Classification of Hot-Rolled Plates Using the Mahalanobis Distance of NMIs in Ti-Stabilized Austenitic Stainless-Steel Produced by Secondary Metallurgy
Abstract
:1. Introduction
2. Methods and Materials
2.1. Sampling
2.2. Chemical Analyses
2.2.1. Steels
2.2.2. Cored Ferrotitanium Wire
2.3. Metallography
Automated Analysis of Inclusions
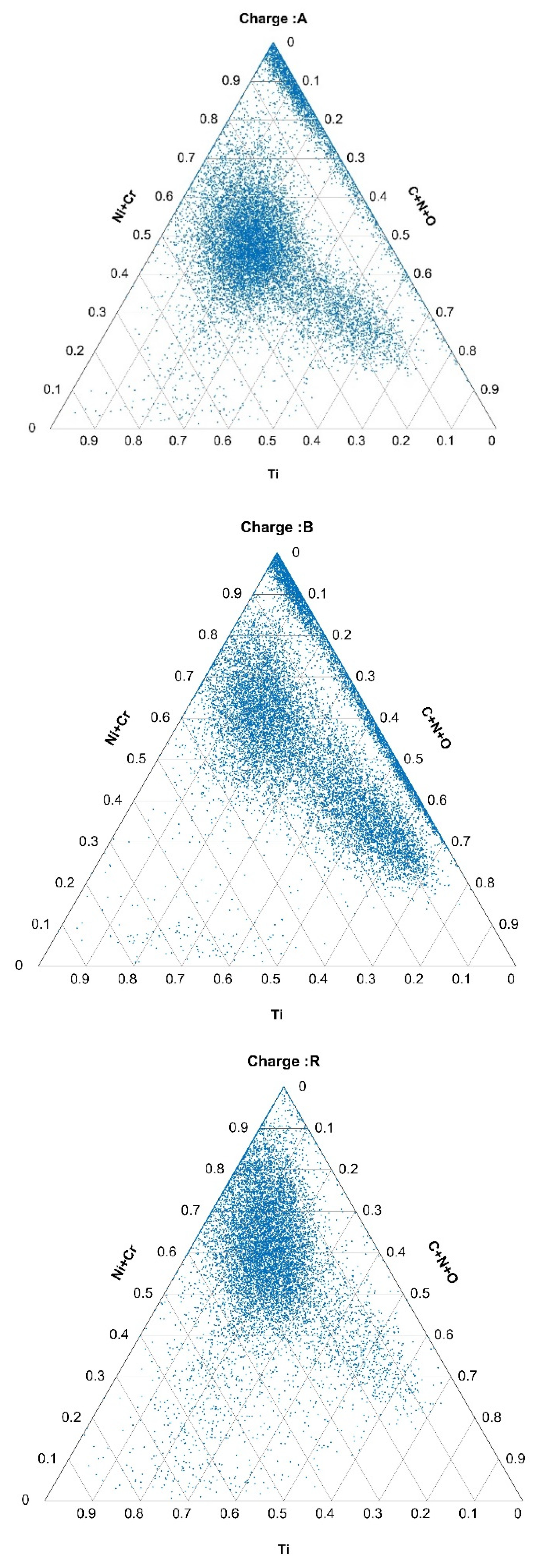
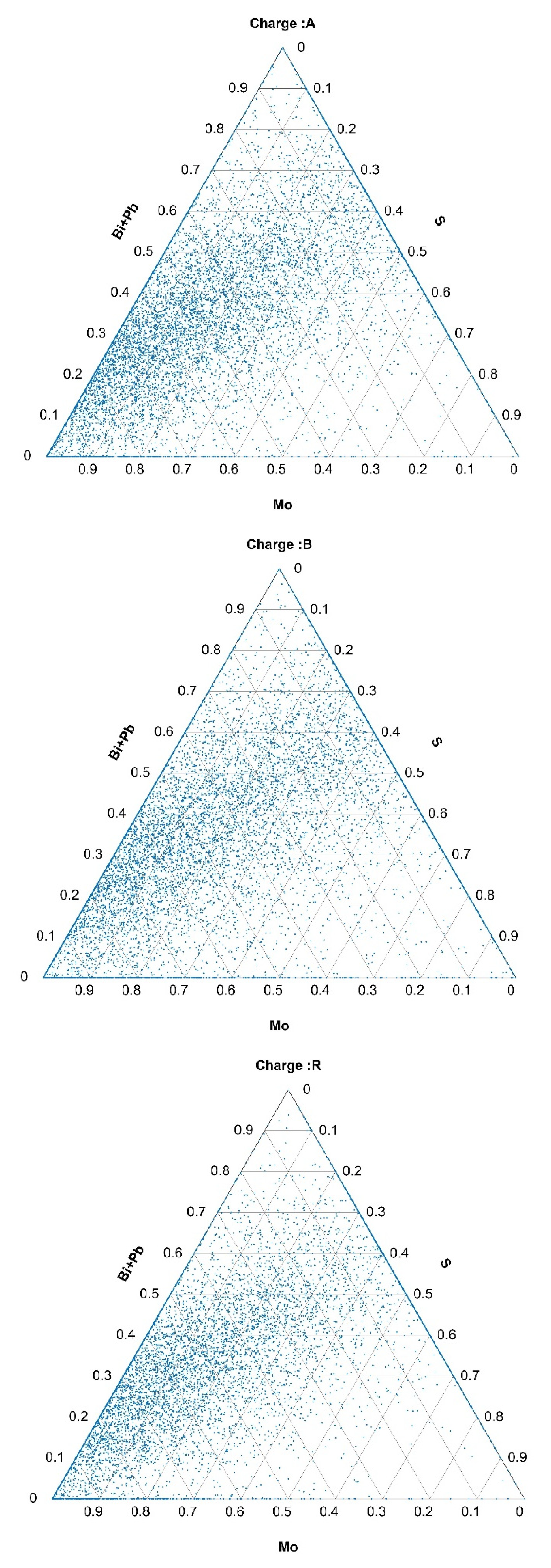
2.4. Composition of Inclusions
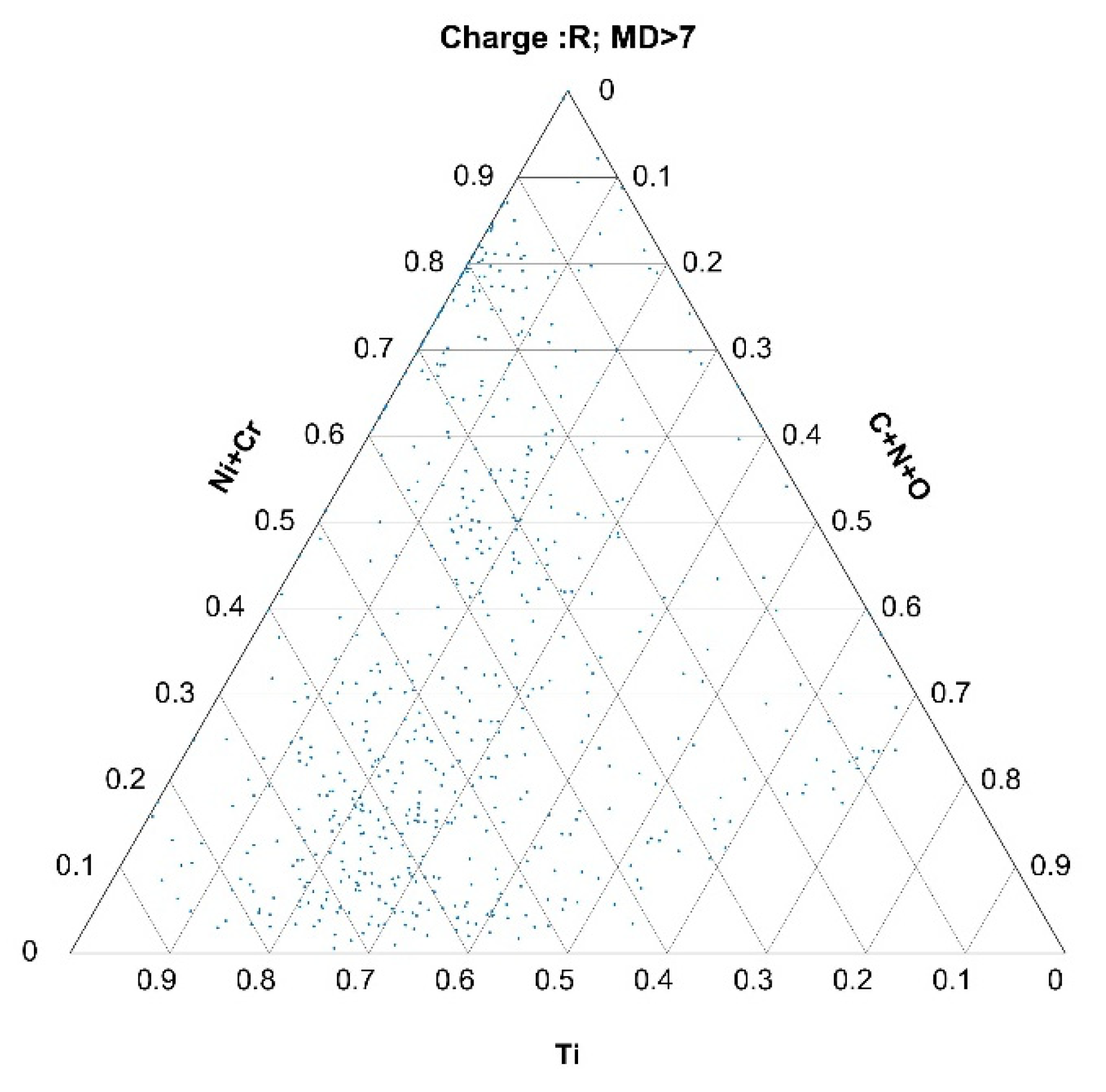
2.5. Calculation of the Mahalanobis Distance
3. Results
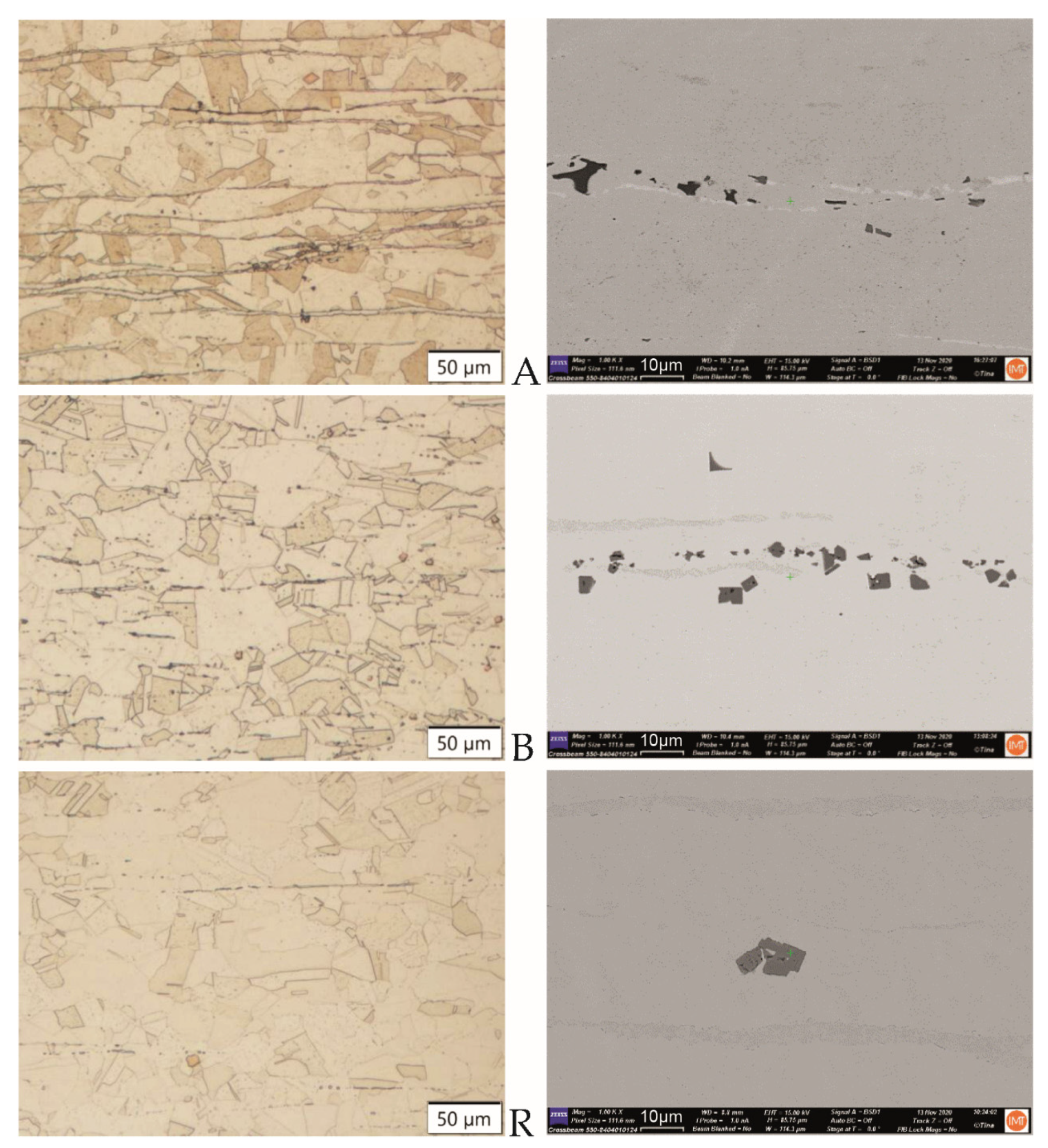
3.1. Microstructures of the Materials
3.1.1. Hot-Rolled Plates of Ti-Stabilized Cr-Ni-Mo Steel
3.1.2. Recycled FeTi-Cored Wire
3.2. Automated Analysis of Inclusions
3.2.1. Quantity, Size, and Composition of Inclusions Based on Data Obtained from Automated Analyses
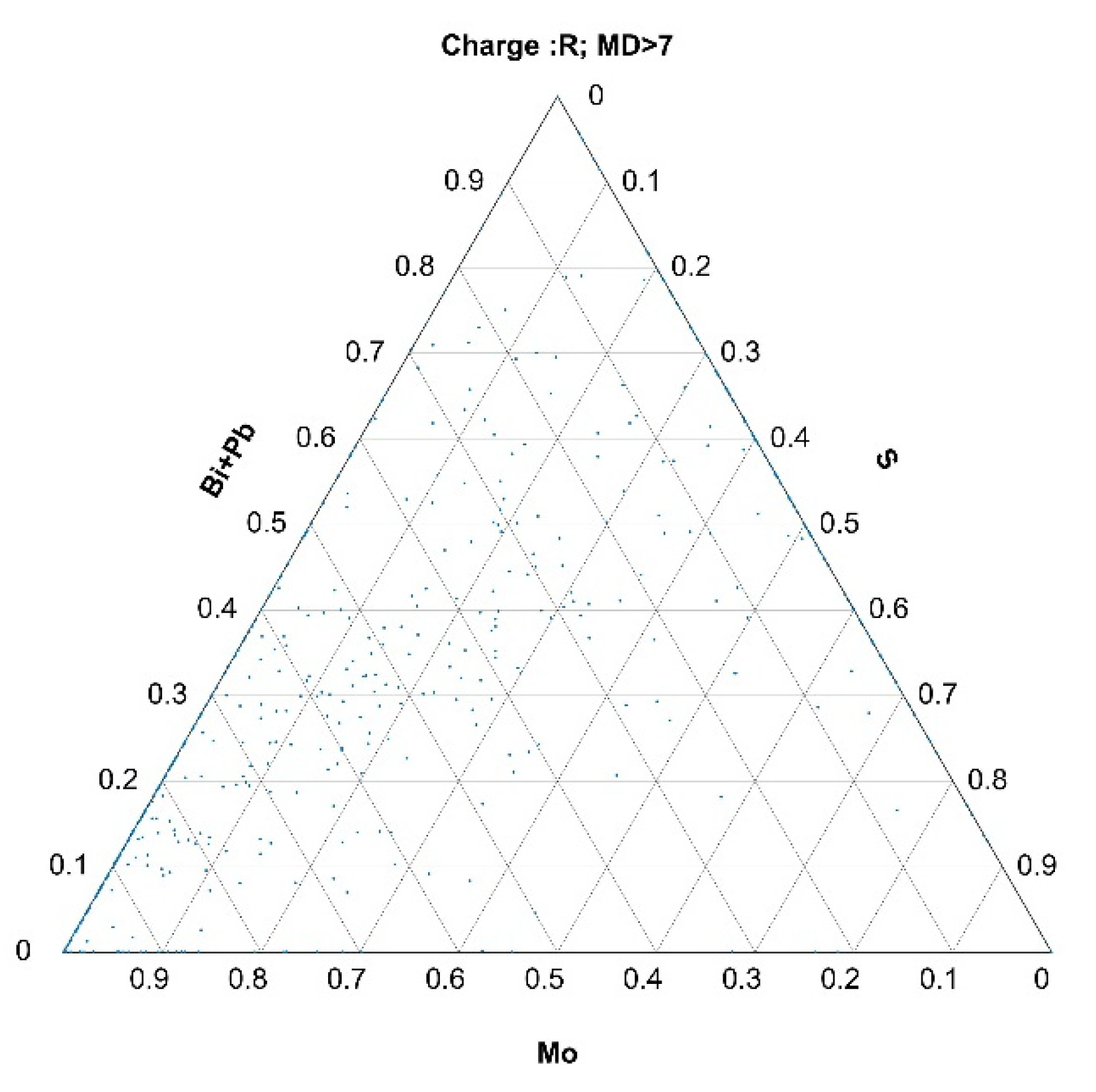
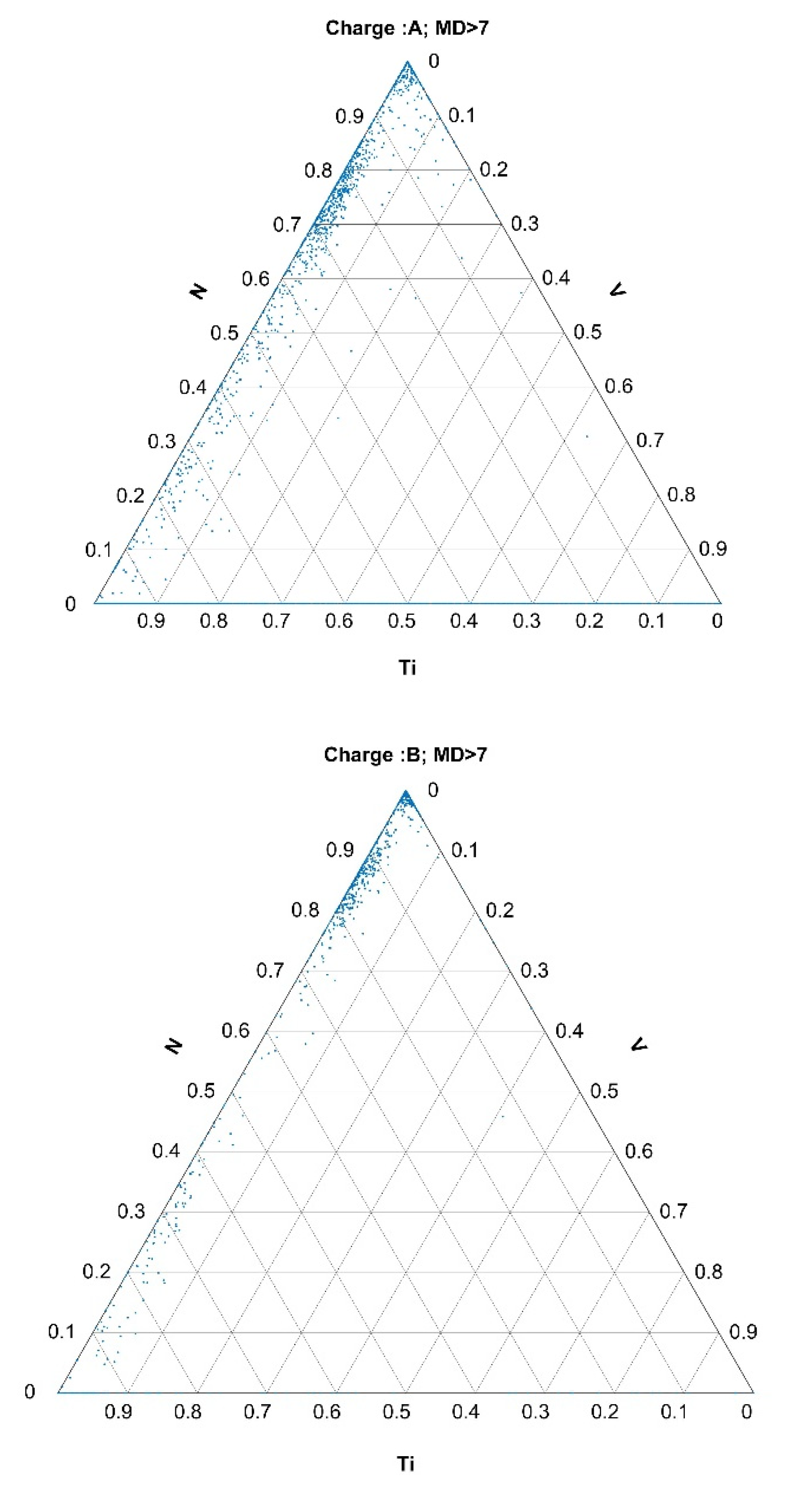
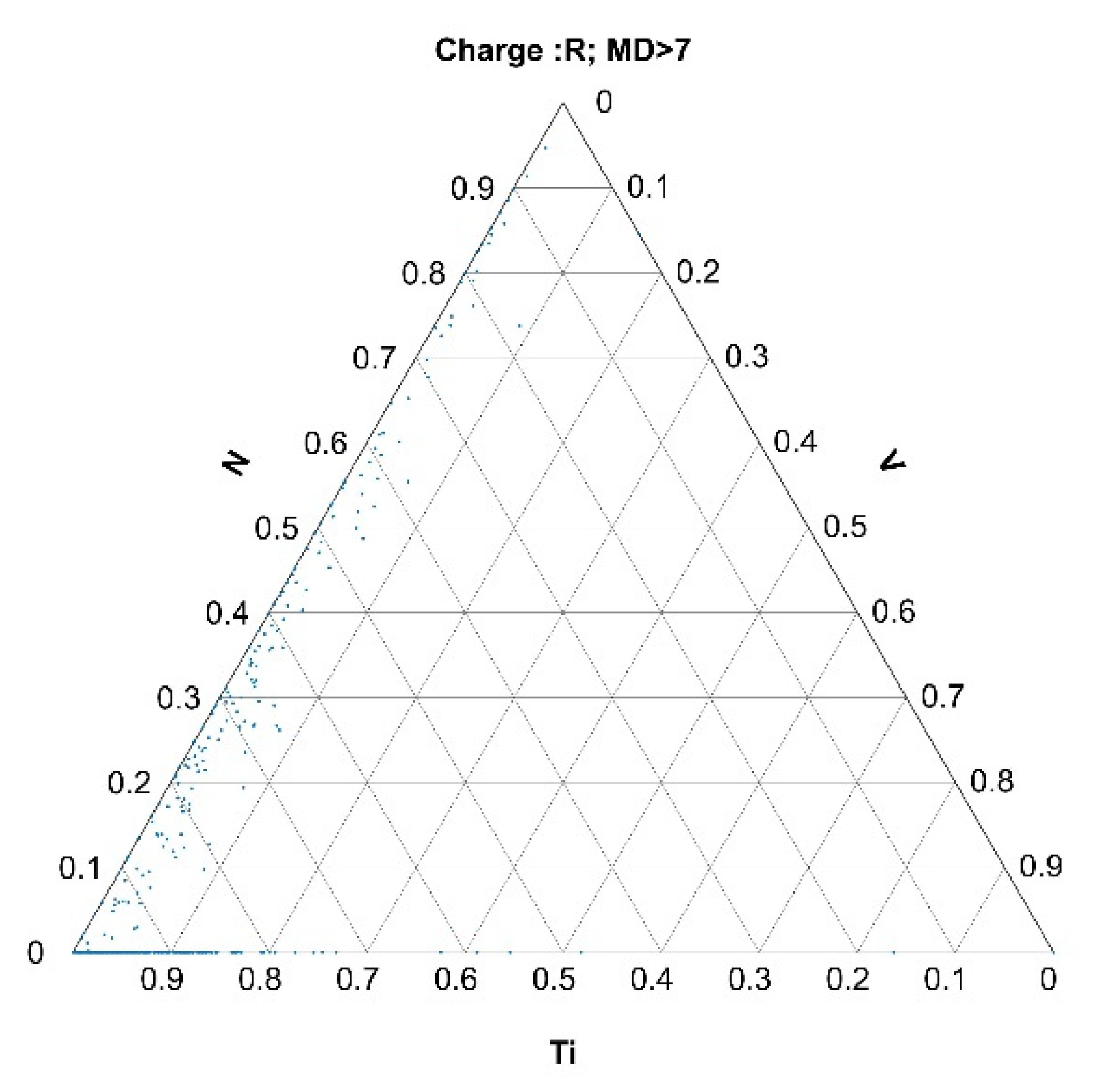
3.2.2. Histograms of Calculated Mahalanobis Distances Based on the Data Obtained from the Automated Analysis of Inclusions for Charges A, B, and R
3.3. Formation of Nonmetallic Inclusions and Segregations
3.3.1. Nonmetallic Inclusions after FeTi Alloying
3.3.2. Nonmetallic Inclusions after FeTi Alloying and Hot Rolling
Segregation of Impurity Elements
4. Discussion
- Bi2(SO4)3 with a melting point of 405 °C;
- Bi2O3 with a melting point of 817 °C;
- Bi2O5, usually stable only in combination with Bi2O3 and not alone;
- in the ternary Bi–Mo–O system, the existence of several bismuth molybdates BixMoyOz with melting points below 1000 °C is possible.
5. Conclusions
- The recycled FeTi-cored wire was the main source of elevated levels of impurities detected in the hot-rolled, Ti-stabilized stainless-steel plates.
- Insoluble impurities that are present in the FeTi wire for alloying of Cr-Ni-Mo stainless steel can greatly affect the final quality of the hot-rolled plates.
- Because of a high oxygen content in the used FeTi wire, the elemental Al could react with TixOy to form complex, nonmetallic inclusions that were very stable at the fabrication temperature of the steel before continuous casting.
- The occurrence of Mo, S, Pb, and Bi segregations was associated with the use of the recycled FeTi wire and the use of MoS2 in the mechanical preparation of the FeTi.
- In calculations of MDs, the steel charge with the smallest number of nonmetallic inclusions and segregations was set as a reference class.
- MDs, as a measure of the difference between only two classes, offer some possibilities for the automatic detection of abnormalities in steel measured indirectly using the data obtained with the automatic detection of inclusions.
- However, a combination of metallographic and mathematical techniques is recommended.
- The selection of outlier inclusions based on their MDs and their back-representation into ternary diagrams gave relevant metallurgical information about the abnormalities.
- The advantage of this technique is that the calculations of the MD and the threshold can be fully automated.
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| AISI | American Iron and Steel Institute |
| AOD | Argon oxygen decarburization |
| ASTM | American Society for Testing and Materials |
| BSE | Back-scattered electrons |
| BSI | Back-scattered electron imaging |
| CE | Circular economy |
| EAF | Electric arc furnace |
| ECD | Equivalent circular diameter |
| EDS | Energy-dispersive spectroscopy |
| EOL | End-of-life |
| FE-SEM | Field emission scanning electron microscopy |
| GOST | Gosudarstvennii Obscesoiuznii Standard (Russian) |
| ICP-OES | Optical emission spectrometer with inductively coupled plasma |
| ISO | The International Organization for Standardization |
| LF | Ladle furnace |
| LN2 | Liquid nitrogen |
| MD | Mahalanobis distance |
| NMI | Nonmetallic inclusion |
| OM | Optical microscopy |
| VOD | Vacuum oxygen decarburization |
| Z | Atomic number of a chemical element |
Appendix A

References
- Ryan, N.A.; Miller, S.A.; Skerlos, S.J.; Cooper, D.R. Reducing CO2 Emissions from US Steel Consumption by 70% by 2050. Environ. Sci. Technol. 2020, 54, 14598–14608. [Google Scholar] [CrossRef] [PubMed]
- Ren, L.; Zhou, S.; Peng, T.; Ou, X. A review of CO2 emissions reduction technologies and low-carbon development in the iron and steel industry focusing on China. Renew. Sustain. Energy Rev. 2021, 143, 110846. [Google Scholar] [CrossRef]
- Rieger, J.; Colla, V.; Matino, I.; Branca, T.A.; Stubbe, G.; Panizza, A.; Brondi, C.; Falsafi, M.; Hage, J.; Wang, X.; et al. Residue Valorization in the Iron and Steel Industries: Sustainable Solutions for a Cleaner and More Competitive Future Europe. Metals 2021, 11, 1202. [Google Scholar] [CrossRef]
- Echterhof, T. Review on the Use of Alternative Carbon Sources in EAF Steelmaking. Metals 2021, 11, 222. [Google Scholar] [CrossRef]
- Harvey, L.D.D. Iron and steel recycling: Review, conceptual model, irreducible mining requirements, and energy implications. Renew. Sustain. Energy Rev. 2021, 138, 110553. [Google Scholar] [CrossRef]
- Ramadan, A.; Shash, A.Y.; El-Mahallawi, I.S.; Senk, D.; Mattar, T. Identification of copper precipitates in scrap based recycled low carbon rebar steel. Mater. Des. 2017, 120, 157–169. [Google Scholar] [CrossRef]
- Sun, G.; Fan, D.; Tao, S. Identification of Antimony precipitates in Sb-bearing C-Mn steel. Metall. Mater. Trans. B 2021, 52, 576–579. [Google Scholar] [CrossRef]
- Arh, B.; Tehovnik, F. The oxidation and reduction of chromium of stainless-steels in an electric arc furnace. Metalurgija 2011, 50, 179–182. [Google Scholar]
- Yin, X.; Sun, Y.H.; Yang, Y.D.; Bai, X.F.; Deng, X.X.; Barati, M.; McLean, A. Inclusion evolution during refining and continuous casting of 316L stainless-steel. Ironmak. Steelmak. 2016, 43, 533–540. [Google Scholar] [CrossRef]
- Yin, X.; Sun, Y.; Yang, Y.; Deng, X.; Barati, M.; McLean, A. Effect of alloy addition on inclusion evolution in stainless-steels. Ironmak. Steelmak. 2017, 44, 152–158. [Google Scholar] [CrossRef]
- Yin, X.; Sun, Y.; Yang, Y.; Bai, X.; Barati, M.; McLean, A. Formation of inclusions in Ti-stabilized 17Cr austenitic stainless-steel. Metall. Mater. Trans. B 2016, 47, 3274–3284. [Google Scholar] [CrossRef]
- Steiner Petrovič, D. Kinetics of Arsenic Surface Segregation in Scrap-Based Silicon Electrical Steel. Metals 2021, 11, 1. [Google Scholar] [CrossRef]
- Vasconcellos da Costa e Silva, A.L. Non-metallic inclusions in steels—Origin and control. J. Mater. Res. Technol. 2018, 7, 283–299. [Google Scholar] [CrossRef]
- Ren, Y.; Wang, Y.; Li, S.; Zhang, L.; Zuo, X.; Lekakh, S.N.; Peaslee, K. Detection of non-metallic inclusions in steel continuous casting billets. Metall. Mater. Trans. B 2014, 45, 1291–1303. [Google Scholar] [CrossRef]
- Wang, H.; Yu, P.; Zhou, X.; Wang, Y.; Lv, X. Three-dimensional stability diagram of Al-Mg-O inclusions in molten steel. J. Mater. Res. Technol. 2021, 12, 43–52. [Google Scholar] [CrossRef]
- Bartosiaki, B.G.; Morales Pereira, J.A.; Bielefeldt, W.V.; Vilela, A.C.F. Assessment of inclusion analysis via manual and automated SEM and total oxygen content of steel. J. Mater. Res. Technol. 2015, 4, 235–240. [Google Scholar] [CrossRef] [Green Version]
- Zhitenev, A.; Salynova, M.; Shamshurin, A.; Ryaboshuk, S.; Kolnyshenko, V. Database Clustering after Automatic Feature Analysis of Nonmetallic Inclusions in Steel. Metals 2021, 11, 1650. [Google Scholar] [CrossRef]
- Cuartas, M.; Ruiz, E.; Ferreño, D.; Setién, J.; Arroyo, V.; Gutiérrez-Solana, F. Machine learning algorithms for the prediction of non-metallic inclusions in steel wires for tire reinforcement. J. Intell. Manuf. 2021, 32, 1739–1751. [Google Scholar] [CrossRef]
- Acerinox Europa, S.A.U. Cr-Ni-Mo Austenitic Stainless-Steel ACX 240. Available online: https://www.acerinox.com (accessed on 13 September 2021).
- Van Bennekom, A.; Wilke, F. Comparison between Stabilised and Low Carbon Austenitic Stainless-Steels. Available online: https://www.edelstahl-rostfrei.de/fileadmin/user_upload/ISER/downloads/Stabilised_LowCarbonAust_EN.pdf (accessed on 1 October 2021).
- Pande, M.M.; Guo, M.; Blanpain, B. Inclusion Formation and Interfacial Reaction between FeTi Alloys and Liquid Steel at an Early Stage. ISIJ Int. 2013, 53, 629–638. [Google Scholar] [CrossRef]
- Gasik, M.; Dashevskii, V.; Bizhanov, A. Metallurgy of Ferrotitanium. In Ferroalloys. Topics in Mining, Metallurgy and Materials Engineering; Springer: Cham, Switzerland, 2020. [Google Scholar]
- Zheng, D.; Li, J.; Shi, C.; Zhang, J.; Geng, R. Evolution of TiN and oxide inclusions in Ti-containing Fe-25Ni-15Cr alloy during electroslag remelting. ISIJ Int. 2020, 60, 1577–1585. [Google Scholar] [CrossRef] [Green Version]
- Wegst, C.W. Stahlschlüssel—Key to Steel, 25th ed.; Verlag Stahlschlüssel Wegst GmbH: Marbach, Germany, 2019. [Google Scholar]
- Baker, T.N. Titanium microalloyed steels. Ironmak. Steelmak. 2019, 46, 1–55. [Google Scholar] [CrossRef]
- Li, J.; Cheng, G.; Ruan, Q.; Pan, J.; Chen, X. Formation and evolution of oxide inclusions in titanium-stabilized 18Cr stainless-steel. ISIJ Int. 2018, 58, 2280–2287. [Google Scholar] [CrossRef] [Green Version]
- Bebbington, R.W. The role of ferroboron and ferrotitanium in steels: Production methods, quality aspects, and addition techniques. In Proceedings of the 1st Int Chromium Steels and Alloys Congress (INFACON 6), Cape Town, South Africa, 8–11 March 1992; SAIMM: Johannesburg, South Africa, 1992; Volume 2, pp. 263–267. [Google Scholar]
- Pan, C.; Hu, X.; Lin, P.; Chou, K. Evolution of Inclusions after Cerium and Titanium Addition in Aluminum Deoxidized Fe-17Cr-9Ni Austenitic Stainless-steel. ISIJ Int. 2020, 60, 1878–1885. [Google Scholar] [CrossRef]
- Pan, C.; Hu, X.; Lin, P.; Chou, K. Effects of Ti and Al addition on the Formation and Evolution of Inclusions in Fe-17Cr-9Ni Austenite Stainless-steel. Metall. Mater. Trans. B 2020, 51, 3039–3050. [Google Scholar] [CrossRef]
- Shah, S.J.; Henein, H.; Ivey, D.G. Microstructural characterization of ferrotitanium and ferroniobium. Mater. Charact. 2013, 78, 96–107. [Google Scholar] [CrossRef]
- Xing, L.; Guo, J.; Li, X.; Zhang, Z.; Wang, M.; Bao, Y.; Zeng, F.; Chen, B. Control of TiN precipitation behavior in titanium-containing micro-alloyed steel. Mater. Today Commun. 2020, 25, 101292. [Google Scholar] [CrossRef]
- Cheng, C.; Dou, Z.; Zhang, T. Mechanism of Melt Separation in Preparation of Low-Oxygen High Titanium Ferroalloy Prepared by Multistage and Deep Reduction. Metals 2020, 10, 309. [Google Scholar] [CrossRef] [Green Version]
- Jiao, H.; Song, W.L.; Chen, H.; Wang, M.; Jiao, S.; Fang, D. Sustainable recycling of titanium scraps and purity titanium production via molten salt electrolysis. J. Clean. Prod. 2020, 261, 121314. [Google Scholar] [CrossRef]
- Chang, Z.P.; Yan, W.L.; Nazish, F. A theoretical survey on Mahalanobis-Taguchi system. Measurement 2019, 136, 501–510. [Google Scholar] [CrossRef]
- Ramlie, F.; Muhamad, W.Z.A.W.; Harudin, N.; Abu, M.Y.; Yahaya, H.; Jamaludin, K.R.; Abdul Talib, H.H. Classification Performance of Thresholding Methods in the Mahalanobis–Taguchi System. Appl. Sci. 2021, 11, 3906. [Google Scholar] [CrossRef]
- Cui, S.; Hu, B.; Ouyang, B.; Zhao, D. Thermodynamic assessment of the Mo-S system and its application in thermal decomposition of MoS2. Thermochim. Acta 2018, 660, 44–55. [Google Scholar] [CrossRef]
- Sales, M.G.; Herweyer, L.; Opila, E.; McDonell, S. MoS2 impurities: Chemical identification and spatial resolution of bismuth impurities in geological material. Appl. Surf. Sci. 2020, 508, 145256. [Google Scholar] [CrossRef] [Green Version]
- RamReddy, K.; Nandha Kumar, E.; Jeyaraam, R.; Janak Ram, G.D.; Subramanya Sarma, V. Effect of grain boundary character distribution on weld heat-affected zone liquation cracking behaviour of AISI 316Ti stainless-steel. Mater. Charact. 2018, 142, 115–123. [Google Scholar]
- Tehovnik, F.; Vodopivec, F.; Celin, R.; Arzenšek, B.; Gontarev, J. Effect of δ-ferrite, lead and sulphur on hot workability of austenitic stainless-steels with solidification structure. Mater. Sci. Technol. 2011, 27, 774–782. [Google Scholar] [CrossRef]
- Shankar, V.; Gill, T.P.; Mannan, S.L.; Sundaresan, S. Solidification cracking in austenitic stainless-steel welds. Sadhana 2003, 28, 359–382. [Google Scholar] [CrossRef]
- Liu, H.T.; Chen, W.Q. Research on recovery for adding low melting point metal bismuth to eco-friendly Bi–S based free cutting steel. Ironmak. Steelmak. 2014, 41, 355–359. [Google Scholar] [CrossRef]
- Xie, J.; Fan, T.; Zeng, Z.; Sun, H.; Fu, J. Bi-sulfide existence in 0Cr18Ni9 steel: Correlation with machinability and mechanical properties. J. Mater. Res. Technol. 2020, 9, 9142–9152. [Google Scholar] [CrossRef]
- Xie, J.; Li, J.; Li, Z.; Wu, L.; Zhang, P.; Fu, J. Forms of Bi-sulphide in 1215MS steel related to machining and mechanical performance. Ironmak. Steelmak. 2021, 48, 927–935. [Google Scholar] [CrossRef]
- Li, Z.; Wu, D. Effect of free-cutting additives on machining characteristics of austenitic stainless-steels. J. Mater. Sci. Technol. 2010, 26, 839–844. [Google Scholar] [CrossRef]
- Kim, M.S.; Lee, H.J.; Chang, Y.W. The effect of Mn/S ratio on hot ductility of Bi bearing free cutting steels. In Key Engineering Materials; Nam, S.W., Chang, Y.W., Lee, S.B., Kim, N.J., Eds.; Trans Tech Publications Ltd.: Bäch, Switzerland, 2007; Volume 345–346, pp. 169–172. [Google Scholar]
- Liu, H.T.; Chen, W.Q. Hot ductility of eco-friendly low carbon resulphurised free cutting steel with bismuth. Ironmak. Steelmak. 2014, 41, 19–25. [Google Scholar] [CrossRef]
- Liu, H.; Yu, Y.; Chen, W.; Wang, Q.; Wang, G. Effect of Mn/S Ratio on the Hot Ductility of Eco-friendly Bi-S based Free Cutting Steel. High Temp. Mater. Processes 2014, 33, 553–561. [Google Scholar] [CrossRef]
- Aiswarya, P.M.; Ghosh, S.; Ganesan, R.; Gnanasekaran, T. Phase diagram studies on ternary Bi–Mo–O system. J. Solid State Chem. 2020, 292, 121657. [Google Scholar] [CrossRef]






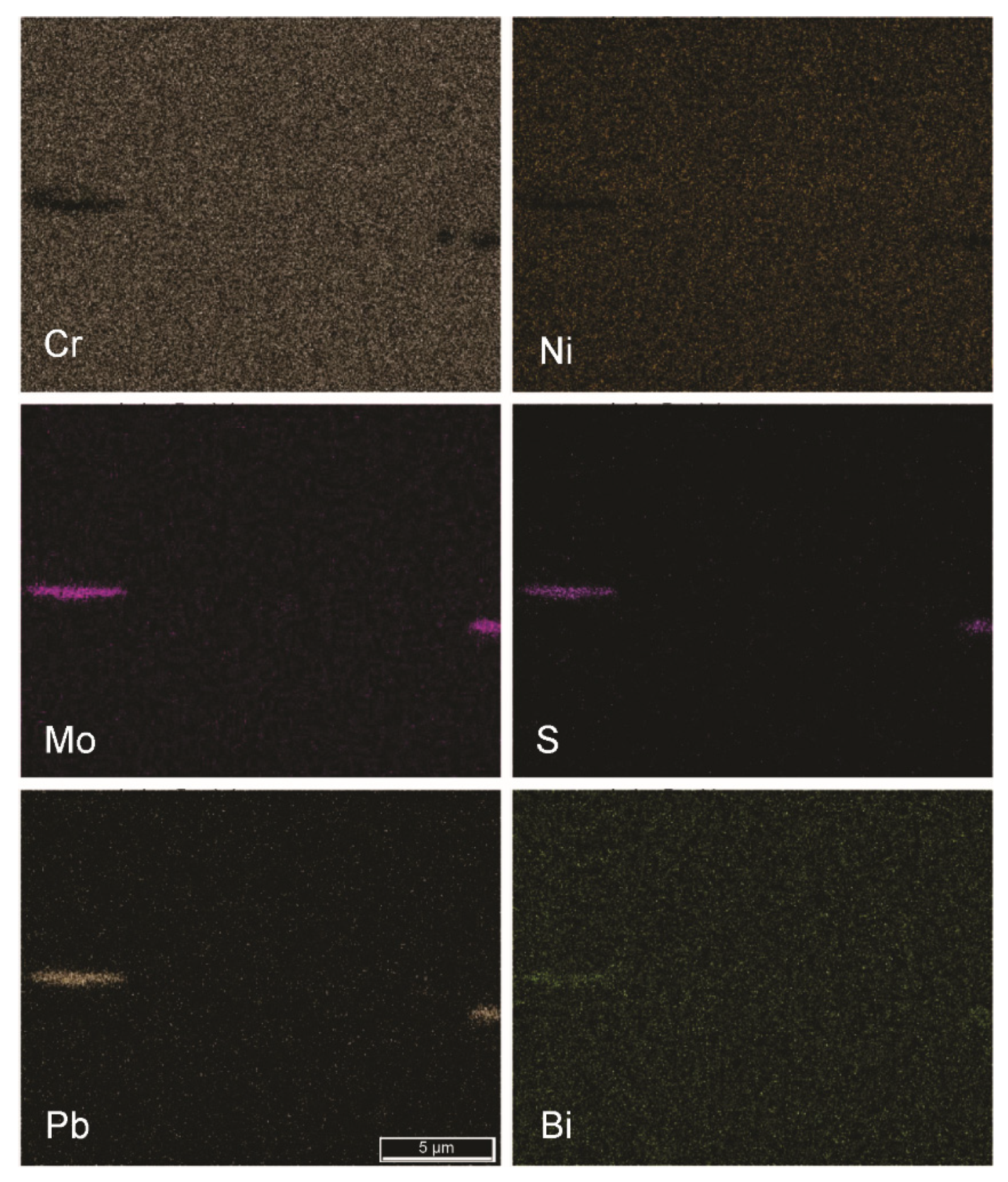

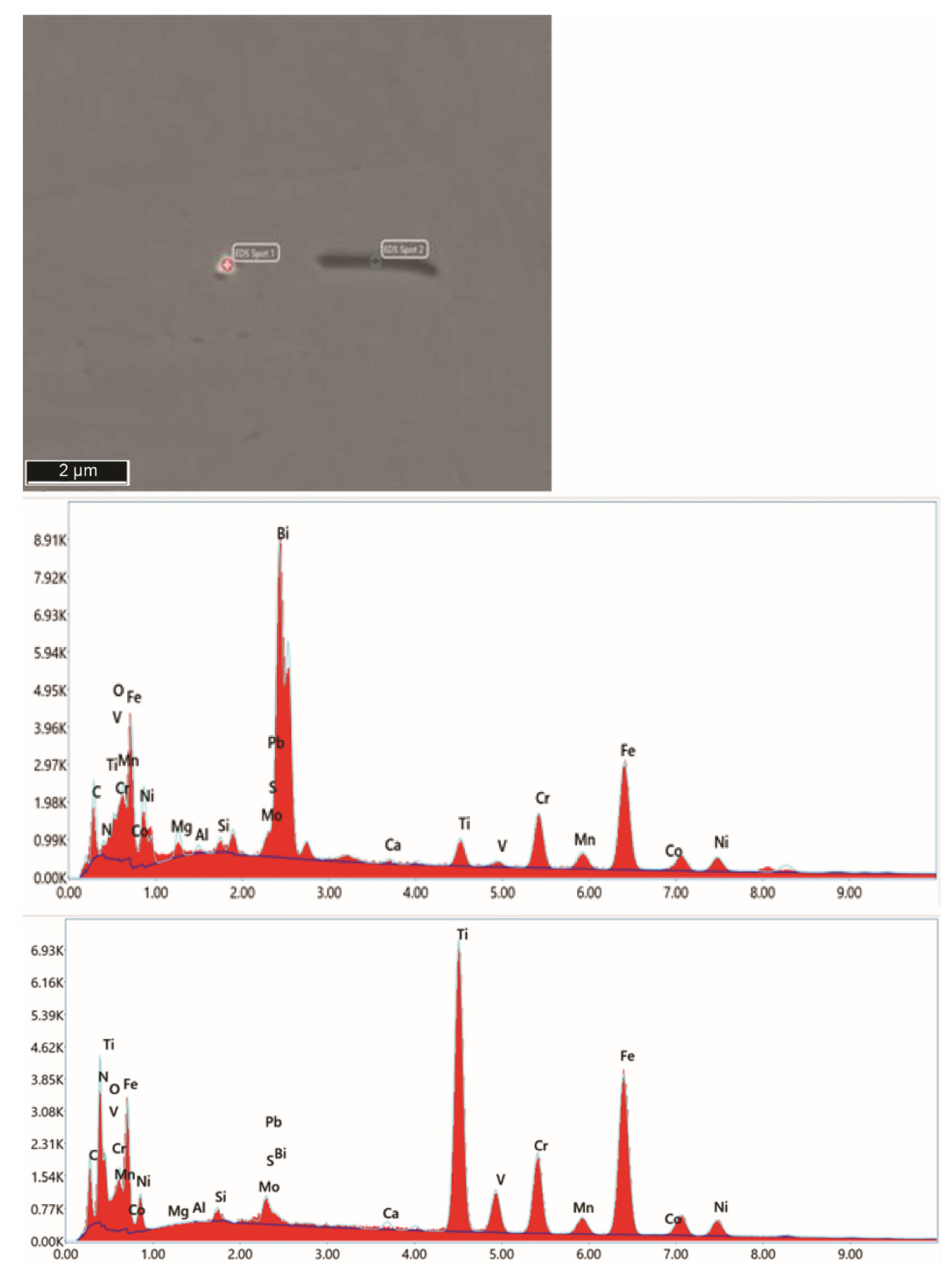
| Chemical Composition/in wt. % | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Sample | Cr | Ni | Mo | Ti | C | O | N | S | Pb | Bi | Fe |
| A | 17.1 | 10.7 | 2.1 | 0.4 | 0.041 | 0.0026 | 0.0104 | <0.001 | <0.01 | <0.01 | Bal. |
| B | 17.1 | 10.7 | 2.1 | 0.3 | 0.024 | 0.0059 | 0.0104 | <0.001 | <0.01 | <0.01 | Bal. |
| R | 17.0 | 11.9 | 2.1 | 0.3 | 0.049 | 0.0013 | 0.0080 | <0.001 | <0.01 | <0.01 | Bal. |
| Tolerance wt. % | ±0.2 | ±0.2 | ±0.05 | ±0.001 | ±0.003 | ±0.00002 | ±0.0005 | ±0.0005 | ±0.005 | ±0.005 | / |
| Charpy V-notch impact toughness/in J | |||||||||||
| Sample | 25 °C | −45 °C | −196 °C | ||||||||
| A | 216.5 ± 0.5 | 219.0 ± 1.0 | 178.0 ± 6.0 | ||||||||
| B | 264.0 ± 0.0 | 266.0 ± 0.0 | 212.0 ± 2.0 | ||||||||
| R | 185.5 ± 0.5 | 163.5 ± 0.5 | 136.0 ± 2.0 | ||||||||
| Vickers hardness (HV10) | |||||||||||
| A | 152 ± 4 | ||||||||||
| B | 150 ± 12 | ||||||||||
| R | 147 ± 1 | ||||||||||
| Element | Sample 1 | Sample 2 | Sample 3 | Tolerance/wt. % |
|---|---|---|---|---|
| Al | 2.7 | 3.1 | 3.2 | ±0.001 |
| Bi | 0.10 | 0.08 | 0.13 | ±0.005 |
| Cr | 0.38 | 0.35 | 0.34 | ±0.2 |
| Fe | 17.1 | 16.1 | 15.0 | ±0.2 |
| Mn | 0.10 | 0.14 | 0.09 | ±0.05 |
| Mo | 0.33 | 0.67 | 0.44 | ±0.05 |
| Ni | 0.42 | 0.55 | 0.39 | ±0.2 |
| Pb | <0.01 | <0.01 | <0.01 | ±0.005 |
| Sb | <0.01 | <0.01 | <0.01 | ±0.005 |
| Sn | 0.21 | 0.24 | 0.34 | ±0.005 |
| V | 1.9 | 1.7 | 2.0 | ±0.01 |
| Zr | 0.22 | 0.25 | 0.36 | ±0.005 |
| Si | 0.29 | 0.29 | 0.30 | ±0.02 |
| C | 0.17 | 0.16 | 0.17 | ±0.003 |
| S | 0.009 | 0.010 | 0.010 | ±0.0005 |
| N | 1.03 | 0.75 | 0.77 | ±0.005 |
| O | 2.02 | 1.47 | 2.06 | ±0.0005 |
| Ti | Bal. | Bal. | Bal. | — |
| Class Frequency | |||
|---|---|---|---|
| Size Class | Sample A | Sample B | Sample R |
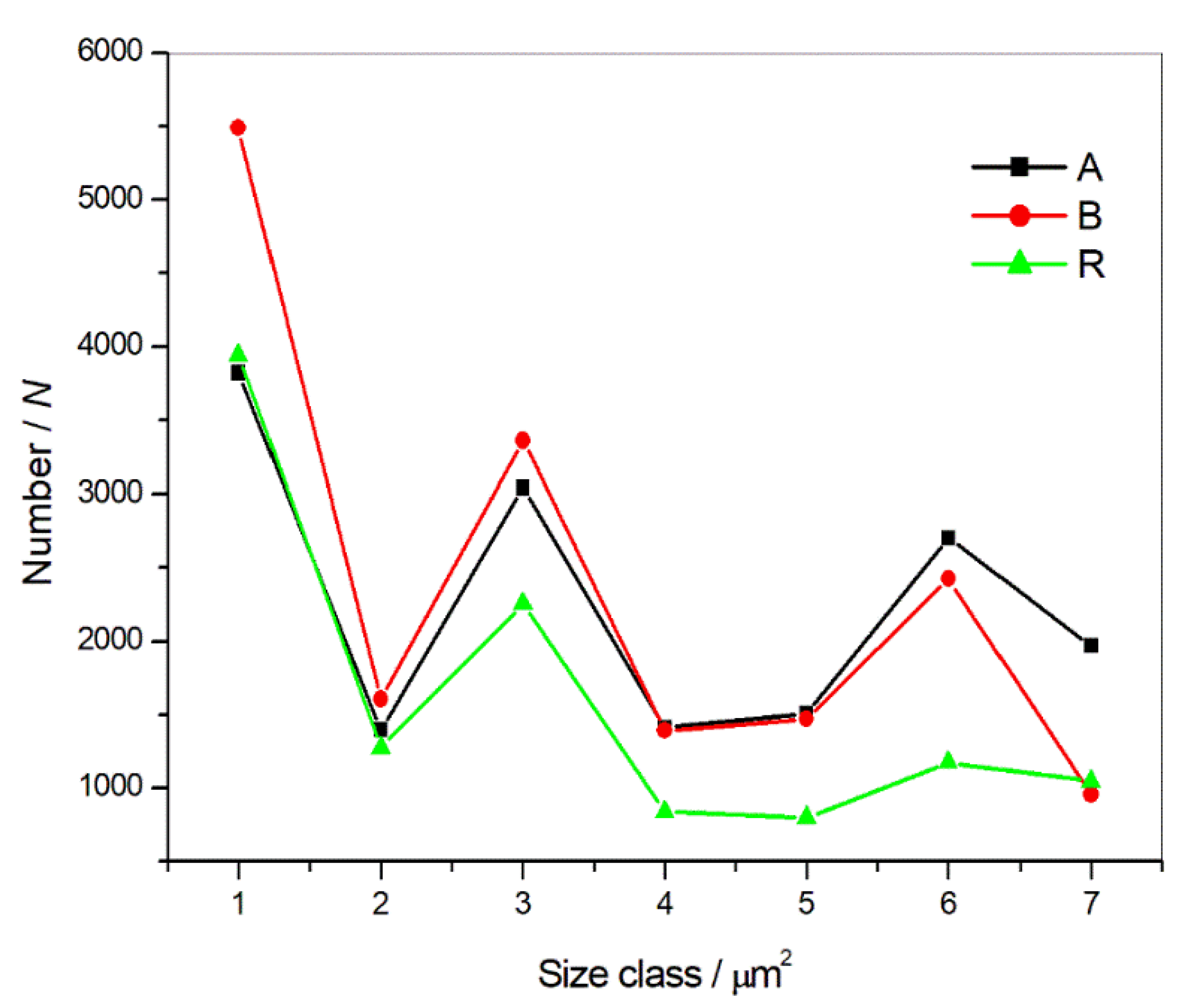
| 1 < 0.015 µm2 | 3824 | 5490 | 3943 |
| 0.015 < 2 < 0.02 µm2 | 1400 | 1607 | 1276 |
| 0.02 < 3 < 0.03 µm2 | 3043 | 3361 | 2259 |
| 0.03 < 4 < 0.04 µm2 | 1411 | 1393 | 839 |
| 0.04 < 5 < 0.05 µm2 | 1506 | 1471 | 799 |
| 0.05 < 6 < 0.1 µm2 | 2705 | 2425 | 1176 |
| 0.1 < 7 < 200 µm2 | 1969 | 958 | 1049 |
| Inclusions in total: | 15,858 | 16,705 | 11,341 |
| Average size of inclusions/µm2: | 0.20 ± 2.36 | 0.13 ± 1.46 | 0.25 ± 2.54 |
| % C | % S | % Al—Soluble | % Al—Insoluble | % Si |
|---|---|---|---|---|
| 0.16 ± 0.01 | 0.01 ± 0.001 | 3.1 ± 0.2 | 0.18 ± 0.03 | 0.29 ± 0.02 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Vode, F.; Tehovnik, F.; Kosec, G.; Steiner Petrovič, D. Classification of Hot-Rolled Plates Using the Mahalanobis Distance of NMIs in Ti-Stabilized Austenitic Stainless-Steel Produced by Secondary Metallurgy. Materials 2022, 15, 684. https://doi.org/10.3390/ma15020684
Vode F, Tehovnik F, Kosec G, Steiner Petrovič D. Classification of Hot-Rolled Plates Using the Mahalanobis Distance of NMIs in Ti-Stabilized Austenitic Stainless-Steel Produced by Secondary Metallurgy. Materials. 2022; 15(2):684. https://doi.org/10.3390/ma15020684
Chicago/Turabian StyleVode, Franci, Franc Tehovnik, Gorazd Kosec, and Darja Steiner Petrovič. 2022. "Classification of Hot-Rolled Plates Using the Mahalanobis Distance of NMIs in Ti-Stabilized Austenitic Stainless-Steel Produced by Secondary Metallurgy" Materials 15, no. 2: 684. https://doi.org/10.3390/ma15020684
APA StyleVode, F., Tehovnik, F., Kosec, G., & Steiner Petrovič, D. (2022). Classification of Hot-Rolled Plates Using the Mahalanobis Distance of NMIs in Ti-Stabilized Austenitic Stainless-Steel Produced by Secondary Metallurgy. Materials, 15(2), 684. https://doi.org/10.3390/ma15020684




