Al0.25CoCrFeNiV High Entropy Alloy Coating Deposited by Laser Cladding on Stainless Steel
Abstract
1. Introduction
2. Materials and Methods
3. Results and Discussion
3.1. Microstructure
3.2. Distribution of Elements According to EDS
3.3. Microhardness
3.4. XRD Analysis
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Cantor, B.; Chang, I.T.H.; Knight, P.; Vincent, A.J.B. Microstructural development in equiatomic multicomponent alloys. Mater. Sci. Eng. A 2004, 375–377, 213–218. [Google Scholar] [CrossRef]
- Yeh, J.W.; Chen, S.K.; Lin, S.J.; Gan, J.Y.; Chin, T.S.; Shun, T.T.; Tsau, C.H.; Chang, S.Y. Nanostructured high-entropy alloys with multiple principal elements: Novel alloy design concepts and outcomes. Adv. Eng. Mater. 2004, 6, 299–303. [Google Scholar] [CrossRef]
- Li, Z.; Pradeep, K.G.; Deng, Y.; Raabe, D.; Tasan, C.C. Metastable high-entropy dual-phase alloys overcome the strength-ductility trade-off. Nature 2016, 534, 227–230. [Google Scholar] [CrossRef] [PubMed]
- George, E.P.; Curtin, W.A.; Tasan, C.C. High entropy alloys: A focused review of mechanical properties and deformation mechanisms. Acta Mater. 2020, 188, 435–474. [Google Scholar] [CrossRef]
- Kasar, A.K.; Scalaro, K.; Menezes, P.L. Tribological properties of high-entropy alloys under dry conditions for a wide temperature range—A review. Materials 2021, 14, 5814. [Google Scholar] [CrossRef]
- Ostovari Moghaddam, A.; Samodurova, M.N.; Pashkeev, K.; Doubenskaia, M.; Sova, A.; Trofimov, E.A. A novel intermediate temperature self-lubricating CoCrCu1-xFeNix high entropy alloy fabricated by direct laser cladding. Tribol. Intern. 2021, 156, 106857. [Google Scholar] [CrossRef]
- Veselkov, S.; Samoilova, O.; Shaburova, N.; Trofimov, E. High-temperature oxidation of high-entropic alloys: A review. Materials 2021, 14, 2595. [Google Scholar] [CrossRef]
- Shi, Y.; Yang, B.; Liaw, P.K. Corrosion-resistant high-entropy alloys: A review. Metals 2017, 7, 43. [Google Scholar] [CrossRef]
- Li, Q.H.; Yue, T.M.; Guo, Z.N.; Lin, X. Microstructure and corrosion properties of AlCoCrFeNi high entropy alloy coatings deposited on AISI 1045 steel by the electrospark process. Metall. Mater. Trans. A 2013, 44, 1767–1778. [Google Scholar] [CrossRef]
- Chen, J.H.; Hua, P.H.; Chen, P.N.; Chang, C.M.; Chen, M.C.; Wu, W. Characteristics of multi-element alloy cladding produced by TIG process. Mater. Lett. 2008, 62, 2490–2492. [Google Scholar] [CrossRef]
- Chen, J.H.; Chen, P.N.; Lin, C.M.; Chang, C.M.; Chang, Y.Y.; Wu, W. Microstructure and wear properties of multicomponent alloy cladding formed by gas tungsten arc welding (GTAW). Surf. Coat. Technol. 2009, 203, 3231–3234. [Google Scholar] [CrossRef]
- Huo, W.Y.; Shi, H.F.; Ren, X.; Zhang, J.Y. Microstructure and wear behavior of CoCrFeMnNbNi high-entropy alloy coating by TIG cladding. Adv. Mater. Sci. Eng. 2015, 2015, 647351. [Google Scholar] [CrossRef]
- Meghwal, A.; Anupam, A.; Murty, B.S.; Berndt, C.C.; Kottada, R.S.; Ang, A.S.M. Thermal spray high-entropy alloy coatings: A review. J. Therm. Spray Technol. 2020, 29, 857–893. [Google Scholar] [CrossRef]
- Tian, L.; Wang, J.; Zhang, Q.; Li, R.; Liu, C. Microstructure characterization of AlCoCrFeNiTi high-entropy alloy coating produced by atmospheric plasma spraying. Mater. Res. Express 2019, 6, 116416. [Google Scholar] [CrossRef]
- Löbel, M.; Lindner, T.; Mehner, T.; Lampke, T. Microstructure and wear resistance of AlCoCrFeNiTi high-entropy alloy coatings produced by HVOF. Coatings 2017, 7, 144. [Google Scholar] [CrossRef]
- Straumal, B.B.; Klinger, L.; Kuzmin, A.; Lopez, G.A.; Korneva, A.; Straumal, A.B.; Vershinin, N.; Gornakova, A.S. High Entropy Alloys Coatings Deposited by Laser Cladding: A Review of Grain Boundary Wetting Phenomena. Coatings 2022, 12, 343. [Google Scholar] [CrossRef]
- Ye, X.; Ma, M.; Cao, Y.; Liu, W.; Ye, X.; Gu, Y. The property research on high-entropy alloy AlxFeCoNiCuCr coating by laser cladding. Phys. Proc. 2011, 12, 303–312. [Google Scholar] [CrossRef]
- Qiu, X.W.; Liu, C.G. Microstructure and properties of Al2CrFeCoCuTiNix high-entropy alloys prepared by laser cladding. J. Alloys Compd. 2013, 553, 216–220. [Google Scholar] [CrossRef]
- Wu, W.; Jiang, L.; Jiang, H.; Pan, X.; Cao, Z.; Deng, D.; Wang, T.; Li, T. Phase evolution and properties of Al2CrFeNiMox high-entropy alloys coatings by laser cladding. J. Therm. Spray Technol. 2015, 24, 1333–1340. [Google Scholar] [CrossRef]
- Liu, J.; Liu, H.; Chen, P.; Hao, J. Microstructural characterization and corrosion behaviour of AlCoCrFeNiTix high-entropy alloy coatings fabricated by laser cladding. Surf. Coat. Technol. 2019, 361, 63–74. [Google Scholar] [CrossRef]
- Zhang, S.; Wu, C.L.; Zhang, C.H.; Guan, M.; Tan, J.Z. Laser surface alloying of FeCoCrAlNi high-entropy alloy on 304 stainless steel to enhance corrosion and cavitation erosion resistance. Optics Laser Technol. 2016, 84, 23–31. [Google Scholar] [CrossRef]
- Zhang, S.; Wu, C.L.; Yi, J.Z.; Zhang, C.H. Synthesis and characterization of FeCoCrAlCu high-entropy alloy coating by laser surface alloying. Surf. Coat. Technol. 2015, 262, 64–69. [Google Scholar] [CrossRef]
- Samoilova, O.; Shaburova, N.; Samodurova, M.; Pashkeev, K.; Ostovari Moghaddam, A.; Doubenskaia, M.; Sova, A.; Trofimov, E. Microstructural evolution of Al0.25CoCrFeNiCu and Al0.45CoCrFeNiSi0.45 high-entropy alloys during laser cladding. Proc. Inst. Mech. Eng. Part L J. Mater. Des. Appl. 2022, 236, 1806–1813. [Google Scholar] [CrossRef]
- Salishchev, G.A.; Tikhonovsky, M.A.; Shaysultanov, D.G.; Stepanov, N.D.; Kuznetsov, A.V.; Kolodiy, I.V.; Tortika, A.S.; Senkov, O.N. Effect of Mn and V on structure and mechanical properties of high-entropy alloys based on CoCrFeNi system. J. Alloys Compd. 2014, 591, 11–21. [Google Scholar] [CrossRef]
- Dong, Y.; Zhou, K.; Lu, Y.; Gao, X.; Wang, T.; Li, T. Effect of vanadium addition on the microstructure and properties of AlCoCrFeNi high entropy alloy. Mater. Des. 2014, 57, 67–72. [Google Scholar] [CrossRef]
- Samoilova, O.; Shaburova, N.; Krymsky, V.; Myasoedov, V.; Ostovari Moghaddam, A.; Trofimov, E. Effect of electromagnetic pulses on the microstructure and abrasive gas wear resistance of Al0.25CoCrFeNiV high entropy alloy. Coatings 2022, 12, 688. [Google Scholar] [CrossRef]
- Pratskova, S.; Samoilova, O.; Ageenko, E.; Shaburova, N.; Ostovari Moghaddam, A.; Trofimov, E. Corrosion resistance of AlxCoCrFeNiM (M = Ti, V, Si, Mn, Cu) high entropy alloys in NaCl and H2SO4 solutions. Metals 2022, 12, 352. [Google Scholar] [CrossRef]
- Shaburova, N.A.; Ostovari Moghaddam, A.; Veselkov, S.N.; Sudarikov, M.V.; Samoilova, O.V.; Trofimov, E.A. High-temperature oxidation behavior of AlxCoCrFeNiM (M = Cu, Ti, V) high-entropy alloys. Phys. Mesomech. 2021, 24, 653–662. [Google Scholar] [CrossRef]
- Samodurova, M.; Logachev, I.; Shaburova, N.; Samoilova, O.; Radionova, L.; Zakirov, R.; Pashkeev, K.; Myasoedov, V.; Trofimov, E. A study of the structural characteristics of titanium alloy products manufactured using additive technologies by combining the selective laser melting and direct metal deposition methods. Materials 2019, 12, 3269. [Google Scholar] [CrossRef]
- Samodurova, M.; Shaburova, N.; Samoilova, O.; Radionova, L.; Zakirov, R.; Pashkeev, K.; Myasoedov, V.; Erdakov, I.; Trofimov, E. A study of characteristics of aluminum bronze coatings applied to steel using additive technologies. Materials 2020, 13, 461. [Google Scholar] [CrossRef]
- Joseph, J.; Haghdadi, N.; Shamlaye, K.; Hodgson, P.; Barnett, M.; Fabijanic, D. The sliding wear behaviour of CoCrFeMnNi and AlxCoCrFeNi high entropy alloys at elevated temperatures. Wear 2019, 428–429, 32–44. [Google Scholar] [CrossRef]
- Du, L.M.; Lan, L.W.; Zhu, S.; Yang, H.J.; Shi, X.H.; Liaw, P.K.; Qiao, J.W. Effects of temperature on the tribological behavior of Al0.25CoCrFeNi high-entropy alloy. J. Mater. Sci. Technol. 2019, 35, 917–925. [Google Scholar] [CrossRef]
- Swalin, R.A. Thermodynamics of Solid, 2nd ed.; Wiley: New York, NY, USA, 1991. [Google Scholar]

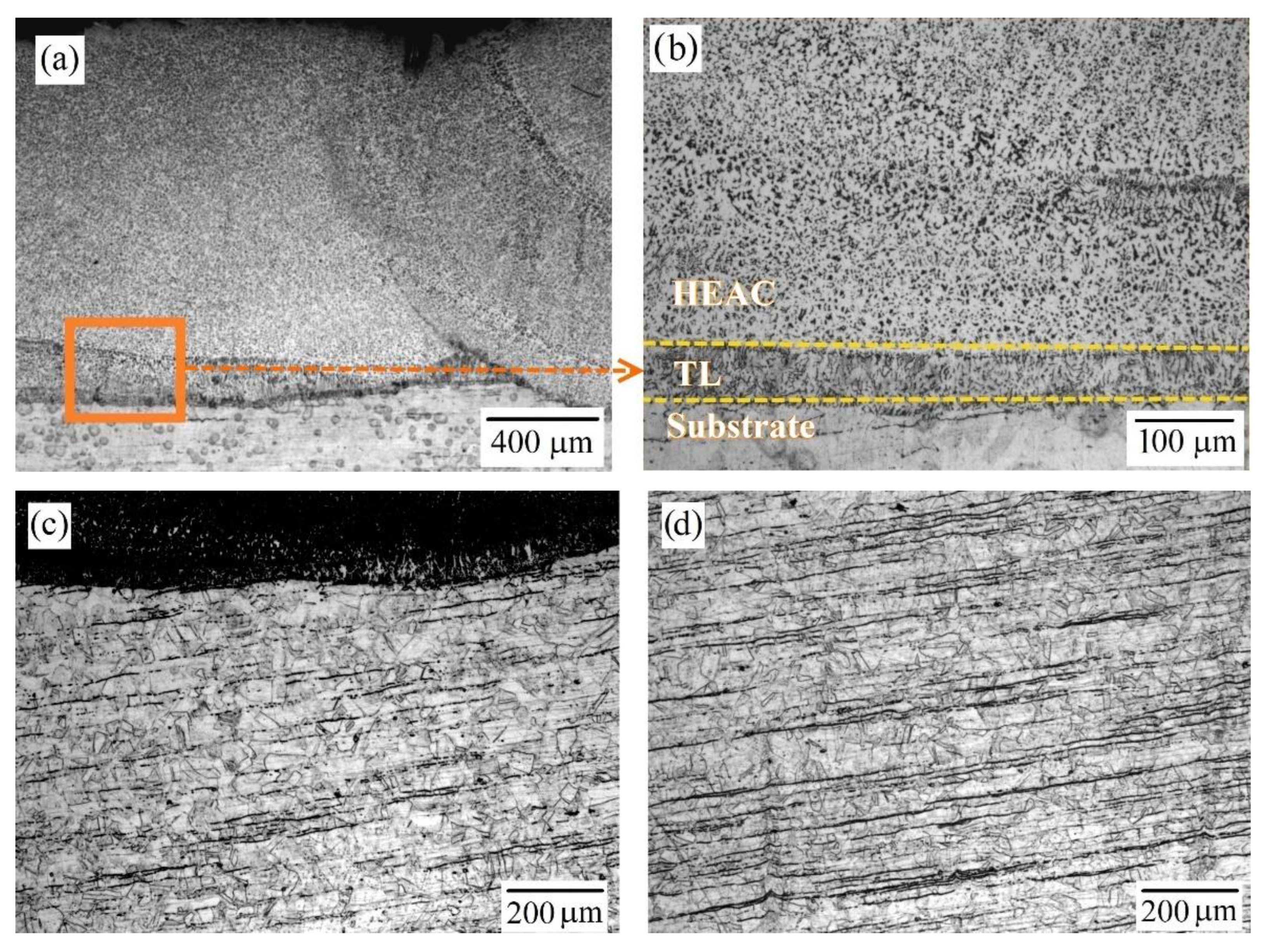
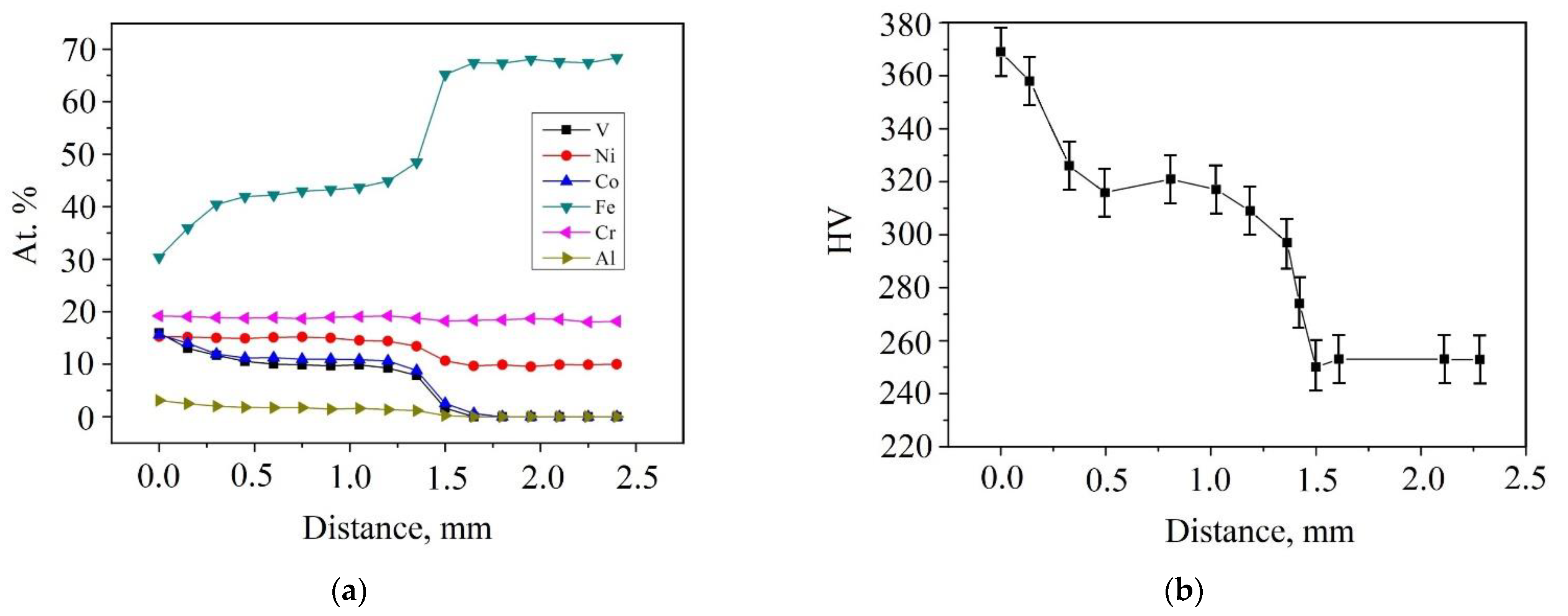
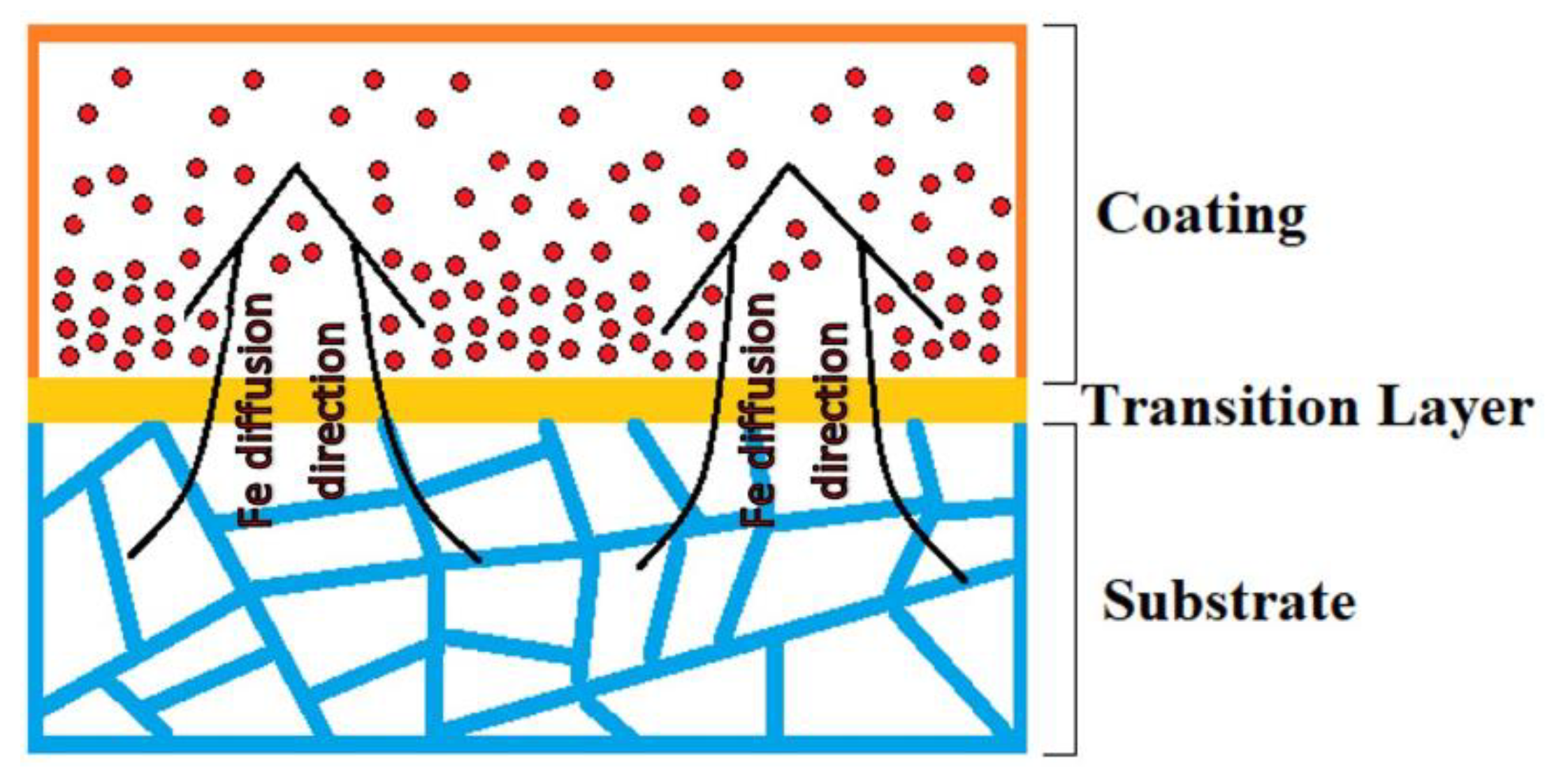

| Alloy | Al | Cr | Fe | Co | Ni | V | Si | Mn | Mo | Ti | |
|---|---|---|---|---|---|---|---|---|---|---|---|
| Al0.25CoCrFeNiV in as-cast state | Av | 4.69 | 19.26 | 18.98 | 19.01 | 18.94 | 19.12 | - | - | - | - |
| D | 4.34 | 18.96 | 21.15 | 21.35 | 21.52 | 12.68 | - | - | – | - | |
| V-rich | 0.87 | 8.78 | 1.45 | 1.49 | 1.78 | 85.63 | - | - | - | - | |
| Al0.25CoCrFeNiV in as-cladded state | Av | 1.44 | 18.98 | 42.69 | 10.61 | 15.18 | 9.29 | 0.58 * | 0.51 * | 0.64 | 0.08 * |
| D | 1.29 | 18.31 | 45.06 | 10.20 | 15.53 | 7.63 | 0.56 * | 0.81 | 0.61 | – | |
| V-rich | 1.10 | 15.41 | 21.10 | 8.22 | 8.56 | 43.23 | - | - | - | 2.38 | |
| Phase | As-Cast State | As-Cladded State | ||
|---|---|---|---|---|
| D (FCC) | V-Rich (BCCV-rich) | D (FCC) | V-Rich (BCCV-rich) | |
| 14.15 | 4.85 | 12.83 | 12.75 | |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Samoilova, O.; Shaburova, N.; Pashkeev, K.; Samodurova, M.; Trofimov, E. Al0.25CoCrFeNiV High Entropy Alloy Coating Deposited by Laser Cladding on Stainless Steel. Materials 2022, 15, 7058. https://doi.org/10.3390/ma15207058
Samoilova O, Shaburova N, Pashkeev K, Samodurova M, Trofimov E. Al0.25CoCrFeNiV High Entropy Alloy Coating Deposited by Laser Cladding on Stainless Steel. Materials. 2022; 15(20):7058. https://doi.org/10.3390/ma15207058
Chicago/Turabian StyleSamoilova, Olga, Nataliya Shaburova, Kirill Pashkeev, Marina Samodurova, and Evgeny Trofimov. 2022. "Al0.25CoCrFeNiV High Entropy Alloy Coating Deposited by Laser Cladding on Stainless Steel" Materials 15, no. 20: 7058. https://doi.org/10.3390/ma15207058
APA StyleSamoilova, O., Shaburova, N., Pashkeev, K., Samodurova, M., & Trofimov, E. (2022). Al0.25CoCrFeNiV High Entropy Alloy Coating Deposited by Laser Cladding on Stainless Steel. Materials, 15(20), 7058. https://doi.org/10.3390/ma15207058








