Influence of Heat Treatment on Microstructure and Mechanical Properties of AZ61 Magnesium Alloy Prepared by Selective Laser Melting (SLM)
Abstract
1. Introduction
2. Material and Methods
2.1. Material and SLM Processing
2.2. Solid Solution Heat Treatment
2.3. Microstructure and Mechanical Properties
3. Results and Discussion
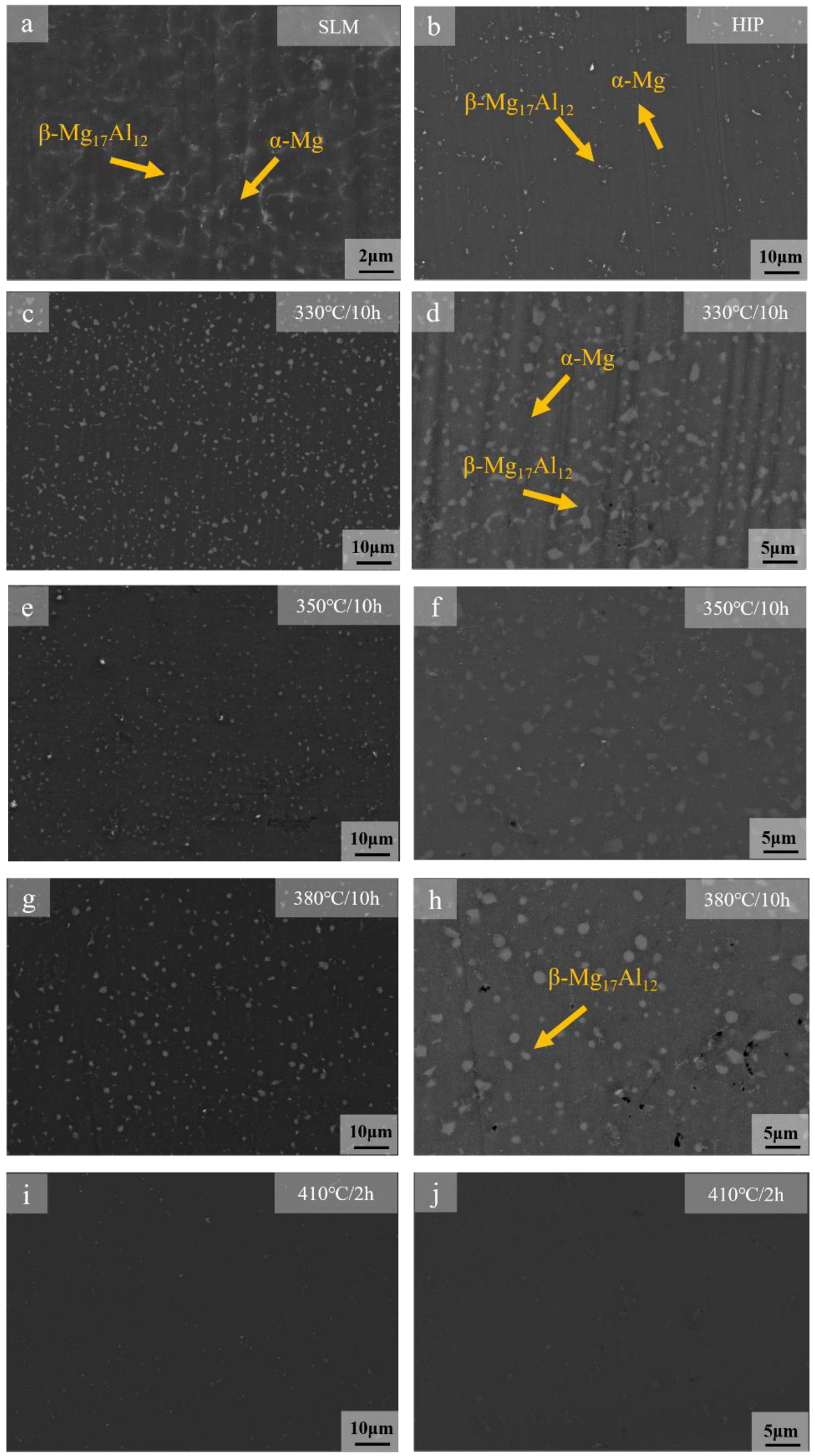
3.1. Microstructure
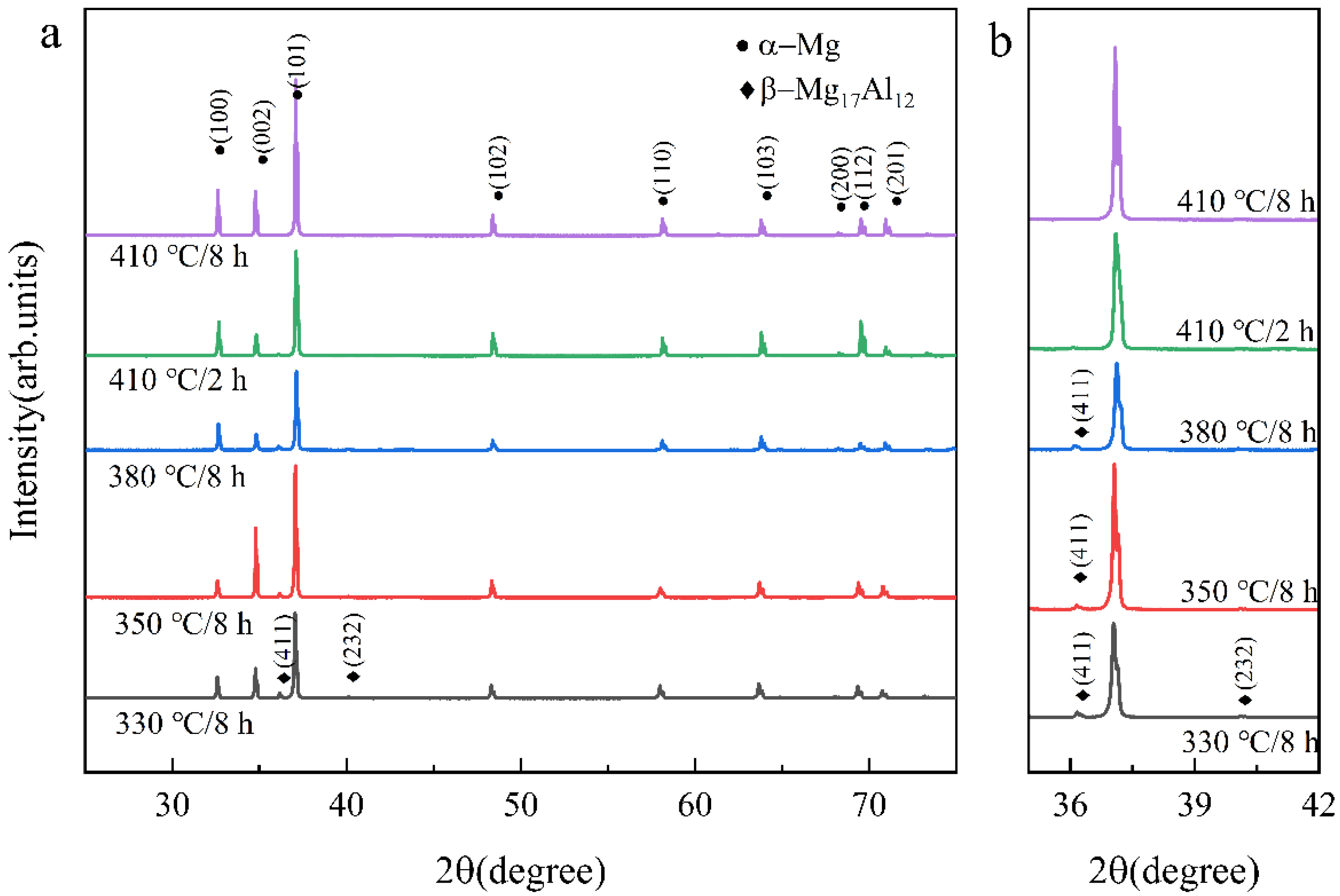
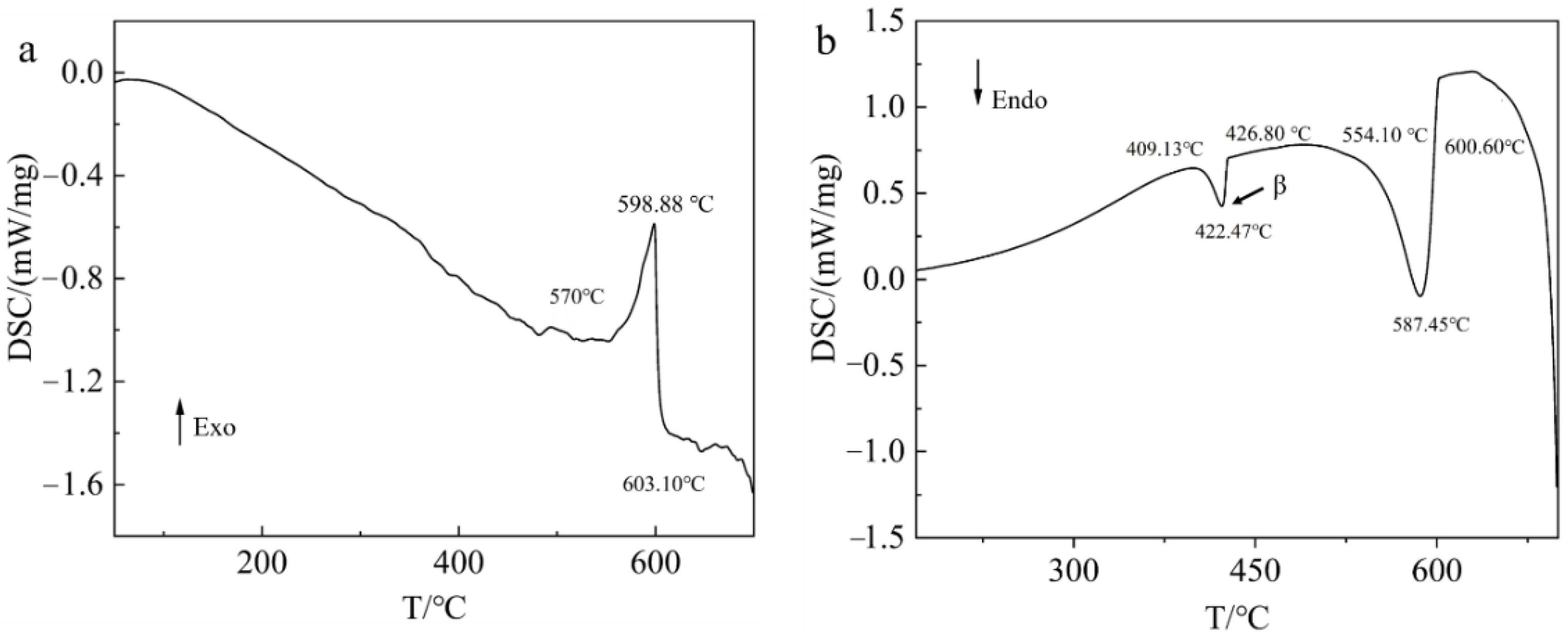
3.2. Thermal Stability of SLMed AZ61 Magnesium Alloy
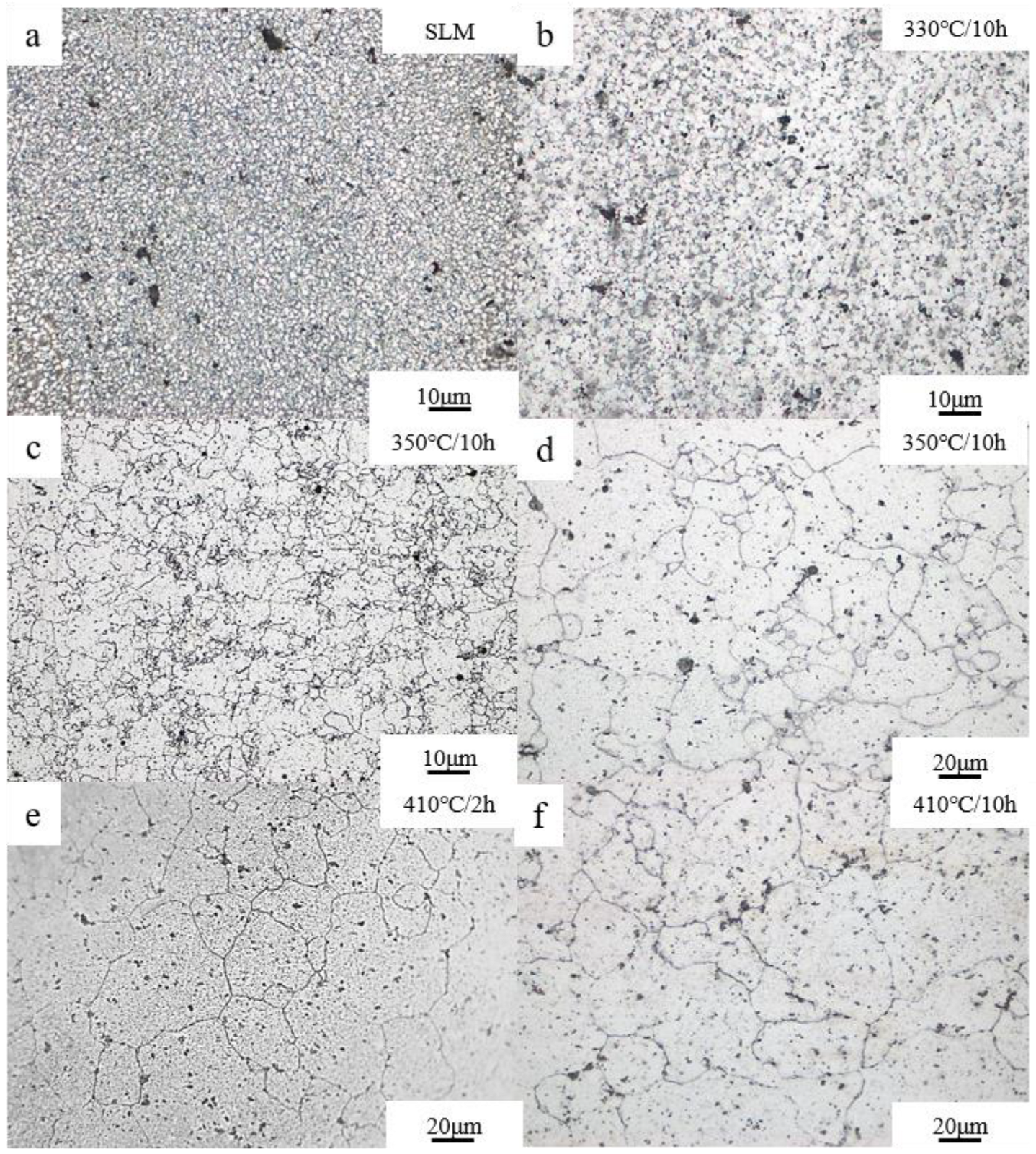
3.3. Evolution of Grain Size
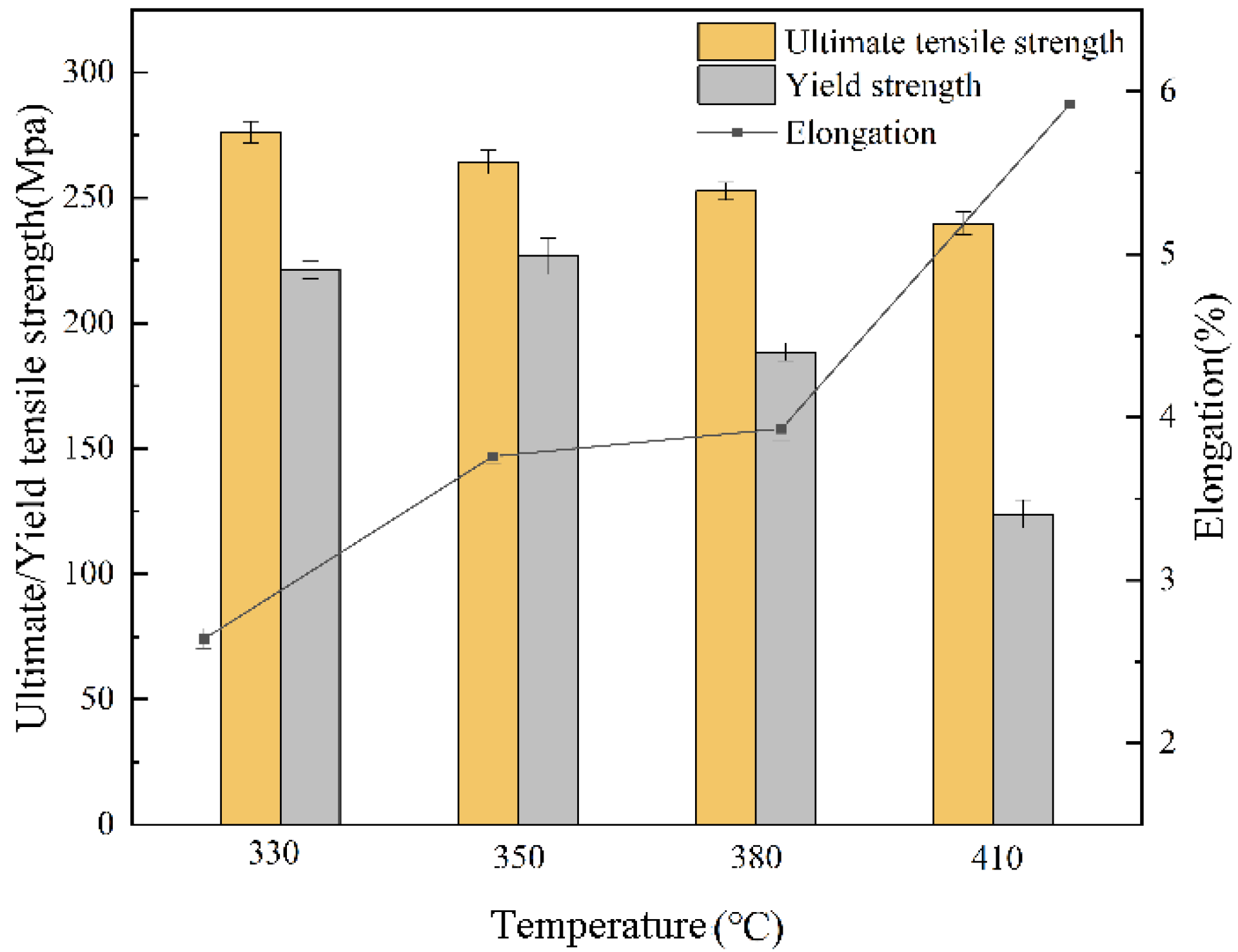
3.4. Mechanical Properties
3.5. Fracture Behavior Analysis
3.6. Mechanism of the β-Mg17Al12 Dissolution
3.7. Kinetic Model of Dissolution
4. Outlook
5. Conclusions
- (1)
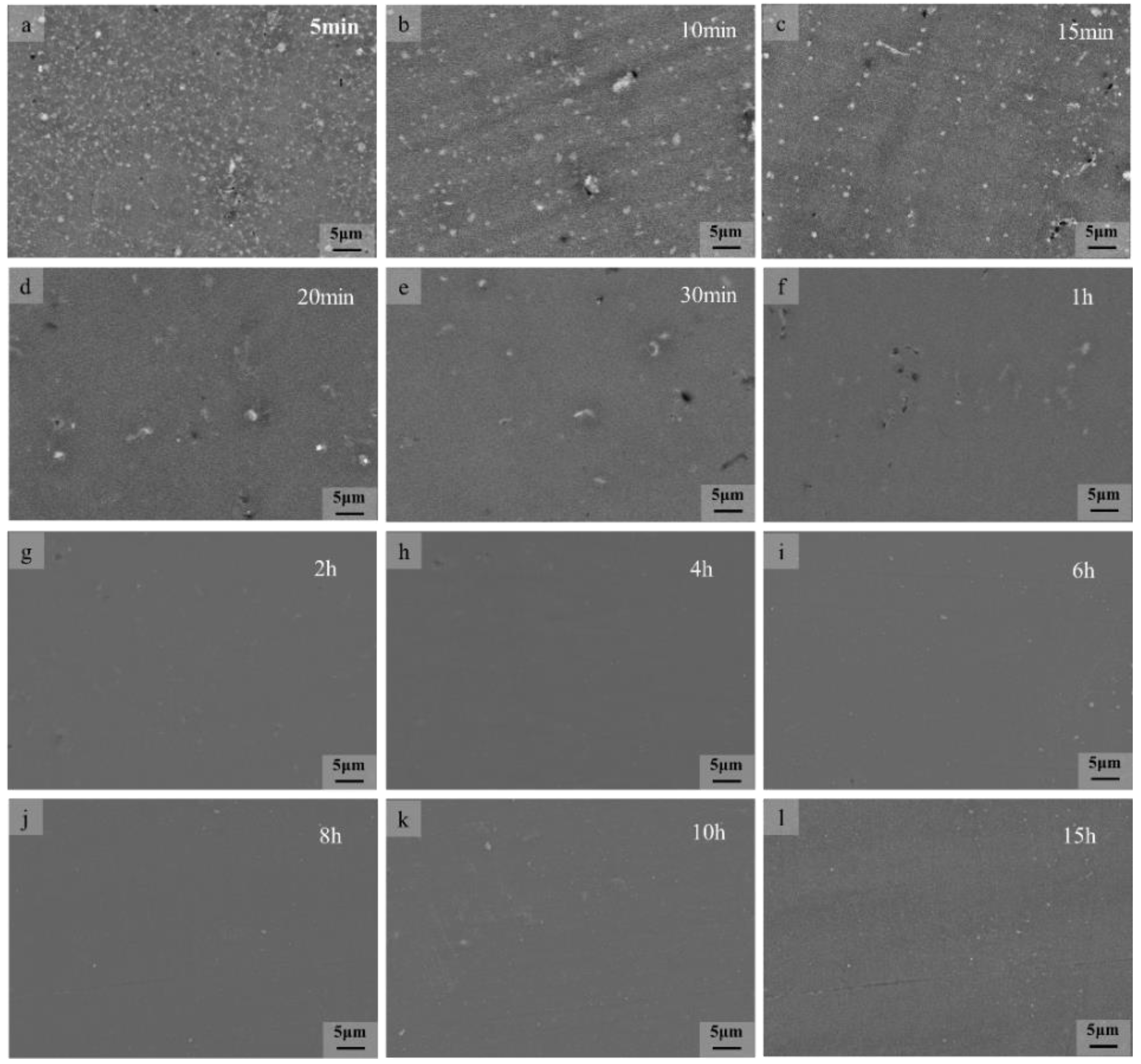
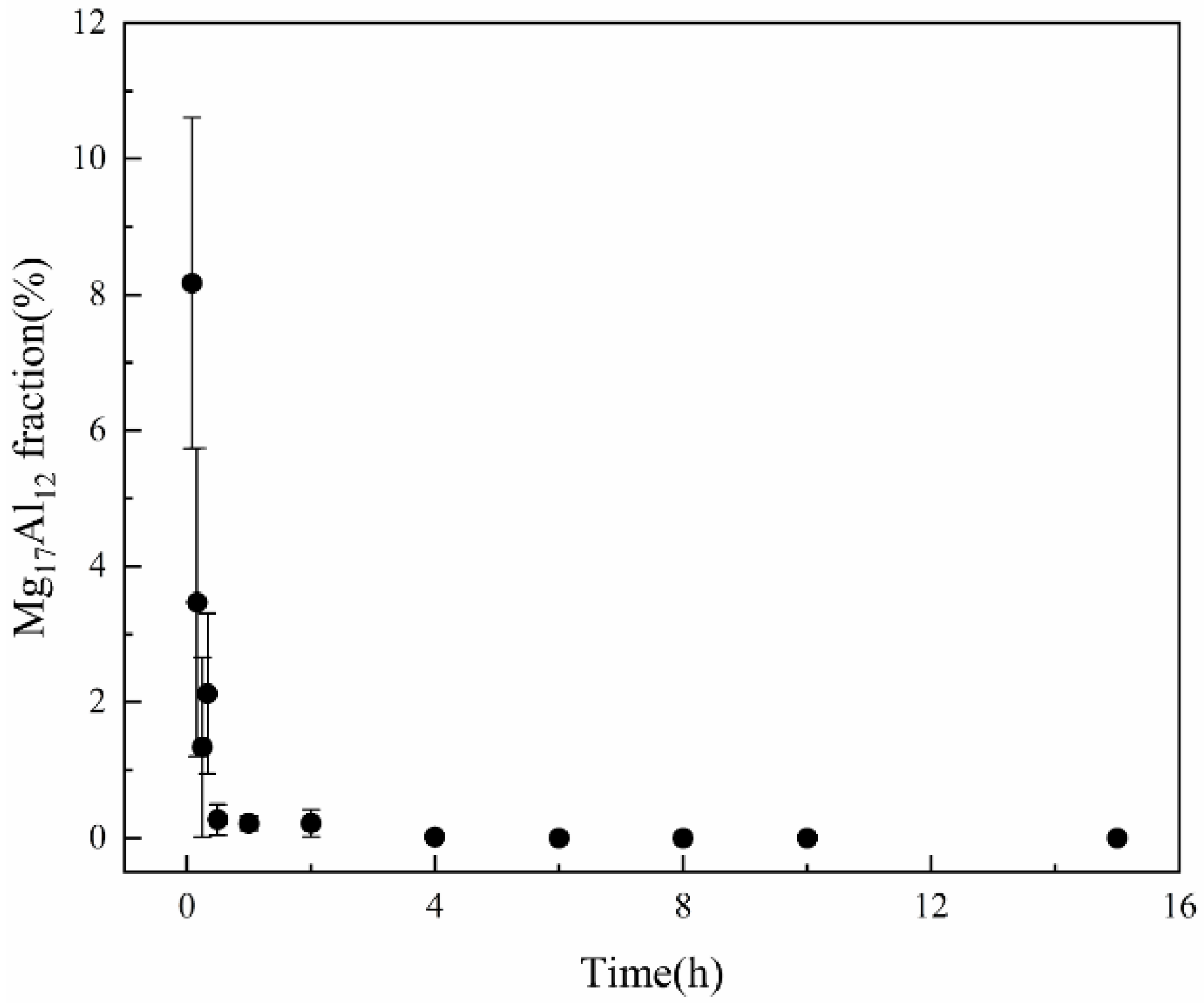
- The dissolution of the β-Mg17Al12 and grain sizes were related to the solid solution temperature and time. At temperatures below 410 °C, β-Mg17Al12 did not dissolve significantly with changes in solid solution temperature and time (even if 10 h), and the decomposition of the net-like β-Mg17Al12 precipitated along the grain boundaries formed a bulk structure from 330 °C. The almost complete dissolution of β-Mg17Al12 occurred after just 2 h at 410 °C and the dissolution rate was significantly higher at 410 °C.
- (2)
- The grain size increased from 3.3 ± 1.3 μm to 29.2 ± 3.7 μm at 330~410 °C. The grain size was without significant growth (29.4 ± 2.5 μm) after 10 h, but was still much smaller than that of the as-cast specimen.
- (3)
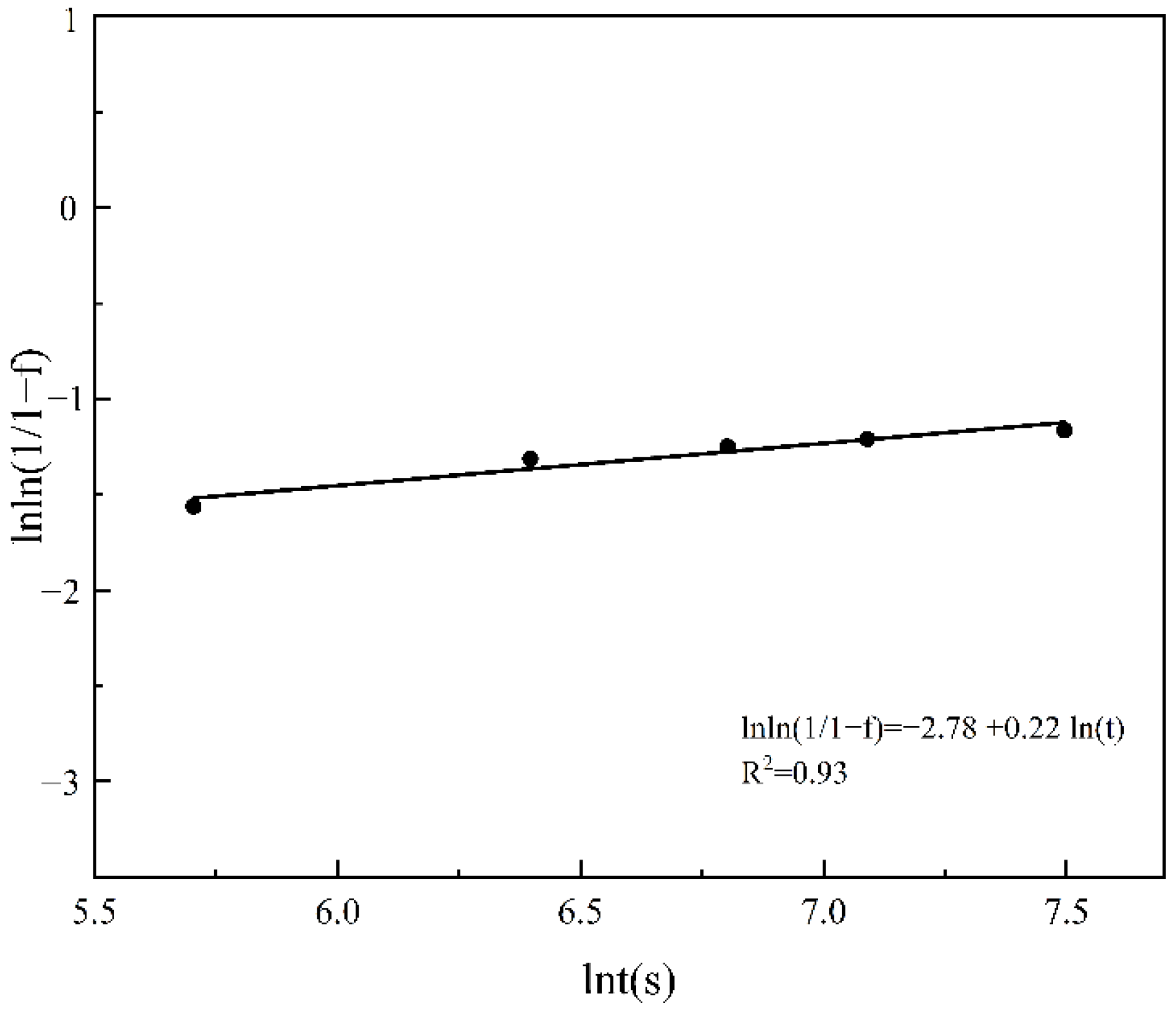
- The kinetic model of β-Mg17Al12 dissolution of AZ61 prepared by SLM was established, and the dissolution characteristics of β-Mg17Al12 at 410 °C were analyzed theoretically. The dissolution rate of β-Mg17Al12 decreased with the increase of dissolution time. Moreover, the optimal solid solution heat treatment temperature and time were 410 °C and 2 h.
- (4)
- The mechanical properties study showed that the strength of SLMed AZ61 magnesium alloy decreased and the ductility increased as the solution heat treatment temperature increased. Additionally, β-Mg17Al12 dissolution was beneficial to improving elongation. At 410 °C, the UTS was 240 ± 5 MPa and the YS decreased to 124 ± 6 MPa under the combined effect of solid solution strengthening and grain coarsening. However, the EL increased to 5.9%, which was due to the reduction of crack generation caused by the mismatch between the β-Mg17Al12 and the α-Mg matrix after the dissolution of the β-Mg17Al12, and the EL increased by 90% compared to SLMed AZ61 specimens. In addition, HIP can further close the internal pores, leading to greater elongation.
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Yeganeh, M.; Mohammadi, N. Superhydrophobic surface of Mg alloys: A review. J. Magnes. Alloys 2018, 6, 59–70. [Google Scholar] [CrossRef]
- Luo, K.; Zhang, L.; Wu, G.; Liu, W.; Ding, W. Effect of Y and Gd content on the microstructure and mechanical properties of Mg–Y–RE alloys. J. Magnes. Alloys 2019, 7, 345–354. [Google Scholar] [CrossRef]
- Ali, Y.; Qiu, D.; Jiang, B.; Pan, F.; Zhang, M. Current research progress in grain refinement of cast magnesium alloys: A review article. J. Alloys Compd. 2015, 619, 639–651. [Google Scholar] [CrossRef]
- Song, J.; She, J.; Chen, D.; Pan, F. Latest research advances on magnesium and magnesium alloys worldwide. J. Magnes. Alloys 2020, 8, 1–41. [Google Scholar] [CrossRef]
- Caiazzo, F.; Campanelli, S.L.; Cardaropoli, F.; Contuzzi, N.; Sergi, V.; Ludovico, A.D. Manufacturing and characterization of similar to foam steel components processed through selective laser melting. Int. J. Adv. Manuf. Technol. 2017, 92, 2121–2130. [Google Scholar] [CrossRef]
- Zhang, B.; Dembinski, L.; Coddet, C. The study of the laser parameters and environment variables effect on mechanical properties of high compact parts elaborated by selective laser melting 316L powder. Mater. Sci. Eng. A 2013, 584, 21–31. [Google Scholar] [CrossRef]
- Herzog, D.; Seyda, V.; Wycisk, E.; Emmelmann, C. Additive manufacturing of metals. Acta. Mater. 2016, 117, 371–392. [Google Scholar] [CrossRef]
- DebRoy, T.; Wei, H.L.; Zuback, J.S.; Mukherjee, T.; Elmer, J.W.; Milewski, J.O.; Beese, A.M.; Wilson-Heid, A.; De, A.; Zhang, W. Additive manufacturing of metallic components–Process, structure and properties. Prog. Mater. Sci. 2018, 92, 112–224. [Google Scholar] [CrossRef]
- Prashanth, K.G.; Scudino, S.; Klauss, H.J.; Surreddi, K.B.; Löber, L.; Wang, Z.; Chaubey, A.K.; Kühn, U.; Eckert, J. Microstructure and mechanical properties of Al–12Si produced by selective laser melting: Effect of heat treatment. Mater. Sci. Eng. A 2014, 590, 153–160. [Google Scholar] [CrossRef]
- Ansari, P.; Salamci, M.U. On the selective laser melting based additive manufacturing of AlSi10Mg: The process parameter investigation through multiphysics simulation and experimental validation. J. Alloys Compd. 2022, 890, 161873. [Google Scholar] [CrossRef]
- Dong, D.D.; Chang, C.; Wang, H.; Yan, X.C.; Ma, W.Y.; Liu, M.; Deng, S.H.; Gardan, J.L.; Bolot, R.; Liao, H.L. Selective laser melting (SLM) of CX stainless steel: Theoretical calculation, process optimization and strengthening mechanism. J. Mater. Sci. Technol. 2021, 73, 151–164. [Google Scholar] [CrossRef]
- Yadroitsev, I.; Bertrand, P.; Smurov, I. Parametric analysis of the selective laser melting process. Appl. Surf. Sci. 2007, 253, 8064–8069. [Google Scholar] [CrossRef]
- Gu, D.; Shen, Y. Balling phenomena in direct laser sintering of stainless steel powder: Metallurgical mechanisms and control methods. Mater. Des. 2009, 30, 2903–2910. [Google Scholar] [CrossRef]
- Brandl, E.; Heckenberger, U.; Holzinger, V.; Buchbinder, D. Additive manufactured AlSi10Mg specimens using Selective Laser Melting (SLM): Microstructure, high cycle fatigue, and fracture behavior. Mater. Des. 2012, 34, 159–169. [Google Scholar] [CrossRef]
- Xia, M.; Gu, D.; Yu, G.; Dai, D.; Chen, H.; Shi, Q. Influence of hatch spacing on heat and mass transfer, thermodynamics and laser processability during additive manufacturing of Inconel 718 alloy. Int. J. Mach. Tools Manuf. 2016, 109, 147–157. [Google Scholar] [CrossRef]
- Boschetto, A.; Bottini, L.; Pilone, D. Effect of laser remelting on surface roughness and microstructure of AlSi10Mg selective laser melting manufactured parts. Int. J. Adv. Maunf. Technol. 2021, 113, 2739–2759. [Google Scholar] [CrossRef]
- Zhao, J.; Easton, M.; Qian, M.; Leary, M.; Brandt, M. Effect of building direction on porosity and fatigue life of selective laser melted AlSi12Mg alloy. Mater. Sci. Eng. A 2018, 729, 76–85. [Google Scholar] [CrossRef]
- Wei, P.; Wei, Z.; Chen, Z.; Du, J.; He, Y.; Li, J.; Zhou, Y. The AlSi10Mg specimens produced by selective laser melting: Single track, densification, microstructure and mechanical behavior. Appl. Surf. Sci. 2017, 408, 38–50. [Google Scholar] [CrossRef]
- Song, B.; Dong, S.; Zhang, B.; Liao, H.; Coddet, C. Effects of processing parameters on microstructure and mechanical property of selective laser melted Ti6Al4V. Mater. Des. 2012, 35, 120–125. [Google Scholar] [CrossRef]
- Cai, C.; Song, B.; Xue, P.; Wei, Q.; Yan, C.; Shi, Y. A novel near α-Ti alloy prepared by hot isostatic pressing: Microstructure evolution mechanism and high temperature tensile properties. Mater. Des. 2016, 106, 371–379. [Google Scholar] [CrossRef]
- Campanelli, S.; Contuzzi, N.; Ludovico, A.; Caiazzo, F.; Cardaropoli, F.; Sergi, V. Manufacturing and Characterization of Ti6Al4V Lattice Components Manufactured by Selective Laser Melting. Materials 2014, 7, 4803–4822. [Google Scholar] [CrossRef] [PubMed]
- Shuai, C.; He, C.; Feng, P.; Guo, W.; Gao, C.; Wu, P.; Yang, Y.; Bin, S. Biodegradation mechanisms of selective laser-melted Mg–xAl–Zn alloy: Grain size and intermetallic phase. Virtual Phys. Prototyp. 2018, 13, 59–69. [Google Scholar] [CrossRef]
- Hu, D.; Wang, Y.; Zhang, D.; Hao, L.; Jiang, J.; Li, Z.; Chen, Y. Experimental investigation on selective laser melting of bulk net-shape pure magnesium. Mater. Manuf. Process. 2015, 30, 1297–1298. [Google Scholar] [CrossRef]
- Zhang, B.; Liao, H.; Coddet, C. Effects of processing parameters on properties of selective laser melting Mg–9%Al powder mixture. Mater. Des. 2012, 34, 753–758. [Google Scholar] [CrossRef]
- Campanelli, S.L.; Contuzzi, N.; Posa, P.; Angelastro, A. Printability and Microstructure of Selective Laser Melting of WC/Co/Cr Powder. Materials 2019, 12, 2397. [Google Scholar] [CrossRef]
- Wei, K.; Gao, M.; Wang, Z.; Zeng, X. Effect of energy input on formability, microstructure and mechanical properties of selective laser melted AZ91D magnesium alloy. Mater. Sci. Eng. A 2014, 611, 212–222. [Google Scholar] [CrossRef]
- Saeidi, K.; Alvi, S.; Lofaj, F.; Petkov, V.I.; Akhtar, F. Advanced Mechanical Strength in Post Heat Treated SLM 2507 at Room and High Temperature Promoted by Hard/Ductile Sigma Precipitates. Metals 2019, 9, 199. [Google Scholar] [CrossRef]
- Tascioglu, E.; Karabulut, Y.; Kaynak, Y. Influence of heat treatment temperature on the microstructural, mechanical, and wear behavior of 316L stainless steel fabricated by laser powder bed additive manufacturing. Int. J. Adv. Maunf. Technol. 2020, 107, 1947–1956. [Google Scholar] [CrossRef]
- Lee, G.M.; Lee, J.U.; Park, S.H. Effects of post-heat treatment on microstructure, tensile properties, and bending properties of extruded AZ80 alloy. J. Mater. Res. Technol.-JMRT 2021, 12, 1039–1050. [Google Scholar] [CrossRef]
- Campanelli, S.L.; Contuzzi, N.; Posa, P.; Angelastro, A. Study of the aging treatment on selective laser melted maraging 300 steel. Mater. Res. Express 2019, 6, 66580. [Google Scholar] [CrossRef]
- Shin, W.; Son, B.; Song, W.; Sohn, H.; Jang, H.; Kim, Y.; Park, C. Heat treatment effect on the microstructure, mechanical properties, and wear behaviors of stainless steel 316L prepared via selective laser melting. Mater. Sci. Eng. A 2021, 806, 140805. [Google Scholar] [CrossRef]
- He, C.; Bin, S.; Wu, P.; Gao, C.; Feng, P.; Yang, Y.; Liu, L.; Zhou, Y.; Zhao, M.; Yang, S.; et al. Microstructure evolution and biodegradation behavior of laser rapid solidified Mg–Al–Zn alloy. Metals 2017, 7, 105. [Google Scholar] [CrossRef]
- Liu, S.; Yang, W.; Shi, X.; Li, B.; Duan, S.; Guo, H.; Guo, J. Influence of laser process parameters on the densification, microstructure, and mechanical properties of a selective laser melted AZ61 magnesium alloy. J. Alloys Compd. 2019, 808, 151160. [Google Scholar] [CrossRef]
- Guo, Y.; Quan, G.; Celikin, M.; Ren, L.; Zhan, Y.; Fan, L.; Pan, H. Effect of heat treatment on the microstructure and mechanical properties of AZ80M magnesium alloy fabricated by wire arc additive manufacturing. J. Magnes. Alloys 2021, 10, 1930–1940. [Google Scholar] [CrossRef]
- Wang, P.; Li, H.C.; Prashanth, K.G.; Eckert, J.; Scudino, S. Selective laser melting of Al-Zn-Mg-Cu: Heat treatment, microstructure and mechanical properties. J. Alloys Compd. 2017, 707, 287–290. [Google Scholar] [CrossRef]
- Chowdary, S.; Dumpala, R.; Kondaiah, V.V. Influence of heat treatment on the machinability and corrosion behavior of AZ91 Mg alloy. J. Magnes. Alloys 2018, 6, 52–58. [Google Scholar]
- Liu, S.; Guo, H. Influence of hot isostatic pressing (HIP) on mechanical properties of magnesium alloy produced by selective laser melting (SLM). Mater. Lett. 2020, 265, 127463. [Google Scholar] [CrossRef]
- Feng, H.; Liu, S.; Du, Y.; Lei, T.; Zeng, R.; Yuan, T. Effect of the second phases on corrosion behavior of the Mg-Al-Zn alloys. J. Alloys Compd. 2017, 695, 2330–2338. [Google Scholar] [CrossRef]
- Jiang, M.G.; Yan, H.; Chen, R.S. Microstructure, texture and mechanical properties in an as-cast AZ61 Mg alloy during multi-directional impact forging and subsequent heat treatment. Mater. Des. 2015, 87, 891–900. [Google Scholar] [CrossRef]
- Mordike, B.L.; Ebert, T. Magnesium: Properties—Applications—Potential. Mater. Sci. Eng. A 2001, 302, 37–45. [Google Scholar] [CrossRef]
- Haitao, T.; Tingju, L.; Xiaoli, Z.; Zhongtao, Z. Influence of sub-rapid solidification on microstructure and mechanical properties of AZ61A magnesium alloy. Trans. Nonferrous Met. Soc. China 2008, 18, s86–s90. [Google Scholar]
- Labusch, R. A statistical theory of solid solution hardening. Phys. Status Solidi (B) 1970, 41, 6–669. [Google Scholar] [CrossRef]
- Aboulkhair, N.T.; Tuck, C.; Ashcroft, I.; Maskery, I.; Everitt, N.M. On the Precipitation Hardening of Selective Laser Melted AlSi10Mg. Metall. Mater. Trans. A 2015, 46, 3337–3341. [Google Scholar] [CrossRef]
- Zhou, B.; Zhou, J.; Li, H.; Lin, F. A study of the microstructures and mechanical properties of Ti6Al4V fabricated by SLM under vacuum. Mater. Sci. Eng. A 2018, 724, 1–10. [Google Scholar] [CrossRef]
- Foley, D.L.; Leff, A.C.; Lang, A.C.; Taheri, M.L. Evolution of β-phase precipitates in an aluminum-magnesium alloy at the nanoscale. Acta. Mater. 2020, 185, 279–286. [Google Scholar] [CrossRef]
- Wang, M.; Song, B.; Wei, Q.; Zhang, Y.; Shi, Y. Effects of annealing on the microstructure and mechanical properties of selective laser melted AlSi7Mg alloy. Mater. Sci. Eng. A 2019, 739, 463–472. [Google Scholar] [CrossRef]
- Kadali, K.; Dubey, D.; Sarvesha, R.; Kancharla, H.; Jain, J.; Mondal, K.; Singh, S.S. Dissolution kinetics of Mg17Al12 eutectic phase and its effect on corrosion behavior of as-cast AZ80 magnesium alloy. JOM 2019, 71, 2209–2218. [Google Scholar] [CrossRef]
- Kulkarni, K.N.; Luo, A.A. Interdiffusion and Phase Growth Kinetics in Magnesium-Aluminum Binary System. J. Phase Equilib. Diffus. 2013, 34, 104–115. [Google Scholar] [CrossRef]
- Rodriguez, P. Serrated plastic flow. Bull. Mater. Sci. 1984, 6, 653–663. [Google Scholar] [CrossRef]
- Geiselman, D.; Guy, A.G. Yield Phenomena in magnesium single crystals containing nitrogen. Trans. Am. Inst. Min. Metall. Eng. 1959, 215, 814–820. [Google Scholar]
- Chiao, W.F.; Gordon, R.B. Simultaneous measurement of ultrasonic attenuation and yield strength of Mg single crystals. Appl. Phys. Lett. 1963, 3, 88–89. [Google Scholar] [CrossRef]


| Elements | Mg | Al | Zn | Mn | Si | Fe | Cu | Ni |
|---|---|---|---|---|---|---|---|---|
| Wt.% | balance | 6.25 | 1.24 | 0.27 | 0.06 | 0.03 | 0.01 | 0.01 |
| Processing Parameters | Value |
|---|---|
| Laser power, P | 150 W |
| Scanning speed, v | 400 mm/s |
| Laser beam spot size, D | 70 mm |
| Hatch spacing, H | 60 mm |
| Powder layer thickness, T | 40 mm |
| Methods | SLM | HIP | T4 Heat Treatment | |||
|---|---|---|---|---|---|---|
| T/°C | - | 450 | 330 | 350 | 380 | 410 |
| P/MPa | - | 103 | - | - | - | - |
| t/h | - | 3 | 2~10 | 2~10 | 2~10 | 2~10 h 5 min, 10 min, 15 min, 20 min, 30 min, 1 h, 15 h |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Liu, S.; Guo, H. Influence of Heat Treatment on Microstructure and Mechanical Properties of AZ61 Magnesium Alloy Prepared by Selective Laser Melting (SLM). Materials 2022, 15, 7067. https://doi.org/10.3390/ma15207067
Liu S, Guo H. Influence of Heat Treatment on Microstructure and Mechanical Properties of AZ61 Magnesium Alloy Prepared by Selective Laser Melting (SLM). Materials. 2022; 15(20):7067. https://doi.org/10.3390/ma15207067
Chicago/Turabian StyleLiu, Shuai, and Hanjie Guo. 2022. "Influence of Heat Treatment on Microstructure and Mechanical Properties of AZ61 Magnesium Alloy Prepared by Selective Laser Melting (SLM)" Materials 15, no. 20: 7067. https://doi.org/10.3390/ma15207067
APA StyleLiu, S., & Guo, H. (2022). Influence of Heat Treatment on Microstructure and Mechanical Properties of AZ61 Magnesium Alloy Prepared by Selective Laser Melting (SLM). Materials, 15(20), 7067. https://doi.org/10.3390/ma15207067






