Remineralization of Artificially Demineralized Human Enamel and Dentin Samples by Zinc-Carbonate Hydroxyapatite Nanocrystals
Abstract
:1. Introduction
2. Materials and Methods
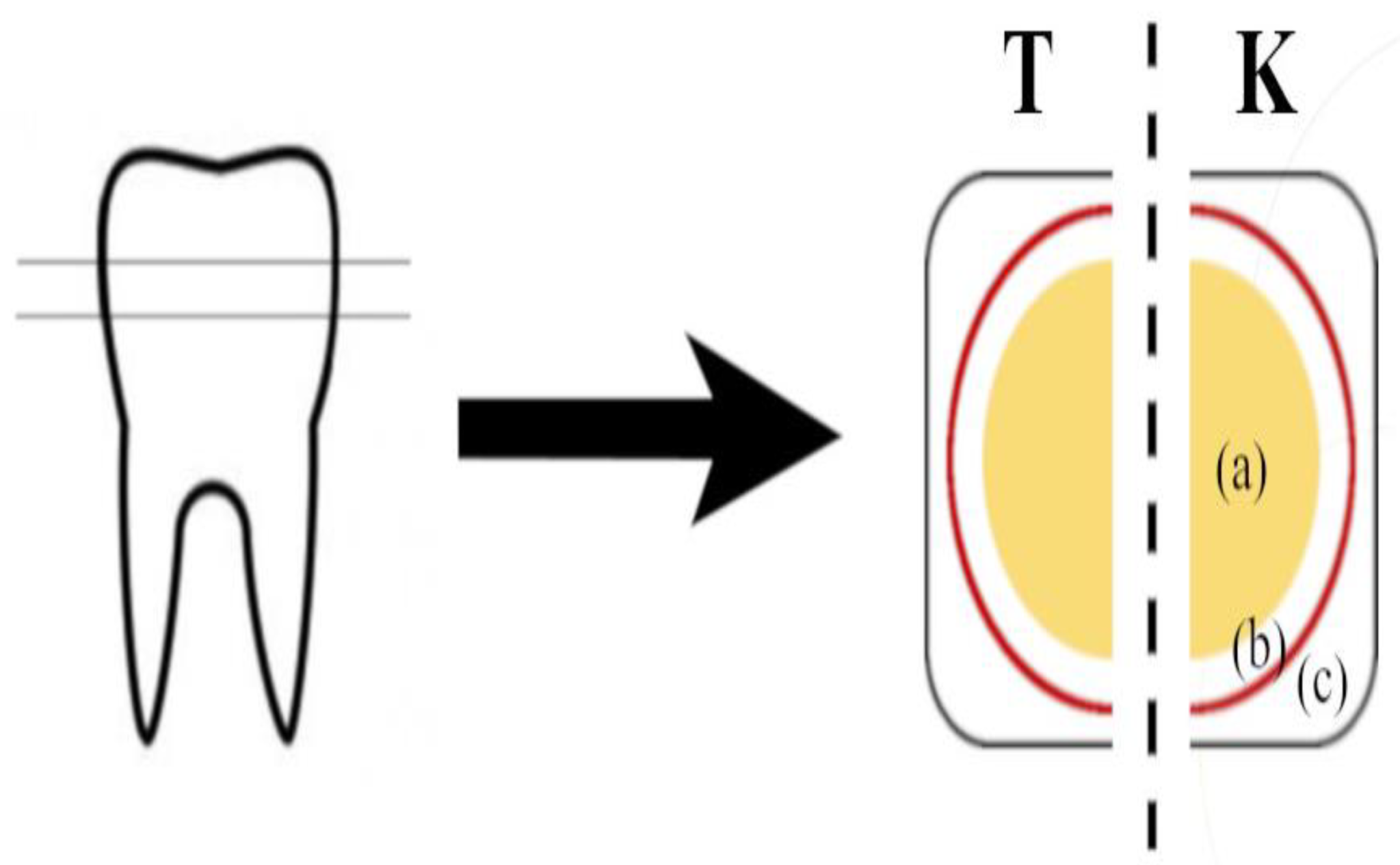
2.1. Sample Preparation
2.2. Experimental Set Up
2.3. Raman Spectroscopy
2.4. Energy-Dispersive X-ray Micro-Analysis (EDX)
2.5. White-Light Interferometry (Optical 3D Profilometry)
2.6. Profilometry of Dentin Surfaces (Mechanical Profilometry)
2.7. Statistical Analysis
3. Results
3.1. Raman Spectroscopy
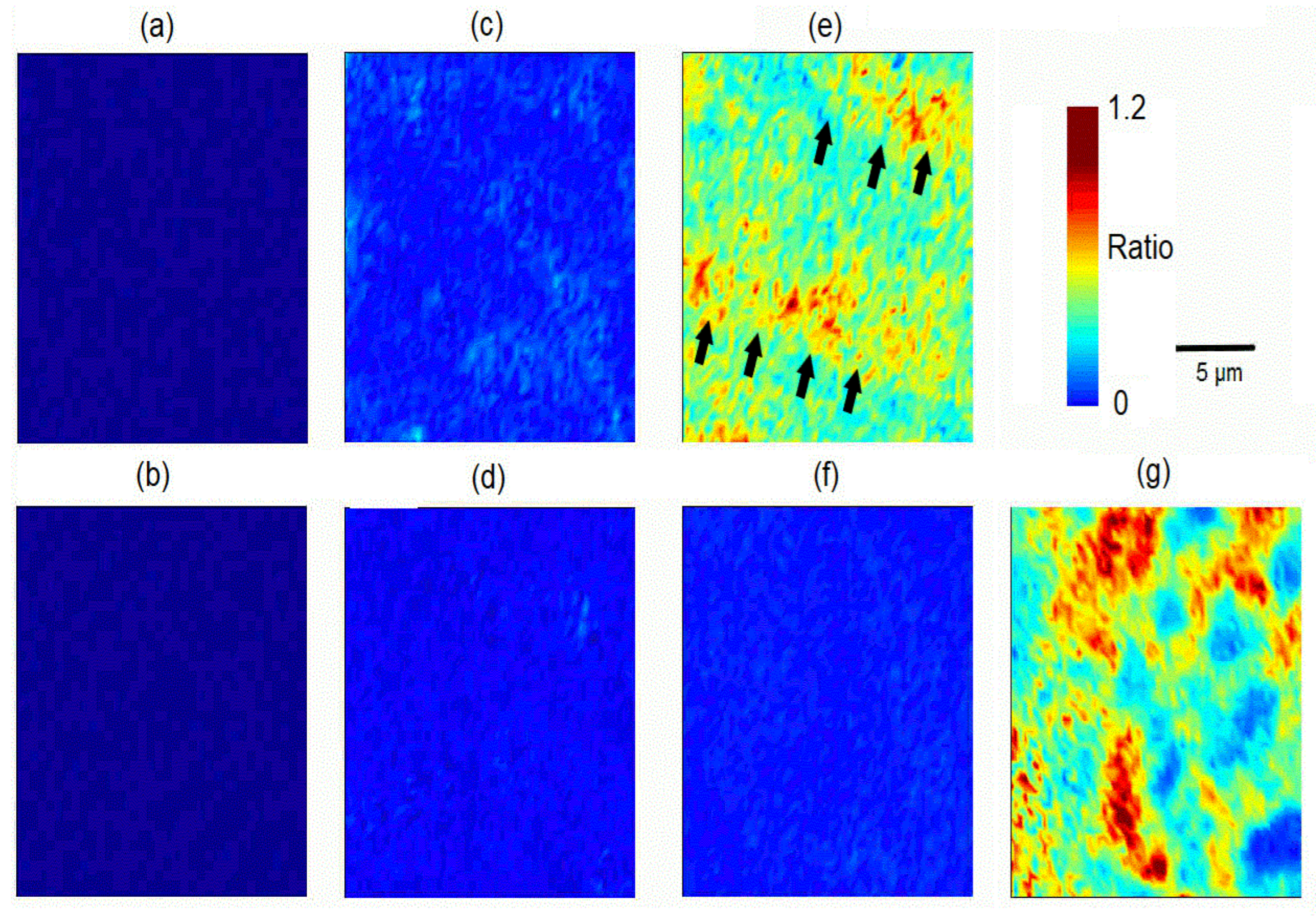
3.2. Energy-Dispersive X-ray Micro-Analysis (EDX)
3.3. White-Light Interferometry (Optical 3D-Profilometry)
3.4. Profilometry of Dentin Surface (Mechanical Profilometry)
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Saads Carvalho, T.; Lussi, A. Chapter 9: Acidic Beverages and Foods Associated with Dental Erosion and Erosive Tooth Wear. Monogr. Oral. Sci. 2020, 28, 91–98. [Google Scholar] [CrossRef] [PubMed]
- Warreth, A.; Abuhijleh, E.; Almaghribi, M.A.; Mahwal, G.; Ashawish, A. Tooth surface loss: A review of literature. Saudi. Dent. J. 2020, 32, 53–60. [Google Scholar] [CrossRef] [PubMed]
- Lynch, R.J.; Smith, S.R. Remineralization agents—New and effective or just marketing hype? Adv. Dent. Res. 2012, 24, 63–67. [Google Scholar] [CrossRef] [PubMed]
- Moreno, E.C.; Kresak, M.; Zahradnik, R.T. Fluoridated hydroxyapatite solubility and caries formation. Nature 1974, 247, 64–65. [Google Scholar] [CrossRef]
- Cury, J.A.; Tenuta, L.M. Enamel remineralization: Controlling the caries disease or treating early caries lesions? Braz. Oral. Res. 2009, 23 (Suppl. 1), 23–30. [Google Scholar] [CrossRef] [Green Version]
- Pandya, M.; Diekwisch, T.G.H. Enamel biomimetics-fiction or future of dentistry. Int. J. Oral. Sci. 2019, 11, 8. [Google Scholar] [CrossRef] [Green Version]
- Buzalaf, M.A.R.; Pessan, J.P.; Honório, H.M.; Ten Cate, J.M. Mechanisms of action of fluoride for caries control. Monogr. Oral. Sci. 2011, 22, 97–114. [Google Scholar] [CrossRef]
- Kim, M.J.; Lee, M.J.; Kim, K.M.; Yang, S.Y.; Seo, J.Y.; Choi, S.H.; Kwon, J.S. Enamel Demineralization Resistance and Remineralization by Various Fluoride-Releasing Dental Restorative Materials. Materials 2021, 14, 4554. [Google Scholar] [CrossRef]
- Qeli, E.; Toti, Ç.; Odorici, A.; Blasi, E.; Tragaj, E.; Tepedino, M.; Masedu, F.; Kaçani, G.; Hysi, D.; Meto, A.; et al. Effectiveness of Two Different Fluoride-Based Agents in the Treatment of Dentin Hypersensitivity: A Prospective Clinical Trial. Materials 2022, 15, 1266. [Google Scholar] [CrossRef]
- Petersson, L.G. The role of fluoride in the preventive management of dentin hypersensitivity and root caries. Clin. Oral. Investig. 2013, 17 (Suppl. 1), S63–S71. [Google Scholar] [CrossRef]
- Philip, N. State of the Art Enamel Remineralization Systems: The Next Frontier in Caries Management. Caries Res. 2019, 53, 284–295. [Google Scholar] [CrossRef] [PubMed]
- Zafar, M.S.; Amin, F.; Fareed, M.A.; Ghabbani, H.; Riaz, S.; Khurshid, Z.; Kumar, N. Biomimetic Aspects of Restorative Dentistry Biomaterials. Biomimetics 2020, 5, 34. [Google Scholar] [CrossRef] [PubMed]
- Reise, M.; Kranz, S.; Heyder, M.; Jandt, K.D.; Sigusch, B.W. Effectiveness of Casein Phosphopeptide-Amorphous Calcium Phosphate (CPP-ACP) Compared to Fluoride Products in an In-Vitro Demineralization Model. Materials 2021, 14, 5974. [Google Scholar] [CrossRef] [PubMed]
- Guentsch, A.; Fahmy, M.D.; Wehrle, C.; Nietzsche, S.; Popp, J.; Watts, D.C.; Kranz, S.; Krafft, C.; Sigusch, B.W. Effect of biomimetic mineralization on enamel and dentin: A Raman and EDX analysis. Dent. Mater. 2019, 35, 1300–1307. [Google Scholar] [CrossRef]
- Chen, L.; Al-Bayatee, S.; Khurshid, Z.; Shavandi, A.; Brunton, P.; Ratnayake, J. Hydroxyapatite in Oral Care Products-A Review. Materials 2021, 14, 4865. [Google Scholar] [CrossRef]
- Arifa, M.K.; Ephraim, R.; Rajamani, T. Recent Advances in Dental Hard Tissue Remineralization: A Review of Literature. Int. J. Clin. Pediatr. Dent. 2019, 12, 139–144. [Google Scholar] [CrossRef]
- Bordea, I.R.; Candrea, S.; Alexescu, G.T.; Bran, S.; Băciuț, M.; Băciuț, G.; Lucaciu, O.; Dinu, C.M.; Todea, D.A. Nano-hydroxyapatite use in dentistry: A systematic review. Drug. Metab. Rev. 2020, 52, 319–332. [Google Scholar] [CrossRef]
- Anil, A.; Ibraheem, W.I.; Meshni, A.A.; Preethanath, R.S.; Anil, S. Nano-Hydroxyapatite (nHAp) in the Remineralization of Early Dental Caries: A Scoping Review. Int. J. Environ. Res. Public Health 2022, 19, 5629. [Google Scholar] [CrossRef]
- Verma, P.; Muthuswamy Pandian, S. Bionic effects of nano hydroxyapatite dentifrice on demineralised surface of enamel post orthodontic debonding: In-vivo split mouth study. Prog. Orthod. 2021, 22, 39. [Google Scholar] [CrossRef]
- Martins, C.C.; Riva, J.J.; Firmino, R.T.; Schünemann, H.J. Formulations of desensitizing toothpastes for dentin hypersensitivity: A scoping review. J. Appl. Oral. Sci. 2022, 30, e20210410. [Google Scholar] [CrossRef]
- O’Hagan-Wong, K.; Enax, J.; Meyer, F.; Ganss, B. The use of hydroxyapatite toothpaste to prevent dental caries. Odontology 2022, 110, 223–230. [Google Scholar] [CrossRef] [PubMed]
- Pajor, K.; Pajchel, L.; Kolmas, J. Hydroxyapatite and Fluorapatite in Conservative Dentistry and Oral Implantology—A Review. Materials 2019, 12, 2683. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Salinovic, I.; Schauperl, Z.; Marcius, M.; Miletic, I. The Effects of Three Remineralizing Agents on the Microhardness and Chemical Composition of Demineralized Enamel. Materials 2021, 14, 6051. [Google Scholar] [CrossRef] [PubMed]
- Pepla, E.; Besharat, L.K.; Palaia, G.; Tenore, G.; Migliau, G. Nano-hydroxyapatite and its applications in preventive, restorative and regenerative dentistry: A review of literature. Ann. Stomatol. 2014, 5, 108–114. [Google Scholar] [CrossRef]
- Creeth, J.; Bosma, M.L.; Govier, K. How much is a ‘pea-sized amount’? A study of dentifrice dosing by parents in three countries. Int. Dent. J. 2013, 63 (Suppl. 2), 25–30. [Google Scholar] [CrossRef]
- Alebrahim, M.A.; Krafft, C.; Sekhaneh, W.; Sigusch, B.; Popp, J. ATR-FTIR and Raman spectroscopy of primary and permanent teeth. Biomed. Spectrosc. Imaging 2014, 3, 15–27. [Google Scholar] [CrossRef]
- Iafisco, M.; Degli Esposti, L.; Ramírez-Rodríguez, G.B.; Carella, F.; Gómez-Morales, J.; Ionescu, A.C.; Brambilla, E.; Tampieri, A.; Delgado-López, J.M. Fluoride-doped amorphous calcium phosphate nanoparticles as a promising biomimetic material for dental remineralization. Sci. Rep. 2018, 8, 17016. [Google Scholar] [CrossRef] [Green Version]
- Shi, Y.; Shen, D.; Zheng, H.; Wu, Z.; Shao, C.; Zhang, L.; Pan, H.; Tang, R.; Fu, B. Therapeutic Management of Demineralized Dentin Surfaces Using a Mineralizing Adhesive To Seal and Mineralize Dentin, Dentinal Tubules, and Odontoblast Processes. ACS Biomater. Sci. Eng. 2019, 5, 5481–5488. [Google Scholar] [CrossRef]
- Zhang, Y.; Wang, Z.; Jiang, T.; Wang, Y. Biomimetic regulation of dentine remineralization by amino acid in vitro. Dent. Mater. 2019, 35, 298–309. [Google Scholar] [CrossRef]
- Peetsch, A.; Epple, M. Characterization of the solid components of threedesensitizing toothpastes and a mouth wash. Mater. Werkstofftech. 2011, 42, 131–135. [Google Scholar] [CrossRef]
- Huang, S.B.; Gao, S.S.; Yu, H.Y. Effect of nano-hydroxyapatite concentration on remineralization of initial enamel lesion in vitro. Biomed. Mater. 2009, 4, 034104. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Adams, D. Calculus-Inhibition Agents: A Review of Recent Clinical Trials. Adv. Dent. Res. 1995, 9, 410–418. [Google Scholar] [CrossRef]
- Steinert, S.; Zwanzig, K.; Doenges, H.; Kuchenbecker, J.; Meyer, F.; Enax, J. Daily Application of a Toothpaste with Biomimetic Hydroxyapatite and Its Subjective Impact on Dentin Hypersensitivity, Tooth Smoothness, Tooth Whitening, Gum Bleeding, and Feeling of Freshness. Biomimetics 2020, 5, 17. [Google Scholar] [CrossRef] [PubMed]
- Chandru, T.P.; Yahiya, M.B.; Peedikayil, F.C.; Dhanesh, N.; Srikant, N.; Kottayi, S. Comparative evaluation of three different toothpastes on remineralization potential of initial enamel lesions: A scanning electron microscopic study. Indian J. Dent. Res. 2020, 31, 217–223. [Google Scholar] [CrossRef]
- Roveri, N.; Iafisco, M. Evolving application of biomimetic nanostructured hydroxyapatite. Nanotechnol. Sci. Appl. 2010, 3, 107–125. [Google Scholar] [CrossRef] [Green Version]
- Kono, T.; Sakae, T.; Nakada, H.; Kaneda, T.; Okada, H. Confusion between Carbonate Apatite and Biological Apatite (Carbonated Hydroxyapatite) in Bone and Teeth. Minerals 2022, 12, 170. [Google Scholar] [CrossRef]
- Bossù, M.; Matassa, R.; Relucenti, M.; Iaculli, F.; Salucci, A.; Di Giorgio, G.; Familiari, G.; Polimeni, A.; Di Carlo, S. Morpho-Chemical Observations of Human Deciduous Teeth Enamel in Response to Biomimetic Toothpastes Treatment. Materials 2020, 13, 1803. [Google Scholar] [CrossRef] [Green Version]
- Kim, H.J.; Mo, S.Y.; Kim, D.S. Effect of Bioactive Glass-Containing Light-Curing Varnish on Enamel Remineralization. Materials 2021, 14, 3745. [Google Scholar] [CrossRef]
- Hsu, S.M.; Alsafadi, M.; Vasconez, C.; Fares, C.; Craciun, V.; O’Neill, E.; Ren, F.; Clark, A.; Esquivel-Upshaw, J. Qualitative Analysis of Remineralization Capabilities of Bioactive Glass (NovaMin) and Fluoride on Hydroxyapatite (HA) Discs: An In Vitro Study. Materials 2021, 14, 3813. [Google Scholar] [CrossRef]
- Imataki, R.; Shinonaga, Y.; Nishimura, T.; Abe, Y.; Arita, K. Mechanical and Functional Properties of a Novel Apatite-Ionomer Cement for Prevention and Remineralization of Dental Caries. Materials 2019, 12, 3998. [Google Scholar] [CrossRef]
- Poggio, C.; Gulino, C.; Mirando, M.; Colombo, M.; Pietrocola, G. Protective effect of zinc-hydroxyapatite toothpastes on enamel erosion: An in vitro study. J. Clin. Exp. Dent. 2017, 9, e118–e122. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ganss, C.; Lussi, A.; Grunau, O.; Klimek, J.; Schlueter, N. Conventional and anti-erosion fluoride toothpastes: Effect on enamel erosion and erosion-abrasion. Caries Res. 2011, 45, 581–589. [Google Scholar] [CrossRef] [PubMed]
- Poggio, C.; Lombardini, M.; Colombo, M.; Bianchi, S. Impact of two toothpastes on repairing enamel erosion produced by a soft drink: An AFM in vitro study. J. Dent. 2010, 38, 868–874. [Google Scholar] [CrossRef] [PubMed]
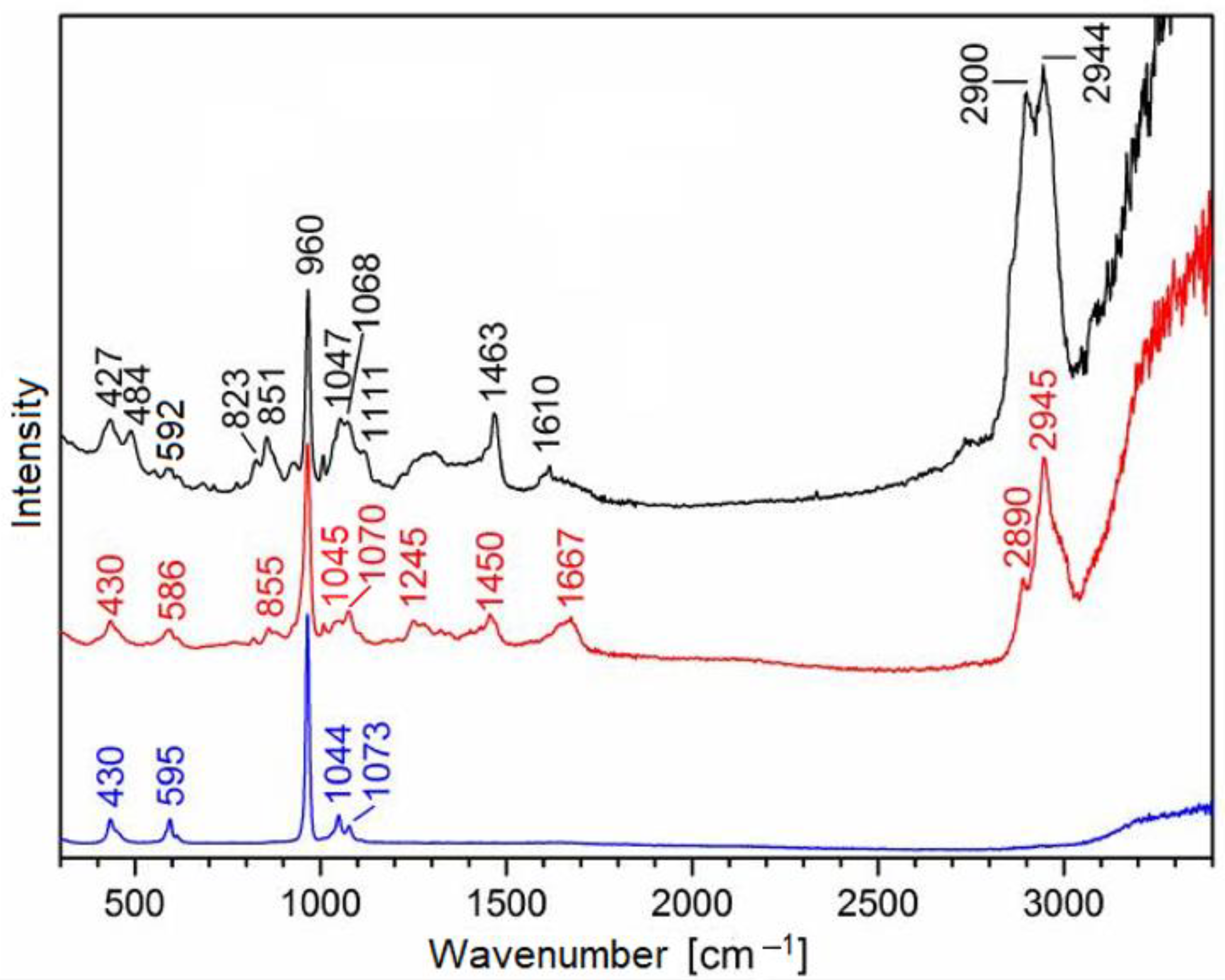



| Raman Band [cm−1] | Assignment | Origin |
|---|---|---|
| 427–430 | δs PO43− | biorepair®, enamel, dentin |
| 484 | additive | biorepair® |
| 586–595 | δas PO43− | biorepair®enamel, dentin |
| 851 | additive | biorepair® |
| 855 | collagen | dentin |
| 960 | νs PO43− | biorepair®, enamel, dentin |
| 1044–1047 | νas PO43− | biorepair®, enamel, dentin |
| 1068 | additive | biorepair® |
| 1070–1073 | νs CO32− | enamel, dentin, biorepair® |
| 1245 | amide III (collagen) | dentin |
| 1450 | δ CH2 (collagen) | dentin |
| 1463 | additive | biorepair® |
| 1610 | additive | biorepair® |
| 1667 | amide I (collagen), δ OH | dentin, H2O |
| 2890–2900 | νs CH3 | biorepair®, dentin |
| 2944–2945 | νas CH3 | biorepair®, dentin |
| 3200–3400 | ν OH | H2O |
| White Light Interferometry Ra in µm (Optical Profilometry) | Profilometry Ra in µm (Mechanical Profilometry) | ||||
|---|---|---|---|---|---|
| Enamel | Dentin | Dentin | |||
| test | control | Test | control | test | control |
| 0.57 | 0.57 | 0.49 | 0.50 | 0.30 | 0.43 |
| 0.56 | 0.56 | 0.58 | 0.54 | 0.39 | 0.60 |
| 0.60 | 0.60 | 0.80 | 0.49 | 0.30 | 0.36 |
| 0.58 ± 0.02 | 0.58 ± 0.02 | 0.62 ± 0.16 | 0.51 ± 0.03 (*) | 0.33 ± 0.05 | 0.46 ± 0.12 (*) |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kranz, S.; Heyder, M.; Mueller, S.; Guellmar, A.; Krafft, C.; Nietzsche, S.; Tschirpke, C.; Herold, V.; Sigusch, B.; Reise, M. Remineralization of Artificially Demineralized Human Enamel and Dentin Samples by Zinc-Carbonate Hydroxyapatite Nanocrystals. Materials 2022, 15, 7173. https://doi.org/10.3390/ma15207173
Kranz S, Heyder M, Mueller S, Guellmar A, Krafft C, Nietzsche S, Tschirpke C, Herold V, Sigusch B, Reise M. Remineralization of Artificially Demineralized Human Enamel and Dentin Samples by Zinc-Carbonate Hydroxyapatite Nanocrystals. Materials. 2022; 15(20):7173. https://doi.org/10.3390/ma15207173
Chicago/Turabian StyleKranz, Stefan, Markus Heyder, Stephan Mueller, André Guellmar, Christoph Krafft, Sandor Nietzsche, Caroline Tschirpke, Volker Herold, Bernd Sigusch, and Markus Reise. 2022. "Remineralization of Artificially Demineralized Human Enamel and Dentin Samples by Zinc-Carbonate Hydroxyapatite Nanocrystals" Materials 15, no. 20: 7173. https://doi.org/10.3390/ma15207173
APA StyleKranz, S., Heyder, M., Mueller, S., Guellmar, A., Krafft, C., Nietzsche, S., Tschirpke, C., Herold, V., Sigusch, B., & Reise, M. (2022). Remineralization of Artificially Demineralized Human Enamel and Dentin Samples by Zinc-Carbonate Hydroxyapatite Nanocrystals. Materials, 15(20), 7173. https://doi.org/10.3390/ma15207173









