Photocatalytic Activity of Sulfanyl Porphyrazine/Titanium Dioxide Nanocomposites in Degradation of Organic Pollutants
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Preparation of Photocatalyst
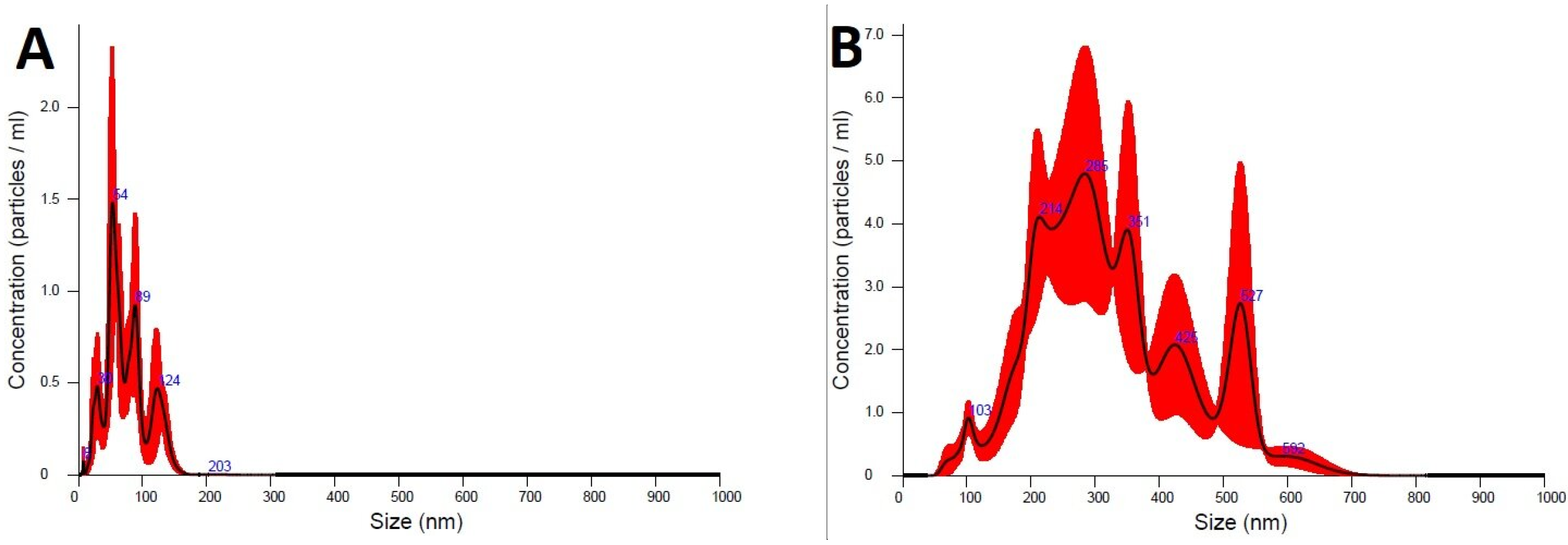
2.3. Size and Distribution Measurements
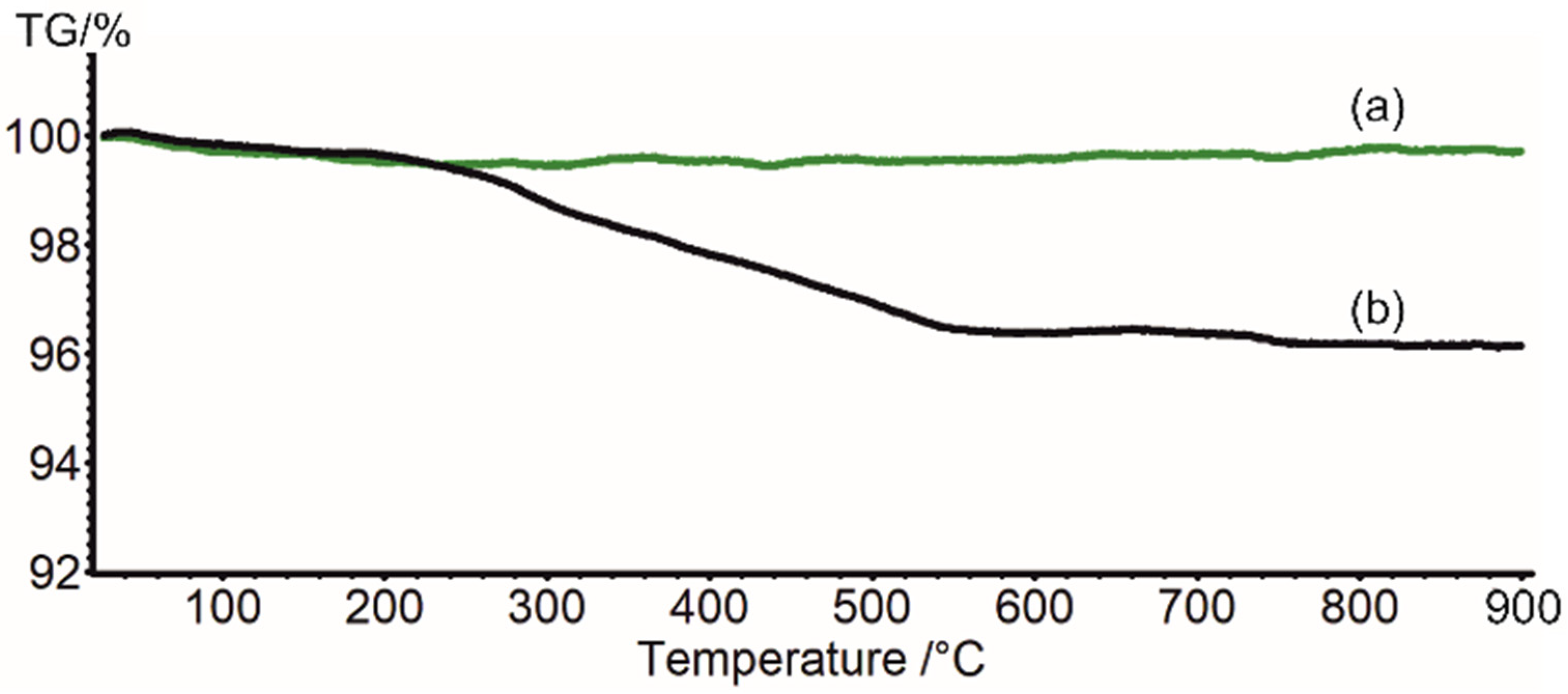
2.4. Thermogravimetry (TGA)
2.5. X-ray Powder Diffraction
2.6. SEM
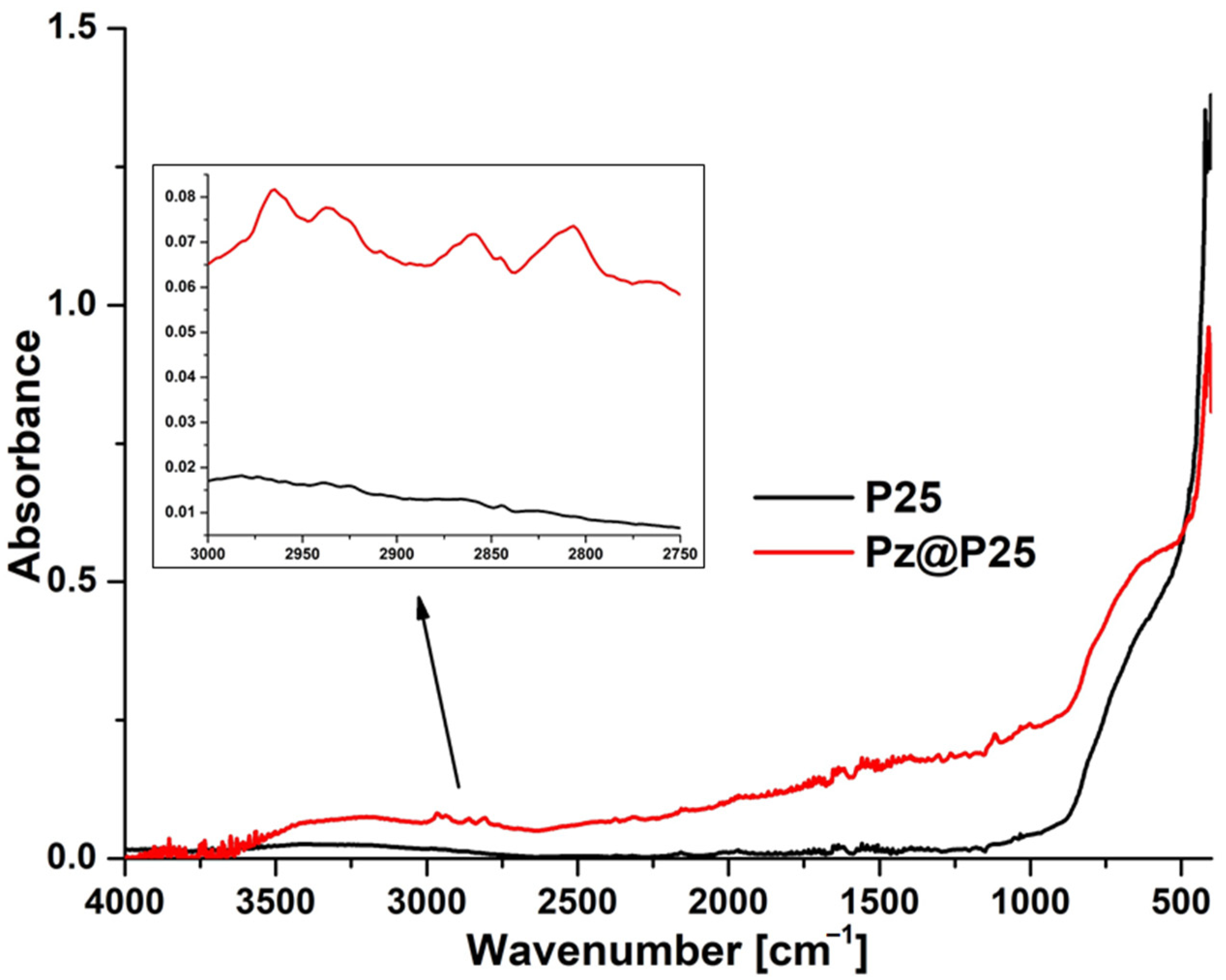
2.7. FTIR–ATR Measurements
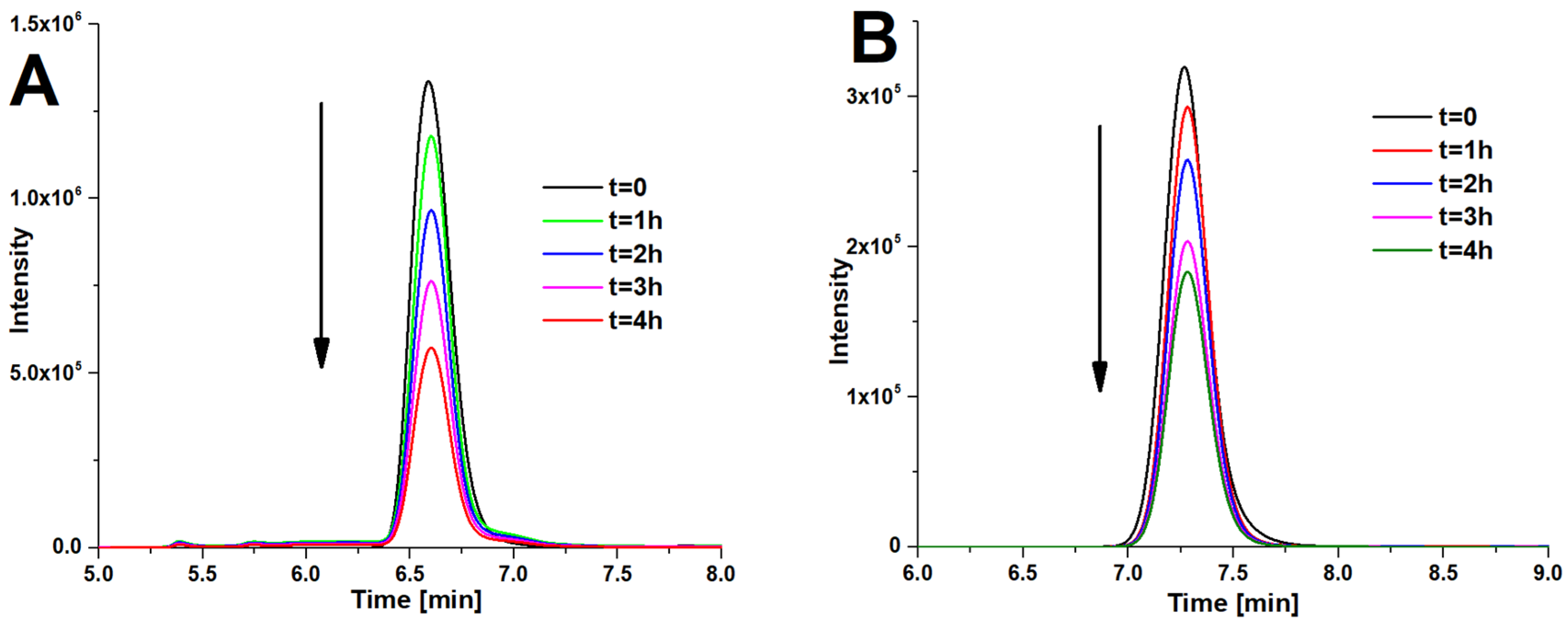
2.8. Photocatalytic Studies
3. Results and Discussion
3.1. Preparation of Photocatalyst
3.2. Physicochemical Characterization of Photocatalyst
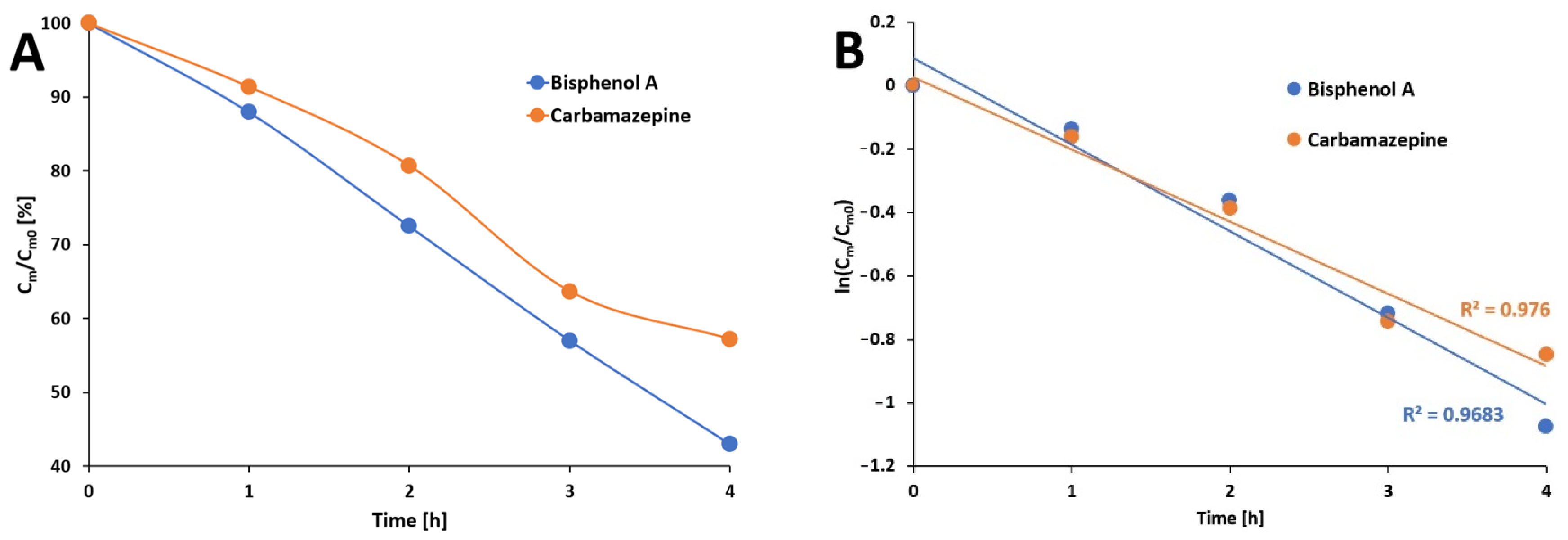
3.3. Photocatalytic Studies
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Gavrilescu, M.; Demnerová, K.; Aamand, J.; Agathos, S.; Fava, F. Emerging Pollutants in the Environment: Present and Future Challenges in Biomonitoring, Ecological Risks and Bioremediation. New Biotechnol. 2015, 32, 147–156. [Google Scholar] [CrossRef] [PubMed]
- Kidd, K.A.; Blanchfield, P.J.; Mills, K.H.; Palace, V.P.; Evans, R.E.; Lazorchak, J.M.; Flick, R.W. Collapse of a Fish Population after Exposure to a Synthetic Estrogen. Proc. Natl. Acad. Sci. USA 2007, 104, 8897–8901. [Google Scholar] [CrossRef]
- Petrie, B.; Camacho-Muñoz, D. Analysis, Fate and Toxicity of Chiral Non-Steroidal Anti-Inflammatory Drugs in Wastewaters and the Environment: A Review; Springer International Publishing: Berlin/Heidelberg, Germany, 2021; Volume 19, ISBN 0123456789. [Google Scholar]
- Wojcieszyńska, D.; Guzik, U. Naproxen in the Environment: Its Occurrence, Toxicity to Nontarget Organisms and Biodegradation. Appl. Microbiol. Biotechnol. 2020, 104, 1849–1857. [Google Scholar] [CrossRef] [PubMed]
- López-Pacheco, I.Y.; Silva-Núñez, A.; Salinas-Salazar, C.; Arévalo-Gallegos, A.; Lizarazo-Holguin, L.A.; Barceló, D.; Iqbal, H.M.N.; Parra-Saldívar, R. Anthropogenic Contaminants of High Concern: Existence in Water Resources and Their Adverse Effects. Sci. Total Environ. 2019, 690, 1068–1088. [Google Scholar] [CrossRef]
- Behera, S.K.; Kim, H.W.; Oh, J.E.; Park, H.S. Occurrence and Removal of Antibiotics, Hormones and Several Other Pharmaceuticals in Wastewater Treatment Plants of the Largest Industrial City of Korea. Sci. Total Environ. 2011, 409, 4351–4360. [Google Scholar] [CrossRef] [PubMed]
- Sui, Q.; Huang, J.; Deng, S.; Yu, G.; Fan, Q. Occurrence and Removal of Pharmaceuticals, Caffeine and DEET in Wastewater Treatment Plants of Beijing, China. Water Res. 2010, 44, 417–426. [Google Scholar] [CrossRef]
- Papageorgiou, M.; Kosma, C.; Lambropoulou, D. Seasonal Occurrence, Removal, Mass Loading and Environmental Risk Assessment of 55 Pharmaceuticals and Personal Care Products in a Municipal Wastewater Treatment Plant in Central Greece. Sci. Total Environ. 2016, 543, 547–569. [Google Scholar] [CrossRef]
- Kim, S.D.; Cho, J.; Kim, I.S.; Vanderford, B.J.; Snyder, S.A. Occurrence and Removal of Pharmaceuticals and Endocrine Disruptors in South Korean Surface, Drinking, and Waste Waters. Water Res. 2007, 41, 1013–1021. [Google Scholar] [CrossRef]
- Ziylan, A.; Ince, N.H. The Occurrence and Fate of Anti-Inflammatory and Analgesic Pharmaceuticals in Sewage and Fresh Water: Treatability by Conventional and Non-Conventional Processes. J. Hazard. Mater. 2011, 187, 24–36. [Google Scholar] [CrossRef]
- Rogowska, J.; Zimmermann, A.; Muszyńska, A.; Ratajczyk, W.; Wolska, L. Pharmaceutical Household Waste Practices: Preliminary Findings from a Case Study in Poland. Environ. Manag. 2019, 64, 97–106. [Google Scholar] [CrossRef]
- Dudziak, M. The Impact of Complex Oxidizing Process on Toxicity of Water Containg Bisphenol A. Proc. ECOpole 2015, 9, 15–17. [Google Scholar] [CrossRef]
- Liu, C.; Mao, S.; Wang, H.; Wu, Y.; Wang, F.; Xia, M.; Chen, Q. Peroxymonosulfate-Assisted for Facilitating Photocatalytic Degradation Performance of 2D/2D WO3/BiOBr S-Scheme Heterojunction. Chem. Eng. J. 2022, 430, 132806. [Google Scholar] [CrossRef]
- Liu, C.; Mao, S.; Shi, M.; Hong, X.; Wang, D.; Wang, F.; Xia, M.; Chen, Q. Enhanced Photocatalytic Degradation Performance of BiVO4/BiOBr through Combining Fermi Level Alteration and Oxygen Defect Engineering. Chem. Eng. J. 2022, 449, 137757. [Google Scholar] [CrossRef]
- Liu, C.; Mao, S.; Shi, M.; Wang, F.; Xia, M.; Chen, Q.; Ju, X. Peroxymonosulfate Activation through 2D/2D Z-Scheme CoAl-LDH/BiOBr Photocatalyst under Visible Light for Ciprofloxacin Degradation. J. Hazard. Mater. 2021, 420, 126613. [Google Scholar] [CrossRef]
- Krakowiak, R.; Musial, J.; Bakun, P.; Spychała, M.; Czarczynska-Goslinska, B.; Mlynarczyk, D.T.; Koczorowski, T.; Sobotta, L.; Stanisz, B.; Goslinski, T. Titanium Dioxide-Based Photocatalysts for Degradation of Emerging Contaminants Including Pharmaceutical Pollutants. Appl. Sci. 2021, 11, 8674. [Google Scholar] [CrossRef]
- Krakowiak, R.; Musial, J.; Frankowski, R.; Spychala, M.; Mielcarek, J.; Dobosz, B.; Krzyminiewski, R.; Sikorski, M.; Bendzinska-Berus, W.; Tykarska, E.; et al. Phthalocyanine-Grafted Titania Nanoparticles for Photodegradation of Ibuprofen. Catalysts 2020, 10, 1328. [Google Scholar] [CrossRef]
- Musial, J.; Krakowiak, R.; Frankowski, R.; Spychala, M.; Dlugaszewska, J.; Dobosz, B.; Bendzinska-Berus, W.; Krzyminiewski, R.; Tykarska, E.; Zgoła-Grześkowiak, A.; et al. Simple Modification of Titanium(IV) Oxide for the Preparation of a Reusable Photocatalyst. Mater. Sci. Eng. B Solid-State Mater. Adv. Technol. 2022, 276, 115559. [Google Scholar] [CrossRef]
- Chen, D.; Cheng, Y.; Zhou, N.; Chen, P.; Wang, Y.; Li, K.; Huo, S.; Cheng, P.; Peng, P.; Zhang, R.; et al. Photocatalytic Degradation of Organic Pollutants Using TiO2-Based Photocatalysts: A Review. J. Clean. Prod. 2020, 268, 121725. [Google Scholar] [CrossRef]
- Eslami, A.; Amini, M.M.; Asadi, A.; Safari, A.A.; Daglioglu, N. Photocatalytic Degradation of Ibuprofen and Naproxen in Water over NS-TiO2 Coating on Polycarbonate: Process Modeling and Intermediates Identification. Inorg. Chem. Commun. 2020, 115, 107888. [Google Scholar] [CrossRef]
- Gopinath, K.P.; Madhav, N.V.; Krishnan, A.; Malolan, R.; Rangarajan, G. Present Applications of Titanium Dioxide for the Photocatalytic Removal of Pollutants from Water: A Review. J. Environ. Manag. 2020, 270, 110906. [Google Scholar] [CrossRef]
- Spychała, M.; Błażejewski, R.; Mlynarczyk, D.T.; Musiał, J.; Krakowiak, R.; Stanisz, B.J.; Goslinski, T.; Nguyen, H.T. Effects of Ultraviolet Irradiation and Titanium(IV) Oxide on Greywater and Domestic Wastewater with a Low Propensity for Bio-Disintegration. Desalination Water Treat. 2021, 243, 143–155. [Google Scholar] [CrossRef]
- Linsebigler, A.L.; Lu, G.; Yates, J.T. Photocatalysis on TiO2 Surfaces: Principles, Mechanisms, and Selected Results. Chem. Rev. 1995, 95, 735–758. [Google Scholar] [CrossRef]
- Michel, S.L.J.; Hoffman, B.M.; Baum, S.M.; Barrett, A.G.M. Peripherally Functionalized Porphyrazines: Novel Metallomacrocycles with Broad, Untapped Potential; J. Wiley & Sons: New York, NY, USA, 2001; Volume 50, ISBN 0471435104. [Google Scholar]
- Yoshida, T.; Furuyama, T.; Kobayashi, N. Synthesis and Optical Properties of Tetraazaporphyrin Phosphorus(V) Complexes with Electron-Rich Heteroatoms. Tetrahedron Lett. 2015, 56, 1671–1674. [Google Scholar] [CrossRef]
- Fernández-Ariza, J.; Urbani, M.; Rodríguez-Morgade, M.S.; Torres, T. Panchromatic Photosensitizers Based on Push–Pull, Unsymmetrically Substituted Porphyrazines. Chem.-A Eur. J. 2018, 24, 2618–2625. [Google Scholar] [CrossRef] [PubMed]
- Koczorowski, T.; Rębiś, T.; Szczolko, W.; Antecka, P.; Teubert, A.; Milczarek, G.; Goslinski, T. Reduced Graphene Oxide/Iron(II) Porphyrazine Hybrids on Glassy Carbon Electrode for Amperometric Detection of NADH and L-Cysteine. J. Electroanal. Chem. 2019, 848, 113322. [Google Scholar] [CrossRef]
- Rębiś, T.; Falkowski, M.; Milczarek, G.; Goslinski, T. Electrocatalytic NADH Sensing Using Electrodes Modified with 2-[2-(4-Nitrophenoxy)Ethoxy]Ethylthio-Substituted Porphyrazine/Single-Walled Carbon Nanotube Hybrids. ChemElectroChem 2020, 7, 2838–2850. [Google Scholar] [CrossRef]
- Piskorz, J.; Mlynarczyk, D.T.; Szczolko, W.; Konopka, K.; Düzgüneş, N.; Mielcarek, J. Liposomal Formulations of Magnesium Sulfanyl Tribenzoporphyrazines for the Photodynamic Therapy of Cancer. J. Inorg. Biochem. 2018, 184, 34–41. [Google Scholar] [CrossRef] [PubMed]
- Sobotta, L.; Skupin-Mrugalska, P.; Piskorz, J.; Mielcarek, J. Porphyrinoid Photosensitizers Mediated Photodynamic Inactivation against Bacteria. Eur. J. Med. Chem. 2019, 175, 72–106. [Google Scholar] [CrossRef]
- Skupin-Mrugalska, P.; Koczorowski, T.; Szczolko, W.; Dlugaszewska, J.; Teubert, A.; Piotrowska-Kempisty, H.; Goslinski, T.; Sobotta, L. Cationic Porphyrazines with Morpholinoethyl Substituents–Syntheses, Optical Properties, and Photocytotoxicities. Dye. Pigment. 2022, 197, 109937. [Google Scholar] [CrossRef]
- Wieczorek, E.; Mlynarczyk, D.T.; Kucinska, M.; Dlugaszewska, J.; Piskorz, J.; Popenda, L.; Szczolko, W.; Jurga, S.; Murias, M.; Mielcarek, J.; et al. Photophysical Properties and Photocytotoxicity of Free and Liposome-Entrapped Diazepinoporphyrazines on LNCaP Cells under Normoxic and Hypoxic Conditions. Eur. J. Med. Chem. 2018, 150, 64–73. [Google Scholar] [CrossRef] [PubMed]
- Kabay, N.; Baygu, Y.; Alpoguz, H.K.; Kaya, A.; Gök, Y. Synthesis and Characterization of Porphyrazines as Novel Extractants for the Removal of Ag(I) and Hg(II) from Aqueous Solution. Dye. Pigment. 2013, 96, 372–376. [Google Scholar] [CrossRef]
- Koczorowski, T.; Szczolko, W.; Teubert, A.; Goslinski, T. Sulfanyl Porphyrazines with Morpholinylethyl Periphery—Synthesis, Electrochemistry, and Photocatalytic Studies after Deposition on Titanium(Iv) Oxide P25 Nanoparticles. Molecules 2021, 26, 2280. [Google Scholar] [CrossRef] [PubMed]
- Lü, X.F.; Li, J.; Wang, C.; Duan, M.Y.; Luo, Y.; Yao, G.P.; Wang, J.L. Enhanced Photoactivity of CuPp-TiO2 Photocatalysts under Visible Light Irradiation. Appl. Surf. Sci. 2010, 257, 795–801. [Google Scholar] [CrossRef]
- Cullity, B.D. Elements of X-ray Diffraction, 2nd ed.; Addison-Wesley: London, UK, 1978. [Google Scholar]
- Arbiol, J.; Cerdà, J.; Dezanneau, G.; Cirera, A.; Peiró, F.; Cornet, A.; Morante, J.R. Effects of Nb Doping on the TiO2 Anatase-to-Rutile Phase Transition. J. Appl. Phys. 2002, 92, 853–861. [Google Scholar] [CrossRef]
- Marinescu, C.; Sofronia, A.; Rusti, C.; Piticescu, R.; Badilita, V.; Vasile, E.; Baies, R.; Tanasescu, S. DSC Investigation of Nanocrystalline TiO2 Powder. J. Therm. Anal. Calorim. 2011, 103, 49–57. [Google Scholar] [CrossRef]
- Jiménez-Salcedo, M.; Monge, M.; Tena, M.T. Photocatalytic Degradation of Ibuprofen in Water Using TiO2/UV and g-C3N4/Visible Light: Study of Intermediate Degradation Products by Liquid Chromatography Coupled to High-Resolution Mass Spectrometry. Chemosphere 2019, 215, 605–618. [Google Scholar] [CrossRef] [PubMed]
- Vignesh, K.; Rajarajan, M.; Suganthi, A. Photocatalytic Degradation of Erythromycin under Visible Light by Zinc Phthalocyanine-Modified Titania Nanoparticles. Mater. Sci. Semicond. Process. 2014, 23, 98–103. [Google Scholar] [CrossRef]
- Mowafy, H.A.; Alanazi, F.K.; El Maghraby, G.M. Development and Validation of an HPLC-UV Method for the Quantification of Carbamazepine in Rabbit Plasma. Saudi Pharm. J. 2012, 20, 29–34. [Google Scholar] [CrossRef]
- Tsai, W.T.; Lee, M.K.; Su, T.Y.; Chang, Y.M. Photodegradation of Bisphenol-A in a Batch TiO2 Suspension Reactor. J. Hazard. Mater. 2009, 168, 269–275. [Google Scholar] [CrossRef] [PubMed]
- Lee, J.M.; Kim, M.S.; Kim, B.W. Photodegradation of Bisphenol-A with TiO2 Immobilized on the Glass Tubes Including the UV Light Lamps. Water Res. 2004, 38, 3605–3613. [Google Scholar] [CrossRef] [PubMed]
- Lu, N.; Lu, Y.; Liu, F.; Zhao, K.; Yuan, X.; Zhao, Y.; Li, Y.; Qin, H.; Zhu, J. H3PW12O40/TiO2 Catalyst-Induced Photodegradation of Bisphenol A (BPA): Kinetics, Toxicity and Degradation Pathways. Chemosphere 2013, 91, 1266–1272. [Google Scholar] [CrossRef] [PubMed]
- Mei, P.; Wang, H.; Guo, H.; Zhang, N.; Ji, S.; Ma, Y.; Xu, J.; Li, Y.; Alsulami, H.; Alhodaly, M.S.; et al. The Enhanced Photodegradation of Bisphenol A by TiO2/C3N4 Composites. Environ. Res. 2020, 182, 2–8. [Google Scholar] [CrossRef]
- Carabin, A.; Drogui, P.; Robert, D. Photo-Degradation of Carbamazepine Using TiO2 Suspended Photocatalysts. J. Taiwan Inst. Chem. Eng. 2015, 54, 109–117. [Google Scholar] [CrossRef]
- Fu, H.; Gray, K.A. TiO2 (Core)/Crumpled Graphene Oxide (Shell) Nanocomposites Show Enhanced Photodegradation of Carbamazepine. Nanomaterials 2021, 11, 2087. [Google Scholar] [CrossRef] [PubMed]
- Cravanzola, S.; Sarro, M.; Cesano, F.; Calza, P.; Scarano, D. Few-Layer MoS2 Nanodomains Decorating TiO2 Nanoparticles: A Case Study for the Photodegradation of Carbamazepine. Nanomaterials 2018, 8, 207. [Google Scholar] [CrossRef] [PubMed]




| k [s−1] | Δk [s−1] | |
|---|---|---|
| Bisphenol A | 5.75 × 10−5 | 1.44 × 10−5 |
| Carbamazepine | 5.66 × 10−5 | 1.42 × 10−5 |
| Type of Photocatalytic Material | Photodegradation Efficiency [% of Initial Conc.] | Source of Light | Ref. | |
|---|---|---|---|---|
| Bisphenol A | Bare P25 TiO2 | 7% after 4 h | UV lamp | [43] |
| H3PW12O40/TiO2 composite | 23% after 12 h | Xe lamp | [44] | |
| Bare P25 TiO2 | 68% after 1 h | UV lamp | [42] | |
| TiO2/C3N4 composite | 0% after 0.5 h | Xe arc lamp | [45] | |
| Porphyrazine/P25 TiO2 | 43% after 4 h | White LED lamp | This work | |
| Carbamazepine | Bare P90 TiO2 | 1% after 1.2 h | UV lamp | [46] |
| TiO2/crumpled graphene oxide composite | 0% after 2 h | UV + Xe arc lamps | [47] | |
| Hybrid MoS2/TiO2 systems | 0% after 0.25 h | UV lamp | [48] | |
| Porphyrazine/P25 TiO2 | 57% after 4 h | White LED lamp | This work |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Koczorowski, T.; Wicher, B.; Krakowiak, R.; Mylkie, K.; Marusiak, A.; Tykarska, E.; Ziegler-Borowska, M. Photocatalytic Activity of Sulfanyl Porphyrazine/Titanium Dioxide Nanocomposites in Degradation of Organic Pollutants. Materials 2022, 15, 7264. https://doi.org/10.3390/ma15207264
Koczorowski T, Wicher B, Krakowiak R, Mylkie K, Marusiak A, Tykarska E, Ziegler-Borowska M. Photocatalytic Activity of Sulfanyl Porphyrazine/Titanium Dioxide Nanocomposites in Degradation of Organic Pollutants. Materials. 2022; 15(20):7264. https://doi.org/10.3390/ma15207264
Chicago/Turabian StyleKoczorowski, Tomasz, Barbara Wicher, Rafal Krakowiak, Kinga Mylkie, Aleksandra Marusiak, Ewa Tykarska, and Marta Ziegler-Borowska. 2022. "Photocatalytic Activity of Sulfanyl Porphyrazine/Titanium Dioxide Nanocomposites in Degradation of Organic Pollutants" Materials 15, no. 20: 7264. https://doi.org/10.3390/ma15207264
APA StyleKoczorowski, T., Wicher, B., Krakowiak, R., Mylkie, K., Marusiak, A., Tykarska, E., & Ziegler-Borowska, M. (2022). Photocatalytic Activity of Sulfanyl Porphyrazine/Titanium Dioxide Nanocomposites in Degradation of Organic Pollutants. Materials, 15(20), 7264. https://doi.org/10.3390/ma15207264






