Electrochemical SEIRAS Analysis of Imidazole-Ring-Functionalized Self-Assembled Monolayers
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Electroless Au Plating
2.3. Attenuated Total Reflection (ATR)-SEIRAS Setup and Au-Film Activation
2.4. Self-Assembled Monolayer (SAM) Formation
2.5. Attenuated Total Reflection Spectroscopy (ATR-FTIR) Measurements
2.6. Scanning Electron Microscopy (SEM)
2.7. Density Functional Theory (DFT) Modeling
3. Results and Discussion
3.1. Assignment of Spectral Bands of IMHA and Molecular Fragment
3.2. SEIRAS Analysis of the Formation of Self-Assembled Monolayer
3.3. SEIRAS Analysis of the Structure of IMHA SAMs
3.4. Hydrogen-Bonding Interactions Revealed by Potential-Dependent SEIRAS
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Iyer, A.H.; Deepak, R.N.V.K.; Sankararamakrishnan, R. Imidazole Nitrogens of Two Histidine Residues Participating in N-H···N Hydrogen Bonds in Protein Structures: Structural Bioinformatics Approach Combined with Quantum Chemical Calculations. J. Phys. Chem. B 2018, 122, 1205–1212. [Google Scholar] [CrossRef] [PubMed]
- Liao, S.-M.; Du, Q.-S.; Meng, J.-Z.; Pang, Z.-W.; Huang, R.-B. The multiple roles of histidine in protein interactions. Chem. Cent. J. 2013, 7, 44. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ashikawa, I.; Itoh, K. Raman spectra of polypeptides containing L-histidine residues and tautomerism of imidazole side chain. Biopolymers 1979, 18, 1859–1876. [Google Scholar] [CrossRef]
- Matulaitienė, I.; Kuodis, Z.; Eicher-Lorka, O.; Niaura, G. SERS characterization of imidazole ring terminated self-assembled monolayer formed from lipoic acid histamide on silver electrode. J. Electroanal. Chem. 2013, 700, 77–85. [Google Scholar] [CrossRef]
- Ashikawa, I.; Itoh, K. Raman Scattering Study on Tautomerism of L-Histidine. Chem. Lett. 1978, 7, 681–684. [Google Scholar] [CrossRef]
- Matulaitienė, I.; Pociūtė, E.; Kuodis, Z.; Eicher-Lorka, O.; Niaura, G. Interaction of 4-imidazolemethanol with a copper electrode revealed by isotope-edited SERS and theoretical modeling. Phys. Chem. Chem. Phys. 2015, 17, 16483–16493. [Google Scholar] [CrossRef]
- Garfinkel, D.; Edsall, J.T. Raman Spectra of Amino Acids and Related Compounds. VIII. Raman and Infrared Spectra of Imidazole, 4-Methylimidazole and Histidine. J. Am. Chem. Soc. 1958, 80, 3807–3812. [Google Scholar] [CrossRef]
- Mesu, J.G.; Visser, T.; Soulimani, F.; Weckhuysen, B.M. Infrared and Raman spectroscopic study of pH-induced structural changes of L-histidine in aqueous environment. Vib. Spectrosc. 2005, 39, 114–125. [Google Scholar] [CrossRef]
- Martusevičius, S.; Niaura, G.; Talaikyté, Z.; Razumas, V. Adsorption of L-histidine on copper surface as evidenced by surface-enhanced Raman scattering spectroscopy. Vib. Spectrosc. 1996, 10, 271–280. [Google Scholar] [CrossRef]
- Carter, D.A.; Pemberton, J.E. Raman spectroscopy and vibrational assignments of 1- and 2-methylimidazole. J. Raman Spectrosc. 1997, 28, 939–946. [Google Scholar] [CrossRef]
- Takeuchi, H. Raman structural markers of tryptophan and histidine side chains in proteins. Biopolym.-Biospectroscopy Sect. 2003, 72, 305–317. [Google Scholar] [CrossRef] [PubMed]
- Miura, T.; Satoh, T.; Hori-i, A.; Takeuchi, H. Raman marker bands of metal coordination sites of histidine side chains in peptides and proteins. J. Raman Spectrosc. 1998, 29, 41–47. [Google Scholar] [CrossRef]
- Suzuki, Y.; Osawa, M.; Hatta, A.; Suëtaka, W. Mechanism of absorption enhancement in infrared ATR spectra observed in the Kretschmann configuration. Appl. Surf. Sci. 1988, 33–34, 875–881. [Google Scholar] [CrossRef]
- Ataka, K.; Heberle, J. Biochemical applications of surface-enhanced infrared absorption spectroscopy. Anal. Bioanal. Chem. 2007, 388, 47–54. [Google Scholar] [CrossRef] [Green Version]
- Sigrist, J.A.; Lins, E.S.; Morhart, T.A.; Briggs, J.L.; Burgess, I.J. Optimization of a Commercial Variable Angle Accessory for Entry Level Users of Electrochemical Attenuated Total Reflection Surface-Enhanced Infrared Absorption Spectroscopy (ATR-SEIRAS). Appl. Spectrosc. 2019, 73, 1394–1402. [Google Scholar] [CrossRef]
- Morhart, T.A.; Unni, B.; Lardner, M.J.; Burgess, I.J. Electrochemical ATR-SEIRAS Using Low-Cost, Micromachined Si Wafers. Anal. Chem. 2017, 89, 11818–11824. [Google Scholar] [CrossRef] [PubMed]
- Delgado, J.M.; Orts, J.M.; Pérez, J.M.; Rodes, A. Sputtered thin-film gold electrodes for in situ ATR-SEIRAS and SERS studies. J. Electroanal. Chem. 2008, 617, 130–140. [Google Scholar] [CrossRef]
- Andvaag, I.R.; Morhart, T.A.; Clarke, O.J.R.; Burgess, I.J. Hybrid Gold-Conductive Metal Oxide Films for Attenuated Total Reflectance Surface Enhanced Infrared Absorption Spectroscopy. ACS Appl. Nano Mater. 2019, 2, 1274–1284. [Google Scholar] [CrossRef]
- Miyake, H.; Ye, S.; Osawa, M. Electroless deposition of gold thin films on silicon for surface-enhanced infrared spectroelectrochemistry. Electrochem. Commun. 2002, 4, 973–977. [Google Scholar] [CrossRef]
- Tantardini, C. When Does a Hydrogen Bond Become a van der Waals Interaction? A Topological Answe. J. Comput. Chem. 2019, 40, 937–943. [Google Scholar] [CrossRef]
- Zdaniauskienė, A.; Talaikis, M.; Charkova, T.; Sadzevičienė, R.; Labanauskas, L.; Niaura, G. Electrochemical Shell-Isolated Nanoparticle-Enhanced Raman Spectroscopy of Imidazole Ring Functionalized Monolayer on Smooth Gold Electrode. Molecules 2022, 27, 6531. [Google Scholar] [CrossRef] [PubMed]
- Frisch, D.J.; Trucks, M.J.; Schlegel, G.W.; Scuseria, H.B.; Robb, G.E.; Cheeseman, M.A.; Scalmani, J.R.; Barone, G.; Mennucci, V.; Petersson, B.; et al. Gaussian 09, Revision D.01; Gaussian Inc.: Wallingford, CT, USA, 2013. [Google Scholar]
- Talaikis, M.; Eicher-Lorka, O.; Valincius, G.; Niaura, G. Water-induced structural changes in the membrane-anchoring monolayers revealed by isotope-edited SERS. J. Phys. Chem. C 2016, 120, 22489–22499. [Google Scholar] [CrossRef]
- Kim, M.; Hohman, J.N.; Serino, A.C.; Weiss, P.S. Structural manipulation of hydrogen-bonding networks in amide-containing alkanethiolate monolayers via electrochemical processing. J. Phys. Chem. C 2010, 114, 19744–19751. [Google Scholar] [CrossRef]
- Kuodis, Z.; Matulaitienė, I.; Špandyreva, M.; Labanauskas, L.; Stončius, S.; Eicher-Lorka, O.; Sadzevičienė, R.; Niaura, G. Reflection Absorption Infrared Spectroscopy Characterization of SAM Formation from 8-Mercapto-N-(phenethyl)octanamide Thiols with Phe Ring and Amide Groups. Molecules 2020, 25, 5633. [Google Scholar] [CrossRef] [PubMed]
- Saini, G.; Kaur, S.; Tripathi, S.; Dogra, S.D.; Abbas, J.M.; Mahajan, C. Vibrational spectroscopic and density functional theory studies of chloranil–imidazole interaction. Vib. Spectrosc. 2011, 56, 66–73. [Google Scholar] [CrossRef]
- Richmond, W.N.; Faguy, P.; Weibel, S.C. An in situ infrared spectroscopic study of imidazole films on copper electrodes. J. Electroanal. Chem. 1998, 448, 237–244. [Google Scholar] [CrossRef]
- Kumar, S.; Rai, A.K.; Rai, S.B.; Rai, D.K. Infrared and Raman spectra of Histidine: An ab initio DFT calculations of Histidine molecule and its different protonated forms. Indian J. Phys. 2010, 84, 563–573. [Google Scholar] [CrossRef]
- Hasegawa, K.; Ono, T.-A.; Noguchi, T. Vibrational Spectra and Ab Initio DFT Calculations of 4-Methylimidazole and Its Different Protonation Forms: Infrared and Raman Markers of the Protonation State of a Histidine Side Chain. J. Phys. Chem. B 2000, 104, 4253–4265. [Google Scholar] [CrossRef]
- Barth, A. Infrared Spectroscopy of Proteins. Biochim. Biophys. Acta-Bioenerg. 2007, 1767, 1073–1101. [Google Scholar] [CrossRef] [Green Version]
- Niaura, G. Raman Spectroscopy in Analysis of Biomolecules. Encycl. Anal. Chem. 2014, 1–34. [Google Scholar] [CrossRef]
- And, N.S.M.; Asher, S.A. Peptide bond vibrational coupling. J. Phys. Chem. B 2007, 111, 4271–4279. [Google Scholar] [CrossRef]
- Gotoh, R.; Takenada, T. Inductive Effect of Polar Substituents on Carbon-Hydrogen Stretching Vibrations of Aliphatic Hydrocarbons. Bull. Inst. Chem. Res. 1961, 39, 202–214. [Google Scholar] [CrossRef] [Green Version]
- Lee, S.-H.; Mirkin, N.G.; Krimm, S. A quantitative anharmonic analysis of the amide A band in alpha-helical poly(L-alanine). Biopolymers 1998, 49, 195–207. [Google Scholar] [CrossRef]
- Lee, S.-H.; Krimm, S. Ab initio-based vibrational analysis of α-poly(l-alanine). Biopolymers 1998, 46, 283–317. [Google Scholar] [CrossRef]
- Osawa, M.; Ataka, K.-I.; Yoshii, K.; Nishikawa, Y. Surface-Enhanced Infrared Spectroscopy: The Origin of the Absorption Enhancement and Band Selection Rule in the Infrared Spectra of Molecules Adsorbed on Fine Metal Particles. Appl. Spectrosc. 1993, 47, 1497–1502. [Google Scholar] [CrossRef]
- Valiokas, R.; Svedhem, S.; Svensson, A.S.C.T.; Liedberg, B. Self-assembled monolayers of oligo(ethylene glycol)-terminated and amide group containing alkanethiolates on gold. Langmuir 1999, 15, 3390–3394. [Google Scholar] [CrossRef]
- Lee, H.-H.; Gavutis, M.; Ruželė, Ž.; Valiokas, R.; Liedberg, B. Mixed Self-Assembled Monolayers with Terminal Deuterated Anchors: Characterization and Probing of Model Lipid Membrane Formation. J. Phys. Chem. B 2018, 122, 8201–8210. [Google Scholar] [CrossRef]
- Javorskis, T.; Rakickas, T.; Jankūnaitė, A.; Talaikis, M.; Niaura, G.; Ulčinas, A.; Orentas, E. Meso-scale surface patterning of self-assembled monolayers with water. Colloids Surf. A Physicochem. Eng. Asp. 2021, 628, 127353. [Google Scholar] [CrossRef]
- Clegg, R.S.; Hutchison, J.E. Hydrogen-bonding, self-assembled monolayers: Ordered molecular films for study of through-peptide electron transfer. Langmuir 1996, 12, 5239–5243. [Google Scholar] [CrossRef]
- Myshakina, N.S.; Ahmed, Z.; Asher, S.A. Dependence of amide vibrations on hydrogen bonding. J. Phys. Chem. B 2008, 112, 11873–11877. [Google Scholar] [CrossRef]
- Kocherbitov, V.; Latynis, J.; Misiūnas, A.; Barauskas, J.; Niaura, G. Hydration of lysozyme studied by Raman spectroscopy. J. Phys. Chem. B 2013, 117, 4981–4992. [Google Scholar] [CrossRef]
- Nelson, P.N. Chain Length and Thermal Sensitivity of the Infrared Spectra of a Homologous Series of Anhydrous Silver(I) n -Alkanoates. Int. J. Spectrosc. 2016, 2016, 1–9. [Google Scholar] [CrossRef] [Green Version]
- Allara, D.L.; Nuzzo, R.G. Spontaneously organized molecular assemblies. 2. Quantitative infrared spectroscopic determination of equilibrium structures of solution-adsorbed n-alkanoic acids on an oxidized aluminum surface. Langmuir 1985, 1, 52–66. [Google Scholar] [CrossRef]
- Budvytyte, R.; Valincius, G.; Niaura, G.; Voiciuk, V.; Mickevicius, M.; Chapman, H.; Goh, H.-Z.; Shekhar, P.; Heinrich, F.; Shenoy, S.; et al. Structure and Properties of Tethered Bilayer Lipid Membranes with Unsaturated Anchor Molecules. Langmuir 2013, 29, 8645. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Clegg, R.S.; Hutchison, J.E. Control of monolayer assembly structure by hydrogen bonding rather than by adsorbate-substrate templating. J. Am. Chem. Soc. 1999, 121, 5319–5327. [Google Scholar] [CrossRef]
- Toyama, A.; Ono, K.; Hashimoto, S.; Takeuchi, H. Raman spectra and normal coordinate analysis of the N1-H and N3-H tautomers of 4-methylimidazole: Vibrational modes of histidine tautomer markers. J. Phys. Chem. A 2002, 106, 3403–3412. [Google Scholar] [CrossRef]
- Zischang, J.; Lee, J.J.; Suhm, M.A. Communication: Where does the first water molecule go in imidazole? J. Chem. Phys. 2011, 135, 061102. [Google Scholar] [CrossRef] [Green Version]
- Varghese, S.; Kannam, S.K.; Hansen, J.; Sathian, S.P. Effect of Hydrogen Bonds on the Dielectric Properties of Interfacial Water. Langmuir 2019, 35, 8159–8166. [Google Scholar] [CrossRef] [Green Version]
- Montenegro, A.; Dutta, C.; Mammetkuliev, M.; Shi, H.; Hou, B.; Bhattacharyya, D.; Zhao, B.; Cronin, S.B.; Benderskii, A.V. Asymmetric response of interfacial water to applied electric fields. Nature 2021, 594, 62–65. [Google Scholar] [CrossRef]








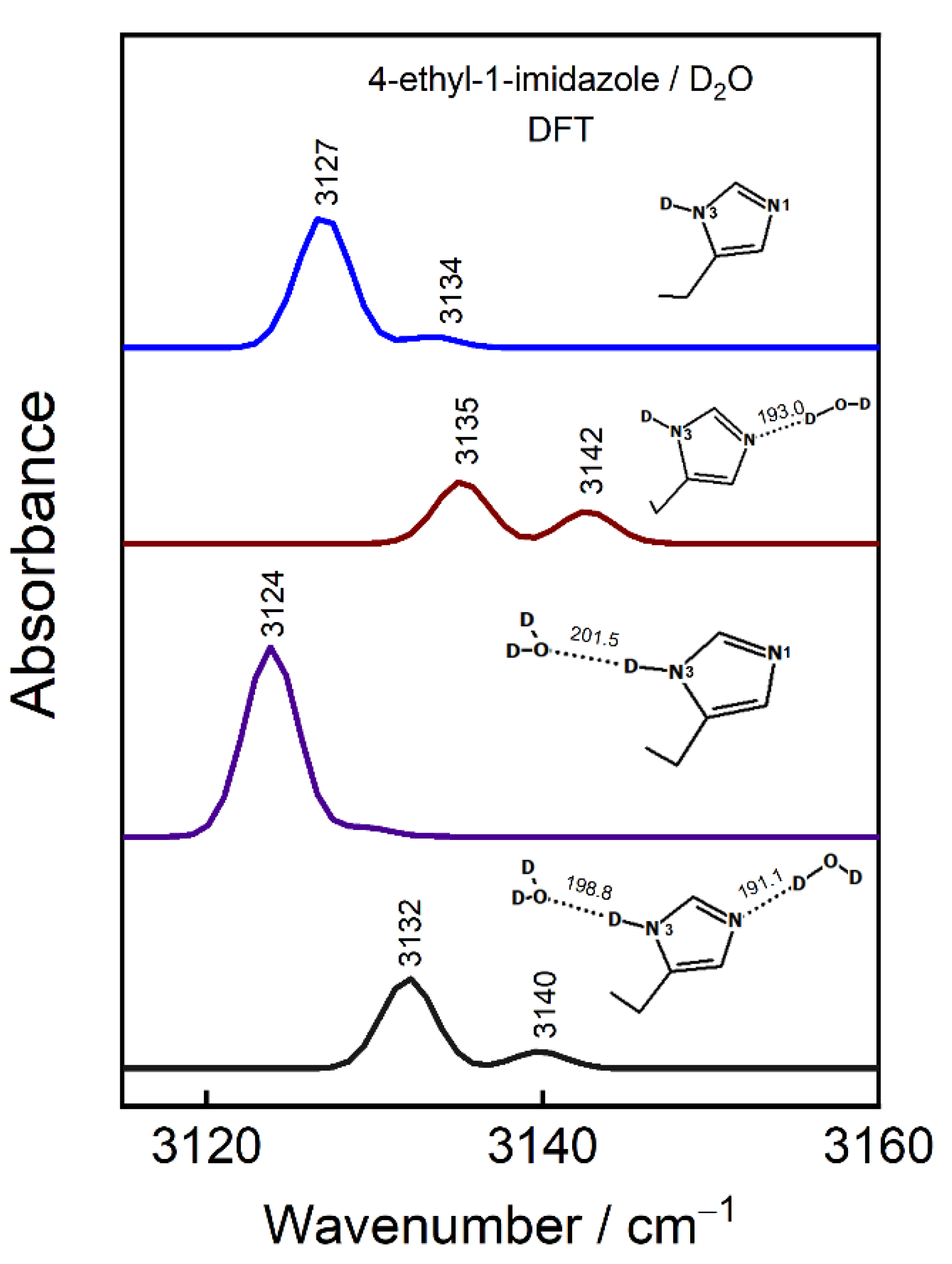
| ATR-FTIR | SEIRAS | DFT | Assignement | ||
|---|---|---|---|---|---|
| IMHA | IMHA-d | IMHA | IMHA-d | IMHA-d (T-I) | |
| Im-related modes | |||||
| 3138 vw | 3152 m | 3158 vw | ν(C5–H) | ||
| 3117 vw | 3115 m | 3139 vw | ν(C2–H) | ||
| 1560 vw | 1564 vw | ν(C4=C5) | |||
| 1494 w | 1480 sh | 1493 vw | 1484 w | ν(C2–N3) + β(C2H) + ν(C2–N1) + ν(C5–N1) | |
| Amide-bond-related modes | |||||
| 1635 s | 1622 s | 1645 m | 1634 s | Am-I or Am-I’, ν(C=O) | |
| 1573 s | 1467 m | 1558 s | 1434 m | Am-II, ν(C–N) + δ(NH) orAm-II’, ν(C–N) | |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Pudžaitis, V.; Talaikis, M.; Sadzevičienė, R.; Labanauskas, L.; Niaura, G. Electrochemical SEIRAS Analysis of Imidazole-Ring-Functionalized Self-Assembled Monolayers. Materials 2022, 15, 7221. https://doi.org/10.3390/ma15207221
Pudžaitis V, Talaikis M, Sadzevičienė R, Labanauskas L, Niaura G. Electrochemical SEIRAS Analysis of Imidazole-Ring-Functionalized Self-Assembled Monolayers. Materials. 2022; 15(20):7221. https://doi.org/10.3390/ma15207221
Chicago/Turabian StylePudžaitis, Vaidas, Martynas Talaikis, Rita Sadzevičienė, Linas Labanauskas, and Gediminas Niaura. 2022. "Electrochemical SEIRAS Analysis of Imidazole-Ring-Functionalized Self-Assembled Monolayers" Materials 15, no. 20: 7221. https://doi.org/10.3390/ma15207221
APA StylePudžaitis, V., Talaikis, M., Sadzevičienė, R., Labanauskas, L., & Niaura, G. (2022). Electrochemical SEIRAS Analysis of Imidazole-Ring-Functionalized Self-Assembled Monolayers. Materials, 15(20), 7221. https://doi.org/10.3390/ma15207221








