The Use of Iron(II,III) Oxide (Fe3O4) as a Cross-Linking Agent for Unfilled and Filled Chlorosulfonated Polyethylene (CSM) and Study of the Vulcanizates Properties
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
- Hypalon 20 (CSM29), product of DuPont de Nemours Inc. (Wilmington, Delaware, USA) with bound chlorine content of 29% by weight and a density of 1.12 g/cm3,
- Hypalon 30 (CSM43), product of DuPont de Nemours Inc. (Wilmington, Delaware, USA) with bound chlorine content of 43% by weight and a density of 1.26 g/cm3,
- Hypalon 40 (CSM35), product of DuPont de Nemours Inc. (Wilmington, Delaware, USA) with bound chlorine content of 35% by weight and a density of 1.18 g/cm3.
- precipitated silica Arsil (Z. Ch. Rudniki S.A., Rudniki, Poland), with a bulk density ~0.15 g/cm3 and pureness > 95%,
- technical kaolin (POCH S.A., Gliwice, Poland), with a density of 2.60 g/cm3 and an average grain size of 1.3 µm,
- carbon black, CB (Chemical Worldwide Business Sp. z o. o., Slupca, Poland), with a bulk density of 180 g/cm3,
- chalcedonite (CRUSIL Sp. z o. o., Inowlodz, Poland), with a density of 2.56 g/cm3 and a particle size < 10 µm,
- talc (KOCH Co., Ltd., Seoul, Korea), with a density of 2.78 g/cm3.
2.2. Methods
3. Results and Discussion
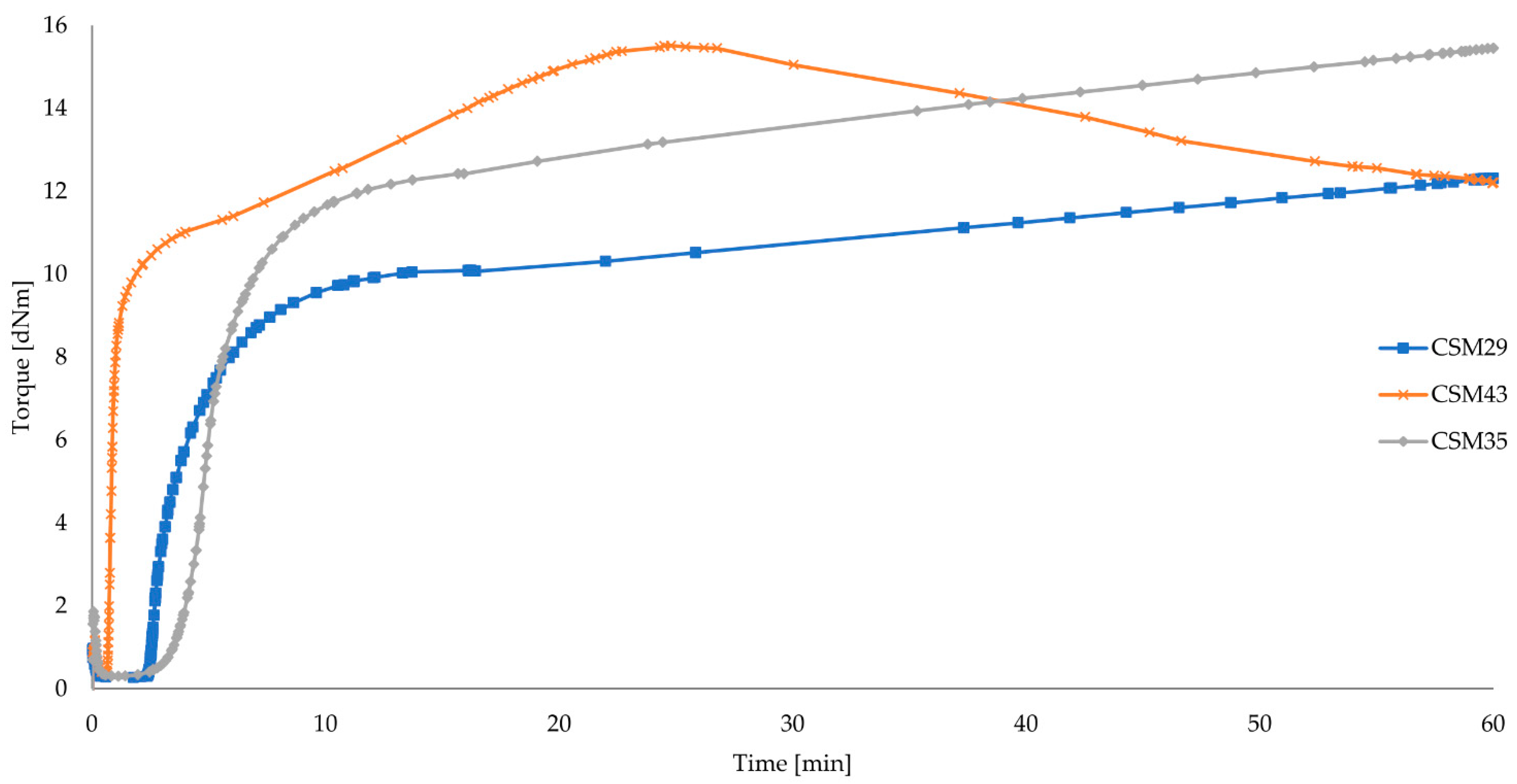
3.1. Influence of Bound Chlorine Content on CSM Cross-Linking with Iron(II,III) Oxide and Properties of Its Vulcanizates
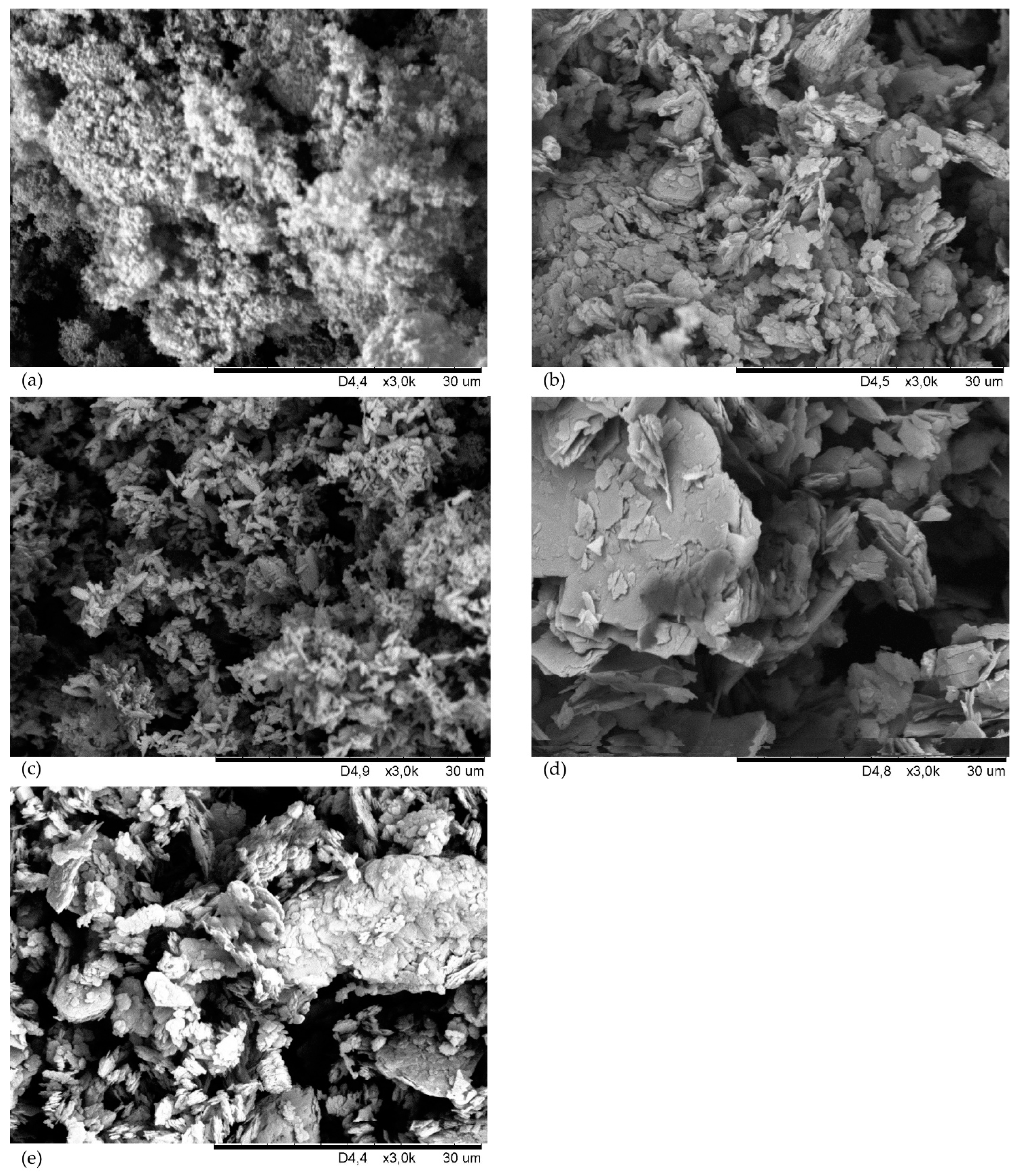
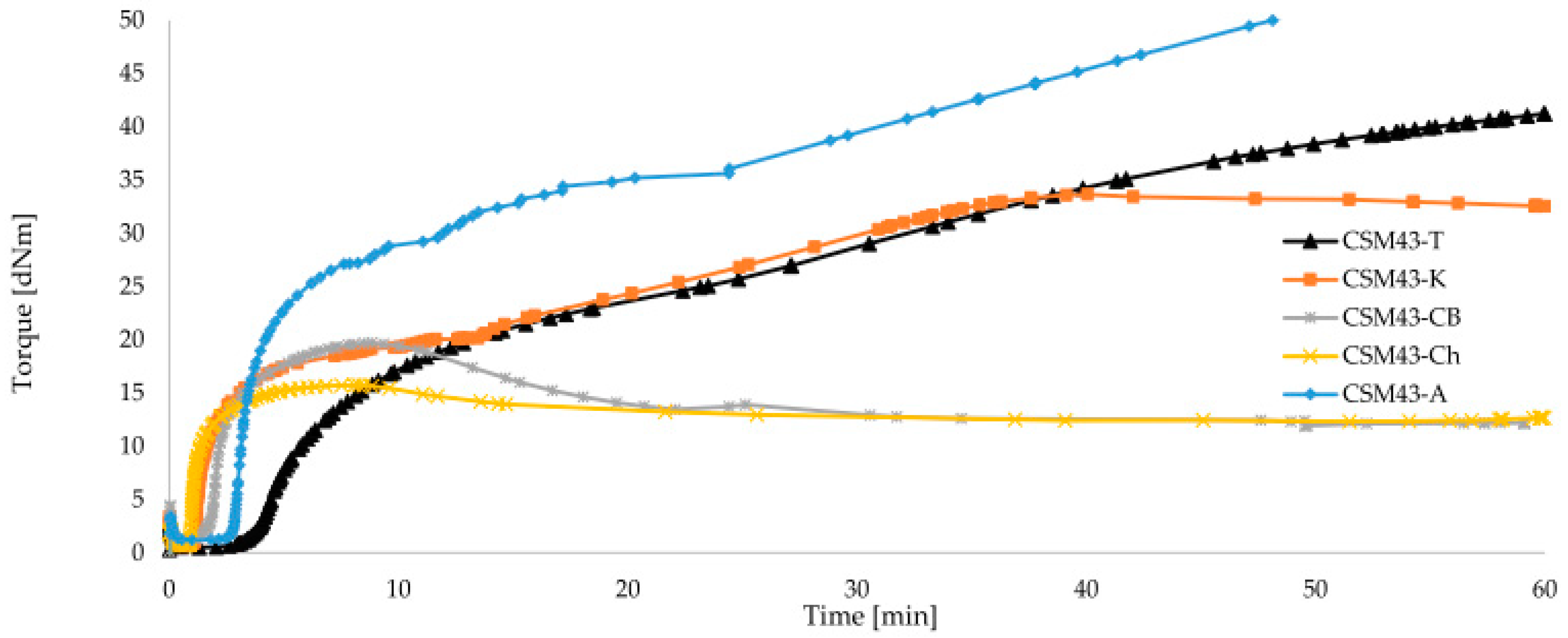
3.2. Cross-Linking Characteristics and Properties of Filled and Cross-Linked CSM with 43% Bound Chlorine
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Koszelew, F.F.; Korniew, A.E.; Klimow, N.S. Ogólna Technologia Gumy; Wydawnictwa Naukowo-Techniczne: Warszawa, Poland, 1972; pp. 150–153. [Google Scholar]
- Nersasian, A.; Andersen, D.E. The structure of chlorosulfonated polyethylene. J. Appl. Polym. Sci. 1960, 4, 74–80. [Google Scholar] [CrossRef]
- Hoffman, W. Rubber Chemistry and Technology; Hanser Publishers: New York, NY, USA, 1964. [Google Scholar]
- Janowska, G.; Rzymski, W.M.; Kmiotek, M.; Kucharska, A.; Kasiczak, A. Właściwości termiczne i palność chlorosulfonowanego polietylenu. Polimery 2009, 54, 243–324. [Google Scholar]
- Janowska, G.; Kucharska, A.; Kawałek, J.; Rzymski, W.M. Właściwości termiczne usieciowanych mieszanin chlorosulfonowanego polietylenu i kauczuku butadienowo-styrenowego. Polimery 2009, 54, 648–653. [Google Scholar] [CrossRef]
- Han, L.; Wang, P.; Feng, Y.; Zhao, J. Preparation of chlorosulfonated polyethylene by liquid-solid method and comparison with industrial products. J. Polym. Res. 2018, 11, 1–9. [Google Scholar] [CrossRef]
- Gu, Z.; Song, G.; Liu, W.; Gao, J.; Don, W.; Ping, L. Preparation and properties of chlorosulfonated polyethylene/organomontmorillonite nanocomposites. J. Appl. Polym. Sci. 2010, 115, 3365–3368. [Google Scholar] [CrossRef]
- Mandal, A.K.; Siddhanta, S.K.; Chakraborty, D. Chlorosulfonated polyethylene–polypropylene thermoplastic vulcanizate: Mechanical, morphological, thermal, and rheological properties. J. Appl. Polym. Sci. 2013, 127, 1268–1274. [Google Scholar] [CrossRef]
- Zhao, P.; Wang, M.; Zhang, J. Influence of chlorosulfonated polyethylene network structure on poly (acrylonitrile-styrene-acrylate)/poly (α-methylstyrene-acrylonitrile) blends: Mechanical properties, morphologies, glass transition behavior and heat resistance. Polym. Test. 2017, 57, 175–183. [Google Scholar] [CrossRef]
- Marković, G.; Marinović-Cincović, M.; Valentova, H.; Ilavsky, M.; Radovanović, B.; Budinski-Simendić, J. Curing characteristics and dynamic mechanical behaviour of reinforced acrylonitrile-butadiene/chlorosulfonated polyethylene rubber blends. In Materials Science Forum; Trans Tech Publications Ltd.: Zurich, Switzerland, 2005; pp. 475–480. [Google Scholar]
- Tanrattanakul, V.; Petchkaew, A. Mechanical properties and blend compatibility of natural rubber–chlorosulfonated polyethylene blends. J. Appl. Polym. Sci. 2006, 99, 127–140. [Google Scholar] [CrossRef]
- Rzymski, W.M.; Kmiotek, M. Reakcje interelastomerowe w niekonwencjonalnych mieszaninach elastomerów. Polimery 2007, 52, 511–516. [Google Scholar] [CrossRef]
- Gillen, K.T.; Bernstein, R.; Celina, M. Non-Arrhenius behavior for oxidative degradation of chlorosulfonated polyethylene materials. Polym. Degrad. Stab. 2005, 87, 335–346. [Google Scholar] [CrossRef]
- A Report of NACE Technical Unit Committee T-6A On Organic Coatings and Linings for Resistance to Chemical Corrosion, and Prepared by Task Group T-6A-18 On Chlorosulfonated Polyethylene. “Chlorosulfonated Polyethylene (Hypalon)”. Corrosion 1961, 17, 313–317. [CrossRef]
- Bentrad, N. Polyvinyl Chloride (PVC), Chlorinated Polyethylene (CPE), Chlorinated Polyvinyl Chloride (CPVC), Chlorosulfonated Polyethylene (CSPE), Polychloroprene Rubber (CR)—Chemistry, Applications and Ecological Impacts—II. In Ecological and Health Effects of Building Materials; Springer: Cham, Switzerland, 2022; pp. 53–66. [Google Scholar]
- Gillen, K.T.; Assink, R.; Bernstein, R.; Celina, M. Condition monitoring methods applied to degradation of chlorosulfonated polyethylene cable jacketing materials. Polym. Degrad. Stab. 2006, 91, 1273–1288. [Google Scholar] [CrossRef]
- Oberoi, S.; Malik, M. Polyvinyl Chloride (PVC), Chlorinated Polyethylene (CPE), Chlorinated Polyvinyl Chloride (CPVC), Chlorosulfonated Polyethylene (CSPE), Polychloroprene Rubber (CR)—Chemistry, Applications and Ecological Impacts—I. In Ecological and Health Effects of Building Materials; Springer: Cham, Switzerland, 2022; pp. 33–52. [Google Scholar]
- Kobędza, P.; Smejda-Krzewicka, A.; Strzelec, K. The use of copper oxides as cross-linking substances for chloroprene rubber and study of the vulcanizates properties. Part I. Materials 2021, 14, 5535. [Google Scholar] [CrossRef]
- Smejda-Krzewicka, A.; Olejnik, A.; Strzelec, K. The effect of metal oxide on the cure, morphology, thermal and mechanical characteristics of chloroprene and butadiene rubber blends. Elastomery 2019, 4, 16–23. [Google Scholar] [CrossRef]
- Rijal, B.; Klipfel, F.; Dez, I.; Colin, J.; Bazin, P.; Arethuse, F.; Tellier, E.; Pluart, L.L. Effect of metal oxides on the thermal degradation of polychloroprene and chlorosulfonated polyethylene. Polym. Degrad. Stab. 2019, 159, 90–97. [Google Scholar] [CrossRef]
- Chmielewski, A.G. Radiation Cross-Linking for the Cable, Rubber and Healthcare Products Industry. In Radiation Effects in Polymeric Materials; Springer: Cham, Switzerland, 2019; pp. 369–391. [Google Scholar]
- Keibal, N.A.; Kablov, V.K.; Tsybul’ko, N.O.; Ismailov, V.V. The Development of Fire-and Heat-Resistant Coatings Based on Chlorosulfonated Polyethylene with Improved Adhesion to Rubber. Polym. Sci. Ser. D 2020, 13, 443–446. [Google Scholar] [CrossRef]
- Zhang, Z.X.; Lang, X.H.; Zhang, X.; Xin, Z.; Prakashan, K. Microcellular foaming of chlorosulfonated polyethylene rubber and its kaolin-filled compounds with supercritical nitrogen. J. Appl. Polym. Sci. 2018, 135, 45656–45667. [Google Scholar] [CrossRef]
- Ganguly, S.; Das, N.C. Chlorosulphonated polyethylene and its composites for electronic applications. In Flexible and Stretchable Electronic Composites; Springer: Cham, Switzerland, 2016; pp. 229–259. [Google Scholar]
- Gumarov, A.K.; Garipov, R.M.; Stoyanov, O.V. Modified coatings based on chlorosulfonated polyethylene. Polym. Sci. Ser. D 2013, 6, 222–227. [Google Scholar] [CrossRef]
- Smejda-Krzewicka, A.; Kobędza, P.; Strzelec, K.; Adamus-Włodarczyk, A. The Use of Copper Oxides as Cross-Linking Substances for Chloroprene Rubber and Study of the Vulcanizates Properties. Part II. The Effect of Filler Type on the Properties of CR Products. Materials 2021, 14, 6528. [Google Scholar] [CrossRef] [PubMed]
- Szadkowski, B.; Marzec, A.; Zaborski, M. Use of carbon black as a reinforcing nano-filler in conductivity-reversible elastomer composites. Polym. Test. 2020, 81, 106222–106230. [Google Scholar] [CrossRef]
- Wolff, S.; Wang, M.J. Carbon black reinforcement of elastomers. In Carbon Black; Routledge: Oxfordshire, UK, 2018; pp. 289–355. [Google Scholar]
- Ibadullayev, A. Composite elastomeric materials filled with modified mineral fillers. In E3S Web of Conferences; EDP Sciences: Les Ulis, France, 2021. [Google Scholar]
- Standard Test Method for Rubber Property—Vulcanization Using Rotorless Cure Meters; American Society for Testing and Materials: West Conshohocken, PA, USA, 2019.
- Guma i Kauczuk Termoplastyczny—OZNACZANIE Właściwości Wytrzymałościowych Przy Rozciąganiu; Polski Komitet Normalizacyjny: Warsaw, Poland, 2007. Available online: https://sklep.pkn.pl/pn-iso-37-2007p.html (accessed on 14 September 2021).
- Slusarski, L.; Janowska, G.; Szkodzinski, A.; Niedomagała, M.; Harwazinski, A. System for Measuring the Oxygen Index of Plastics, Natural Materials or Rubber. Polish Patent No. PL 129411, 30 April 1987. [Google Scholar]
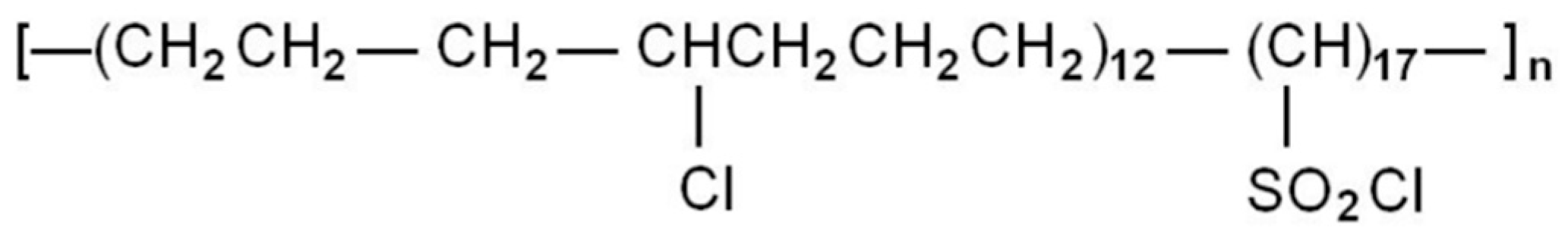


| Component Content (phr) | |||
|---|---|---|---|
| Hypalon 20 | 100 | --- | --- |
| Hypalon 30 | --- | 100 | --- |
| Hypalon 40 | --- | --- | 100 |
| Fe3O4 | 3 | 3 | 3 |
| SA | 1 | 1 | 1 |
| Symbol | CSM29 | CSM43 | CSM35 |
| Symbol | Mmin | ΔM10 | ΔM15 | ΔM20 | t02 | t90 | CRI | tv | Vulcametric Curves |
|---|---|---|---|---|---|---|---|---|---|
| dNm | dNm | dNm | dNm | min | min | min−1 | min | ||
| CSM29 | 0.27 | 9.28 | 9.82 | 10.04 | 2.72 | 37.32 | 2.89 | 15 | marching |
| CSM43 | 0.36 | 12.12 | 13.49 | 14.55 | 0.74 | 16.05 | 6.53 | 15 | reversion |
| CSM35 | 0.30 | 11.37 | 12.12 | 12.42 | 4.12 | 35.32 | 3.20 | 15 | marching |
| Symbol | Toluene | Heptane | |||||
|---|---|---|---|---|---|---|---|
| QvT (cm3/cm3) | –QwT (mg/mg) | VRT | αc (-) | QvH (cm3/cm3) | –QwH (mg/mg) | VrH | |
| CSM29 | 2.71 ± 0.04 | 0.09 ± 0.01 | 0.269 ± 0.003 | 0.37 | 0.62 ± 0.03 | 0.07 ± 0.01 | 0.616 ± 0.011 |
| CSM43 | 1.15 ± 0.03 | 0.03 ± 0.01 | 0.465 ± 0.006 | 0.87 | 0.10 ± 0.02 | 0.04 ± 0.01 | 0.911 ± 0.009 |
| CSM35 | 2.56 ± 0.05 | 0.07 ± 0.01 | 0.281 ± 0.004 | 0.39 | 0.34 ± 0.02 | 0.05 ± 0.01 | 0.747 ± 0.008 |
| Symbol | Se100 [MPa] | TSb [MPa] | Eb [%] |
|---|---|---|---|
| CSM29 | 1.55 ± 0.05 | 1.61 ± 0.10 | 106 ± 9 |
| CSM43 | --- | 6.97 ± 0.56 | 38 ± 12 |
| CSM35 | --- | 1.25 ± 0.10 | 88 ± 4 |
| Component Content (phr) | ||||||
|---|---|---|---|---|---|---|
| Hypalon 30 (CSM43) | 100 | 100 | 100 | 100 | 100 | 100 |
| Fe3O4 | 3 | 3 | 3 | 3 | 3 | 3 |
| SA | 1 | 1 | 1 | 1 | 1 | 1 |
| Arsil | 30 | --- | --- | --- | --- | --- |
| Kaolin | --- | 30 | --- | --- | --- | --- |
| Chalcedonite | --- | --- | 30 | --- | --- | --- |
| Talc | --- | --- | --- | 30 | --- | --- |
| Carbon black | --- | --- | --- | --- | 30 | --- |
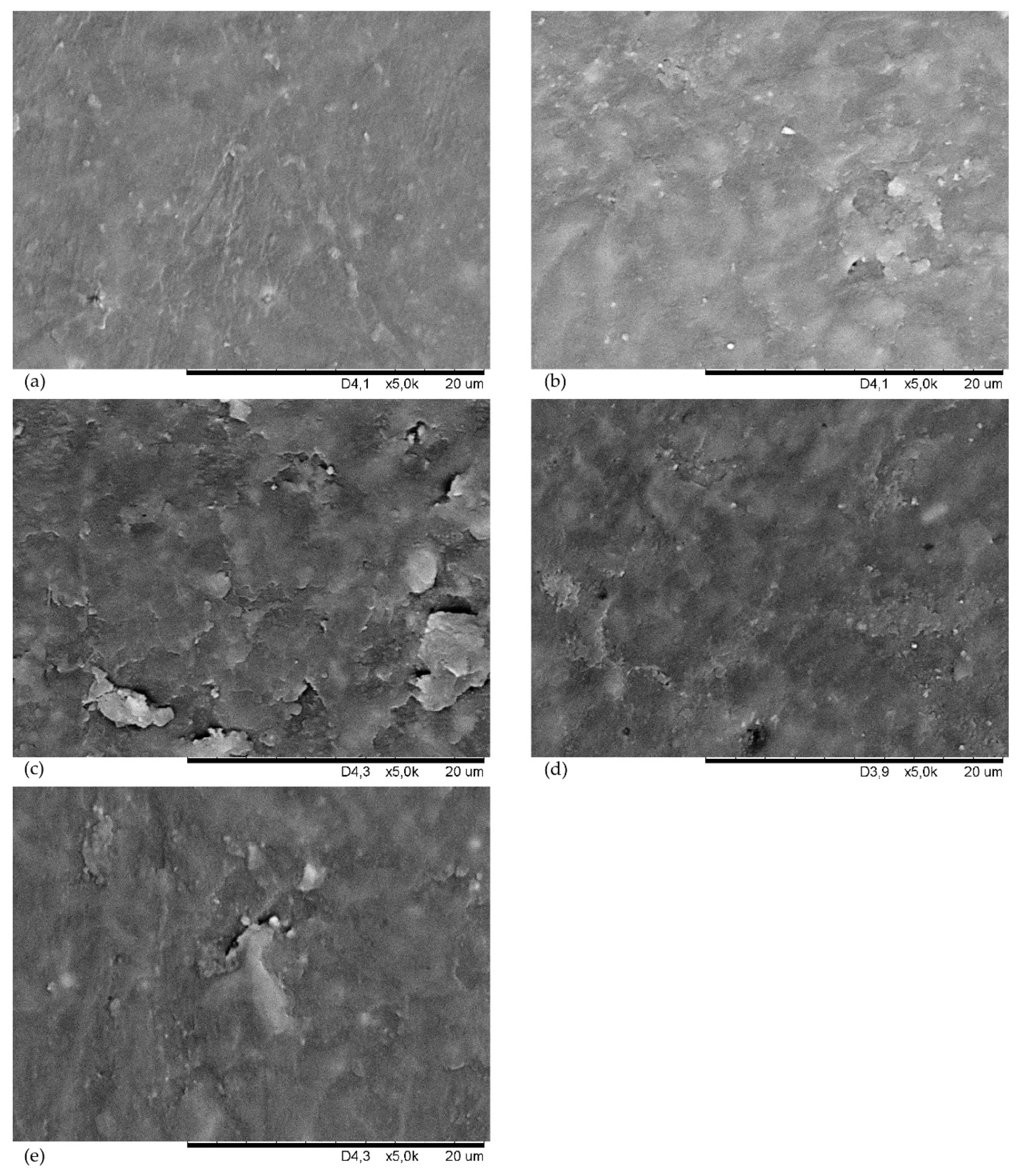
| Symbol | CSM43-A | CSM43-K | CSM43-Ch | CSM43-T | CSM43-CB | CSM43 |
| Symbol | Mmin | ΔM5 | ΔM10 | ΔM20 | t02 | t90 | Vulcametric Curves | CRI | tv |
|---|---|---|---|---|---|---|---|---|---|
| dNm | dNm | dNm | dNm | min | min | --- | min−1 | min | |
| CSM43-A | 1.19 | 21.46 | 26.19 | 32.16 | 2.86 | 47.12 | marching | 2.26 | 5 |
| CSM43-K | 0.63 | 16.75 | 14.68 | 23.72 | 1.16 | 30.94 | reversion | 3.36 | 5 |
| CSM43-Ch | 0.68 | 14.45 | 14.77 | 12.56 | 0.95 | 3.45 | reversion | 40.0 | 5 |
| CSM43-T | 0.51 | 7.30 | 16.46 | 22.45 | 3.97 | 46.52 | marching | 2.35 | 15 |
| CSM43-CB | 0.97 | 16.55 | 18.43 | 13.11 | 1.79 | 5.17 | reversion | 29.58 | 5 |
| CSM43 | 0.36 | 12.12 | 13.49 | 14.55 | 0.74 | 16.05 | reversion | 6.53 | 15 |
| Symbol | Toluene | Heptane | |||||
|---|---|---|---|---|---|---|---|
| QvT (cm3/cm3) | –QwT (mg/mg) | VRT | QvH (cm3/cm3) | –QwH (mg/mg) | VrH | αc | |
| CSM43-A | 1.57 ± 0.04 | 0.28 ± 0.01 | 0.337 ± 0.009 | 0.13 ± 0.01 | 0.26 ± 0.01 | 0.883 ± 0.004 | 0.64 |
| CSM43-K | 2.04 ± 0.07 | 0.28 ± 0.01 | 0.329 ± 0.008 | 0.20 ± 0.04 | 0.26 ± 0.01 | 0.834 ± 0.024 | 0.49 |
| CSM43-Ch | 2.12 ± 0.05 | 0.28 ± 0.01 | 0.320 ± 0.005 | 0.21 ± 0.04 | 0.26 ± 0.01 | 0.83 ± 0.03 | 0.47 |
| CSM43-T | 1.47 ± 0.05 | 0.26 ± 0.01 | 0.405 ± 0.008 | 0.27 ± 0.02 | 0.26 ± 0.01 | 0.78 ± 0.01 | 0.68 |
| CSM43-CB | 1.62 ± 0.03 | 0.28 ± 0.01 | 0.381 ± 0.004 | 0.14 ± 0.03 | 0.25 ± 0.01 | 0.878 ± 0.023 | 0.62 |
| CSM43 | 1.15 ± 0.03 | 0.03 ± 0.01 | 0.465 ± 0.006 | 0.10 ± 0.01 | 0.04 ± 0.01 | 0.911 ± 0.009 | 0.87 |
| Symbol | Se100 (MPa) | TSb (MPa) | Eb (%) | Ts (N/mm) | HA (°ShA) |
|---|---|---|---|---|---|
| CSM43-A | 7.05 ± 0.09 | 12.40 ± 0.70 | 187 ± 10 | 6.10 ± 0.41 | 82.8 ± 1.4 |
| CSM43-K | 5.90 ± 0.26 | 4.76 ± 0.38 | 82 ± 6 | 6.46 ± 0.78 | 80.7 ± 1.6 |
| CSM43-Ch | 3.83 ± 0.08 | 4.81 ± 0.28 | 140 ± 10 | 6.56 ± 0.74 | 77.1 ± 1.8 |
| CSM43-T | --- | 11.00 ± 0.60 | 46 ± 9 | 7.77 ± 0.35 | 92.5 ± 0.8 |
| CSM43-CB | --- | 7.82 ± 0.82 | 86 ± 9 | 4.35 ± 0.24 | 79.2 ± 1.4 |
| CSM43 | --- | 6.97 ± 0.56 | 46 ± 2 | --- | --- |
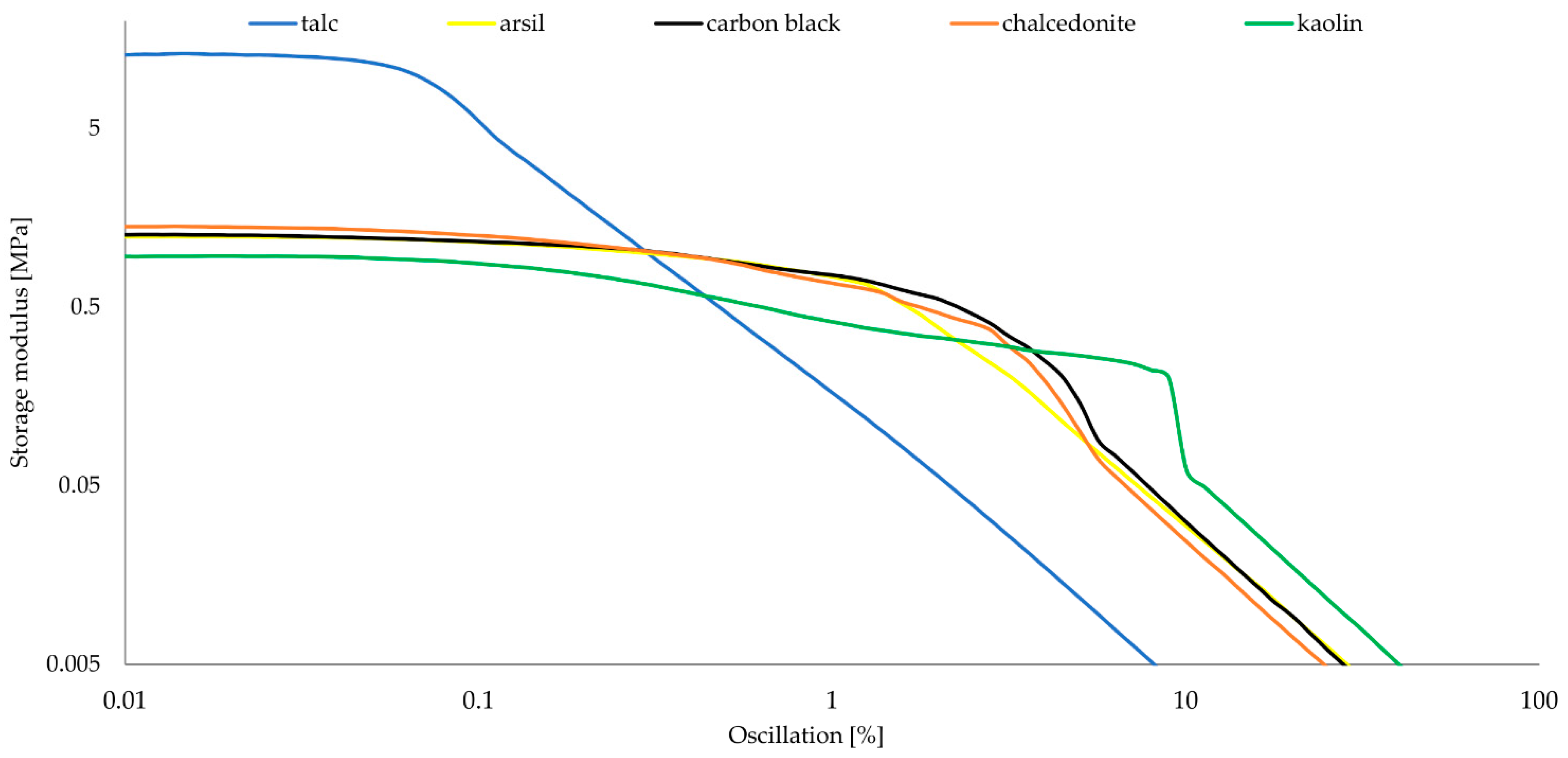
| Symbol | G′max (MPa) | G″max (MPa) | ΔG (MPa) |
|---|---|---|---|
| CSM43-A | 1.24 | 0.55 | 1.24 |
| CSM43-K | 0.96 | 0.88 | 0.96 |
| CSM43-Ch | 1.41 | 1.09 | 1.41 |
| CSM43-T | 13.1 | 10.2 | 13.1 |
| CSM43-CB | 1.27 | 0.73 | 1.27 |
| Symbol | tb (s) | OI (%) | Observation |
|---|---|---|---|
| CSM43-A | <10 | ≥37.5 | When the fire is applied, a “cold fire” effect occurs, when the fire is removed the flame goes out immediately. |
| CSM43-K | <10 | ≥37.5 | The samples do not react to fire (they do not burn), the flame goes out immediately. |
| CSM43-Ch | <10 | ≥37.5 | The samples fade when the fire is removed, a slight surface incandescence that subsides after a while. |
| CSM43-T | <10 | ≥37.5 | The samples did not ignite, no glow, when the fire is removed the sample immediately goes out. |
| CSM43-CB | <10 | ≥37.5 | The samples do not burn, after removal of the flame there is a slight glow which disappears after a while. |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Smejda-Krzewicka, A.; Mrozowski, K.; Kobędza, P.; Adamus-Włodarczyk, A. The Use of Iron(II,III) Oxide (Fe3O4) as a Cross-Linking Agent for Unfilled and Filled Chlorosulfonated Polyethylene (CSM) and Study of the Vulcanizates Properties. Materials 2022, 15, 7276. https://doi.org/10.3390/ma15207276
Smejda-Krzewicka A, Mrozowski K, Kobędza P, Adamus-Włodarczyk A. The Use of Iron(II,III) Oxide (Fe3O4) as a Cross-Linking Agent for Unfilled and Filled Chlorosulfonated Polyethylene (CSM) and Study of the Vulcanizates Properties. Materials. 2022; 15(20):7276. https://doi.org/10.3390/ma15207276
Chicago/Turabian StyleSmejda-Krzewicka, Aleksandra, Konrad Mrozowski, Piotr Kobędza, and Agnieszka Adamus-Włodarczyk. 2022. "The Use of Iron(II,III) Oxide (Fe3O4) as a Cross-Linking Agent for Unfilled and Filled Chlorosulfonated Polyethylene (CSM) and Study of the Vulcanizates Properties" Materials 15, no. 20: 7276. https://doi.org/10.3390/ma15207276
APA StyleSmejda-Krzewicka, A., Mrozowski, K., Kobędza, P., & Adamus-Włodarczyk, A. (2022). The Use of Iron(II,III) Oxide (Fe3O4) as a Cross-Linking Agent for Unfilled and Filled Chlorosulfonated Polyethylene (CSM) and Study of the Vulcanizates Properties. Materials, 15(20), 7276. https://doi.org/10.3390/ma15207276






