Structure and Crystallization of High-Calcium, CMAS Glass Ceramics Synthesized with a High Content of Slag
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials Preparation
2.2. Glass Composition
2.3. Characterizations
3. Results
3.1. Characterization of Basic Glass
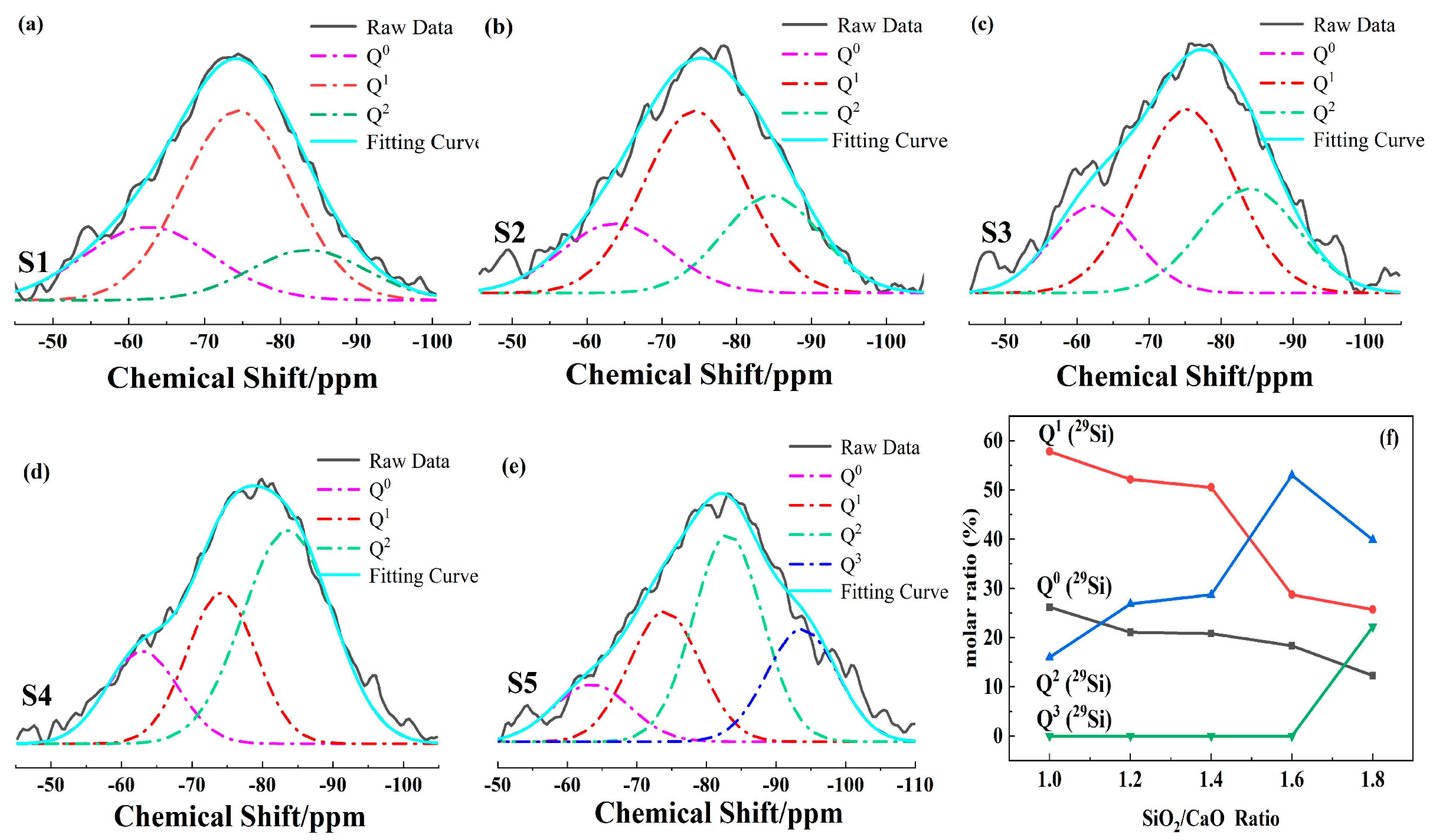
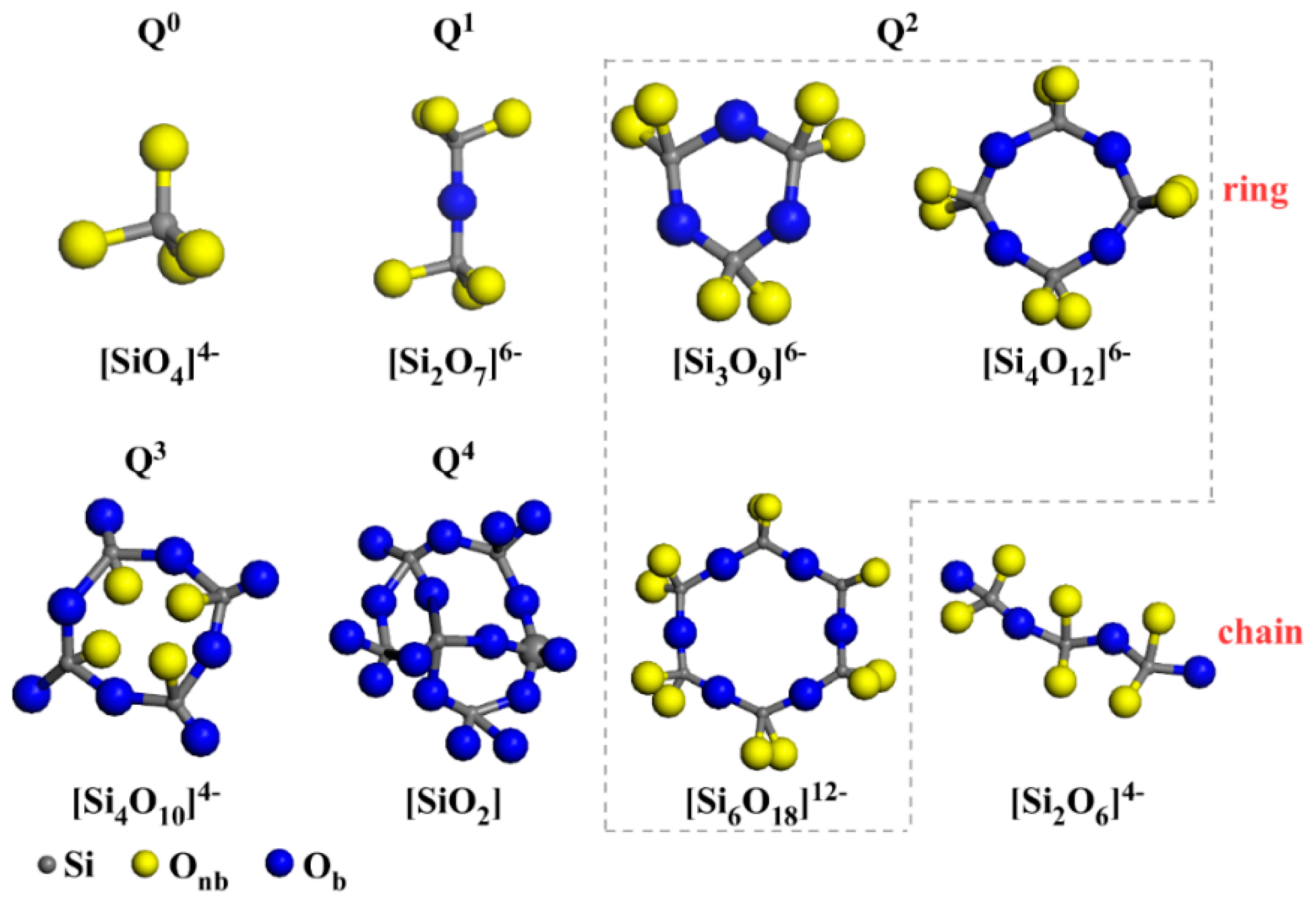
3.2. The Influence of SiO2/CaO on the Silicon–Oxygen Tetrahedron Structure
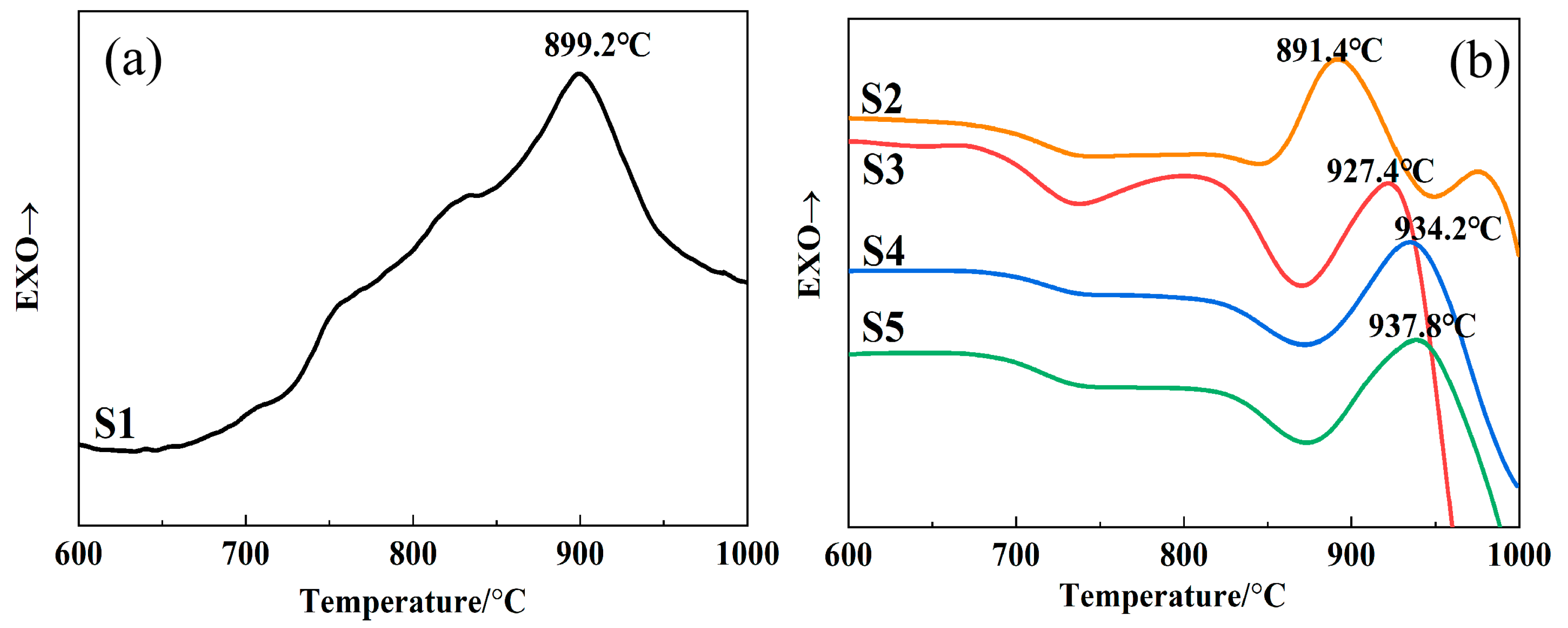
3.3. The Effect of SiO2/CaO on Crystallization Behavior
4. Conclusions
- (1)
- In the high-calcium CMAS systems, the type of crystals precipitated in the sample was closely related to Qn. When the ratio of SiO2/CaO was 0.98, the main forms of Qn were Q0 and Q1 (main units). At this time, only akermanite was precipitated. As the ratio of SiO2/CaO increased, the main forms of Qn changed from Q0 and Q1 (main units) to Q1 (main units) and Q2. The sample began to precipitate diopside–wollastonite. When the SiO2/CaO ratio was greater than 1.6, the main existing form of the silicon-oxygen tetrahedron changed from Q1 (main units) and Q2 to Q1 and Q2 (main units). At this time, akermanite in the sample disappeared, and only diopside–wollastonite was precipitated.
- (2)
- A low SiO2/CaO ratio reduced the stability of the glass, and caused rapid crystallization during the melt–cooling process in high-calcium CMAS systems. As the ratio of SiO2/CaO increased, the anionic groups in the glass changed from a low polymerization degree to a high polymerization degree. The glass stability was obviously improved. The crystallization peak temperature of the sample continuously increased, and the crystallization kinetic constant k and the frequency factor υ gradually decreased.
- (3)
- In high-calcium CMAS systems, the rapid crystallization problem in the cooling process, caused by low SiO2/CaO, caused significant differences in grain size. As the ratio of SiO2/CaO increased, the homogeneity of the crystal improved, and the crystal changed from herringbone shape (akermanite) to dendritic shape (diopside–wollastonite).
Author Contributions
Funding
Conflicts of Interest
References
- Linrui, D. Ultilization of Silicomanganese Slag in Building Materials. China’s Manganese Ind. 2017, 35, 136–138. [Google Scholar]
- Heo, J.H.; Chung, Y.; Park, J.H. Effect of CaF2 Addition on the Silicothermic Reduction of MnO in Ferromanganese Slag. Metall. Mater. Trans. B 2015, 46, 1154–1161. [Google Scholar] [CrossRef]
- Li, X.; Tang, K.; Tangstad, M. Reduction and Dissolution Behaviour of Manganese Slag in the Ferromanganese Process. Minerals 2020, 10, 97. [Google Scholar] [CrossRef] [Green Version]
- Ji, J.; Liu, X.; Nie, J.; Zhang, S.; Wang, M.; Nie, W. Application status and development trend of silico-manganese slag in construction materials field. China Metall. 2018, 28, 1–4. [Google Scholar]
- Choi, S.; Kim, J.; Oh, S.; Han, D. Hydro-thermal reaction according to the CaO/SiO2 mole-ratio in silico-manganese slag. J. Mater. Cycles Waste Manag. 2015, 19, 374–381. [Google Scholar] [CrossRef]
- Meng, X.Z.Z.; Wang, B. On Industry Research of Mn-silicon Alloy. China’s Manganese Ind. 2018, 36, 6–9. [Google Scholar]
- Song, Y.X.; Lan, S.D.; Di, J.H.; Jiang, B.Q. Present situation and development trend of comprehensive utilization of ferroalloy slag in China. China Metall. 2017, 27, 73–77. [Google Scholar]
- Lizhu, W.Y.L.; Jun, W.; Meng, Z.; Zhe, Z. Research status of comprehensive utilization of water-quenched manganese slag in building materials. Dev. Guide Build. Mater. 2017, 15, 75–77. [Google Scholar]
- Frías, M.; Rojas, M.I.S.d.; Rodríguez, C. The influence of SiMn slag on chemical resistance of blended cement pastes. Constr. Build. Mater. 2009, 23, 1472–1475. [Google Scholar] [CrossRef]
- Han, Y.; Jia, X.; Liu, F.; Deng, L.; Zhang, X. Effect of Cr2O3 content on high-temperature dielectric properties and crystallisation of CMAS glass-ceramics. Mater. Res. Express 2019, 6, 075213. [Google Scholar] [CrossRef]
- Guo, X.; Cai, X.; Song, J.; Yang, G.; Yang, H. Crystallization and microstructure of CaO-MgO-Al2O3-SiO2 glass-ceramics containing complex nucleation agents. J. Non-Cryst. Solids 2014, 405, 63–67. [Google Scholar] [CrossRef]
- Deng, L.; Yun, F.; Jia, R.; Li, H.; Jia, X.; Shi, Y.; Zhang, X. Effect of SiO2/MgO ratio on the crystallization behavior, structure, and properties of wollastonite-augite glass-ceramics derived from stainless steel slag. Mater. Chem. Phys. 2020, 239, 122039. [Google Scholar] [CrossRef]
- Zhao, M.; Cao, J.; Wang, Z.; Li, G. Insight into the dual effect of Fe2O3 addition on the crystallization of CaO-MgO-Al2O3-SiO2 glass-ceramics. J. Non-Cryst. Solids 2019, 513, 144–151. [Google Scholar] [CrossRef]
- Sycheva, G.A.; Polyakova, I.G.; Kostyreva, T.G. Volumetric nucleation of crystals catalyzed by Cr2O3 in glass based on furnace slags. Glass Phys. Chem. 2016, 42, 238–245. [Google Scholar] [CrossRef]
- Tong, Z.; Sun, J.; Wang, J.; Tan, Z.; Liu, S. Iron reduction and diopside-based glass ceramic preparation based on mineral carbonation of steel slag. Environ. Sci. Pollut. Res. Int. 2021, 28, 796–804. [Google Scholar] [CrossRef]
- Bai, Z.; Qiu, G.; Yue, C.; Guo, M.; Zhang, M. Crystallization kinetics of glass-ceramics prepared from high-carbon ferrochromium slag. Ceram. Int. 2016, 42, 19329–19335. [Google Scholar] [CrossRef] [Green Version]
- Ljatifi, E.; Kamusheva, A.; Grozdanov, A.; Paunović, P.; Karamanov, A. Optimal thermal cycle for production of glass-ceramic based on wastes from ferronickel manufacture. Ceram. Int. 2015, 41, 11379–11386. [Google Scholar] [CrossRef]
- Miao, X.; Bai, Z.; Qiu, G.; Tang, S.; Guo, M.; Cheng, F.; Zhang, M. Preparation of transparent Mn-doped CaF2 glass-ceramics from silicon-manganese slag: Dependence of colour-controllable change on slag addition and crystallization behaviour. J. Eur. Ceram. Soc. 2020, 40, 3249–3261. [Google Scholar] [CrossRef]
- Kang, J.; Wang, J.; Cheng, J.; Yuan, J.; Hou, Y.; Qian, S. Crystallization behavior and properties of CaO-MgO-Al2O3-SiO2 glass-ceramics synthesized from granite wastes. J. Non-Cryst. Solids 2017, 457, 111–115. [Google Scholar] [CrossRef]
- Zheng, W.; Sheng, L.; Chao, H.; Wang, Z.; Qian, S.; Peng, Z.; Shen, C. Crystallization of CaO-MgO-Al2O3-SiO2 by Float Process. Glass Phys. Chem. 2017, 43, 347–356. [Google Scholar] [CrossRef]
- Li, H.; Wang, C.; Yu, H.; Liang, X.; Yang, S. Effects of Calcium Oxide Addition on the Structure and Thermal Properties of Iron Phosphate Glasses. Spectrosc. Lett. 2015, 48, 184–189. [Google Scholar] [CrossRef]
- Jia, R.; Deng, L.; Yun, F.; Li, H.; Zhang, X.; Jia, X. Effects of SiO2/CaO ratio on viscosity, structure, and mechanical properties of blast furnace slag glass ceramics. Mater. Chem. Phys. 2019, 233, 155–162. [Google Scholar] [CrossRef]
- Wang, Y.; Gui, Y.; Song, C.; Xie, C.; Zhao, J. Effect of Mass Ratio of CaO to SiO2 on Crystallization of CaO-MgO-SiO2-Al2O3 Glass Ceramics. Multipurp. Util. Miner. Resour. 2018, 1, 137–141. [Google Scholar]
- Fu, Y.; Li, P.; Tao, H.; Zhang, L.; Xin, M.; Chang, Y.; Xia, Y.; Zhou, H. The effects of Ca/Si ratio and B2O3 content on the dielectric properties of the CaO–B2O3–SiO2 glass–ceramics. J. Mater. Sci. Mater. Electron. 2019, 30, 14053–14060. [Google Scholar] [CrossRef]
- Goj, P.; Stoch, P. Influence of CaO on structural features of polyphosphate P2O5-Fe2O3-FeO glasses by molecular dynamics simulations. J. Non-Cryst. Solids 2020, 537, 120014. [Google Scholar] [CrossRef]
- Jha, P.; Singh, K. Effect of Field Strength and Electronegativity of CaO and MgO on Structural and Optical Properties of SiO2-K2O-CaO-MgO Glasses. Silicon 2016, 8, 437–442. [Google Scholar] [CrossRef]
- Hanuza, J.; Ptak, M.; Mączka, M.; Hermanowicz, K.; Lorenc, J.; Kaminskii, A.A. Polarized IR and Raman spectra of Ca2MgSi2O7, Ca2ZnSi2O7 and Sr2MgSi2O7 single crystals: Temperature-dependent studies of commensurate to incommensurate and incommensurate to normal phase transitions. J. Solid State Chem. 2012, 191, 90–101. [Google Scholar] [CrossRef]
- Zeng, L.; Huang, S.; Lin, H.; Zhao, H.; Jiang, H.; Ma, Y. Effect of Mixed Alkali on the Structure and Thermal Expansion Properties of Li2O- Al2O3-SiO2 Glass. Bullet. Chinese Ceram. Soc. 2021, 40, 3813–3821. [Google Scholar]
- Silva, A.M.B.; Queiroz, C.M.; Agathopoulos, S.; Correia, R.N.; Fernandes, M.H.V.; Oliveira, J. Structure of SiO2-MgO-Na2O glasses by FTIR, Raman and 29Si MAS NMR. J. Mol. Struct. 2011, 986, 16–21. [Google Scholar] [CrossRef]
- Duer, M.J.; Elliott, S.R.; Gladden, L.F. An investigation of the structural units in sodium disilicate glass: A 2-D 295i NMR study. J. Non-Cryst. Solids 1995, 189, 107–117. [Google Scholar] [CrossRef]
- Elgayar, I.; Aliev, A.E.; Boccaccini, A.R.; Hill, R.G. Structural analysis of bioactive glasses. J. Non-Cryst. Solids 2005, 351, 173–183. [Google Scholar] [CrossRef]
- Zhang, X.; Hu, L.; Ren, J. Structural Studies of Rare Earth-Doped Fluoroborosilicate Glasses by Advanced Solid-State NMR. J. Phys. Chem. C 2020, 124, 8919–8929. [Google Scholar] [CrossRef]
- Zhidong, W.; Jinglin, Y.; Jian, W.; Min, W.; Yingxia, H.; Yejin, Y. NMR Spectroscopic Study on Binary Sodium Silicate Glass Based on the Fine Structure. Chem. J. Chin. Univ. 2019, 40, 108–114. [Google Scholar]
- Li, H.; Liu, S.; Xu, W.; Zhang, Y.; Li, X.; Ouyang, S.; Zhao, G.; Liu, F.; Wu, N. The Effect of Microwave on the Crystallization Behavior of CMAS System Glass-Ceramics. Materials 2020, 13, 4555. [Google Scholar] [CrossRef] [PubMed]
- Liu, S.; Chen, Y.; Ouyang, S.; Li, H.; Li, X.; Li, B. Microstructural transformation of stainless steel slag-based CAMS glass ceramics prepared by SPS. Ceram. Int. 2021, 47, 1284–1293. [Google Scholar] [CrossRef]
- Zhang, L.; Huang, X.; Song, X. Fundamentals of Materials Science; Wuhan University of Technology Press: Wuhan, China, 2008. [Google Scholar]
- Kissinger, H.E. Analytical Chemistry; National Bureau of Standards: Washington, DC, USA, 1957.
- Deng, L.; Wang, S.; Zhang, Z.; Li, Z.; Jia, R.; Yun, F.; Li, H.; Ma, Y.; Wang, W. The viscosity and conductivity of the molten glass and crystallization behavior of the glass ceramics derived from stainless steel slag. Mater. Chem. Phys. 2020, 251, 123159. [Google Scholar] [CrossRef]
- Cheng, J.; Kang, J.; Lou, X.; Wu, H.; Liu, K. Effect of Nucleation Temperature on the Crystallization Behavior of Glass-ceramics. J. Wuhan Univ. Technol. 2014, 36, 1–5. [Google Scholar]
- Hu, L.; Jiang, Z. A New Criterion for Crystallization Of Glass. Bull. Chin. Ceram. Soc. 1990, 18, 315–321. [Google Scholar]
- Schneider, J.; Mastelaro, V.; Panepucci, H.; Zanotto, E. 29Si MAS-NMR studies of Qn structural units in metasilicate glasses and their nucleating ability. J. Non-Cryst. Solids 2000, 273, 8–18. [Google Scholar] [CrossRef]
- Davis, M.C.; Sanders, K.J.; Grandinetti, P.J.; Gaudio, S.J.; Sen, S. Structural investigations of magnesium silicate glasses by 29Si 2D Magic-Angle Flipping NMR. J. Non-Cryst. Solids 2011, 357, 2787–2795. [Google Scholar] [CrossRef]






| Material | LOSS | SiO2 | Al2O3 | CaO | MgO | Fe2O3 | MnO |
|---|---|---|---|---|---|---|---|
| Ferromanganese slag | 0.27 | 40.87 | 4.46 | 41.78 | 6.87 | 0.26 | 3.99 |
| Bauxite | 12.83 | 34.84 | 43.52 | 0.76 | 0.29 | 2.61 | 0.04 |
| Sample | SiO2/CaO | Al2O3 | SiO2 | CaO | MgO | Fe2O3 | MnO |
|---|---|---|---|---|---|---|---|
| S1 | 0.98 | 4.5 | 40.9 | 41.8 | 6.9 | 0.26 | 4.0 |
| S2 | 1.20 | 4.5 | 45.11 | 37.59 | 6.9 | 0.26 | 4.0 |
| S3 | 1.40 | 4.5 | 48.24 | 34.46 | 6.9 | 0.26 | 4.0 |
| S4 | 1.60 | 4.5 | 50.89 | 31.81 | 6.9 | 0.26 | 4.0 |
| S5 | 1.80 | 4.5 | 53.16 | 29.53 | 6.9 | 0.26 | 4.0 |
| Wavenumber/cm−1 | Corresponding Characteristic Vibration |
|---|---|
| 430~550 | Bending vibration of Si-Ob-Si |
| 520 | The Al-O bending vibration in [AlO6] |
| 700 | The Al-O bending vibration in [AlO4] |
| 760 | Symmetric stretching vibration of Si-Ob-Si |
| 780~800 | Symmetric bending vibration of O-Si-O |
| 950 | Anti-symmetric stretching vibration of Si-Onb |
| 1020~1060 | Anti-symmetric stretching vibration of Si-Ob-Si |
| Sample | ||||
|---|---|---|---|---|
| 5 °C·min−1 | 10 °C·min−1 | 15 °C·min−1 | 20 °C·min−1 | |
| S1 | 882.8 | 899.2 | 909.1 | 914.2 |
| S2 | 870.3 | 891.4 | 902.1 | 909.3 |
| S3 | 905.6 | 927.4 | 941.7 | 952.8 |
| S4 | 912.7 | 934.2 | 951.9 | 960.1 |
| S5 | 915.4 | 937.8 | 957.1 | 965.8 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chen, L.; Long, Y.; Zhou, M.; Wang, H. Structure and Crystallization of High-Calcium, CMAS Glass Ceramics Synthesized with a High Content of Slag. Materials 2022, 15, 657. https://doi.org/10.3390/ma15020657
Chen L, Long Y, Zhou M, Wang H. Structure and Crystallization of High-Calcium, CMAS Glass Ceramics Synthesized with a High Content of Slag. Materials. 2022; 15(2):657. https://doi.org/10.3390/ma15020657
Chicago/Turabian StyleChen, Lishun, Yuting Long, Mingkai Zhou, and Huaide Wang. 2022. "Structure and Crystallization of High-Calcium, CMAS Glass Ceramics Synthesized with a High Content of Slag" Materials 15, no. 2: 657. https://doi.org/10.3390/ma15020657





