, H.; Pöykiö, R.; Watkins, G.; Dahl, O. Total and extractable heavy metal, phosphorous and sulfur concentrations in slaker grits from the causticizing process of a pulp mill for use as a soil amendment. Chem. Speciat. Bioavailab. 2010, 22, 87–97. [Google Scholar] [CrossRef]
- Ketterings, Q.; Miyamoto, C.; Mathur, R.R.; Dietzel, K.; Gami, S. A comparison of soil sulfur extraction methods. Soil Sci. Soc. Am. J. 2011, 75, 1578–1583. [Google Scholar] [CrossRef]
- Cancela, R.C.; de Abreu, C.A.; Paz-González, A. DTPA and Mehlich-3 micronutrient extractability in natural soils. Commun. Soil Sci. Plant Anal. 2002, 33, 2879–2893. [Google Scholar] [CrossRef]
- Sahrawat, K.L.; Murthy, K.V.S.; Wani, S.P. Comparative evaluation of Ca chloride and Ca phosphate for extractable sulfur in soils with a wide range in pH. J. Soil Sci. Plant Nutr. 2009, 172, 404–407. [Google Scholar] [CrossRef] [Green Version]
- Sikora, J.; Niemiec, M.; Szeląg-Sikora, A.; Gródek-Szostak, Z.; Kuboń, M.; Komorowska, M. The Impact of a Controlled-Release Fertilizer on Greenhouse Gas Emissions and the Efficiency of the Production of Chinese Cabbage. Energies 2020, 13, 2063. [Google Scholar] [CrossRef] [Green Version]
- Sikora, J.; Niemiec, M.; Tabak, M.; Gródek-Szostak, Z.; Szeląg-Sikora, A.; Kuboń, M.; Komorowska, M. Assessment of the Efficiency of Nitrogen Slow-Release Fertilizers in Integrated Production of Carrot Depending on Fertilization Strategy. Sustainability 2020, 12, 1982. [Google Scholar] [CrossRef] [Green Version]
- Niemiec, M.; Sikora, J.; Szeląg-Sikora, A.; Kuboń, M.; Stuglik, J.; Komorowska, M. Evaluation of a possibility of producing fertilizers based on brown algae from anthropogenically transformed regions of the Black Sea. Przem. Chem. 2019, 98, 94–97. [Google Scholar] [CrossRef]
- Niemiec, M.; Kuboń, M.; Komorowska, M.; Gródek-Szostak, Z.; Stuglik, J.; Mudryk, K. Assessment of selected properties of fertilizers based on dolomite and citric acid. Przem. Chem. 2020, 99, 45–48. [Google Scholar] [CrossRef]
- Tabak, M.; Filipek-Mazur, B. Effect of the Fertilizer Application Method on Soil Abundance in Available Sulfur. Agric. Eng. 2018, 22, 81–88. [Google Scholar] [CrossRef] [Green Version]
- Wróbel, M.; Frączek, J.; Jewiarz, M.; Mudryk, K.; Dziedzic, K. Impact of Selected Properties of Raw Material on Quality Features of Granular Fertilizers Obtained from Digestates and ASH Mixtures. Agric. Eng. 2017, 20, 207–217. [Google Scholar] [CrossRef] [Green Version]
- Sikora, J.; Niemiec, M.; Szelag-Sikora, A. Evaluation of the chemical composition of raw common duckweed (Lemna minor L.) and pulp after methane fermentation. J. Elem. 2018, 23, 685–695. [Google Scholar] [CrossRef]
- Niemiec, M.; Komorowska, M.; Mudryk, K.; Jewiarz, M.; Sikora, J.; Szeląg-Sikora, A.; Rozkosz, A. Evaluation of the Fertilizing Potential of Products Based on Torrefied Biomass and Valorized with Mineral Additives. In Renewable Energy Sources: Engineering, Technology, Innovation; Springer: Cham, Switzerland, 2020; pp. 267–275. [Google Scholar] [CrossRef]
- IUSS Working Group WRB, 2015. World Reference Base for soil resources 2014. In International Soil Classification System for Naming Soils and Creating Legends for Soil Maps; Update 2015. World Soil Resources Report No. 106; FAO: Rome, Italy, 2015; Volume 212. [Google Scholar]
- Niemiec, M.; Szeląg-Sikora, A.; Cupiał, M. Evaluation of the Efficiency of Celeriac Fertilization with the Use of Slow-Acting Fertilizers; Elsevier: Amsterdam, The Netherlands, 2015; Volume 7, pp. 177–183. [Google Scholar] [CrossRef] [Green Version]
- Sikora, J.; Niemiec, M.; Szeląg-Sikora, A.; Gródek-Szostak, Z.; Kuboń, M.; Komorowska, M. Effect of the Addition of a Fat Emulsifier on the Amount and Quality of the Obtained Biogas. Energies 2020, 13, 1825. [Google Scholar] [CrossRef] [Green Version]
- Kulhánek, M.; Černý, J.; Balík, J.; Sedlář, O.; Suran, P. Potential of Mehlich 3 method for extracting plant available sulfur in the Czech agricultural soils. Plant Soil Environ. 2018, 64, 455–462. [Google Scholar] [CrossRef] [Green Version]


| Parameter | Value |
|---|---|
| Soil texture, % | |
| Fraction sand 2–0.05 mm | 88 |
| Fraction dust 0.05–0.002 mm | 10 |
| Fraction loam < 0.002 mm | 2 |
| Maximum water capacity, % | 20.55 |
| pHH2O | 6.01 |
| pHKCl | 5.04 |
| Hydrolityc acidity, mmol (+) kg–1 d.m. | 14.93 |
| Electrical conductivity, μS cm–1 ± SD | 91 |
| Sulfate S—extraction with 0.03 mol L−1 CH3COOH, mg kg–1 d.m | 3.57 |
| Sulfate S—extraction with 0.01 mol L−1 CaCl2, mg kg–1 d.m | 3.86 |
| Sulfate S—extraction with Mehlich 3, mg kg–1 d.m | 22.10 |
| Total N, g kg–1 d.m. | 0.61 |
| Total C, g kg–1 d.m. | 8.23 |
| Total S, mg kg−1 d.m. | 97.5 |
| Total Fe, g kg−1 d.m. | 2.70 |
| Total Cd, mg kg−1 d.m. | 0.683 |
| Total Cr, mg kg−1 d.m. | 3.86 |
| Total Cu, mg kg−1 d.m. | 4.27 |
| Total Mn, mg kg−1 d.m. | 106 |
| Total Ni, mg kg−1 d.m. | 3.18 |
| Total Pb, mg kg−1 d.m. | 7.13 |
| Total Zn, mg kg−1 d.m. | 23.9 |
| Percentage Share of Particular Fractions of Particle Diameters in mm ± SD | |||||||
|---|---|---|---|---|---|---|---|
| Fertilizer | Moisture, % ± SD | <0.25 | 0.25–0.50 | 0.50–1.02 | 1.02–2.0 | >2.0 | Bulk Density, kg m−3 ± SD |
| A | 0.822 ± 0.027 | 48.2 ± 0.9 | 9.55 ± 0.27 | 11.8 ± 0.3 | 20.4 ± 0.1 | 9.84 ± 0.22 | 376 ± 9.77 |
| B | 1.78 ± 0.05 | 8.26 ± 0.02 | 9.97 ± 0.05 | 19.7 ± 0.5 | 34.7 ± 0.8 | 27.2 ± 0.3 | 626 ± 2.18 |
| C | 0.904 ± 0.020 | 5.26 ± 0.15 | 6.34 ± 0.11 | 15.9 ± 0.1 | 33.4 ± 0.2 | 39.0 ± 0.3 | 759 ± 18.3 |
| D | 2.08 ± 0.08 | 13.7 ± 0.3 | 11.6 ± 0.2 | 21.5 ± 0.6 | 30.4 ± 0.3 | 22.5 ± 0.5 | 917 ± 13.2 |
| Treatment | Incubation Time (Days) | |||||
|---|---|---|---|---|---|---|
| 0 | 15 | 30 | 60 | 90 | ||
| 0Ca | C 1 | 4.70 abc 2 | 4.71 bc | 4.68 abc | 4.61 ab | 4.57 ab |
| I | 4.71 bc | 4.81 c | 4.61 ab | 4.63 ab | 4.56 a | |
| II | 4.72 bc | 4.65 abc | 4.66 abc | 4.62 ab | 4.58 ab | |
| III | 4.72 bc | 4.66 abc | 4.63 abc | 4.65 abc | 4.69 abc | |
| IV | 4.71 bc | 4.71 bc | 4.65 abc | 4.69 abc | 4.72 bc | |
| +Ca | C | 5.40 efgh | 5.28 abc | 5.29 abcd | 5.39 defgh | 5.41 fgh |
| I | 5.36 bcdefgh | 5.30 bcde | 5.21 a | 5.31 bcdef | 5.30 bcde | |
| II | 5.37 cdefgh | 5.35 bcdefg | 5.30 bcdef | 5.46 gh | 5.33 bcdef | |
| III | 5.36 cdefgh | 5.26 ab | 5.27 ab | 5.44 gh | 5.35 bcdefg | |
| IV | 5.41 fgh | 5.35 bcdefg | 5.31 bcdef | 5.48 h | 5.34 bcdefg | |
| Treatment | Incubation Time (Days) | |||||
|---|---|---|---|---|---|---|
| 0 | 15 | 30 | 60 | 90 | ||
| 0Ca | C 1 | 129 a 2 ± 8 | 135 a ± 1 | 139 a ± 12 | 245 gh ± 6 | 179 abcdef ± 13 |
| I | 144 ab ± 9 | 153 abc ± 7 | 142 ab ± 18 | 256 h ± 41 | 206 cdefgh ± 8 | |
| II | 139 a ± 13 | 155 abc ± 7 | 141 ab ± 15 | 231 fgh ± 39 | 195 bcdefg ± 11 | |
| III | 133 a ± 10 | 143 ab ± 1 | 148 ab ± 14 | 176 abcde ± 3 | 222 efgh ± 8 | |
| IV | 149 ab ± 13 | 166 abcd ± 3 | 172 abcde ± 26 | 219 defgh ± 34 | 218 defgh ± 12 | |
| +Ca | C | 138 ab ± 6 | 131 ab ± 2 | 142 abc ± 15 | 225 gh ± 20 | 179 abcdefg ± 7 |
| I | 136 ab ± 9 | 143 abc ± 2 | 143 abc ± 24 | 180 bcdefg ± 23 | 200 efgh ± 1 | |
| II | 134 ab ± 18 | 138 ab ± 1 | 140 ab ± 9 | 177 abcdefg ± 19 | 195 defg ± 5 | |
| III | 129 a ± 13 | 150 abcde ± 11 | 157 abcdef ± 17 | 277 i ± 33 | 192 cdefg ± 14 | |
| IV | 145 abcd ± 4 | 154 abcdef ± 4 | 203 fgh ± 26 | 250 hi ± 33 | 192 cdefg ± 7 | |
| Extraction with 0.03 mol L−1 CH3COOH | |||||||
|---|---|---|---|---|---|---|---|
| Treatment | Incubation Time (Days) | ||||||
| 0 | 15 | 30 | 60 | 90 | |||
| 0Ca | C 1 | 4.58 a 2 ± 0.67 | 7.78 abc ± 0.21 | 12.73 cd ± 0.55 | 6.85 abc ± 0.57 | 11.05 bc ± 0.25 | |
| I | 6.68 ab ± 6.68 | 23.68 fg ± 2.32 | 34.59 i ± 3.29 | 27.92 gh ± 3.79 | 17.03 de ± 1.68 | ||
| II | 7.13 abc ± 7.13 | 21.32 ef ± 0.94 | 31.30 hi ± 2.48 | 31.37 hi ± 2.42 | 18.53 ef ± 1.79 | ||
| III | 7.85 abc ± 0.89 | 20.79 ef ± 0.28 | 32.79 hi ± 2.82 | 35.18 i ± 3.23 | 19.09 ef ± 0.96 | ||
| IV | 7.22 abc ± 0.42 | 19.93 ef ± 0.84 | 32.67 hi ± 1.28 | 30.63 hi ± 2.42 | 18.00 de ± 0.37 | ||
| +Ca | C | 5.19 ab ± 0.49 | 9.53 abcd ± 0.60 | 12.40 bcde ± 0.49 | 11.79 bcde ± 0.84 | 2.97 a ± 0.40 | |
| I | 12.06 bcde ± 0.69 | 24.92 fgh ± 1.64 | 41.59 j ± 6.56 | 41.67 j ± 2.02 | 15.50 cde ± 0.94 | ||
| II | 8.23 abc ± 0.44 | 14.13 bcde ± 0.29 | 20.40 efg ± 0.77 | 33.72 hij ± 0.83 | 15.88 cde ± 0.77 | ||
| III | 11.02 abcd ± 0.83 | 17.69 def ± 1.56 | 32.47 hi ± 5.52 | 34.59 ij± 5.03 | 12.05 bcde ± 0.37 | ||
| IV | 11.77 abcde ± 0.53 | 16.38 cdef ± 2.15 | 28.90 ghi ± 7.58 | 33.52 hij ± 4.26 | 9.93 abcd ± 1.60 | ||
| Extraction with 0.01 mol L−1 CaCl2 | Extraction with Mehlich 3 | ||||||
| Treatment | Incubation time (days) | Treatment | Incubation time (days) | ||||
| 0 | 90 | 0 | 90 | ||||
| 0Ca | C 1 | 7.95 a ± 1.02 | 10.41 a ± 1.15 | 0Ca | C 1 | 23.55 a ± 1.60 | 28.49 a ± 0.17 |
| I | 11.72 a ± 0.12 | 32.07 b ± 3.30 | I | 33.04 a ± 3.98 | 56.32 b ± 3.42 | ||
| II | 13.08 a ± 0.30 | 35.26 bc ± 2.82 | II | 28.58 a ± 0.45 | 68.58 c ± 6.58 | ||
| III | 11.03 a ± 1.10 | 40.05 c ± 1.78 | III | 29.18 a ± 2.44 | 71.74 c ± 1.28 | ||
| IV | 13.31 a ± 2.22 | 32.97 b ± 2.64 | IV | 31.50 a ± 3.40 | 62.60 bc ± 7.64 | ||
| +Ca | C | 7.76 a ± 0.65 | 11.97 a ± 1.72 | +Ca | C | 28.20 ab ± 0.53 | 30.77 ab ± 2.81 |
| I | 14.03 a ± 0.39 | 40.39 c ± 1.54 | I | 35.98 b ± 1.79 | 77.29 d ± 2.76 | ||
| II | 12.69 a ± 0.58 | 36.61 c ± 4.98 | II | 29.72 ab ± 2.21 | 76.05 d ± 4.45 | ||
| III | 11.00 a ± 1.30 | 35.40 bc ± 2.61 | III | 26.60 ab ± 0.92 | 85.38 d ± 5.09 | ||
| IV | 11.97 a ± 0.76 | 28.80 b ± 3.69 | IV | 22.16 a ± 0.49 | 61.69 c ± 6.57 | ||
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lisowska, A.; Filipek-Mazur, B.; Sołtys, J.; Niemiec, M.; Gorczyca, O.; Bar-Michalczyk, D.; Komorowska, M.; Gródek-Szostak, Z.; Szeląg-Sikora, A.; Sikora, J.; et al. Preparation, Characterization of Granulated Sulfur Fertilizers and Their Effects on a Sandy Soils. Materials 2022, 15, 612. https://doi.org/10.3390/ma15020612
Lisowska A, Filipek-Mazur B, Sołtys J, Niemiec M, Gorczyca O, Bar-Michalczyk D, Komorowska M, Gródek-Szostak Z, Szeląg-Sikora A, Sikora J, et al. Preparation, Characterization of Granulated Sulfur Fertilizers and Their Effects on a Sandy Soils. Materials. 2022; 15(2):612. https://doi.org/10.3390/ma15020612
Chicago/Turabian StyleLisowska, Aneta, Barbara Filipek-Mazur, Józef Sołtys, Marcin Niemiec, Olga Gorczyca, Dominika Bar-Michalczyk, Monika Komorowska, Zofia Gródek-Szostak, Anna Szeląg-Sikora, Jakub Sikora, and et al. 2022. "Preparation, Characterization of Granulated Sulfur Fertilizers and Their Effects on a Sandy Soils" Materials 15, no. 2: 612. https://doi.org/10.3390/ma15020612








