Covalent Organic Frameworks as Nanocarriers for Improved Delivery of Chemotherapeutic Agents
Abstract
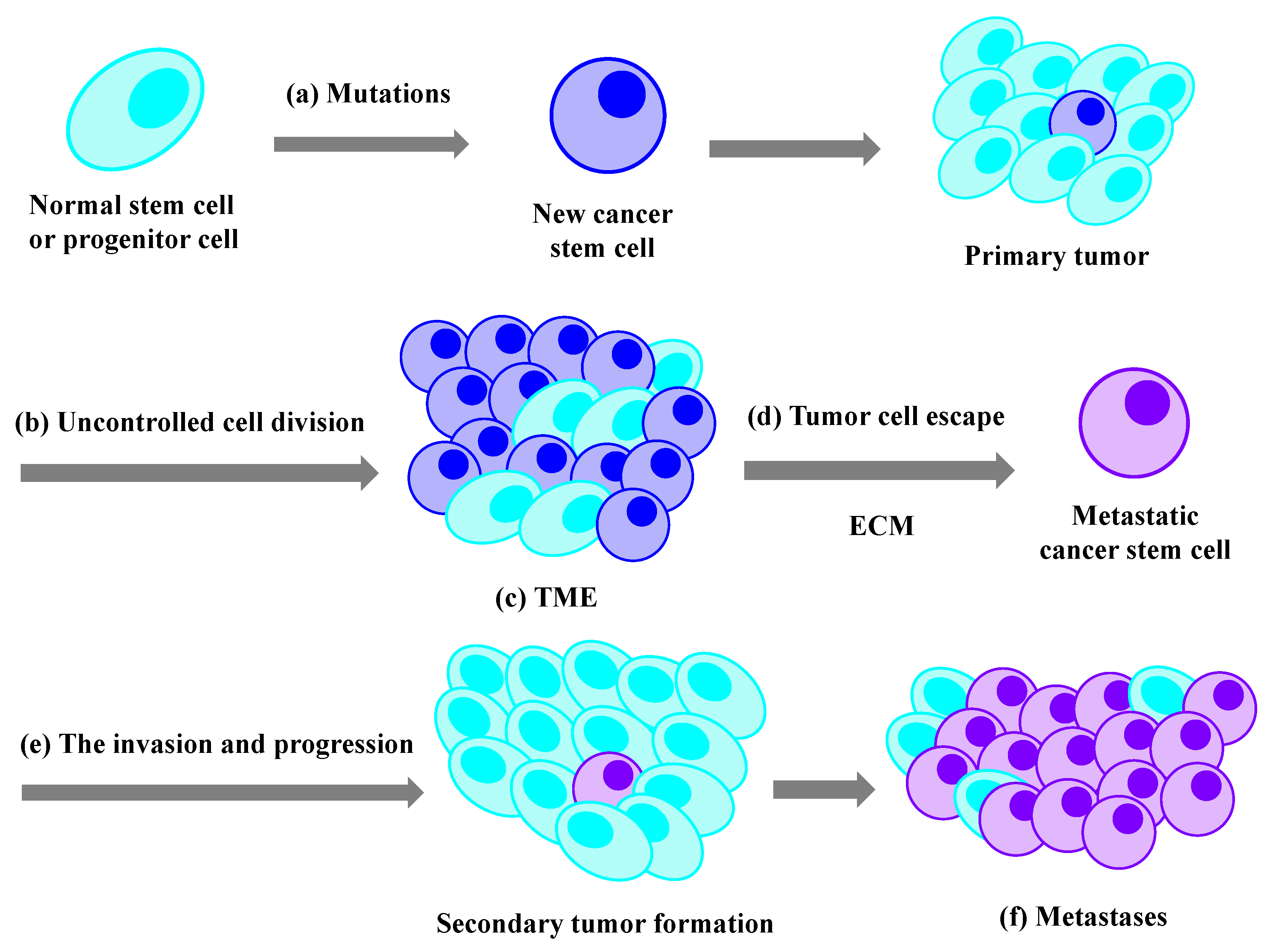
1. Introduction
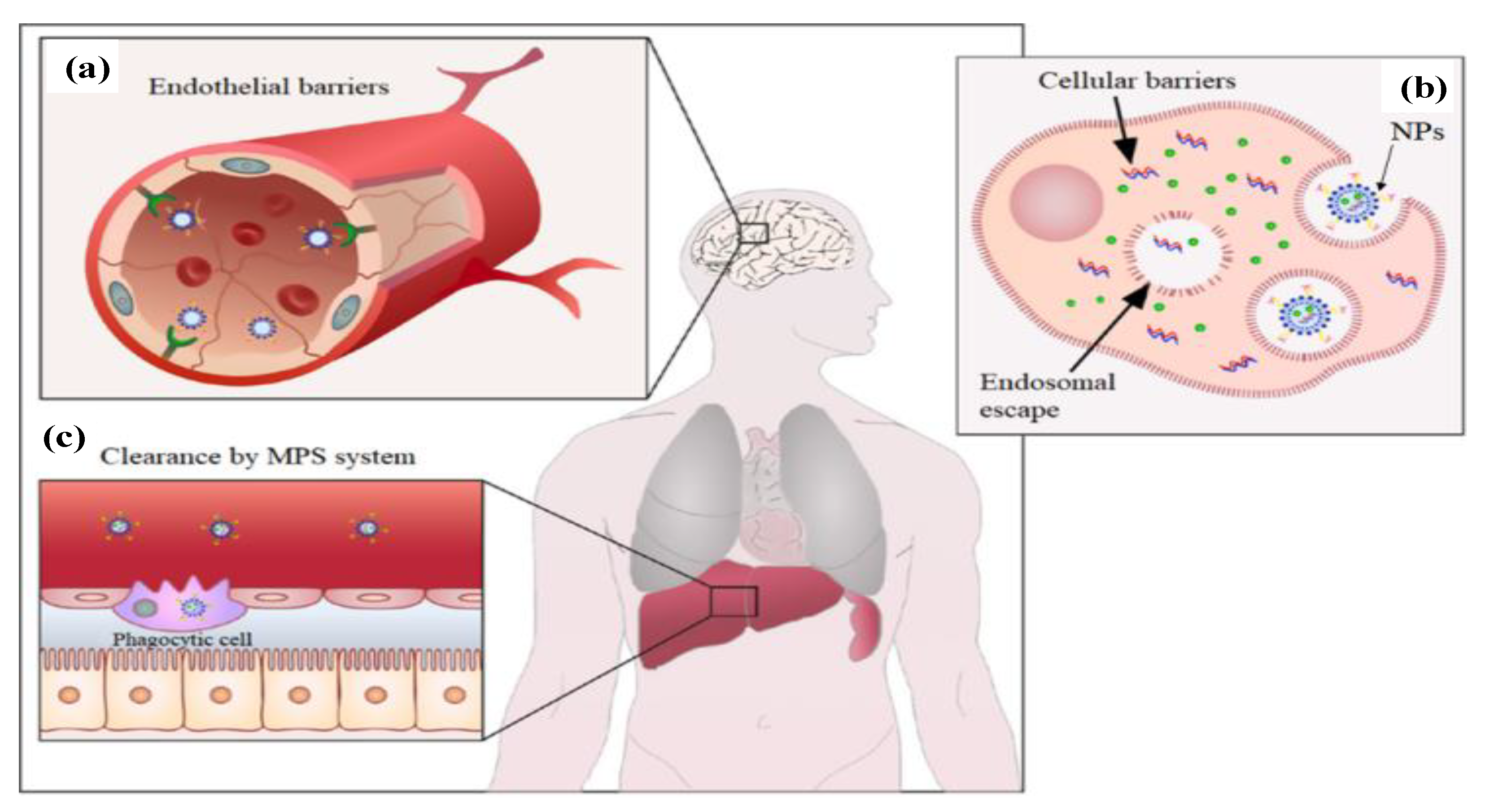
2. Nanoparticles for Chemotherapy
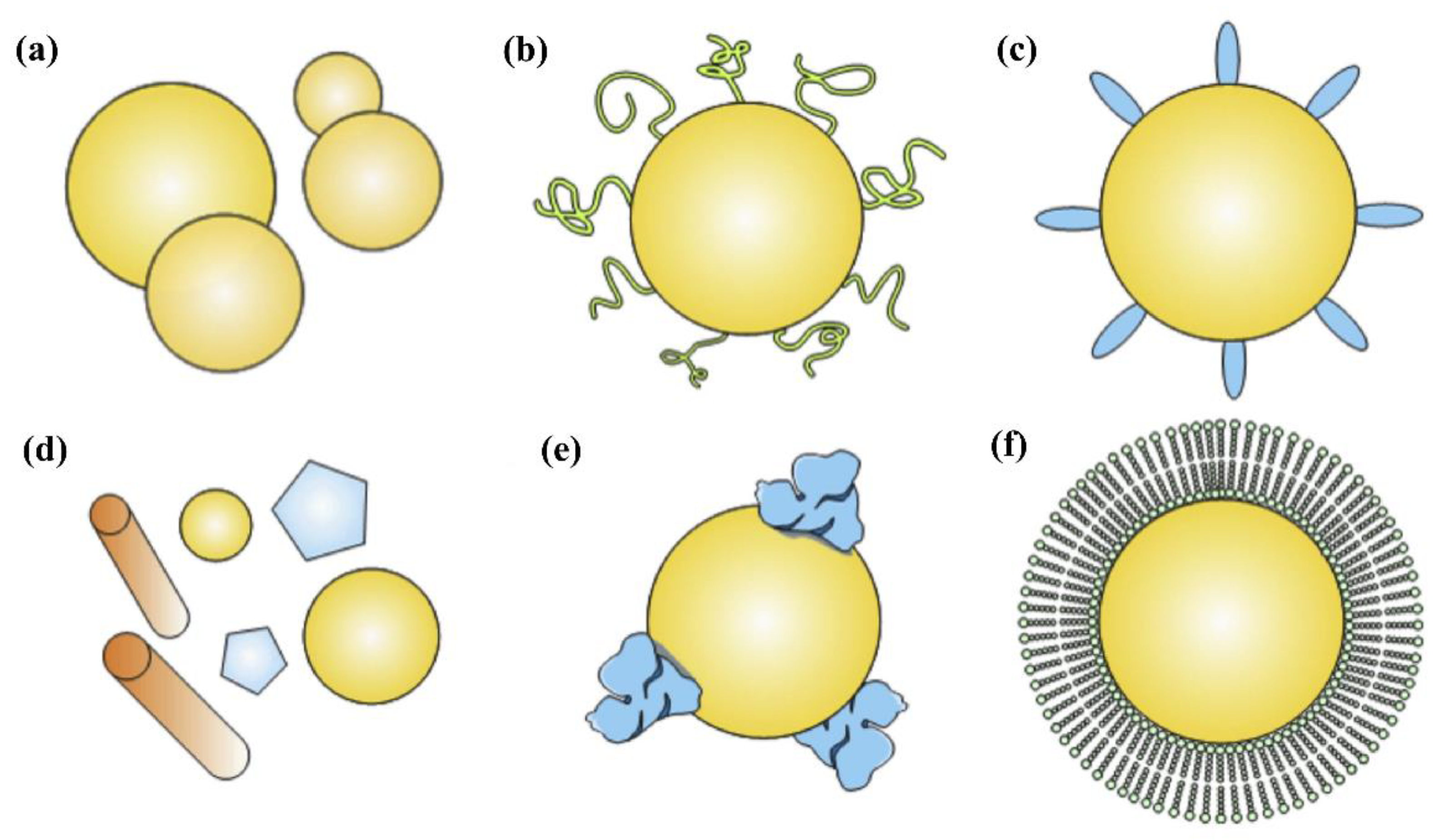
3. Functionalization Strategies of Nanocarriers
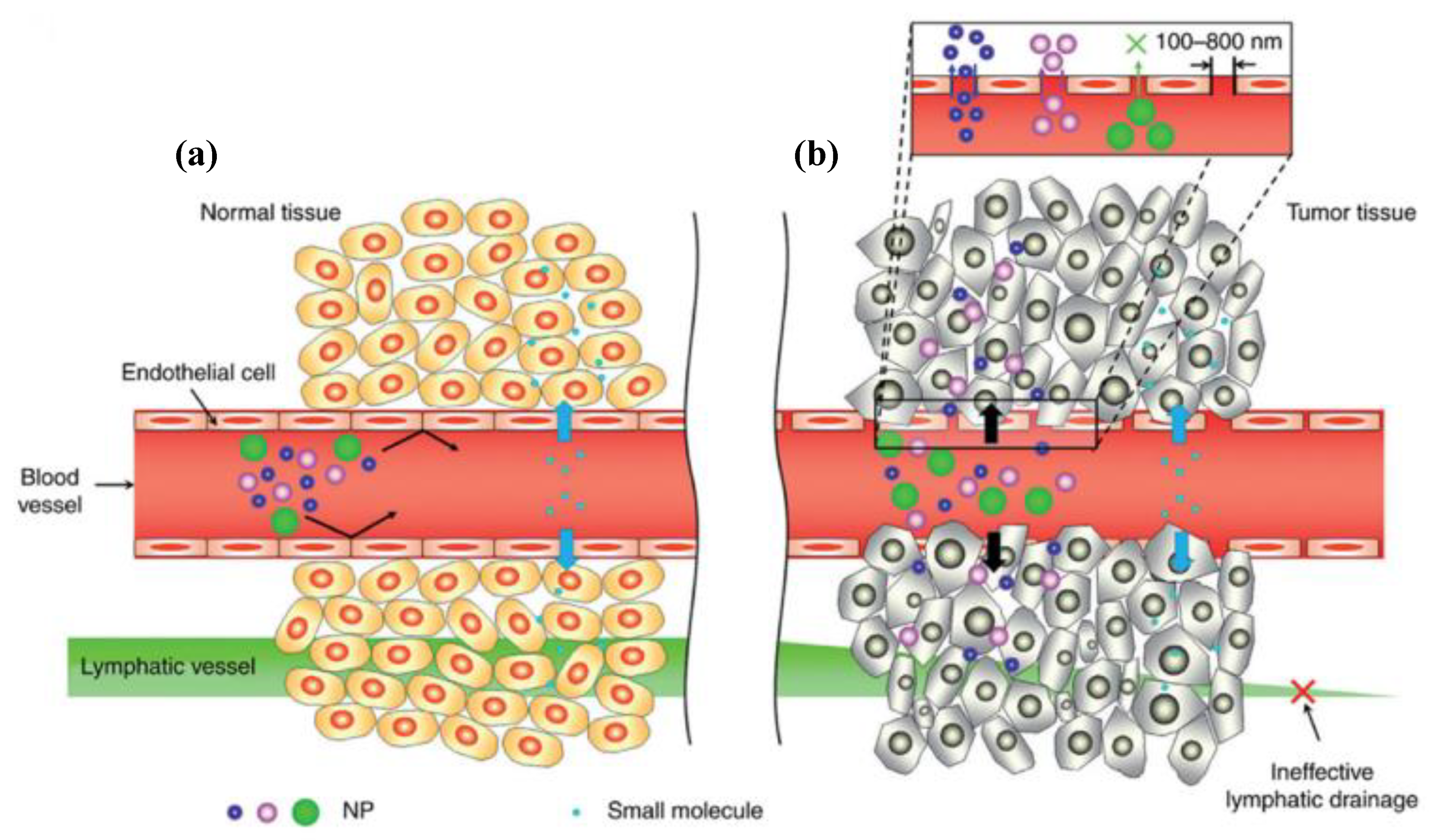
3.1. Passive Targeting Strategies
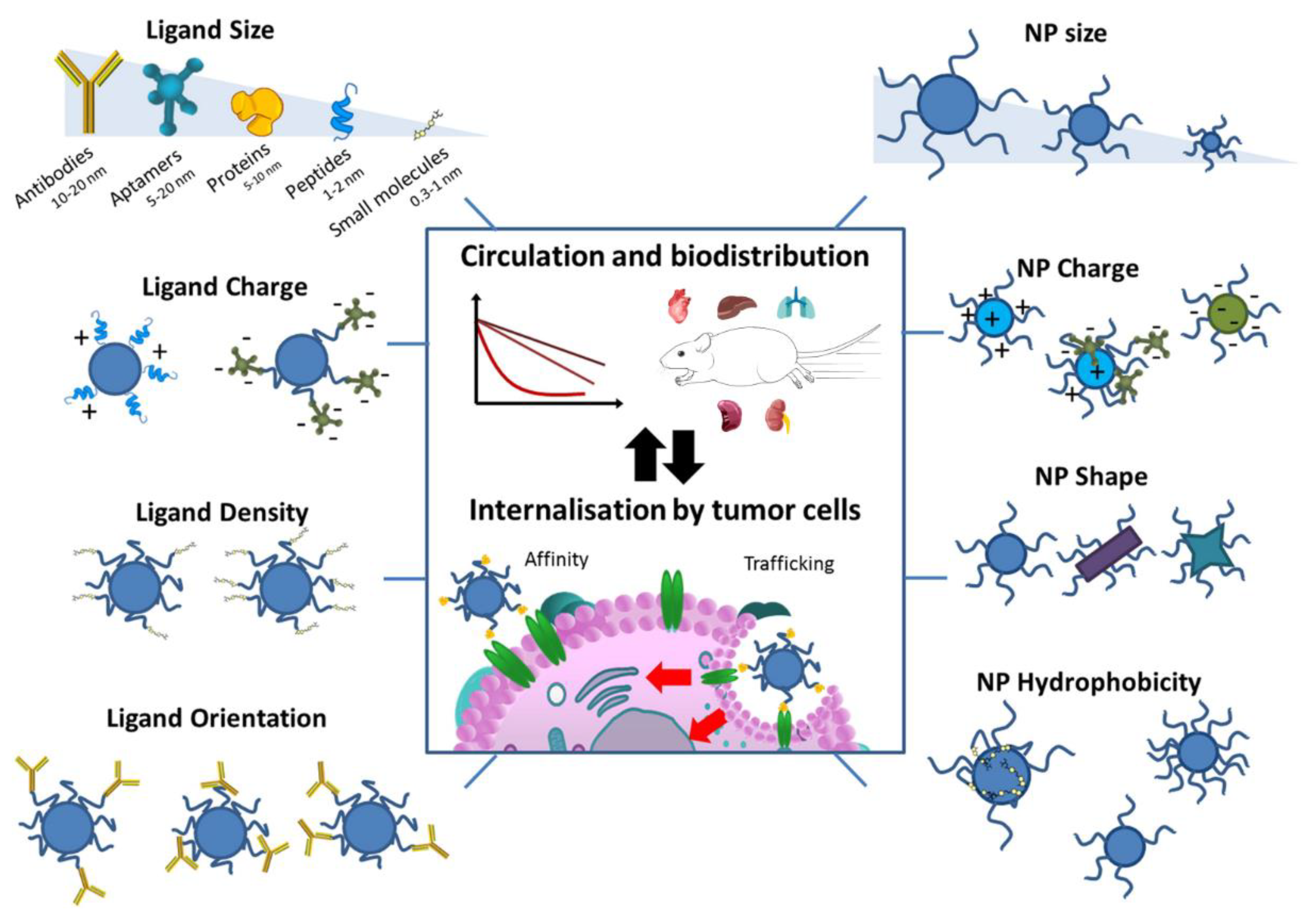
3.2. Active Targeting Strategies
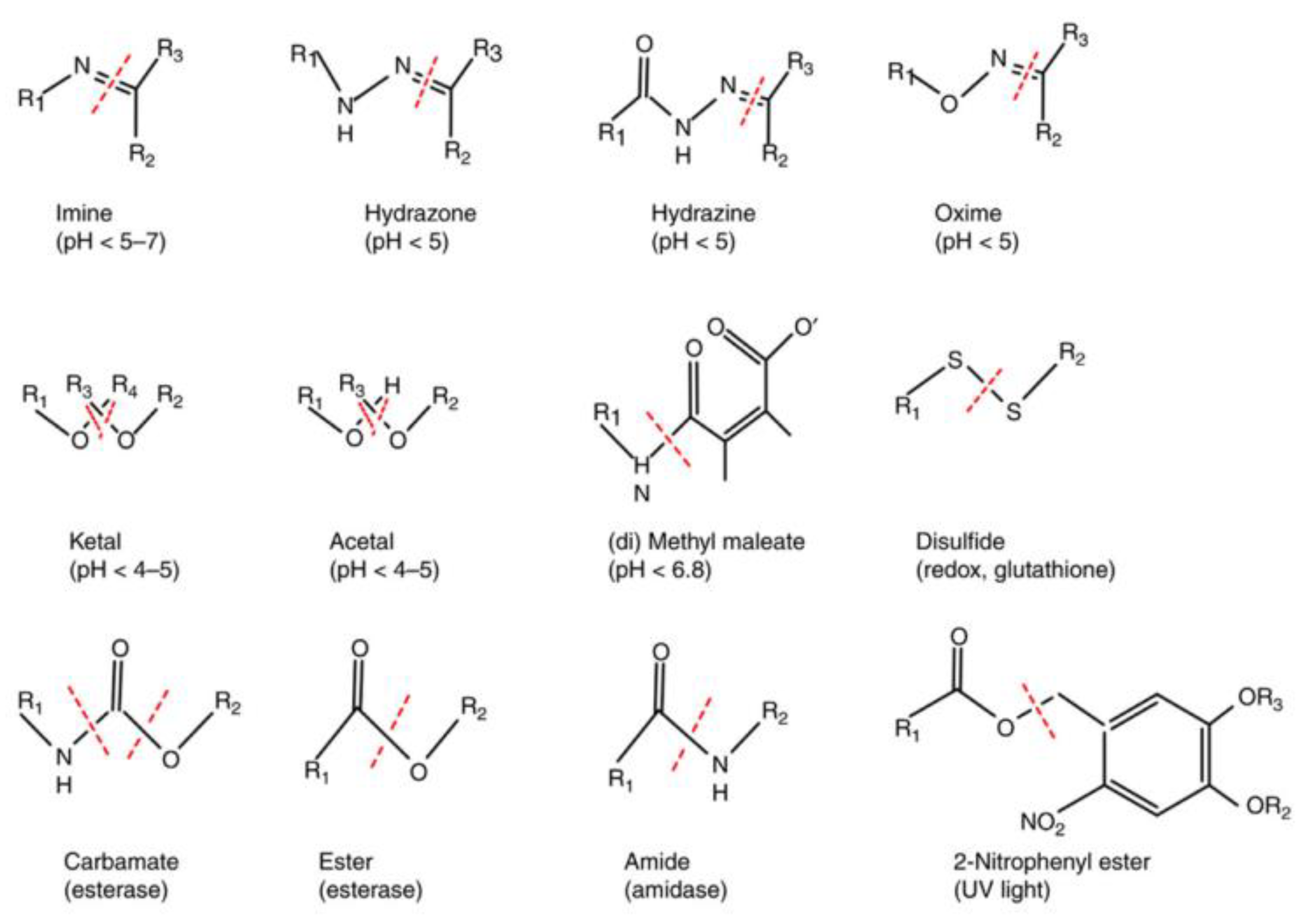
3.3. Stimuli-Responsive Strategies
4. Covalent Organic Frameworks in Antitumor Drug Delivery
4.1. Unique Properties of COFs as Nanocarriers
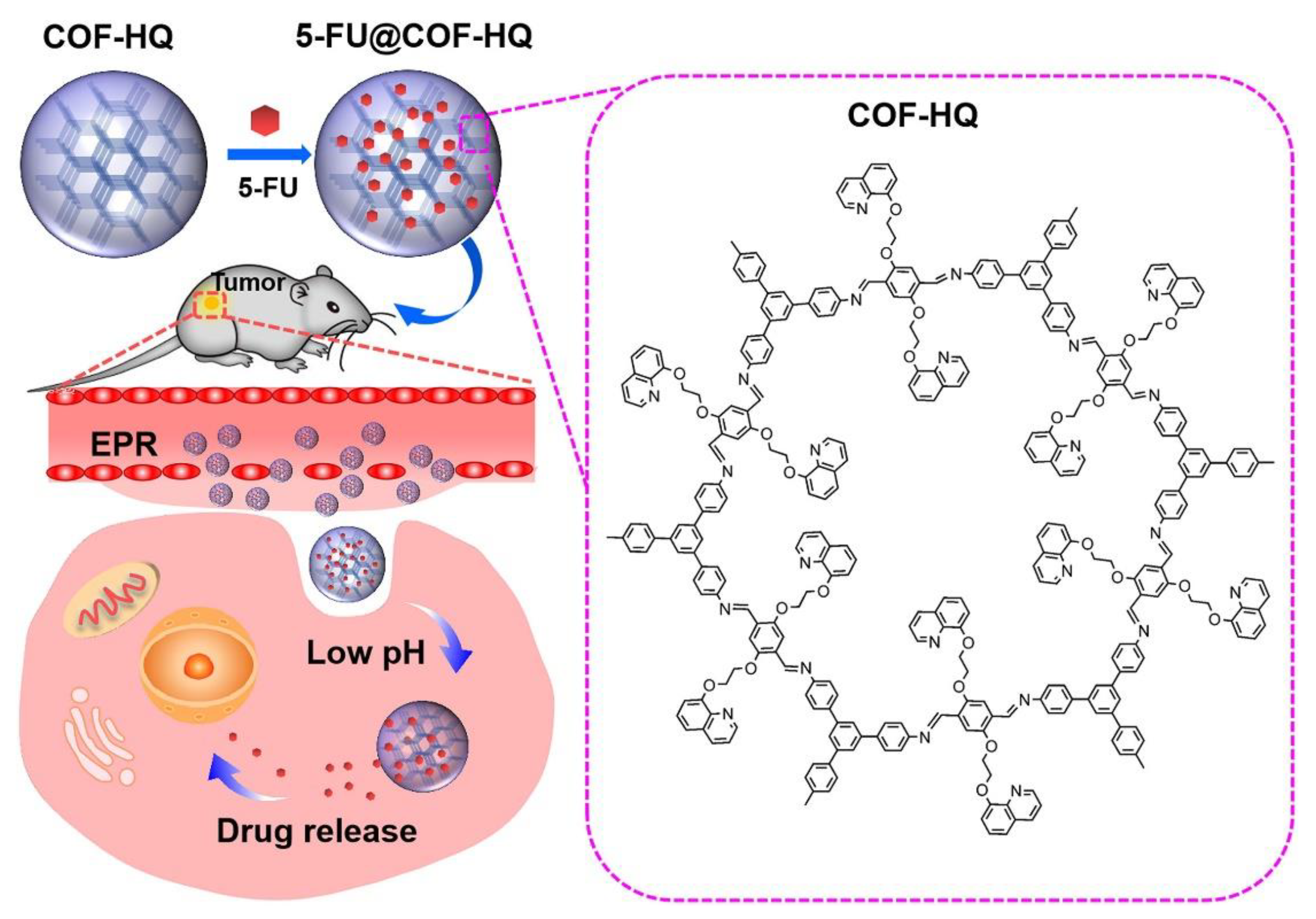
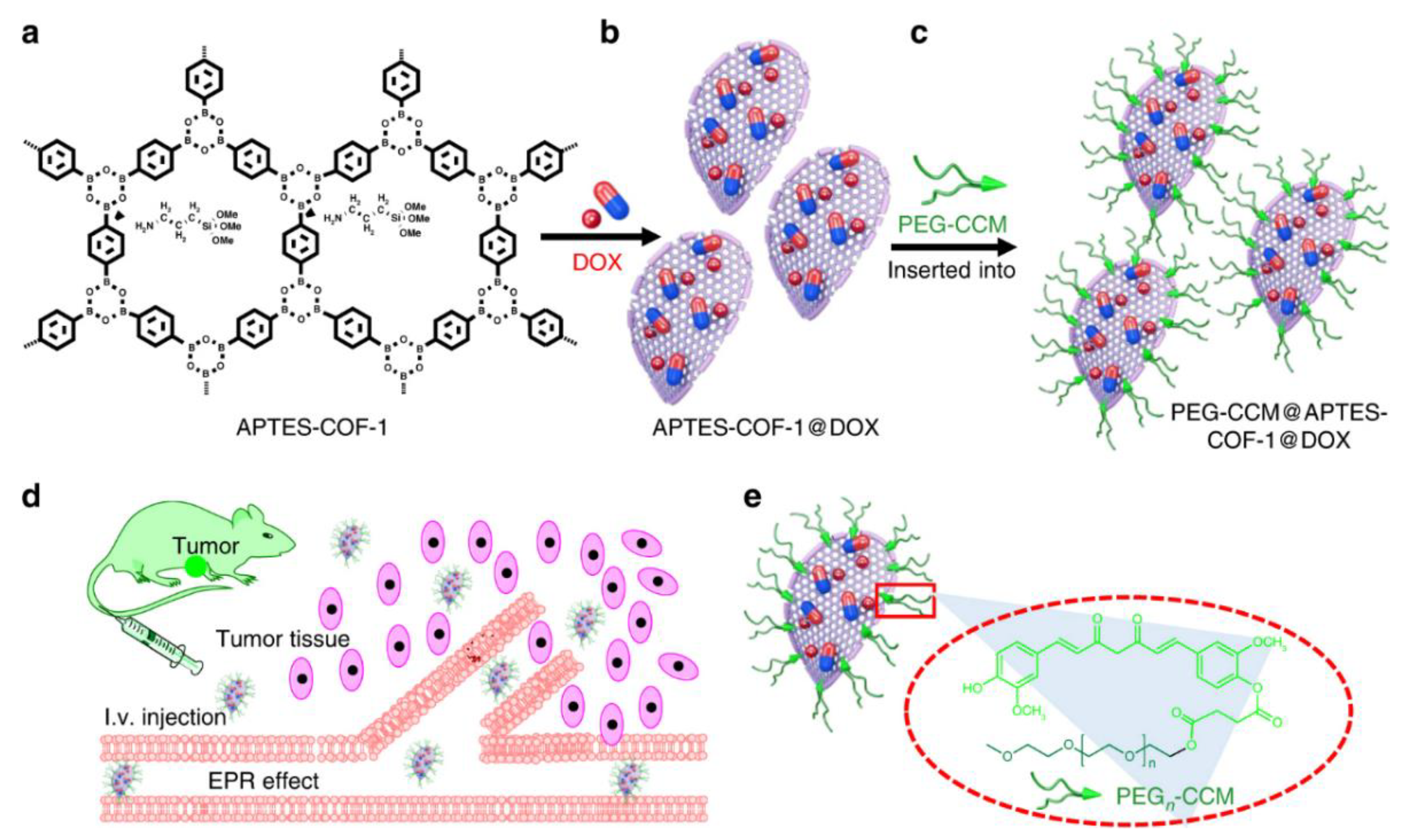
4.2. COFs for Efficient Drug Loading
4.3. COFs for Controlled Drug Release
4.4. COFs for Targeted Drug Delivery
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Wicki, A.; Witzigmann, D.; Balasubramanian, V.; Huwyler, J. Nanomedicine in Cancer Therapy: Challenges, Opportunities, and Clinical Applications. J. Control. Release 2015, 200, 138–157. [Google Scholar] [CrossRef]
- Siegel, R.L.; Miller, K.D.; Jemal, A. Cancer Statistics, 2019. CA Cancer J. Clin. 2019, 69, 7–34. [Google Scholar] [CrossRef] [PubMed]
- Youn, Y.S.; Bae, Y.H. Perspectives on the Past, Present, and Future of Cancer Nanomedicine. Adv. Drug Deliv. Rev. 2018, 130, 3–11. [Google Scholar] [CrossRef] [PubMed]
- Blanco, E.; Shen, H.; Ferrari, M. Principles of Nanoparticle Design for Overcoming Biological Barriers to Drug Delivery. Nat. Biotechnol. 2015, 33, 941–951. [Google Scholar] [CrossRef] [PubMed]
- Luo, F.; Fan, Z.; Yin, W.; Yang, L.; Li, T.; Zhong, L.; Li, Y.; Wang, S.; Yan, J.; Hou, Z.; et al. PH-Responsive Stearic Acid-O-Carboxymethyl Chitosan Assemblies as Carriers Delivering Small Molecular Drug for Chemotherapy. Mater. Sci. Eng. C 2019, 105, 110107. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Zhou, K.; Huang, G.; Hensley, C.; Huang, X.; Ma, X.; Zhao, T.; Sumer, B.D.; DeBerardinis, R.J.; Gao, J. A Nanoparticle-Based Strategy for the Imaging of a Broad Range of Tumours by Nonlinear Amplification of Microenvironment Signals. Nat. Mater. 2014, 13, 204–212. [Google Scholar] [CrossRef] [PubMed]
- Kim, M.; Lee, J.; Nam, J. Plasmonic Photothermal Nanoparticles for Biomedical Applications. Adv. Sci. 2019, 6, 1900471. [Google Scholar] [CrossRef] [PubMed]
- Wang, L.; Huo, M.; Chen, Y.; Shi, J. Tumor Microenvironment-Enabled Nanotherapy. Adv. Healthc. Mater. 2018, 7, 1701156. [Google Scholar] [CrossRef] [PubMed]
- Ji, T.; Zhao, Y.; Ding, Y.; Nie, G. Using Functional Nanomaterials to Target and Regulate the Tumor Microenvironment: Diagnostic and Therapeutic Applications. Adv. Mater. 2013, 25, 3508–3525. [Google Scholar] [CrossRef] [PubMed]
- Bae, Y.H.; Mrsny, R.J.; Park, K. (Eds.) Cancer Targeted Drug Delivery: An Elusive Dream; Springer: London, UK, 2013; ISBN 978-1-4614-7875-1. [Google Scholar]
- Bor, G.; Mat Azmi, I.D.; Yaghmur, A. Nanomedicines for Cancer Therapy: Current Status, Challenges and Future Prospects. Ther. Deliv. 2019, 10, 113–132. [Google Scholar] [CrossRef]
- Zhao, F.; Yao, D.; Guo, R.; Deng, L.; Dong, A.; Zhang, J. Composites of Polymer Hydrogels and Nanoparticulate Systems for Biomedical and Pharmaceutical Applications. Nanomaterials 2015, 5, 2054–2130. [Google Scholar] [CrossRef] [PubMed]
- Blanco, E.; Kessinger, C.W.; Sumer, B.D.; Gao, J. Multifunctional Micellar Nanomedicine for Cancer Therapy. Exp. Biol. Med. 2009, 234, 123–131. [Google Scholar] [CrossRef]
- Hashemzadeh, H.; Raissi, H. Understanding Loading, Diffusion and Releasing of Doxorubicin and Paclitaxel Dual Delivery in Graphene and Graphene Oxide Carriers as Highly Efficient Drug Delivery Systems. Appl. Surf. Sci. 2020, 500, 144220. [Google Scholar] [CrossRef]
- Liong, M.; Lu, J.; Kovochich, M.; Xia, T.; Ruehm, S.G.; Nel, A.E.; Tamanoi, F.; Zink, J.I. Multifunctional Inorganic Nanoparticles for Imaging, Targeting, and Drug Delivery. ACS Nano 2008, 2, 889–896. [Google Scholar] [CrossRef] [PubMed]
- Mitra, S.; Sasmal, H.S.; Kundu, T.; Kandambeth, S.; Illath, K.; Díaz Díaz, D.; Banerjee, R. Targeted Drug Delivery in Covalent Organic Nanosheets (CONs) via Sequential Postsynthetic Modification. J. Am. Chem. Soc. 2017, 139, 4513–4520. [Google Scholar] [CrossRef]
- Gadekar, V.; Borade, Y.; Kannaujia, S.; Rajpoot, K.; Anup, N.; Tambe, V.; Kalia, K.; Tekade, R.K. Nanomedicines accessible in the market for clinical interventions. J. Control. Release 2021, 330, 372–397. [Google Scholar] [CrossRef] [PubMed]
- Farjadian, F.; Ghasemi, A.; Gohari, O.; Roointan, A.; Karimi, M.; Hamblin, M.R. Nanopharmaceuticals and nanomedicines currently on the market: Challenges and opportunities. Nanomedicine 2019, 14, 93–126. [Google Scholar] [CrossRef] [PubMed]
- Wu, Y.; Chen, W.; Meng, F.; Wang, Z.; Cheng, R.; Deng, C.; Liu, H.; Zhong, Z. Core-Crosslinked PH-Sensitive Degradable Micelles: A Promising Approach to Resolve the Extracellular Stability versus Intracellular Drug Release Dilemma. J. Control. Release 2012, 164, 338–345. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Zhang, T.; Liu, Q.; He, J. PEG-Derivatized Dual-Functional Nanomicelles for Improved Cancer Therapy. Front. Pharmacol. 2019, 10, 808. [Google Scholar] [CrossRef] [PubMed]
- Rosenblum, D.; Joshi, N.; Tao, W.; Karp, J.M.; Peer, D. Progress and Challenges towards Targeted Delivery of Cancer Therapeutics. Nat. Commun. 2018, 9, 1410. [Google Scholar] [CrossRef]
- Danhier, F.; Feron, O.; Préat, V. To Exploit the Tumor Microenvironment: Passive and Active Tumor Targeting of Nanocarriers for Anti-Cancer Drug Delivery. J. Control. Release 2010, 148, 135–146. [Google Scholar] [CrossRef] [PubMed]
- Lammers, T.; Hennink, W.E.; Storm, G. Tumour-Targeted Nanomedicines: Principles and Practice. Br. J. Cancer 2008, 99, 392–397. [Google Scholar] [CrossRef]
- Nichols, J.W.; Bae, Y.H. Odyssey of a Cancer Nanoparticle: From Injection Site to Site of Action. Nano Today 2012, 7, 606–618. [Google Scholar] [CrossRef] [PubMed]
- Alasvand, N.; Urbanska, A.M.; Rahmati, M.; Saeidifar, M.; Gungor-Ozkerim, P.S.; Sefat, F.; Rajadas, J.; Mozafari, M. Chapter 13—Therapeutic Nanoparticles for Targeted Delivery of Anticancer Drugs. Multifunct. Syst. Comb. Deliv. Biosensing Diagn. 2017, 2017, 245–259. [Google Scholar] [CrossRef]
- Wibroe, P.P.; Ahmadvand, D.; Oghabian, M.A.; Yaghmur, A.; Moghimi, S.M. An Integrated Assessment of Morphology, Size, and Complement Activation of the PEGylated Liposomal Doxorubicin Products Doxil®, Caelyx®, DOXOrubicin, and SinaDoxosome. J. Control. Release 2016, 221, 1–8. [Google Scholar] [CrossRef]
- Deng, H.; Song, K.; Zhang, J.; Deng, L.; Dong, A.; Qin, Z. Modulating the Rigidity of Nanoparticles for Tumor Penetration. Chem. Commun. 2018, 54, 3014–3017. [Google Scholar] [CrossRef] [PubMed]
- Blanco, E.; Hsiao, A.; Mann, A.P.; Landry, M.G.; Meric-Bernstam, F.; Ferrari, M. Nanomedicine in Cancer Therapy: Innovative Trends and Prospects. Cancer Sci. 2011, 102, 1247–1252. [Google Scholar] [CrossRef]
- Gerlowski, L.E.; Jain, R.K. Microvascular Permeability of Normal and Neoplastic Tissues. Microvasc. Res. 1986, 31, 288–305. [Google Scholar] [CrossRef]
- Sykes, E.A.; Chen, J.; Zheng, G.; Chan, W.C.W. Investigating the Impact of Nanoparticle Size on Active and Passive Tumor Targeting Efficiency. ACS Nano 2014, 8, 5696–5706. [Google Scholar] [CrossRef] [PubMed]
- Gullotti, E.; Yeo, Y. Extracellularly Activated Nanocarriers: A New Paradigm of Tumor Targeted Drug Delivery. Mol. Pharm. 2009, 6, 1041–1051. [Google Scholar] [CrossRef] [PubMed]
- von Roemeling, C.; Jiang, W.; Chan, C.K.; Weissman, I.L.; Kim, B.Y.S. Breaking Down the Barriers to Precision Cancer Nanomedicine. Trends Biotechnol. 2017, 35, 159–171. [Google Scholar] [CrossRef]
- Rodriguez, P.L.; Harada, T.; Christian, D.A.; Pantano, D.A.; Tsai, R.K.; Discher, D.E. Minimal “Self” Peptides That Inhibit Phagocytic Clearance and Enhance Delivery of Nanoparticles. Science 2013, 339, 971–975. [Google Scholar] [CrossRef]
- Hu, C.-M.J.; Zhang, L.; Aryal, S.; Cheung, C.; Fang, R.H.; Zhang, L. Erythrocyte Membrane-Camouflaged Polymeric Nanoparticles as a Biomimetic Delivery Platform. Proc. Natl. Acad. Sci. USA 2011, 108, 10980–10985. [Google Scholar] [CrossRef]
- Attia, M.F.; Anton, N.; Wallyn, J.; Omran, Z.; Vandamme, T.F. An Overview of Active and Passive Targeting Strategies to Improve the Nanocarriers Efficiency to Tumour Sites. J. Pharm. Pharmacol. 2019, 71, 1185–1198. [Google Scholar] [CrossRef]
- Kamaly, N.; Xiao, Z.; Valencia, P.M.; Radovic-Moreno, A.F.; Farokhzad, O.C. Targeted Polymeric Therapeutic Nanoparticles: Design, Development and Clinical Translation. Chem. Soc. Rev. 2012, 41, 2971. [Google Scholar] [CrossRef]
- Wang, X.; Li, S.; Shi, Y.; Chuan, X.; Li, J.; Zhong, T.; Zhang, H.; Dai, W.; He, B.; Zhang, Q. The Development of Site-Specific Drug Delivery Nanocarriers Based on Receptor Mediation. J. Control. Release 2014, 193, 139–153. [Google Scholar] [CrossRef] [PubMed]
- Panyam, J.; Zhou, W.; Prabha, S.; Sahoo, S.K.; Labhasetwar, V. Rapid Endo-lysosomal Escape of Poly(DL-lactide- Co Glycolide) Nanoparticles: Implications for Drug and Gene Delivery. FASEB J. 2002, 16, 1217–1226. [Google Scholar] [CrossRef]
- Saha, R.N.; Vasanthakumar, S.; Bende, G.; Snehalatha, M. Nanoparticulate Drug Delivery Systems for Cancer Chemotherapy. Mol. Membr. Biol. 2010, 27, 215–231. [Google Scholar] [CrossRef]
- Chen, D.; Qu, X.; Shao, J.; Wang, W.; Dong, X. Anti-Vascular Nano Agents: A Promising Approach for Cancer Treatment. J. Mater. Chem. B 2020, 8, 2990–3004. [Google Scholar] [CrossRef] [PubMed]
- Liang, P.; Huang, X.; Wang, Y.; Chen, D.; Ou, C.; Zhang, Q.; Shao, J.; Huang, W.; Dong, X. Tumor-Microenvironment-Responsive Nanoconjugate for Synergistic Antivascular Activity and Phototherapy. ACS Nano 2018, 12, 11446–11457. [Google Scholar] [CrossRef] [PubMed]
- Bertrand, N.; Wu, J.; Xu, X.; Kamaly, N.; Farokhzad, O.C. Cancer nanotechnology: The impact of passive and active targeting in the era of modern cancer biology. Adv. Drug Deliv. Rev. 2014, 66, 2–25. [Google Scholar] [CrossRef] [PubMed]
- Li, Z.; Song, N.; Yang, Y.-W. Stimuli-Responsive Drug-Delivery Systems Based on Supramolecular Nanovalves. Matter 2019, 1, 345–368. [Google Scholar] [CrossRef]
- Khan, A.R.; Yang, X.; Fu, M.; Zhai, G. Recent progress of drug nanoformulations targeting to brain. J. Control. Release 2018, 291, 37–64. [Google Scholar] [CrossRef]
- de Bree, E.; Romanos, J.; Tsiftsis, D.D. Hyperthermia in Anticancer Treatment. Eur. J. Surg. Onc. 2002, 28, 95. [Google Scholar] [CrossRef]
- Holme, M.N.; Fedotenko, I.A.; Abegg, D.; Althaus, J.; Babel, L.; Favarger, F.; Reiter, R.; Tanasescu, R.; Zaffalon, P.-L.; Ziegler, A.; et al. Shear-Stress Sensitive Lenticular Vesicles for Targeted Drug Delivery. Nat. Nanotechnol. 2012, 7, 536–543. [Google Scholar] [CrossRef]
- Lee, E.S.; Gao, Z.; Bae, Y.H. Recent Progress in Tumor PH Targeting Nanotechnology. J. Control. Release 2008, 132, 164–170. [Google Scholar] [CrossRef] [PubMed]
- Zhou, M.; Wei, W.; Chen, X.; Xu, X.; Zhang, X.; Zhang, X. PH and Redox Dual Responsive Carrier-Free Anticancer Drug Nanoparticles for Targeted Delivery and Synergistic Therapy. Nanomedicine 2019, 20, 102008. [Google Scholar] [CrossRef] [PubMed]
- Meng, F.; Zhong, Y.; Cheng, R.; Deng, C.; Zhong, Z. PH-Sensitive Polymeric Nanoparticles for Tumor-Targeting Doxorubicin Delivery: Concept and Recent Advances. Nanomedicine 2014, 9, 487–499. [Google Scholar] [CrossRef] [PubMed]
- Deng, H.; Liu, J.; Zhao, X.; Zhang, Y.; Liu, J.; Xu, S.; Deng, L.; Dong, A.; Zhang, J. PEG- b -PCL Copolymer Micelles with the Ability of PH-Controlled Negative-to-Positive Charge Reversal for Intracellular Delivery of Doxorubicin. Biomacromolecules 2014, 15, 4281–4292. [Google Scholar] [CrossRef]
- Chen, F.; Cai, W. Nanomedicine for Targeted Photothermal Cancer Therapy: Where Are We Now? Nanomedicine 2015, 10, 1–3. [Google Scholar] [CrossRef]
- Mi, P. Stimuli-Responsive Nanocarriers for Drug Delivery, Tumor Imaging, Therapy and Theranostics. Theranostics 2020, 10, 4557–4588. [Google Scholar] [CrossRef] [PubMed]
- Kwon, S.; Ko, H.; You, D.G.; Kataoka, K.; Park, J.H. Nanomedicines for Reactive Oxygen Species Mediated Approach: An Emerging Paradigm for Cancer Treatment. Acc. Chem. Res. 2019, 52, 1771–1782. [Google Scholar] [CrossRef] [PubMed]
- Chen, D.; Wang, Z.; Dai, H.; Lv, X.; Ma, Q.; Yang, D.; Shao, J.; Xu, Z.; Dong, X. Boosting O 2 •− Photogeneration via Promoting Intersystem-Crossing and Electron-Donating Efficiency of Aza-BODIPY-Based Nanoplatforms for Hypoxic-Tumor Photodynamic Therapy. Small Methods 2020, 4, 2000013. [Google Scholar] [CrossRef]
- Yang, B.; Chen, Y.; Shi, J. Reactive Oxygen Species (ROS)-Based Nanomedicine. Chem. Rev. 2019, 119, 4881–4985. [Google Scholar] [CrossRef]
- Yu, S.; Chen, Z.; Zeng, X.; Chen, X.; Gu, Z. Advances in Nanomedicine for Cancer Starvation Therapy. Theranostics 2019, 9, 8026–8047. [Google Scholar] [CrossRef]
- Trachootham, D.; Alexandre, J.; Huang, P. Targeting Cancer Cells by ROS-Mediated Mechanisms: A Radical Therapeutic Approach? Nat. Rev. Drug Discov. 2009, 8, 579–591. [Google Scholar] [CrossRef]
- Chen, Q.; Xu, L.; Liang, C.; Wang, C.; Peng, R.; Liu, Z. Photothermal Therapy with Immune-Adjuvant Nanoparticles Together with Checkpoint Blockade for Effective Cancer Immunotherapy. Nat. Commun. 2016, 7, 13193. [Google Scholar] [CrossRef]
- Zhou, Z.; Song, J.; Nie, L.; Chen, X. Reactive Oxygen Species Generating Systems Meeting Challenges of Photodynamic Cancer Therapy. Chem. Soc. Rev. 2016, 45, 6597–6626. [Google Scholar] [CrossRef]
- Nosaka, Y.; Nosaka, A.Y. Generation and Detection of Reactive Oxygen Species in Photocatalysis. Chem. Rev. 2017, 117, 11302–11336. [Google Scholar] [CrossRef]
- Agostinis, P.; Berg, K.; Cengel, K.A.; Foster, T.H.; Girotti, A.W.; Gollnick, S.O.; Hahn, S.M.; Hamblin, M.R.; Juzeniene, A.; Kessel, D.; et al. Photodynamic Therapy of Cancer: An Update. CA Cancer J. Clin. 2011, 61, 250–281. [Google Scholar] [CrossRef]
- Lin, J.; Li, Y.; Li, Y.; Wu, H.; Yu, F.; Zhou, S.; Xie, L.; Luo, F.; Lin, C.; Hou, Z. Drug/Dye-Loaded, Multifunctional PEG–Chitosan–Iron Oxide Nanocomposites for Methotraxate Synergistically Self-Targeted Cancer Therapy and Dual Model Imaging. ACS Appl. Mater. Inter. 2015, 7, 11908–11920. [Google Scholar] [CrossRef] [PubMed]
- Dong, Z.; Gong, H.; Gao, M.; Zhu, W.; Sun, X.; Feng, L.; Fu, T.; Li, Y.; Liu, Z. Polydopamine Nanoparticles as a Versatile Molecular Loading Platform to Enable Imaging-Guided Cancer Combination Therapy. Theranostics 2016, 6, 1031–1042. [Google Scholar] [CrossRef] [PubMed]
- Chen, H.; Zhang, W.; Zhu, G.; Xie, J.; Chen, X. Rethinking Cancer Nanotheranostics. Nat. Rev. Mater. 2017, 2, 17024. [Google Scholar] [CrossRef] [PubMed]
- Sun, Q.; Radosz, M.; Shen, Y. Challenges in Design of Translational Nanocarriers. J. Control. Release 2012, 164, 156–169. [Google Scholar] [CrossRef]
- Côté, A.P.; Benin, A.I.; Ockwig, N.W.; O’Keeffe, M.; Matzger, A.J.; Yaghi, O.M. Porous, Crystalline, Covalent Organic Frameworks. Science 2005, 310, 1166–1170. [Google Scholar] [CrossRef]
- Song, Y.; Sun, Q.; Aguila, B.; Ma, S. Opportunities of Covalent Organic Frameworks for Advanced Applications. Adv. Sci. 2019, 6, 1801410. [Google Scholar] [CrossRef]
- Waller, P.J.; Gándara, F.; Yaghi, O.M. Chemistry of Covalent Organic Frameworks. Acc. Chem. Res. 2015, 48, 3053–3063. [Google Scholar] [CrossRef]
- Du, Y.; Calabro, D.; Wooler, B.; Kortunov, P.; Li, Q.; Cundy, S.; Mao, K. One Step Facile Synthesis of Amine-Functionalized COF-1 with Enhanced Hydrostability. Chem. Mater. 2015, 27, 1445–1447. [Google Scholar] [CrossRef]
- Zhao, F.; Liu, H.; Mathe, S.; Dong, A.; Zhang, J. Covalent Organic Frameworks: From Materials Design to Biomedical Application. Nanomaterials 2017, 8, 15. [Google Scholar] [CrossRef]
- Wang, H.; Wang, H.; Wang, Z.; Tang, L.; Zeng, G.; Xu, P.; Chen, M.; Xiong, T.; Zhou, C.; Li, X.; et al. Covalent Organic Framework Photocatalysts: Structures and Applications. Chem. Soc. Rev. 2020, 49, 4135–4165. [Google Scholar] [CrossRef]
- Wang, J.; Li, N.; Xu, Y.; Pang, H. Two-Dimensional MOF and COF Nanosheets: Synthesis and Applications in Electrochemistry. Chem. Eur. J. 2020, 26, 6402–6422. [Google Scholar] [CrossRef] [PubMed]
- Jarju, J.J.; Lavender, A.M.; Espiña, B.; Romero, V.; Salonen, L.M. Covalent Organic Framework Composites: Synthesis and Analytical Applications. Molecules 2020, 25, 5404. [Google Scholar] [CrossRef] [PubMed]
- Wang, W.; Zhao, W.; Xu, H.; Liu, S.; Huang, W.; Zhao, Q. Fabrication of Ultra-Thin 2D Covalent Organic Framework Nanosheets and Their Application in Functional Electronic Devices. Coord. Chem. Rev. 2021, 429, 213616. [Google Scholar] [CrossRef]
- Scicluna, M.C.; Vella-Zarb, L. Evolution of Nanocarrier Drug-Delivery Systems and Recent Advancements in Covalent Organic Framework–Drug Systems. ACS Appl. Nano Mater. 2020, 3, 3097–3115. [Google Scholar] [CrossRef]
- Jia, Y.; Zhang, L.; He, B.; Lin, Y.; Wang, J.; Li, M. 8-Hydroxyquinoline Functionalized Covalent Organic Framework as a PH Sensitive Carrier for Drug Delivery. Mater. Sci. Eng. C 2020, 117, 111243. [Google Scholar] [CrossRef] [PubMed]
- Qian, C.; Qi, Q.-Y.; Jiang, G.-F.; Cui, F.-Z.; Tian, Y.; Zhao, X. Toward Covalent Organic Frameworks Bearing Three Different Kinds of Pores: The Strategy for Construction and COF-to-COF Transformation via Heterogeneous Linker Exchange. J. Am. Chem. Soc. 2017, 139, 6736–6743. [Google Scholar] [CrossRef]
- Ding, X.; Guo, J.; Feng, X.; Honsho, Y.; Guo, J.; Seki, S.; Maitarad, P.; Saeki, A.; Nagase, S.; Jiang, D. Synthesis of Metallophthalocyanine Covalent Organic Frameworks That Exhibit High Carrier Mobility and Photoconductivity. Angew. Chem. Int. Edit. 2011, 50, 1289–1293. [Google Scholar] [CrossRef]
- Vyas, V.S.; Vishwakarma, M.; Moudrakovski, I.; Haase, F.; Savasci, G.; Ochsenfeld, C.; Spatz, J.P.; Lotsch, B.v. Exploiting Noncovalent Interactions in an Imine-Based Covalent Organic Framework for Quercetin Delivery. Adv. Mater. 2016, 28, 8749–8754. [Google Scholar] [CrossRef]
- Kandambeth, S.; Venkatesh, V.; Shinde, D.B.; Kumari, S.; Halder, A.; Verma, S.; Banerjee, R. Self-Templated Chemically Stable Hollow Spherical Covalent Organic Framework. Nat. Commun. 2015, 6, 6786. [Google Scholar] [CrossRef]
- Zhao, F.; Dong, A.; Deng, L.; Guo, R.; Zhang, J. Morphology Control and Property Design of Boronate Dynamic Nanostructures. Polym. Chem. 2019, 10, 2436–2446. [Google Scholar] [CrossRef]
- Guo, J.; Xu, Y.; Jin, S.; Chen, L.; Kaji, T.; Honsho, Y.; Addicoat, M.A.; Kim, J.; Saeki, A.; Ihee, H.; et al. Conjugated Organic Framework with Three-Dimensionally Ordered Stable Structure and Delocalized π Clouds. Nat. Commun. 2013, 4, 2736. [Google Scholar] [CrossRef] [PubMed]
- Yang, Y.; Niu, H.; Xu, L.; Zhang, H.; Cai, Y. Triazine Functionalized Fully Conjugated Covalent Organic Framework for Efficient Photocatalysis. Appl. Catal. B 2020, 269, 118799. [Google Scholar] [CrossRef]
- Zhou, Y.; Liu, S.; Hu, C.; Cai, L.; Pang, M. A Covalent Organic Framework as a Nanocarrier for Synergistic Phototherapy and Immunotherapy. J. Mater. Chem. B 2020, 8, 5451–5459. [Google Scholar] [CrossRef] [PubMed]
- Zeng, J.; Wang, X.; Xie, B.; Li, M.; Zhang, X. Covalent Organic Framework for Improving Near-Infrared Light Induced Fluorescence Imaging through Two-Photon Induction. Angew. Chem. Int. Edit. 2020, 59, 10087–10094. [Google Scholar] [CrossRef] [PubMed]
- Li, W.; Yang, C.-X.; Yan, X.-P. A Versatile Covalent Organic Framework-Based Platform for Sensing Biomolecules. Chem. Commun. 2017, 53, 11469–11471. [Google Scholar] [CrossRef] [PubMed]
- Das, G.; Benyettou, F.; Sharama, S.K.; Prakasam, T.; Gándara, F.; de la Peña-O’Shea, V.A.; Saleh, N.; Pasricha, R.; Jagannathan, R.; Olson, M.A.; et al. Covalent Organic Nanosheets for Bioimaging. Chem. Sci. 2018, 9, 8382–8387. [Google Scholar] [CrossRef] [PubMed]
- Sun, Q.; Fu, C.-W.; Aguila, B.; Perman, J.; Wang, S.; Huang, H.-Y.; Xiao, F.-S.; Ma, S. Pore Environment Control and Enhanced Performance of Enzymes Infiltrated in Covalent Organic Frameworks. J. Am. Chem. Soc. 2018, 140, 984–992. [Google Scholar] [CrossRef]
- Zhang, S.; Zheng, Y.; An, H.; Aguila, B.; Yang, C.; Dong, Y.; Xie, W.; Cheng, P.; Zhang, Z.; Chen, Y.; et al. Covalent Organic Frameworks with Chirality Enriched by Biomolecules for Efficient Chiral Separation. Angew. Chem. Int. Edit. 2018, 57, 16754–16759. [Google Scholar] [CrossRef]
- Wang, J.; Zhao, L.; Yan, B. Indicator Displacement Assay Inside Dye-Functionalized Covalent Organic Frameworks for Ultrasensitive Monitoring of Sialic Acid, an Ovarian Cancer Biomarker. ACS Appl. Mater. Inter. 2020, 12, 12990–12997. [Google Scholar] [CrossRef]
- Bhanja, P.; Mishra, S.; Manna, K.; Mallick, A.; das Saha, K.; Bhaumik, A. Covalent Organic Framework Material Bearing Phloroglucinol Building Units as a Potent Anticancer Agent. ACS Appl. Mater. Inter. 2017, 9, 31411–31423. [Google Scholar] [CrossRef]
- Bi, S.; Yang, C.; Zhang, W.; Xu, J.; Liu, L.; Wu, D.; Wang, X.; Han, Y.; Liang, Q.; Zhang, F. Two-Dimensional Semiconducting Covalent Organic Frameworks via Condensation at Arylmethyl Carbon Atoms. Nat. Commun. 2019, 10, 2467. [Google Scholar] [CrossRef] [PubMed]
- Kong, W.; Wan, J.; Namuangruk, S.; Guo, J.; Wang, C. Water-Soluble Metalated Covalent Organic Nanobelts with Improved Bioavailability for Protein Transportation. Sci. Rep. 2018, 8, 5529. [Google Scholar] [CrossRef] [PubMed]
- Xu, Z.; Hu, L.; Ming, J.; Cui, X.; Zhang, M.; Dou, J.; Zhang, W.; Zhou, B. Self-Gated Porous Organic Polymer as Drug Delivery System for PH Stimuli-Responsive Controlled Quercetin Release. Micropor. Mesopor. Mater. 2020, 303, 110259. [Google Scholar] [CrossRef]
- Valenzuela, C.; Chen, C.; Sun, M.; Ye, Z.; Zhang, J. Strategies and Applications of Covalent Organic Frameworks as Promising Nanoplatforms in Cancer Therapy. J. Mater. Chem. B 2021, 9, 3450–3483. [Google Scholar] [CrossRef]
- Luo, Y.; Liu, J.; Liu, Y.; Lyu, Y. Porphyrin-Based Covalent Triazine Frameworks: Porosity, Adsorption Performance, and Drug Delivery. J. Polym. Sci. A Polym. Chem. 2017, 55, 2594–2600. [Google Scholar] [CrossRef]
- Ghadari, R.; Ghanbari, S.; Mohammadzadeh, Y. A Computational Study on the Interactions between a Layered Imine-Based COF Structure and Selected Anticancer Drugs. J. Mol. Model. 2021, 27, 44. [Google Scholar] [CrossRef] [PubMed]
- Rengaraj, A.; Puthiaraj, P.; Haldorai, Y.; Heo, N.S.; Hwang, S.-K.; Han, Y.-K.; Kwon, S.; Ahn, W.-S.; Huh, Y.S. Porous Covalent Triazine Polymer as a Potential Nanocargo for Cancer Therapy and Imaging. ACS Appl. Mater. Inter. 2016, 8, 8947–8955. [Google Scholar] [CrossRef]
- Sun, T.; Xia, R.; Zhou, J.; Zheng, X.; Liu, S.; Xie, Z. Protein-Assisted Synthesis of Nanoscale Covalent Organic Frameworks for Phototherapy of Cancer. Mater. Chem. Front. 2020, 4, 2346–2356. [Google Scholar] [CrossRef]
- Tan, J.; Namuangruk, S.; Kong, W.; Kungwan, N.; Guo, J.; Wang, C. Manipulation of Amorphous-to-Crystalline Transformation: Towards the Construction of Covalent Organic Framework Hybrid Microspheres with NIR Photothermal Conversion Ability. Angew. Chem. Int. Edit. 2016, 55, 13979–13984. [Google Scholar] [CrossRef]
- Wang, P.; Zhou, F.; Guan, K.; Wang, Y.; Fu, X.; Yang, Y.; Yin, X.; Song, G.; Zhang, X.-B.; Tan, W. In Vivo Therapeutic Response Monitoring by a Self-Reporting Upconverting Covalent Organic Framework Nanoplatform. Chem. Sci. 2020, 11, 1299–1306. [Google Scholar] [CrossRef]
- Liu, S.; Hu, C.; Liu, Y.; Zhao, X.; Pang, M.; Lin, J. One-Pot Synthesis of DOX@Covalent Organic Framework with Enhanced Chemotherapeutic Efficacy. Chem. Eur. J. 2019, 25, 4315–4319. [Google Scholar] [CrossRef] [PubMed]
- Fang, Q.; Wang, J.; Gu, S.; Kaspar, R.B.; Zhuang, Z.; Zheng, J.; Guo, H.; Qiu, S.; Yan, Y. 3D Porous Crystalline Polyimide Covalent Organic Frameworks for Drug Delivery. J. Am. Chem. Soc. 2015, 137, 8352–8355. [Google Scholar] [CrossRef]
- Bai, L.; Phua, S.Z.F.; Lim, W.Q.; Jana, A.; Luo, Z.; Tham, H.P.; Zhao, L.; Gao, Q.; Zhao, Y. Nanoscale Covalent Organic Frameworks as Smart Carriers for Drug Delivery. Chem. Commun. 2016, 52, 4128–4131. [Google Scholar] [CrossRef] [PubMed]
- Modi, S.; MG, S.; Goswami, D.; Gupta, G.D.; Mayor, S.; Krishnan, Y. A DNA Nanomachine That Maps Spatial and Temporal PH Changes inside Living Cells. Nat. Nanotechnol. 2009, 4, 325–330. [Google Scholar] [CrossRef]
- Liu, S.; Yang, J.; Guo, R.; Deng, L.; Dong, A.; Zhang, J. Facile Fabrication of Redox-Responsive Covalent Organic Framework Nanocarriers for Efficiently Loading and Delivering Doxorubicin. Macromol. Rapid Commun. 2020, 41, 1900570. [Google Scholar] [CrossRef] [PubMed]
- Wang, C.; Liu, H.; Liu, S.; Wang, Z.; Zhang, J. PH and Redox Dual-Sensitive Covalent Organic Framework Nanocarriers to Resolve the Dilemma Between Extracellular Drug Loading and Intracellular Drug Release. Front. Chem. 2020, 8, 488. [Google Scholar] [CrossRef] [PubMed]
- Zhang, G.; Li, X.; Liao, Q.; Liu, Y.; Xi, K.; Huang, W.; Jia, X. Water-Dispersible PEG-Curcumin/Amine-Functionalized Covalent Organic Framework Nanocomposites as Smart Carriers for in Vivo Drug Delivery. Nat. Commun. 2018, 9, 2785. [Google Scholar] [CrossRef]
- Gao, P.; Wei, R.; Liu, X.; Chen, Y.; Wu, T.; Shi, M.; Wang, M.; Li, N.; Tang, B. Covalent Organic Framework-Engineered Polydopamine Nanoplatform for Multimodal Imaging-Guided Tumor Photothermal-Chemotherapy. Chem. Commun. 2021, 57, 5646–5649. [Google Scholar] [CrossRef]
- Wang, H.; Zhu, W.; Liu, J.; Dong, Z.; Liu, Z. PH-Responsive Nanoscale Covalent Organic Polymers as a Biodegradable Drug Carrier for Combined Photodynamic Chemotherapy of Cancer. ACS Appl. Mater. Inter. 2018, 10, 14475–14482. [Google Scholar] [CrossRef] [PubMed]
- Yang, Q.; Luo, M.; Liu, K.; Cao, H.; Yan, H. Covalent Organic Frameworks for Photocatalytic Applications. Appl. Catal. B 2020, 276, 119174. [Google Scholar] [CrossRef]
- Liu, M.; Guo, L.; Jin, S.; Tan, B. Covalent Triazine Frameworks: Synthesis and Applications. J. Mater. Chem. A Mater. 2019, 7, 5153–5172. [Google Scholar] [CrossRef]
- Zhao, H.; Jin, Z.; Su, H.; Jing, X.; Sun, F.; Zhu, G. Targeted Synthesis of a 2D Ordered Porous Organic Framework for Drug Release. Chem. Commun. 2011, 47, 6389. [Google Scholar] [CrossRef] [PubMed]
- Cerella, C.; Grandjenette, C.; Dicato, M.; Diederich, M. Roles of Apoptosis and Cellular Senescence in Cancer and Aging. Curr. Drug Targets 2016, 17, 405–415. [Google Scholar] [CrossRef]
- Ge, L.; Qiao, C.; Tang, Y.; Zhang, X.; Jiang, X. Light-Activated Hypoxia-Sensitive Covalent Organic Framework for Tandem-Responsive Drug Delivery. Nano Lett. 2021, 21, 3218–3224. [Google Scholar] [CrossRef]


| Nanomedicines | Nanoformulations | Drug | Oncology |
|---|---|---|---|
| Oncaspar® commercialized by Enzon in 1994 | PEGylated enzyme | L-asparaginase enzyme | Acute lymphocytic leukemia |
| Doxil® commercialized by Johnson and Johnson in 1995 | Liposome | Doxorubicin | Kaposi’s sarcoma, ovarian cancer, breast cancer, multiple myeloma |
| DaunoXom® commercialized by Galen in 1996 | Liposome | Daunorubicin | Kaposi’s sarcoma |
| Lipodox® commercialized by Taiwan Liposome in 1998 | Liposome | Doxorubicin | Kaposi’s sarcoma, breast cancer, ovarian cancer |
| Ontak® commercialized by Eisai in 1999 | Protein-based formulation | Denileukin diftitox | Cutaneous T-cell lymphoma |
| DepoCyt® commercialized by Pacira in 1999 | Liposome | Cytarabine | Neoplastic meningitis |
| Myocet® commercialized by Cephalon in 2000 | Liposome | Doxorubicin | Breast cancer |
| Eligard® commercialized by Tolmar in 2002 | Polymeric nanosuspension | Leuprolide acetate | Prostate cancer |
| Abraxane® commercialized by Abraxis in 2005 | Albumin based NPs | Paclitaxel | Breast cancer, pancreatic cancer, lung cancer |
| Oncaspar® commercialized by Enzon-Sigma-Tau in 2006 | Polymer protein conjugate | L-asparaginase | Leukemia |
| Genexol-PM® commercialized by Samyang in 2007 | PEG-PLA polymeric micelle | Paclitaxel | Breast cancer, lung cancer, ovarian cancer |
| Mepact® commercialized by Takeda in 2009 | Liposome | Mifamurtide | Osteosarcoma |
| NanoTherm® commercialized by Magforce in 2010 | Metallic NPs | Iron oxide | Glioblastoma |
| Marqibo® commercialized by Talon in 2012 | Liposome | Vincristine | Acute lymphoid leukemia |
| Onivyde® commercialized by Merrimack Pharma in 2015 | Liposome | Irinotecan | Pancreatic cancer |
| Vyxeos® commercialized by Celator in 2017 | Liposome | Cytarabine | Acute myeloid leukemia |
| Hensify® commercialized by Nanobiotix in 2019 | Metallic NPs | Hafnium oxide | Locally-advanced soft tissue sarcoma |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Liu, W.; Ma, X.; Kheyr, S.M.; Dong, A.; Zhang, J. Covalent Organic Frameworks as Nanocarriers for Improved Delivery of Chemotherapeutic Agents. Materials 2022, 15, 7215. https://doi.org/10.3390/ma15207215
Liu W, Ma X, Kheyr SM, Dong A, Zhang J. Covalent Organic Frameworks as Nanocarriers for Improved Delivery of Chemotherapeutic Agents. Materials. 2022; 15(20):7215. https://doi.org/10.3390/ma15207215
Chicago/Turabian StyleLiu, Weiming, Xinyu Ma, Shuayb Mohamed Kheyr, Anjie Dong, and Jianhua Zhang. 2022. "Covalent Organic Frameworks as Nanocarriers for Improved Delivery of Chemotherapeutic Agents" Materials 15, no. 20: 7215. https://doi.org/10.3390/ma15207215
APA StyleLiu, W., Ma, X., Kheyr, S. M., Dong, A., & Zhang, J. (2022). Covalent Organic Frameworks as Nanocarriers for Improved Delivery of Chemotherapeutic Agents. Materials, 15(20), 7215. https://doi.org/10.3390/ma15207215







