Removal Mechanism and Electrochemical Milling of (TiB+TiC)/TC4 Composites
Abstract
:1. Introduction
2. Materials and Methods
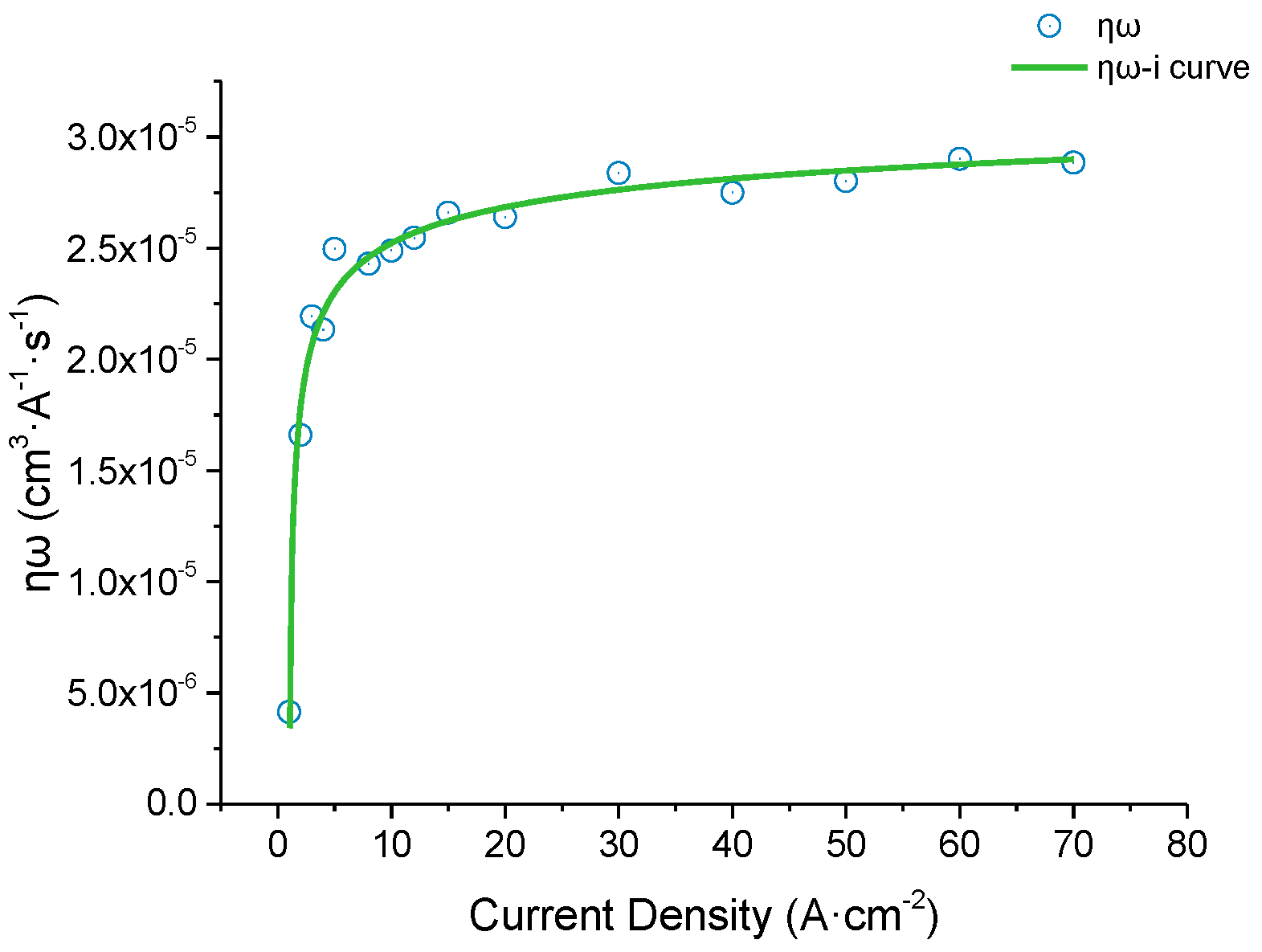
2.1. Current Efficiency Curve Experiments
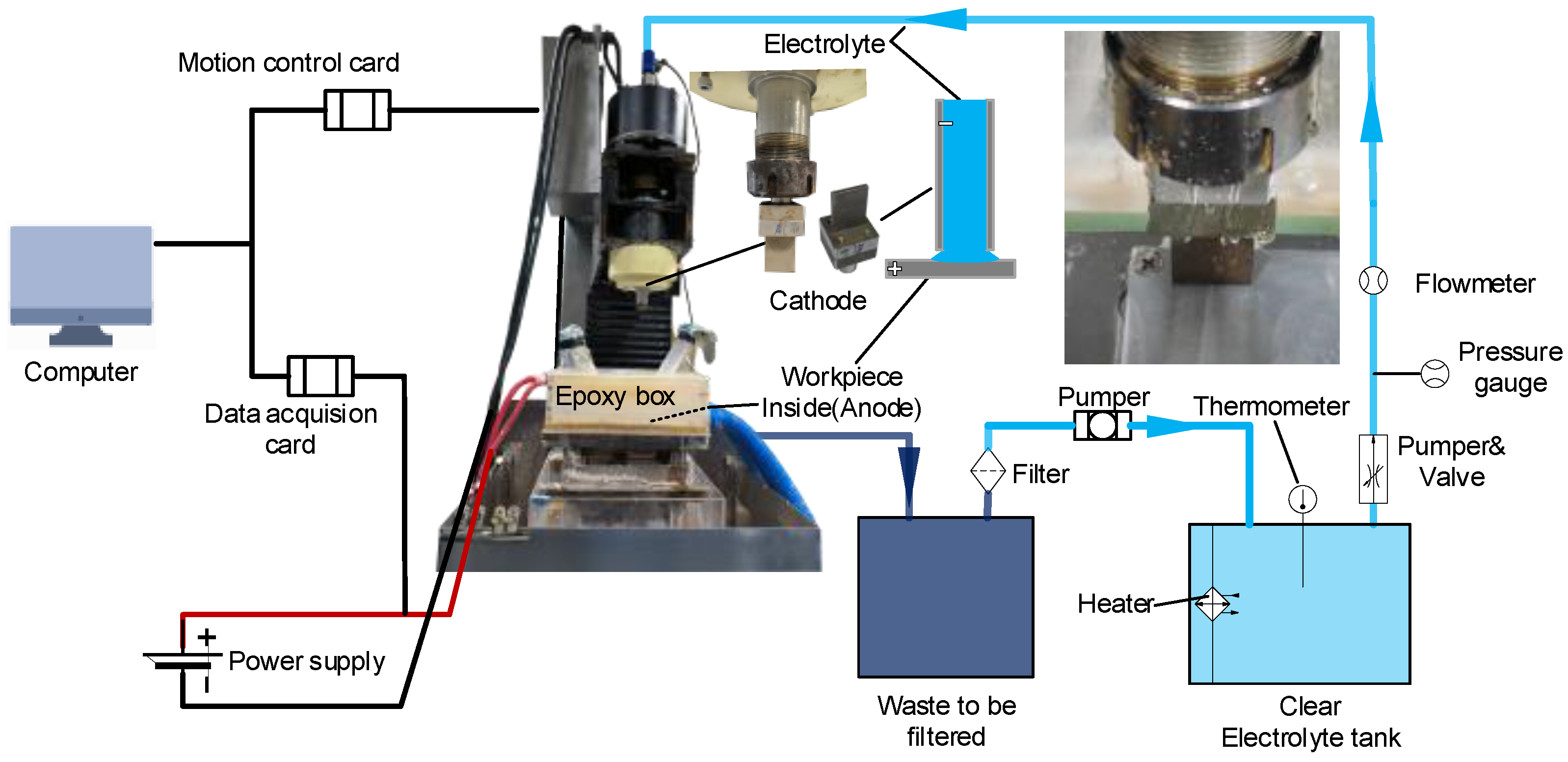
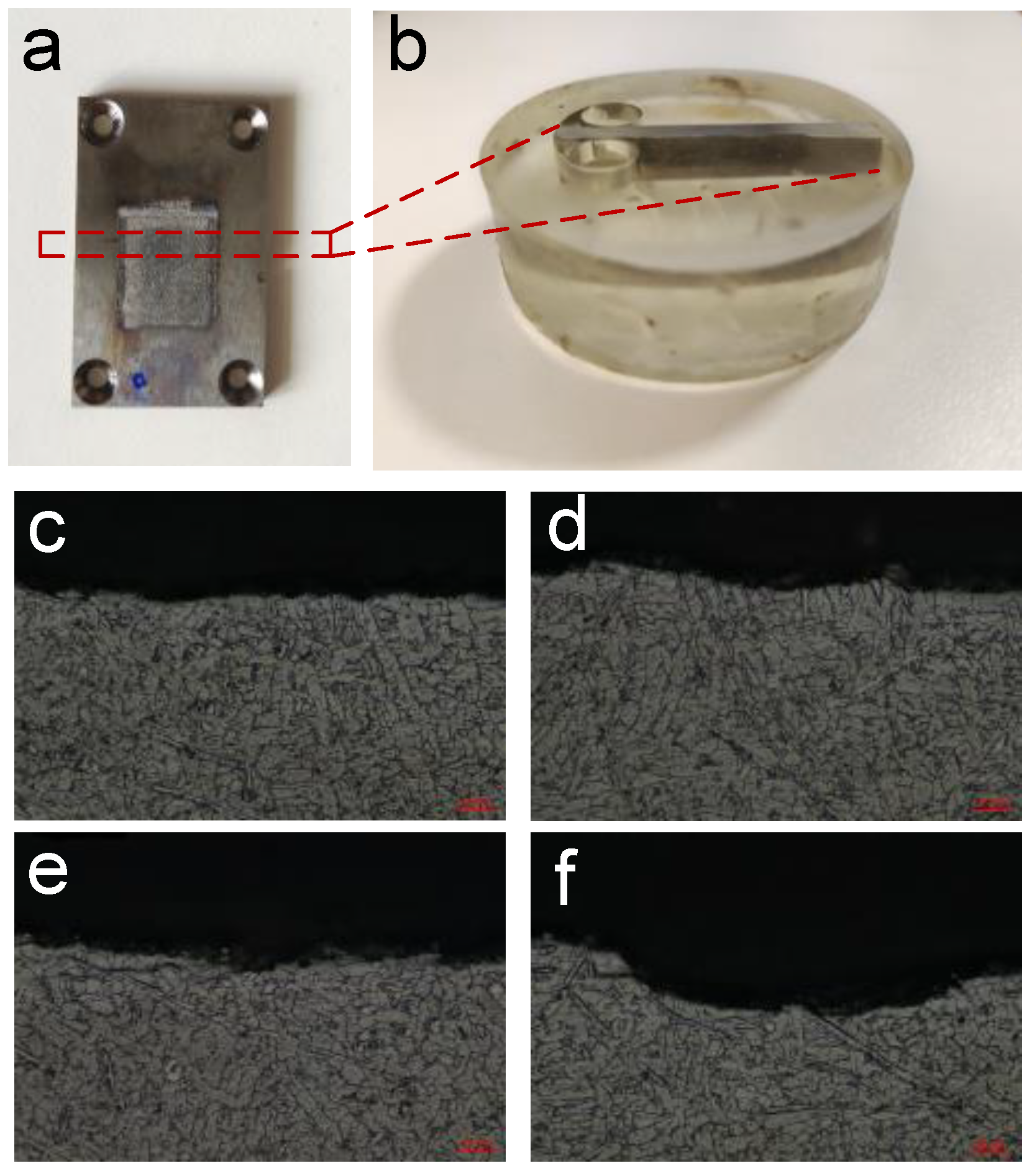
2.2. ECM Experimental Setup
3. Results and Discussion
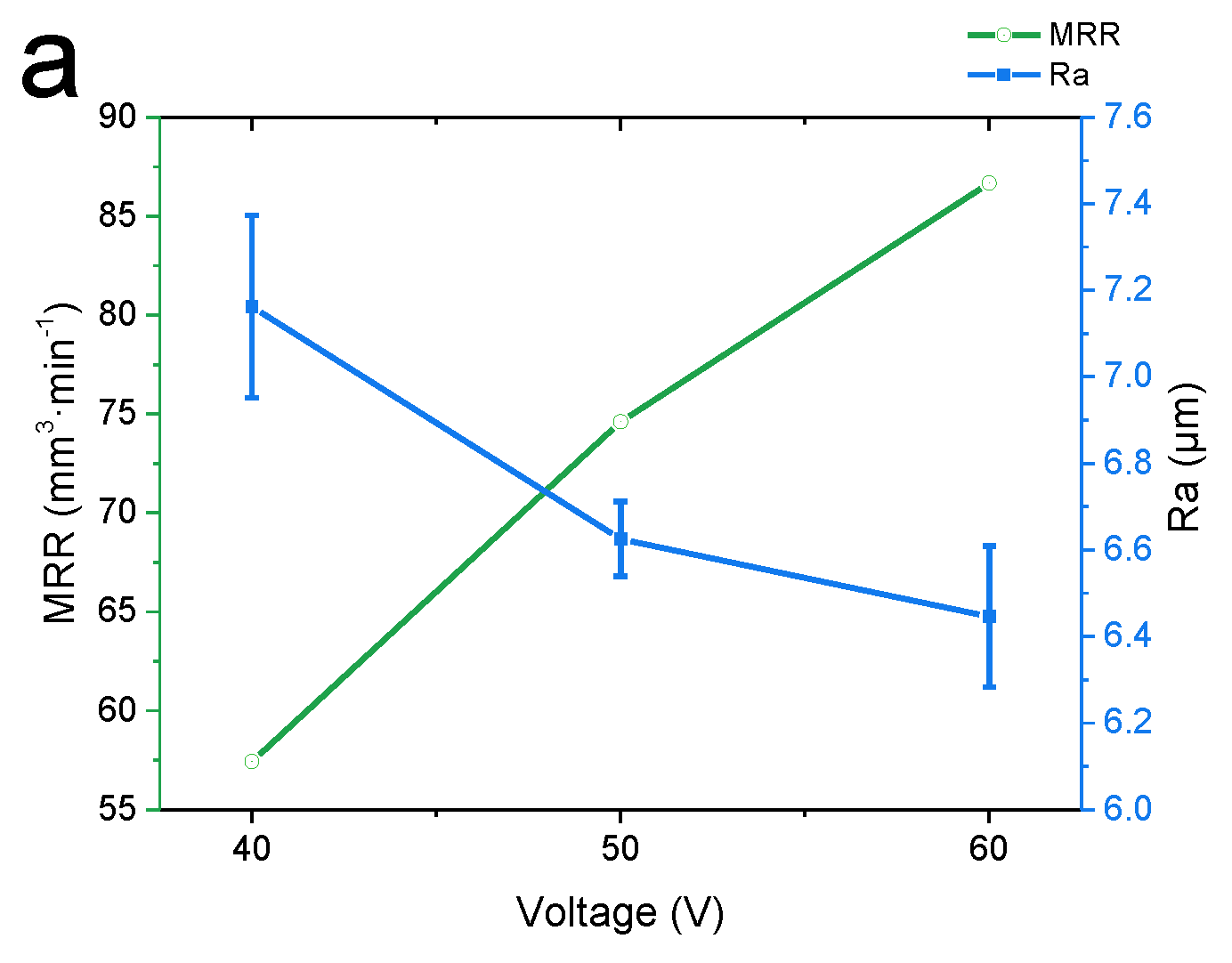
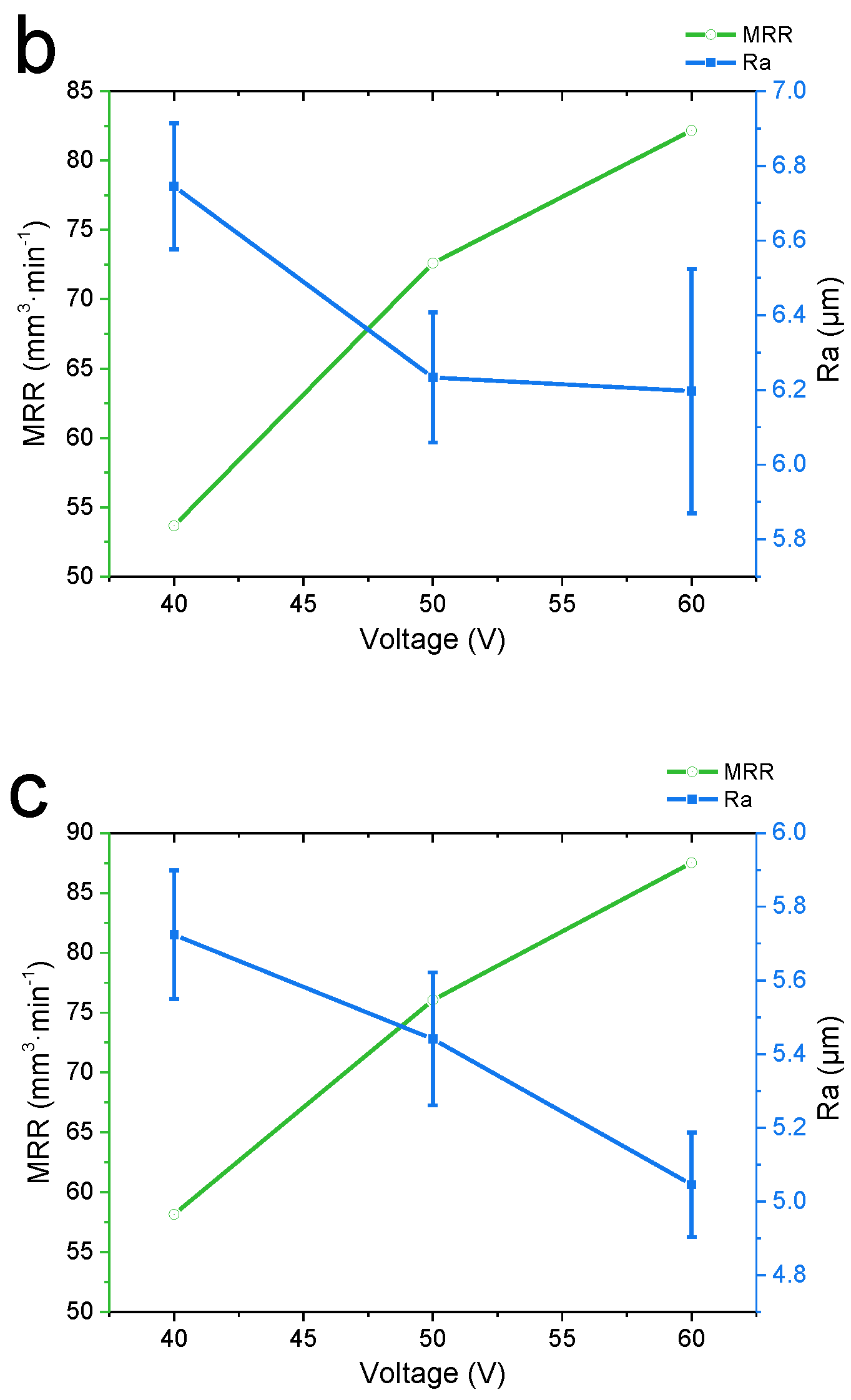
3.1. Effect of the Machining Voltage on the Experimental Results
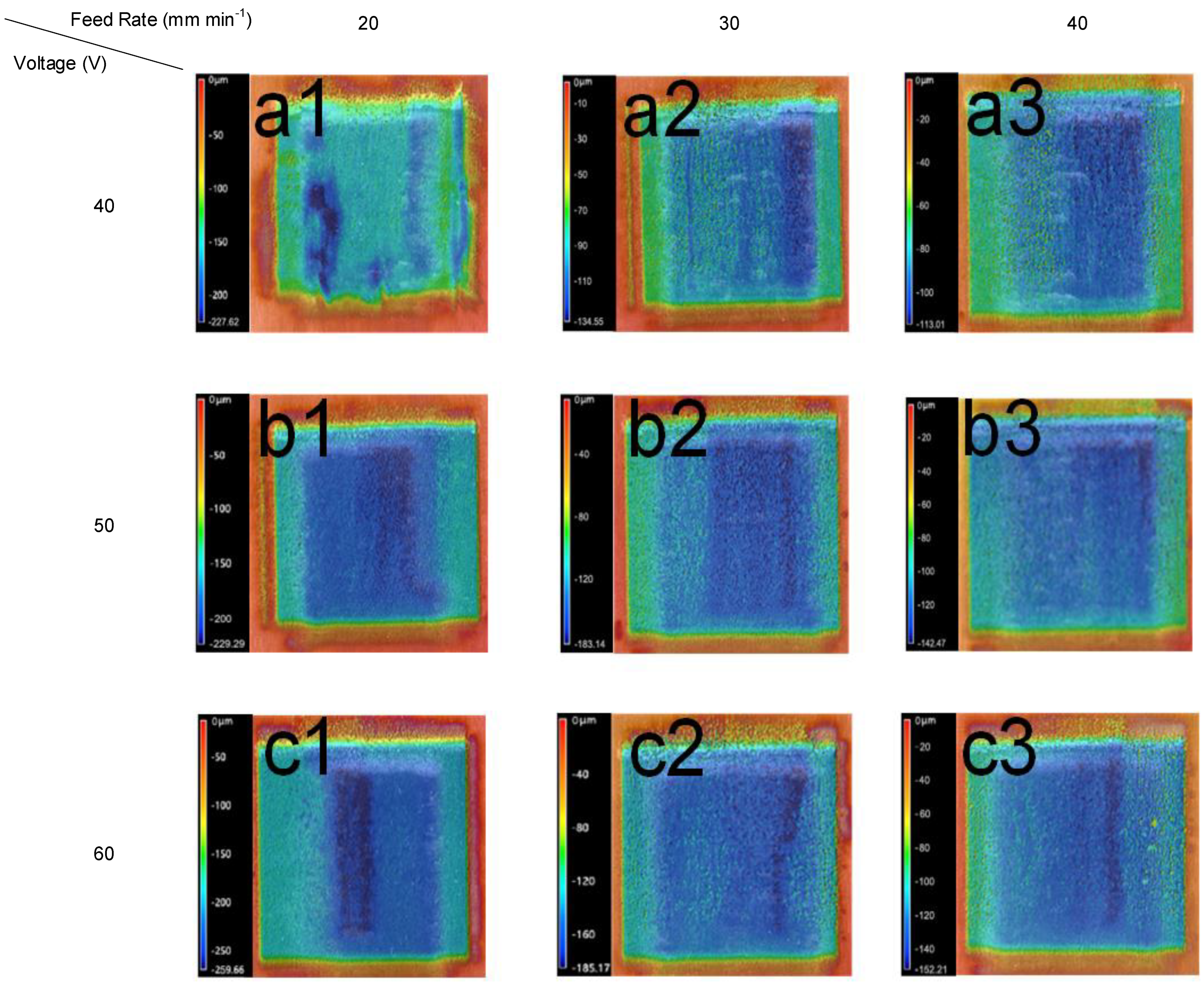
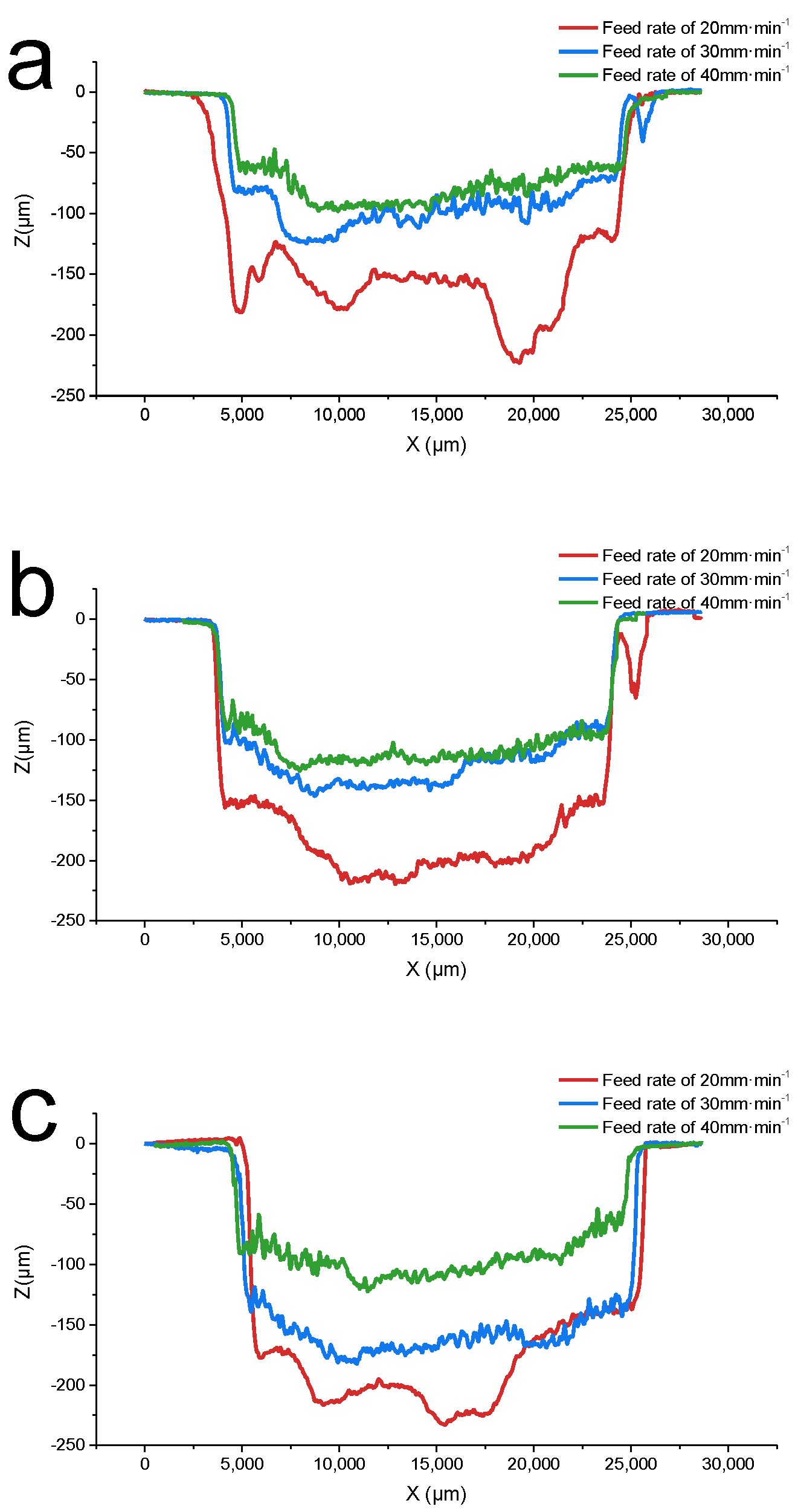
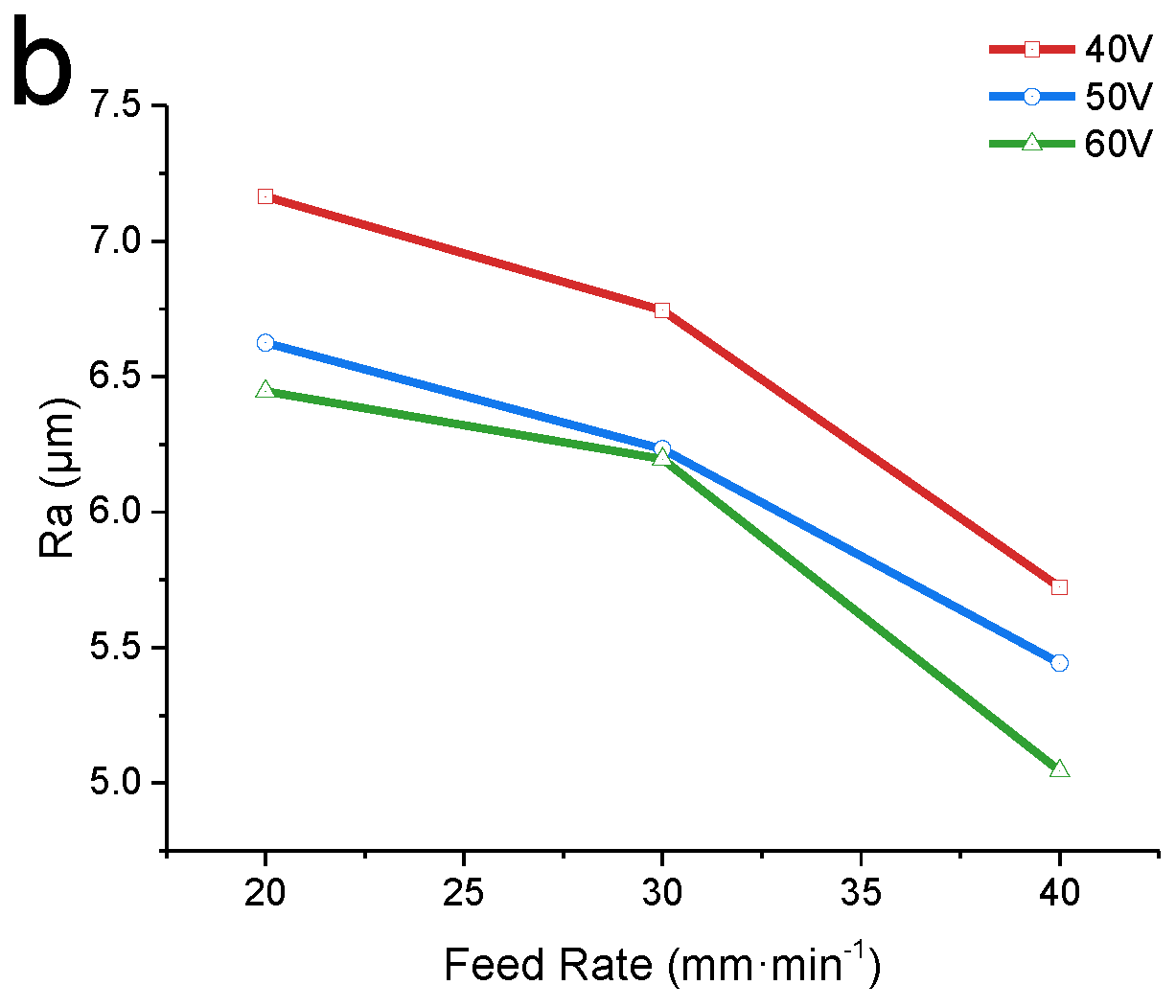
3.2. Effect of the Feed Rate on the Experimental Results
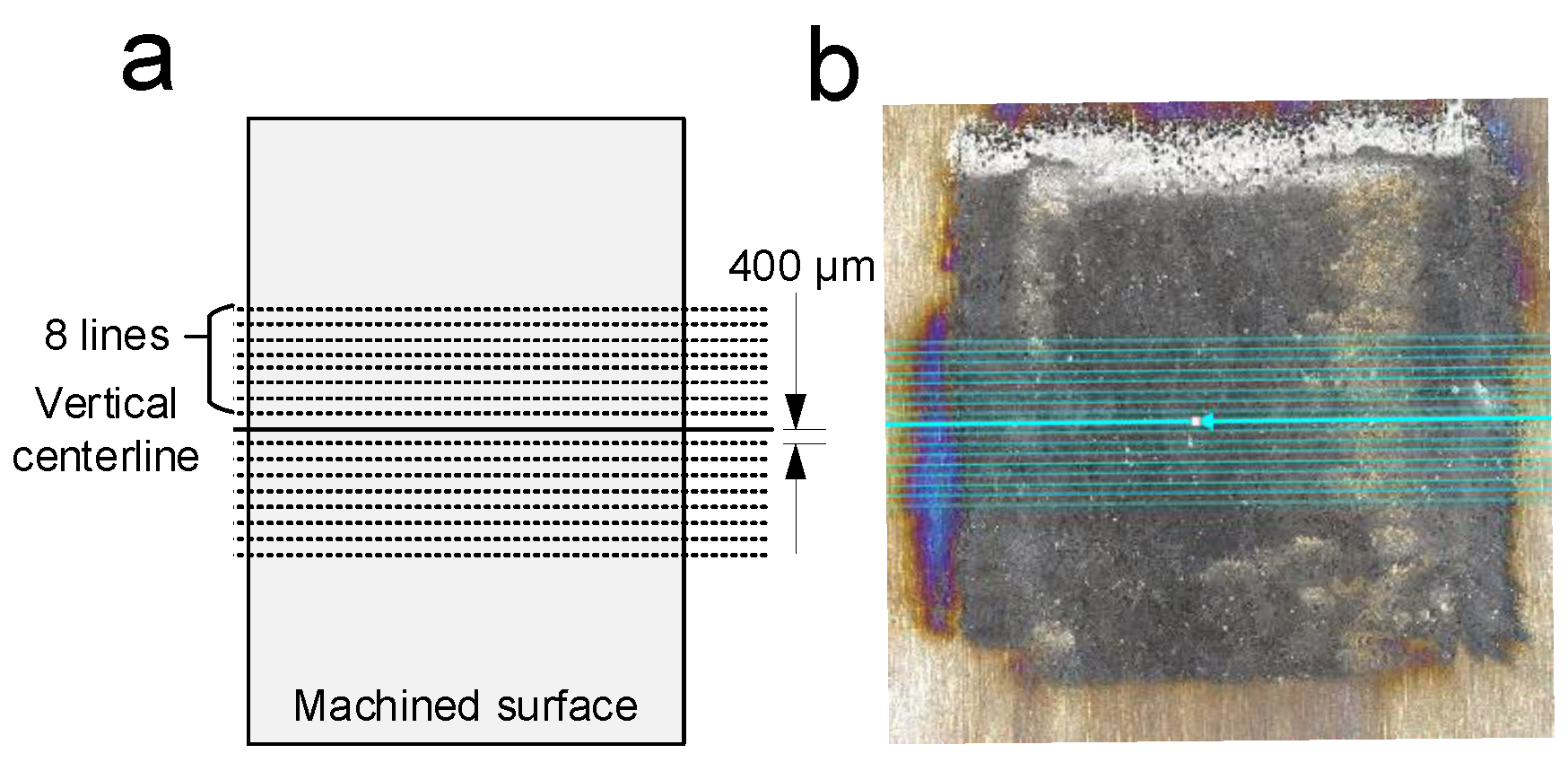
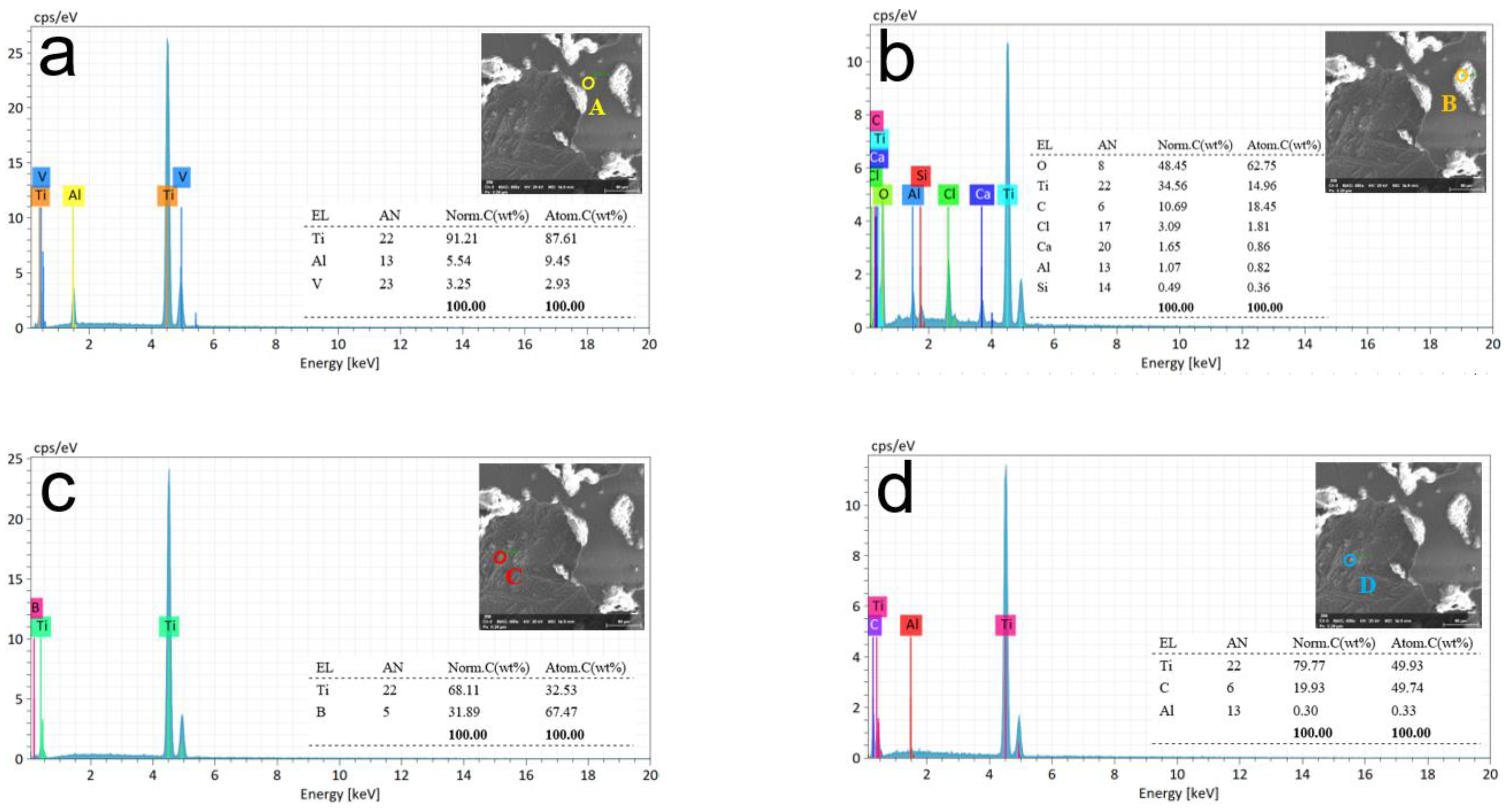
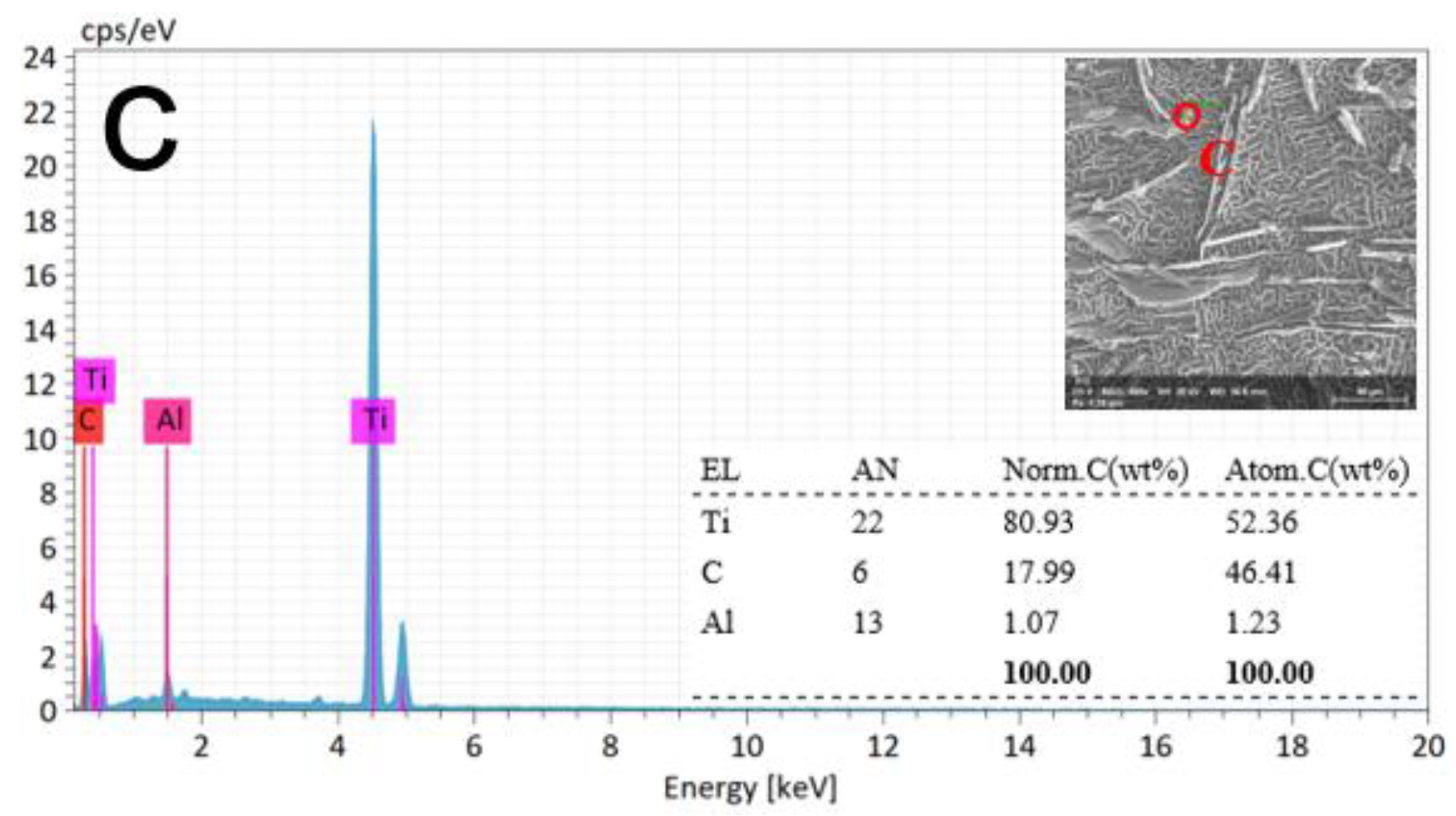
3.3. Metallographic Analysis after ECM
3.4. Experimental Discussion
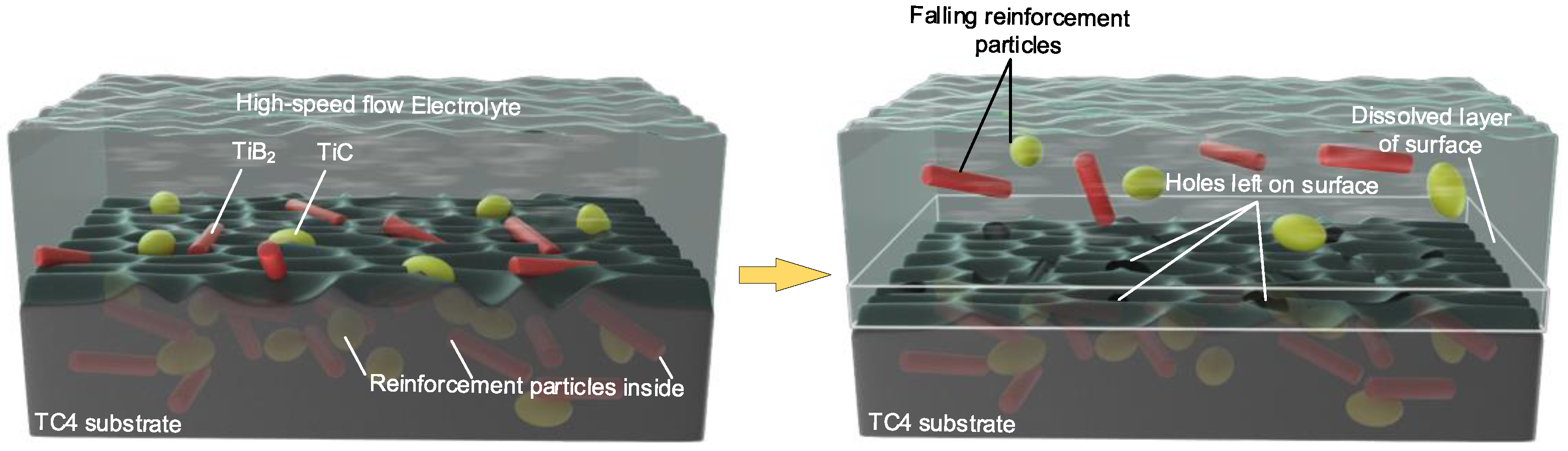
4. Removal Mechanism of (TiB+TiC)/TC4 Composites
5. Conclusions and Outlook
- (1)
- As the current density increased from 1 A cm−2 to 70 A cm−2, the current efficiency curve showed a nonlinear trend, and the actual volume electrochemical equivalent first increased rapidly and then stabilized. So, there is a stable process for the electrolytic removal of this material at a current density higher than 10 A cm-2.
- (2)
- In the voltage and feed speed ranges of 40–60 V and 20–40 mm·min−1, by adjusting the machining parameters, the material removal rate was increased by 52.5%, while the surface roughness was decreased by 27.3%. Under the processing parameters of 60 V (voltage) and 40 mm·min-1 (feed speed), the maximum MRR of 87.51 mm3 min−1 and the minimum surface roughness of 5.045μm were obtained, which met the rough and semi-finishing requirements.
- (3)
- Three stages were observed and summarized in the process of electrolytic dissolution. At the beginning of the electrolysis reaction (0–5 s), the surface metal dissolved, exposing the underlying metal and reinforcing the phase materials. Subsequently, in a brief period (5–20 s), all of the top-surface metals were dissolved, but the reinforcing phase material did not participate in the electrolysis reaction. As the metal near the reinforcing phase particles dissolved, the reinforcing phase particles lost their support and then moved away by the high-speed flow electrolyte. Reinforcement particles have the possibility of reducing the corrosion resistance of the matrix TC4 material, and the reinforcement particles attached to the surface after processing also have a bad effect on the surface quality. From the mechanism and experiments, it is proved that electrolytic milling is an excellent low-cost method for roughing and semi-finishing (TiB+TiC)/TC4 composites.
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Schoon, J.; Hesse, B.; Rakow, A.; Ort, M.J.; Lagrange, A.; Jacobi, D.; Winter, A.; Huesker, K.; Reinke, S.; Cotte, M.; et al. Metal-Specific Biomaterial Accumulation in Human Peri-Implant Bone and Bone Marrow. Adv. Sci. 2020, 7, 2000412. [Google Scholar] [CrossRef]
- Morsi, K. Review: Titanium-titanium boride composites. J. Mater. Sci. 2019, 54, 6753–6771. [Google Scholar] [CrossRef]
- Zhang, D.; Qiu, D.; Gibson, M.A.; Zheng, Y.; Fraser, H.L.; StJohn, D.H.; Easton, M.A. Additive manufacturing of ultrafine-grained high-strength titanium alloys. Nature 2019, 576, 91–95. [Google Scholar] [CrossRef] [PubMed]
- Cotton, J.; Clark, L.P.; Phelps, H. Titanium alloys on the F-22 fighter airframe. Adv. Mater. Processes 2002, 160, 25–28. [Google Scholar]
- Boyer, R. Titanium Airframe Applications: Brief History, Present Applications and Future Trends. Mater. Sci. Forum 2003, 426–432, 643–648. [Google Scholar] [CrossRef]
- Pavlenko, D.; Dvirnyk, Y.; Przysowa, R. Advanced Materials and Technologies for Compressor Blades of Small Turbofan Engines. Aerospace 2021, 8, 1. [Google Scholar] [CrossRef]
- Anil Kumar, V.; Gupta, R.K.; Prasad, M.J.N.V.; Narayana Murty, S.V.S. Recent advances in processing of titanium alloys and titanium aluminides for space applications: A review. J. Mater. Res. 2021, 36, 689–716. [Google Scholar] [CrossRef]
- Liu, S.; Song, X.; Xue, T.; Ma, N.; Wang, Y.; Wang, L. Application and development of titanium alloy and titanium matrix composites in aerospace field. J. Aeronaut. Mater. 2020, 40, 77–94. [Google Scholar]
- Qiu, P.; Le, J.; Han, Y.; Chen, Y.; Huang, G.; Mao, J.; Lei, L.; Lu, W. Superior superplasticity and multiple accommodation mechanisms in TiB reinforced near-α titanium matrix composites. Composites Part B 2022, 238, 109940. [Google Scholar] [CrossRef]
- Song, W.D.; Ning, J.G.; Mao, X.N. Mechanical and microstructural properties of TiC particle-reinforced titanium matrix composite. Int. J. Mater. Prod. Technol. 2011, 42, 183–194. [Google Scholar] [CrossRef]
- Ying, S.; Qian, Z.; Gang, Y.; Yi, Y.; Can, W.; Ling, W.; Jian, L. Compressive Properties of the Grahpene Reinforced Titanium Composites. Rare Met. Mater. Eng. 2017, 46, 3882–3886. [Google Scholar]
- Feng, G.; Yang, Y.; Luo, X.; Lou, J.; Li, M.; Xie, L. Research Progresses of Fatigue of SiC Fiber Reinforced Titanium Matrix Composites. Rare Met. Mater. Eng. 2013, 42, 215–220. [Google Scholar]
- Sousa, L.; Basilio, L.; Alves, A.C.; Toptan, F. Tribocorrosion-resistant biofunctionalized Ti-Al2O3 composites. Surf. Coat. Technol. 2021, 420. [Google Scholar] [CrossRef]
- Han, Y.; Sun, X.; Qiu, P.; Mao, J.; Lu, W.; Zhang, D. Research and development of processing technology on particulate reinforced titanium matrix composites. Acta Mater. Compos. Sin. 2017, 34, 1625–1635. [Google Scholar]
- Huang, X.; Li, Z.; Huang, H. Recent Development of New High-Temperature Titanium Alloys for High Thrust-Weight Ratio Aero-Engines. Mater. China 2011, 30, 21–27. [Google Scholar]
- Elshazli, A.M.; Elshaer, R.N.; Hussein, A.H.A.; Al-Sayed, S.R. Laser Surface Modification of TC21 (alpha/beta) Titanium Alloy Using a Direct Energy Deposition (DED) Process. Micromachines 2021, 12, 739. [Google Scholar] [CrossRef]
- Fereiduni, E.; Ghasemi, A.; Elbestawi, M. Selective Laser Melting of Aluminum and Titanium Matrix Composites: Recent Progress and Potential Applications in the Aerospace Industry. Aerospace 2020, 7, 77. [Google Scholar] [CrossRef]
- Lu, W.J.; Zhang, D.; Zhang, X.N.; Wu, R.J.; Sakata, T.; Mori, H. Microstructure and tensile properties of in situ (TiB+TiC)/Ti6242 (TiB : TiC=1 : 1) composites prepared by common casting technique. Mater. Sci. Eng. a-Struct. Mater. Prop. Microstruct. Processing 2001, 311, 142–150. [Google Scholar] [CrossRef]
- Bejjani, R.; Shi, B.; Attia, H.; Balazinski, M. Laser assisted turning of Titanium Metal Matrix Composite. Cirp Ann. -Manuf. Technol. 2011, 60, 61–64. [Google Scholar] [CrossRef]
- Ge, Y.; Xu, J.; Fu, Y. High-speed Turning of Titanium Matrix Composites with PCD and Carbide Tools. In Proceedings of the 15th International Manufacturing Conference in China, Nanjing, China, 16–18 October 2014. [Google Scholar]
- Huan, H.; Xu, J.; Su, H.; Fu, Y.; Ge, Y. Experimental Study on Milling of Titanium Matrix Composites. In Proceedings of the 12th Conference on Machining and Advanced Manufacturing Technology, Xiamen, China, 24–27 July 2013. [Google Scholar]
- Aramesh, M.; Attia, M.H.; Kishawy, H.A.; Balazinski, M. Estimating the remaining useful tool life of worn tools under different cutting parameters: A survival life analysis during turning of titanium metal matrix composites (Ti-MMCs). CIRP J. Manuf. Sci. Technol. 2016, 12, 35–43. [Google Scholar] [CrossRef]
- Song, W.; Ning, J.; Wang, J.; Mao, X. Mechanical Behavior of Titanium Carbide Particle-Reinforced Titanium Matrix Composite Under Quasi-Static and Dynamic Tension. Adv. Sci. Lett. 2011, 4, 635–640. [Google Scholar] [CrossRef]
- Li, Z.; Ding, W.; Shen, L.; Xi, X.; Fu, Y. Comparative investigation on high-speed grinding of TiCp/Ti-6Al-4V particulate reinforced titanium matrix composites with single-layer electroplated and brazed CBN wheels. Chin. J. Aeronaut. 2016, 29, 1414–1424. [Google Scholar] [CrossRef] [Green Version]
- Ablyaz, T.R.; Shlykov, E.S.; Muratov, K.R.; Sidhu, S.S. Analysis of Wire-Cut Electro Discharge Machining of Polymer Composite Materials. Micromachines 2021, 12, 571. [Google Scholar] [CrossRef] [PubMed]
- Senthilkumar, T.S.; Muralikannan, R.; Ramkumar, T.; Kumar, S.S. Studies of kerf width and surface roughness using the response surface methodology in AA 4032-TiC composites. Proc. Inst. Mech. Eng. Part E-J. Process Mech. Eng. 2021, 235, 2240–2253. [Google Scholar] [CrossRef]
- Smirnov, G.V.; Pronichev, N.D.; Nekhoroshev, M.V.; Bogdanovich, V.I. Experimental and theoretical study of the hydriding behaviour in the pulse ecm of titanium alloys. In Proceedings of the 10th International Conference on Mechanical Engineering, Automation and Control Systems (MEACS), Tomsk Polytechn Univ, Tomsk, Russia, 4–6 December 2017. [Google Scholar]
- Tang, Y.; Xu, Z. The Electrochemical Dissolution Characteristics of GH4169 Nickel Base Super Alloy in the Condition of Electrochemical Machining. Int. J. Electrochem. Sci. 2018, 13, 1105–1119. [Google Scholar] [CrossRef]
- Wang, Y.; Xu, Z.; Zhang, A.; Xu, G.; Zhang, C. Surface morphology and electrochemical behaviour of Ti-48Al-2Cr-2Nb alloy in low-concentration salt solution. Sci. China-Technol. Sci. 2021, 64, 283–296. [Google Scholar] [CrossRef]
- Li, J.; Lin, X.; Zheng, M.; Wang, J.; Guo, P.; Qin, T.; Zhu, M.; Huang, W.; Yang, H. Distinction in anodic dissolution behavior on different planes of laser solid formed Ti-6Al-4V alloy. Electrochim. Acta 2018, 283, 1482–1489. [Google Scholar] [CrossRef]
- Ge, Y.; Zhu, Z.; Wang, D. Electrochemical Dissolution Behavior of the Nickel-Based Cast Superalloy K423A in NaNO3 Solution. Electrochim. Acta 2017, 253, 379–389. [Google Scholar] [CrossRef]
- Feng, Y.; Jia, B.H.; Wang, X.Y.; Zhang, M.; Zhu, Z.H. Research on Microscopic Properties of TiBw/TC4 Composites for Drilling Process. Materials 2019, 12, 2112. [Google Scholar] [CrossRef] [Green Version]
- Li, J.Q.; Yang, Y.; Zhu, G.X.; Sun, C.F.; Chen, Y.Y.; Wang, K.J.; Shi, S.H. The Anisotropic Electrochemical Machinability of Laser Cladding Deposited Ti6Al4V Alloy in NaCl Solution. Materials 2022, 15, 3642. [Google Scholar] [CrossRef]
- Wang, M.; Qu, N. Investigation on material removal mechanism in mechano-electrochemical milling of TC4 titanium alloy. J. Mater. Process. Technol. 2021, 295. [Google Scholar] [CrossRef]
- Liu, Y.; Qu, N.S. Electrochemical Milling of TB6 Titanium Alloy in NaNO3 Solution. J. Electrochem. Soc. 2019, 166, E35–E49. [Google Scholar] [CrossRef]
- He, B.S.; Li, H.S.; Ma, X.; Li, J.; Fan, S.K. Plane Machining by Inner-Jet Electrochemical Milling of TiB2/7050 Aluminum Matrix Composite. Appl. Sci.-Basel 2021, 11, 8087. [Google Scholar] [CrossRef]
- Ma, X.; Li, H.; Yue, X.; Yang, Y.; Wang, L.; Xu, G. Electrochemical Turning of (TiB + TiC)/TC4 Composites Using a Rectangular Cathode. J. Electrochem. Soc. 2022, 169. [Google Scholar] [CrossRef]
- Zhang, S.; Hu, X.; Li, H.; Yang, Y. Electrochemical Properties and Electrochemical Milling of (TiB + TiC)/TC4 Composites. J. Electrochem. Soc. 2022, 169, 063522. [Google Scholar] [CrossRef]
- Chin, D.T.; Wallace, A.J., Jr. Anodic current efficiency and dimensional control in electrochemical machining. J. Electrochem. Soc. 1973, 120, 1487–1493. [Google Scholar] [CrossRef]
- Kotlyar, L.M.; Minazetdinov, N.M. Modeling of electrochemical machining with the use of a curvilinear electrode and a stepwise dependence of the current efficiency on the current density. J. Appl. Mech. Tech. Phys. 2016, 57, 127–135. [Google Scholar] [CrossRef]
- Yue, X.K.; Qu, N.S.; Ma, X.; Li, H.S. Anodic electrochemical behaviors of in situ synthesized (TiB+TiC)/Ti6Al4V composites in NaNO3 and NaCl electrolyte. Corros. Sci. 2022, 204. [Google Scholar] [CrossRef]
- Wang, Y.; Xu, Z.; Zhang, A. Electrochemical dissolution behavior of Ti-45Al-2Mn-2Nb+0.8 vol% TiB2 XD alloy in NaCl and NaNO3 solutions. Corros. Sci. 2019, 157, 357–369. [Google Scholar] [CrossRef]
- Choi, S.H.; Shin, H.S.; Chung, D.K.; Chu, C.N.; Kim, B.H. Micro Electrochemical Machining of Tungsten Carbide. In Proceedings of the 2nd International Conference on Mechanical and Aerospace Engineering (ICMAE 2011), Bangkok, Thailand, 29–31 July 2011; pp. 3696–3704. [Google Scholar]
- Xu, Z.; Chen, X.; Zhou, Z.; Qin, P.; Zhu, D. Electrochemical Machining of High-temperature Titanium Alloy Ti60. Procedia CIRP 2016, 42, 125–130. [Google Scholar] [CrossRef]
- Dikusar, A.I.; Likrizon, E.A.; Dikusar, G.K. High-Rate Pulsed Galvanostatic Anodic Dissolution of Chromium-Nickel Steels in Electrolytes for Electrochemical Machining: The Role of Surface Temperature. Surf. Eng. Appl. Electrochem. 2021, 57, 10–18. [Google Scholar] [CrossRef]
- Li, J.; Lin, X.; Guo, P.; Song, M.; Huang, W. Electrochemical behaviour of laser solid formed Ti–6Al–4V alloy in a highly concentrated NaCl solution. Corros. Sci. 2018, 142, 161–174. [Google Scholar] [CrossRef]
- Neto, J.C.D.; da Silva, E.M.; da Silva, M.B. Intervening variables in electrochemical machining. J. Mater. Process. Technol. 2006, 179, 92–96. [Google Scholar] [CrossRef]
- Aramesh, M.; Shi, B.; Nassef, A.O.; Attia, H.; Balazinski, M.; Kishawy, H.A. Meta-modeling Optimization of the Cutting Process During Turning Titanium Metal Matrix Composites (Ti-MMCs). Procedia CIRP 2013, 8, 576–581. [Google Scholar] [CrossRef]
- Chen, X.; Zhu, D.; Xu, Z.; Liu, J.; Zhu, D. Dissolution Characteristics of New Titanium Alloys in Electrochemical Machining. Trans. Nanjing Univ. Aeronaut. Astronaut. 2016, 33, 610–619. [Google Scholar]
- Zhang, J.; Zhao, C.; Qu, N.; Shen, Z. Improving surface quality through macro electrochemical jet milling with novel cathode tool. J. Mater. Process. Technol. 2022, 309, 117731. [Google Scholar] [CrossRef]
- Li, H.; Fu, S.; Zhang, Q.; Niu, S.; Qu, N. Simulation and experimental investigation of inner-jet electrochemical grinding of GH4169 alloy. Chin. J. Aeronaut. 2018, 31, 608–616. [Google Scholar] [CrossRef]
- Huang, S.; Ma, Q.; Liu, C.; Shi, X.; Wang, C. Research on electrochemical discharge milling of TC4 titanium alloy. Mater. Manuf. Processes 2022, 1–6. [Google Scholar] [CrossRef]





| Element | Ti | Al | V | Fe | C | N | H | O |
|---|---|---|---|---|---|---|---|---|
| wt.% | Balance | 6.75 | 4.5 | 0.3 | 0.08 | 0.05 | 0.015 | 0.2 |
| Density (g/cm3) | Tensile Strength (MPa) | Yield Strength (MPa) | Elongations |
|---|---|---|---|
| 4.5 | >1020 | >920 | >6% |
| Elastic Modulus (GPa) | Thermal Conductivity (W mK−1) | Thermal Expansion Coefficient (°C−1) | Transformation Point (°C) |
| 125 | 6.6 | 9.0 × 10−6 | 1035~1045 |
| Experiment Parameters | Result |
|---|---|
| Electrolyte | Wt. 20% NaCl |
| Electrolyte Pressure (MPa) | 0.2 ± 0.01 |
| Electrolyte Temperature (°C) | 30 ± 1 |
| Machining gap (mm) | 1 |
| Electrolytic corrosion area (cm2) | 0.25 |
| Current Density (A cm−2) | 1–70 |
| Parameter | Value |
|---|---|
| Applied voltage (V) | 40, 50, 60 |
| Feed rate (mm min−1) | 20, 30, 40 |
| Electrolyte | 20% NaCl |
| Electrolyte temperature (°C) | 20 |
| Electrolyte pressure (MPa) | 0.2 |
| Machining gap (mm) | 0.3 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Fan, S.; Hu, X.; Ma, X.; Lu, Y.; Li, H. Removal Mechanism and Electrochemical Milling of (TiB+TiC)/TC4 Composites. Materials 2022, 15, 7046. https://doi.org/10.3390/ma15207046
Fan S, Hu X, Ma X, Lu Y, Li H. Removal Mechanism and Electrochemical Milling of (TiB+TiC)/TC4 Composites. Materials. 2022; 15(20):7046. https://doi.org/10.3390/ma15207046
Chicago/Turabian StyleFan, Shukai, Xiaoyun Hu, Xin Ma, Yuting Lu, and Hansong Li. 2022. "Removal Mechanism and Electrochemical Milling of (TiB+TiC)/TC4 Composites" Materials 15, no. 20: 7046. https://doi.org/10.3390/ma15207046




