Surface Modification of Biomass with Di-(2-Ethylhexyl)phosphoric Acid and Its Use for Vanadium Adsorption
Abstract
:1. Introduction
2. Materials and Experiment Methods
2.1. Materials
2.2. Experimental
2.2.1. Preparation of SICB
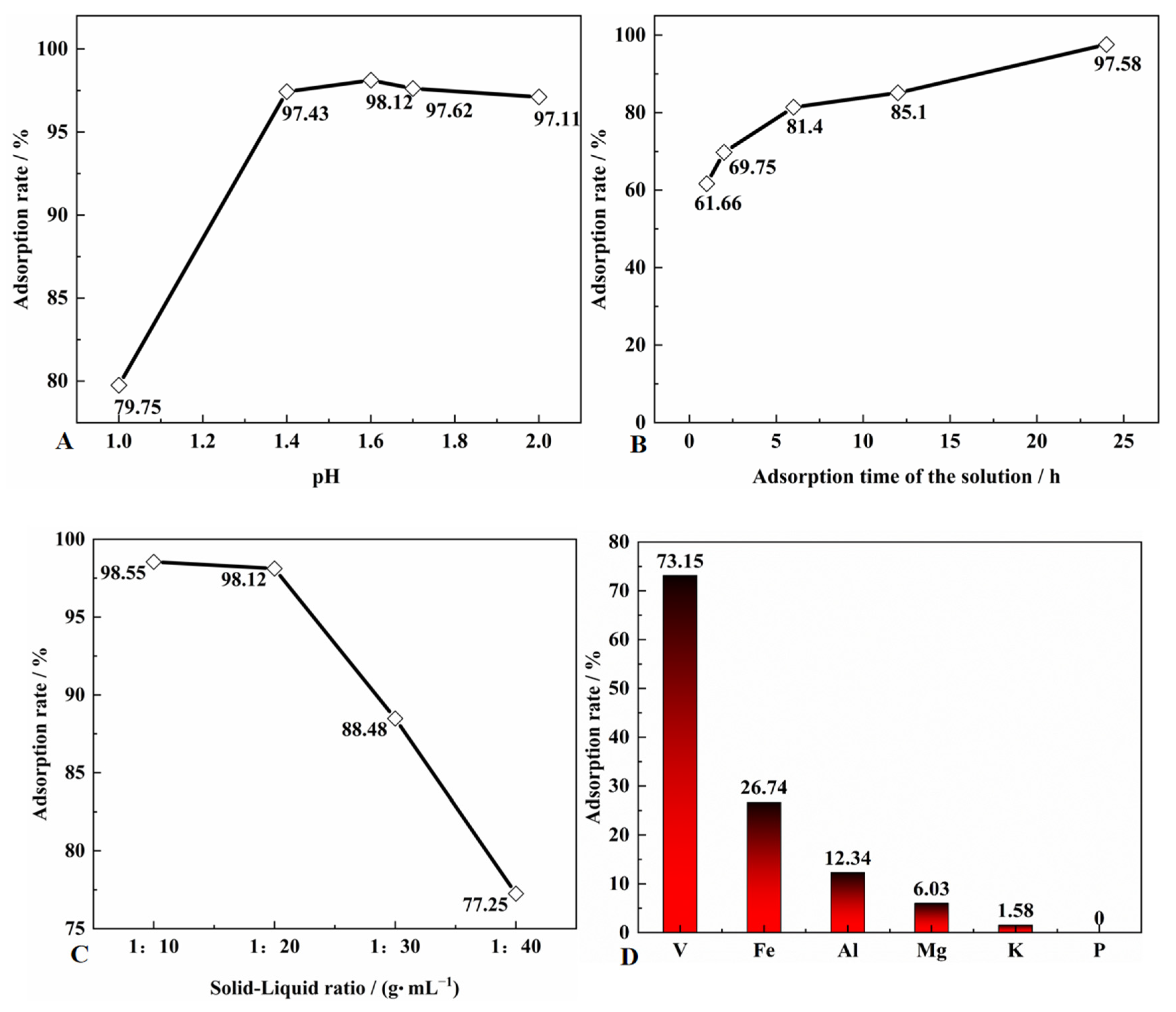
2.2.2. Adsorption Experiments of Vanadium
2.2.3. Analysis Methods
3. Results and Discussion
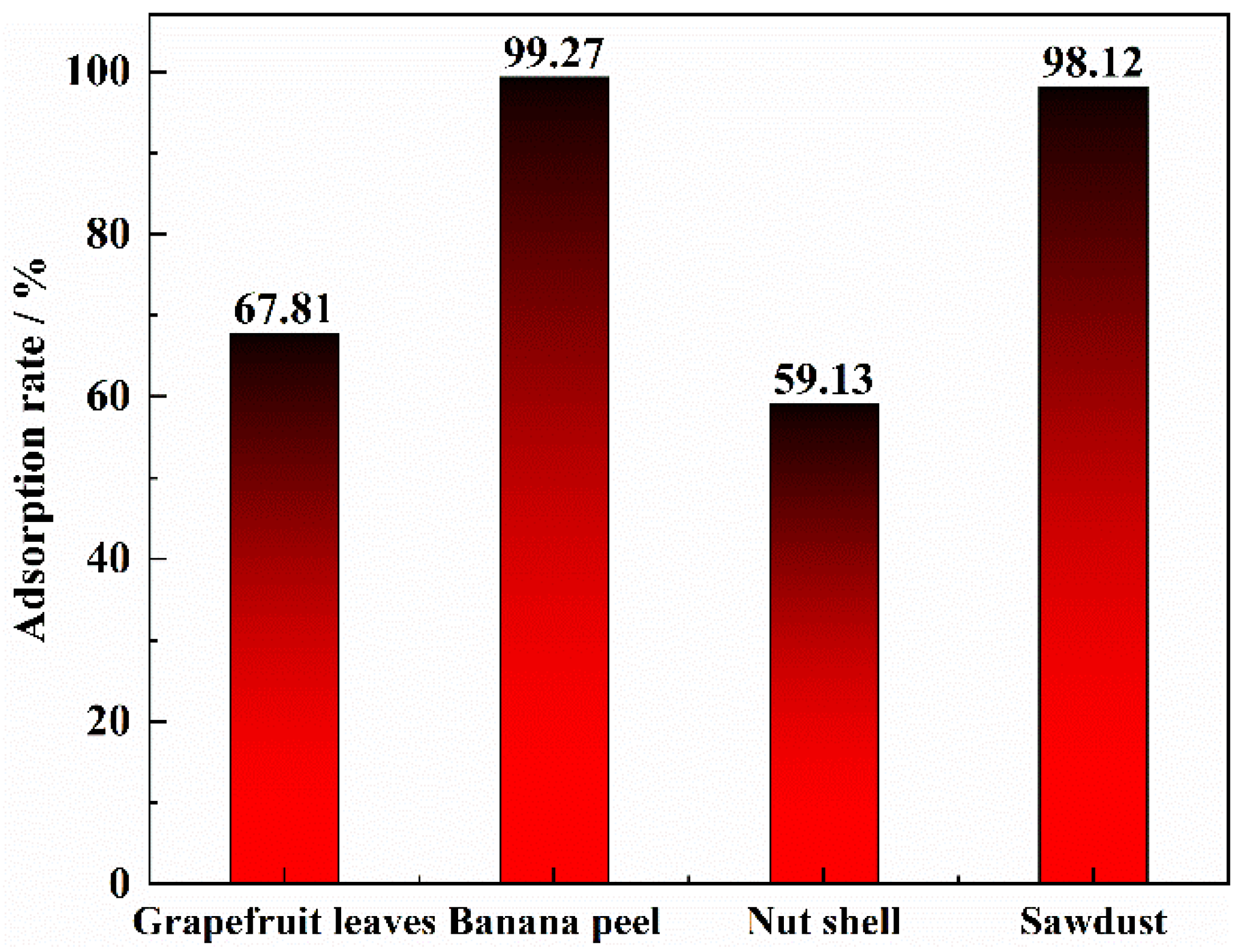
3.1. Effect of Different Biomass Matrices on the Adsorption Effect
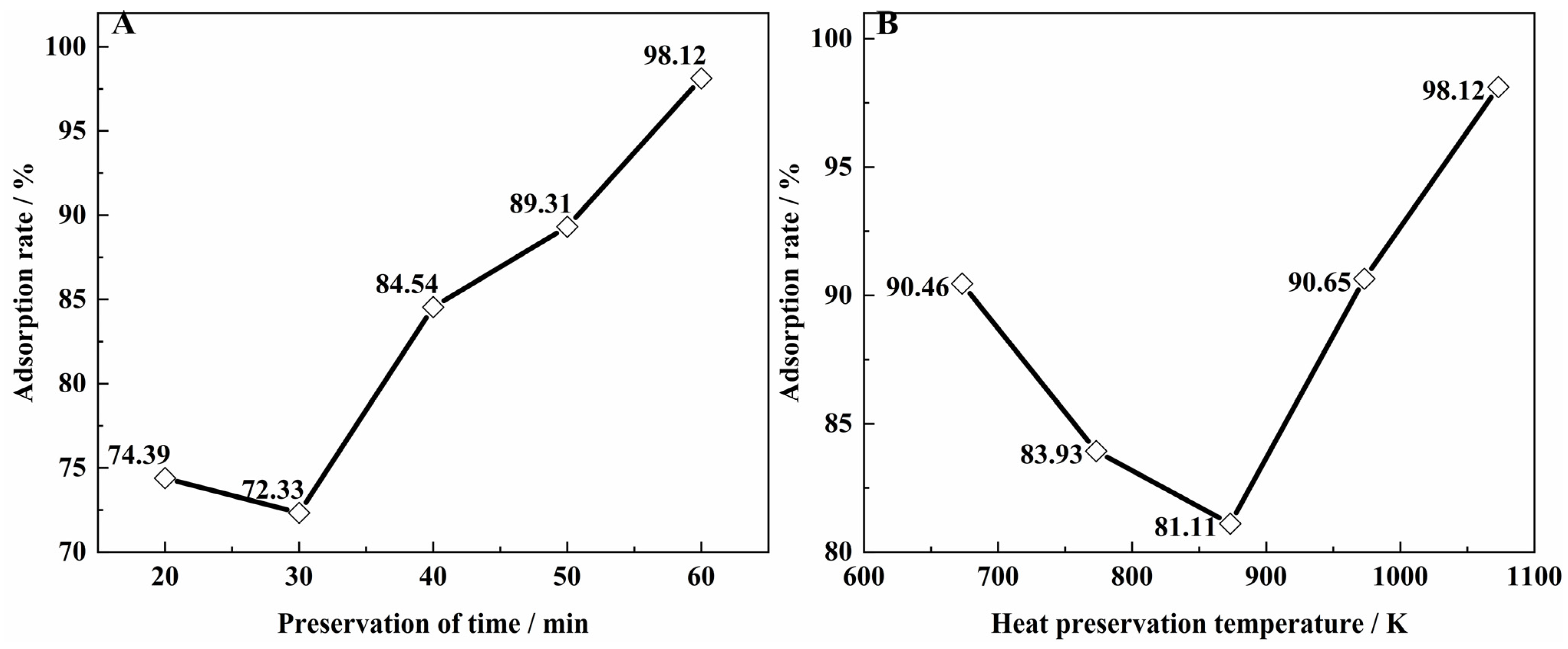
3.2. Effect of Carbonation Temperature and Time on Vanadium Adsorption from Biomass
3.3. Effect of Particle Size of Carbonized Biomass on the Adsorption of Vanadium
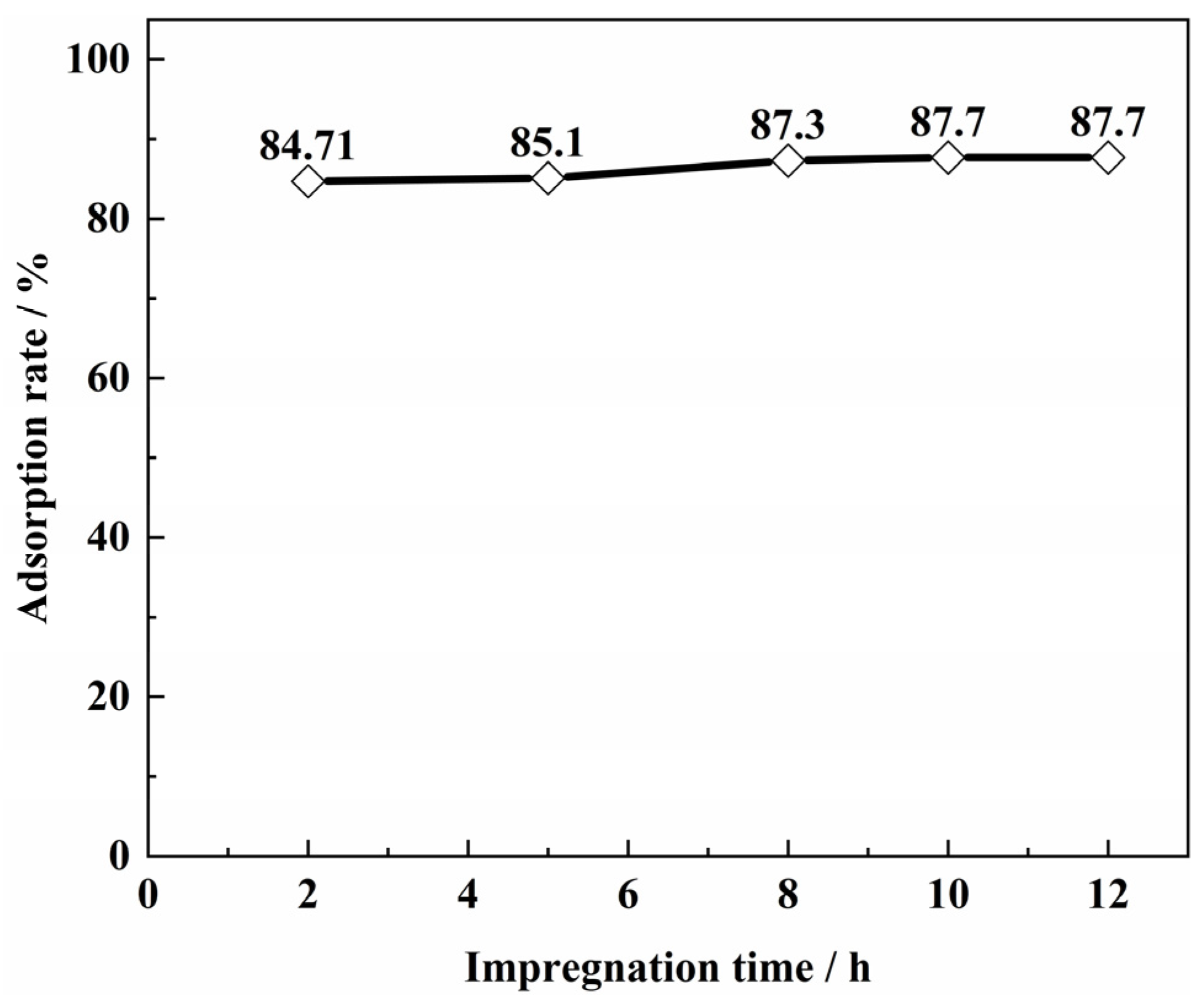
3.4. Effect of SICB Impregnation Time on Vanadium Adsorption
3.5. Characterization of Carbonized Biomass and SICB
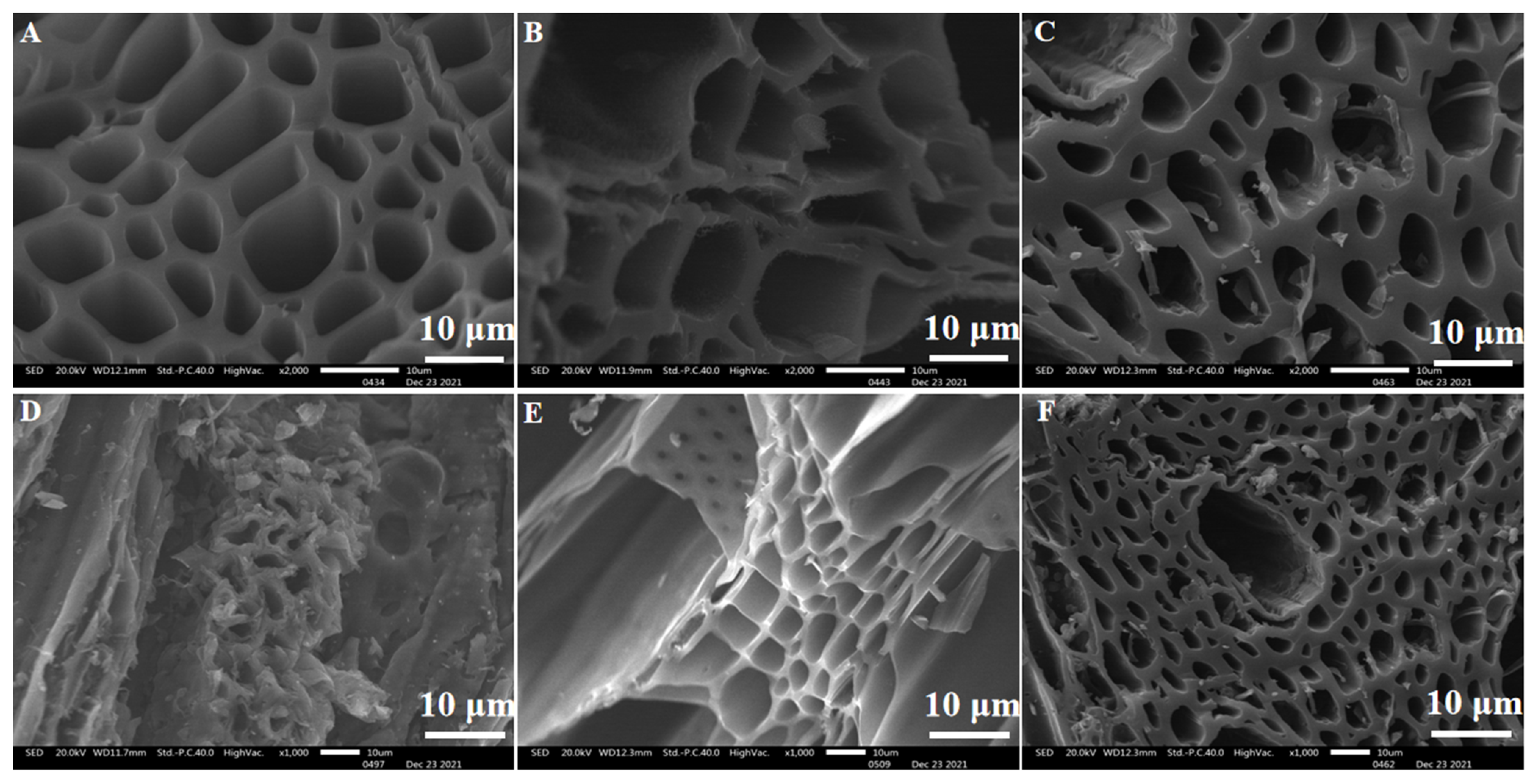
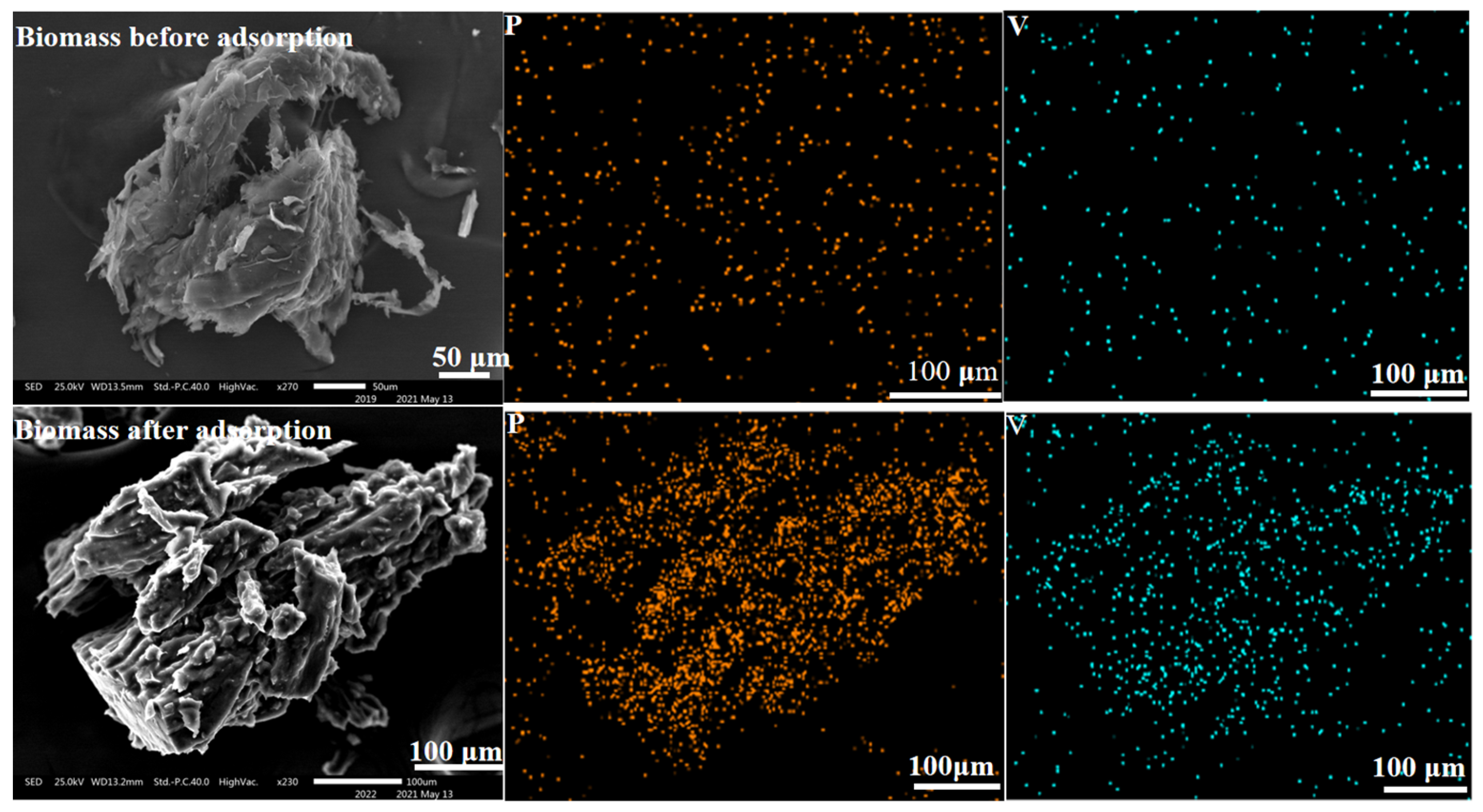
3.5.1. Surface Topography of Carbonized Biomass and SICB
3.5.2. Specific Surface Area Test of Biomass and CB
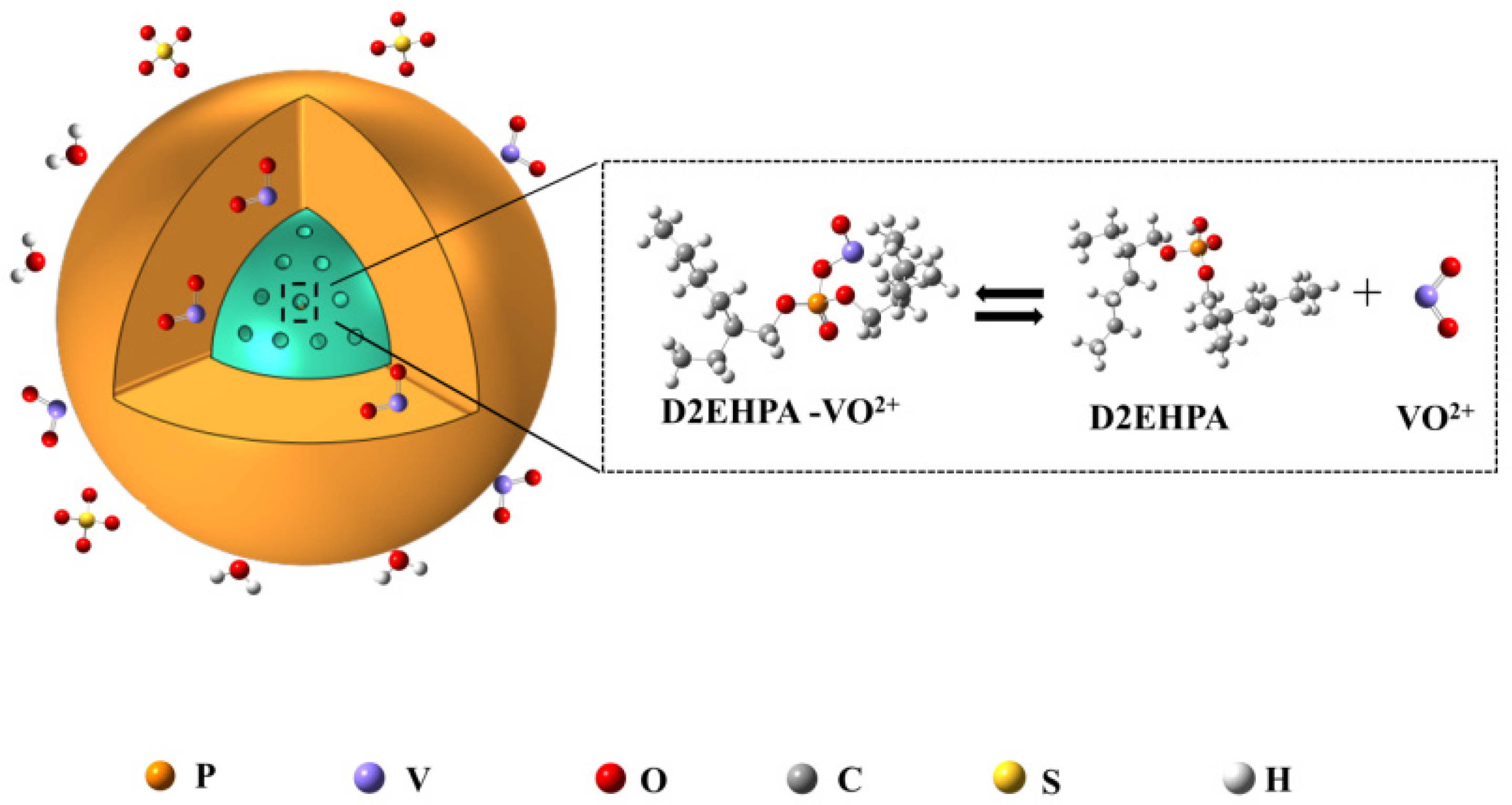
3.6. Effect of Adsorption Process on the Adsorbed Vanadium of SICB
3.7. Element Distribution of SICB before and after Adsorption
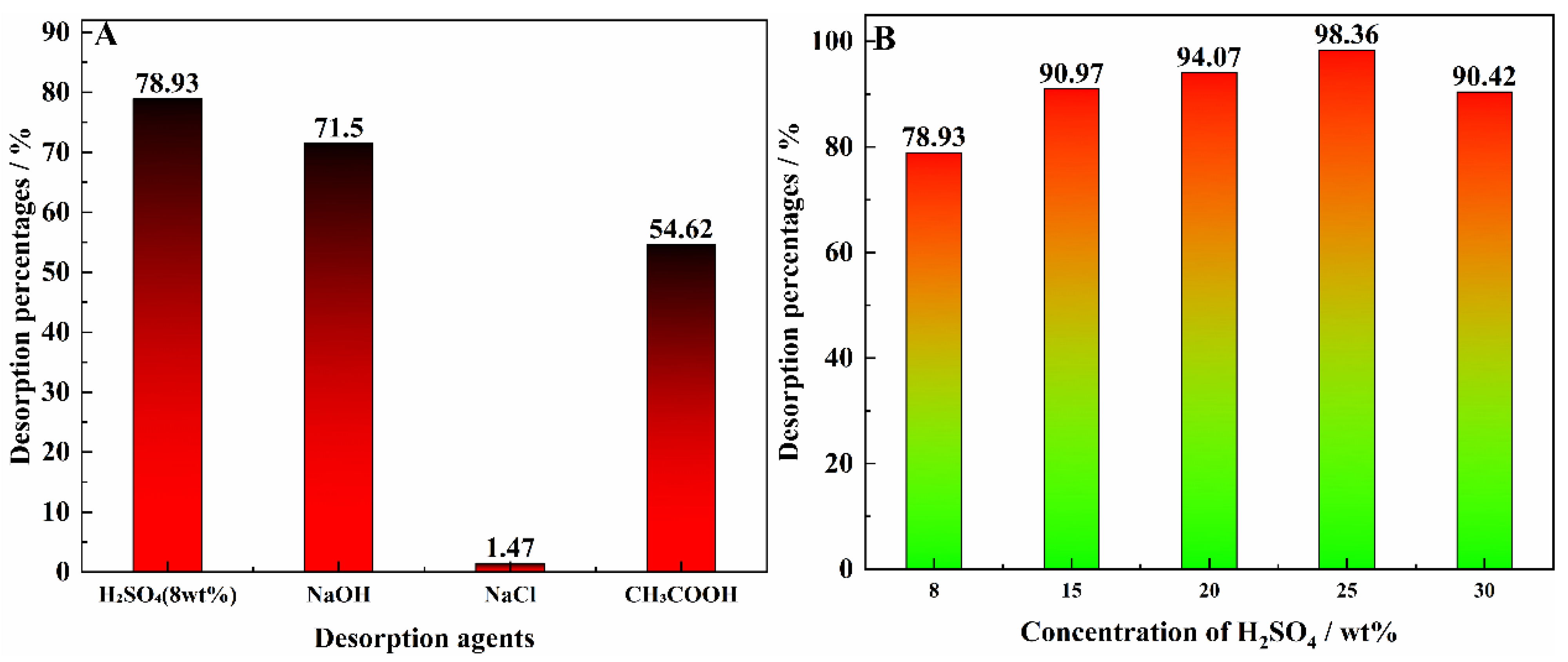
3.8. Effect of Desorption on Vanadium Desorption Agent
3.9. Modeling of Adsorption of V(IV) on SICB
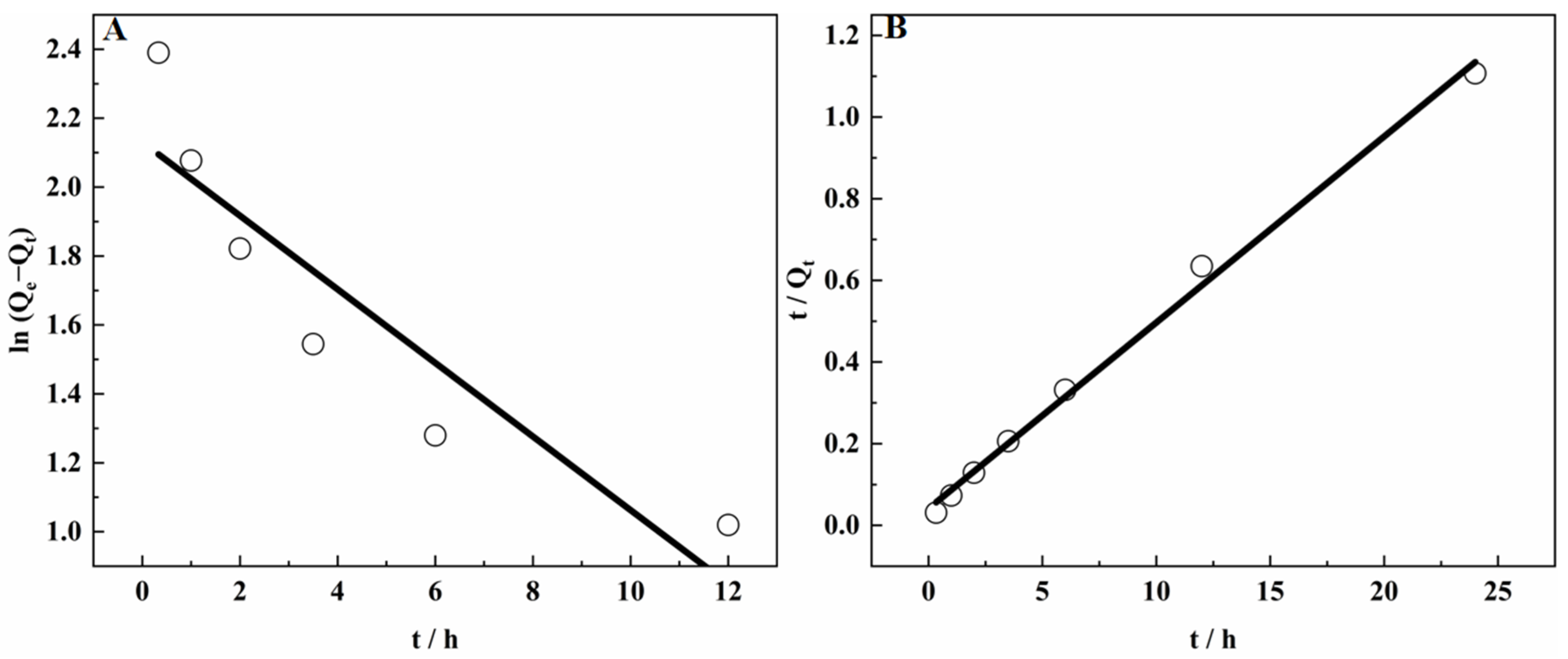
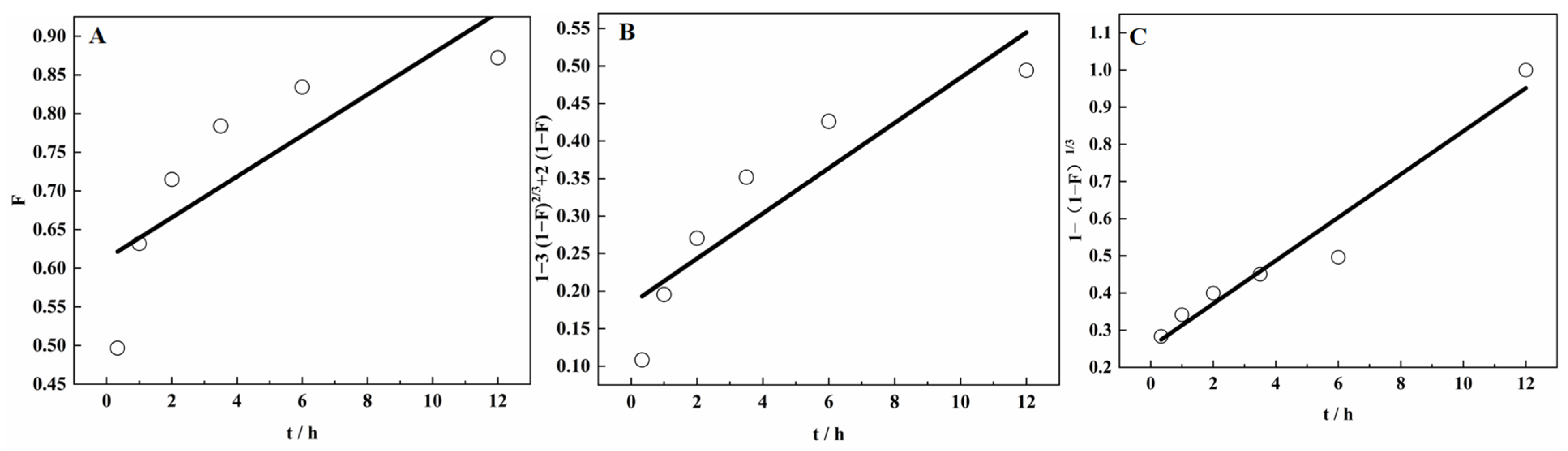
3.9.1. Adsorption Kinetics of SICB on V(IV)
3.9.2. Adsorption Process of V(IV) in SICB
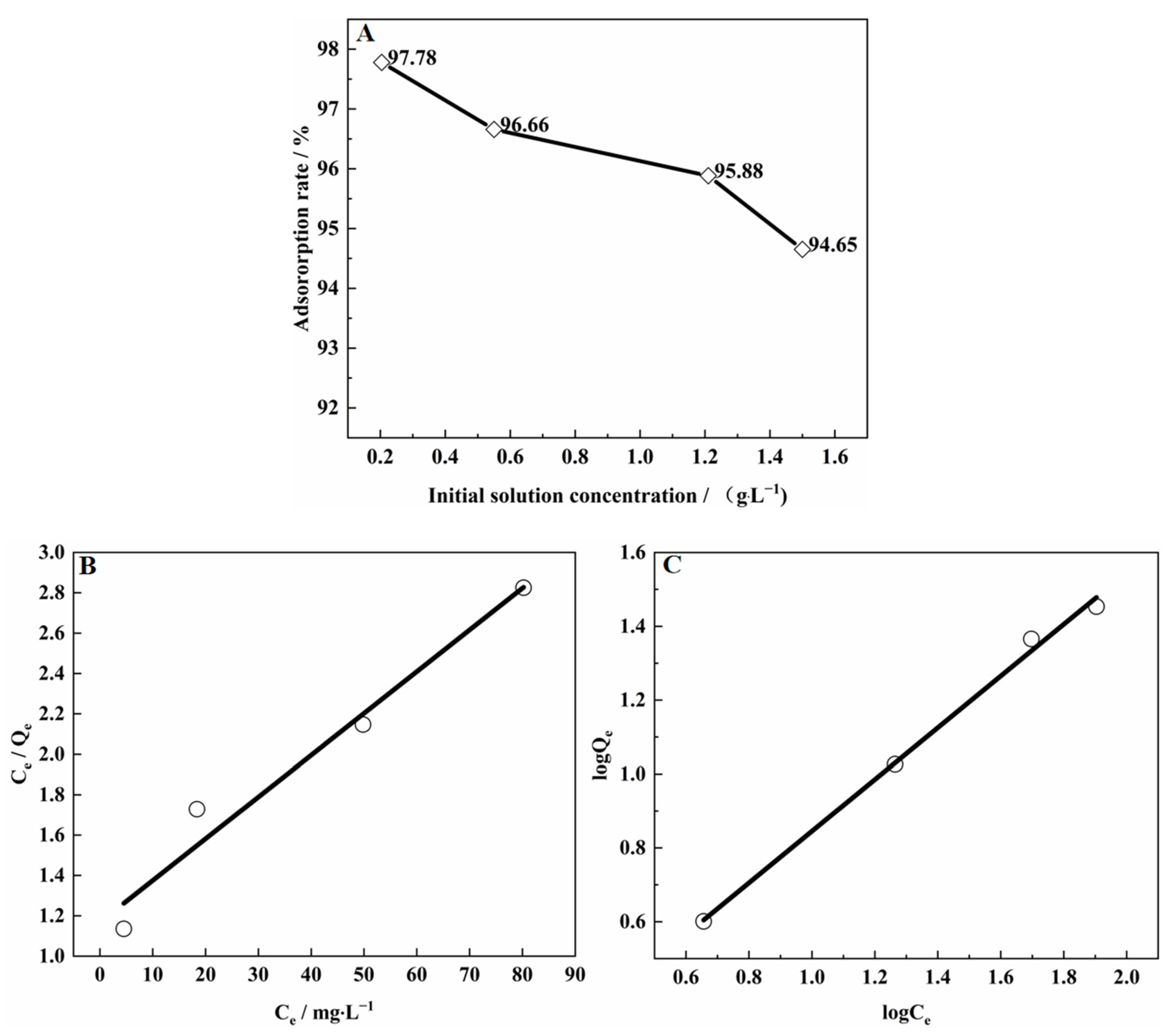
3.9.3. Adsorption Isotherm of V(IV) on SICB
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Zhang, Y.M.; Bao, S.X.; Liu, T.; Chen, T.J.; Huang, J. The technology of extracting vanadium from black shale in China: History, current status, and future prospects. Hydrometallurgy 2011, 109, 116–124. [Google Scholar] [CrossRef]
- Bao, S.X.; Zhang, Y.M.; Chen, B. Research Status and Prospect of Vanadium Extraction Technology for Vanadium-bearing Shale in China. Met. Mine 2020, 10, 20–33. [Google Scholar]
- Nguyen, T.H.; Lee, M.S. Solvent extraction of vanadium(V) from sulfate solution using LIX 63 and PC88A. J. Ind. Eng. Chem. 2015, 31, 118–123. [Google Scholar] [CrossRef]
- Ye, G.H.; Hu, Y.B.; Tong, X.; Lu, L. Extraction of vanadium from direct acid leaching solution of clay vanadium ore using solvent extraction with N235. Hydrometallurgy 2018, 177, 27–33. [Google Scholar] [CrossRef]
- Liu, H.; Zhang, Y.M.; Huang, J. Enhancing the separation performance of vanadium from a black shale leaching solution by supported liquid membrane using trialkyl amine. Chem. Eng. Res. Des. 2018, 136, 262–270. [Google Scholar] [CrossRef]
- Jiang, D.D.; Song, N.Z.; Liao, S.F.; Lian, Y.; Ma, J.T.; Jia, Q. Study on the synergistic extraction of vanadium by mixtures of acidic organophosphorus extractants and primary amine N1923. Sep. Purif. Technol. 2015, 156, 835–840. [Google Scholar] [CrossRef]
- Deng, Z.G.; Wei, C.; Fan, G.; Li, M.T.; Li, C.X.; Li, X.B. Extracting vanadium from stone-coal by oxygen pressure acid leaching and solvent extraction. Trans. Nonferrous Met. Soc. China 2010, 20, 118–122. [Google Scholar] [CrossRef]
- Li, C.L.; Zhou, X.Y.; Wang, H.; Zhang, T.K.; Li, J.; Ou, X.; Jiang, X.D. Effect of oxidation on vanadium extraction from stone coal with calcified roasting. J. Cent. South Univ. (Sci. Technol.) 2011, 42, 7–10. [Google Scholar]
- Zeng, L.; Cheng, C.Y. Recovery of molybdenum and vanadium from synthetic sulphuric acid leach solutions of spent hydro desulphurisation catalysts using solvent extraction. Hydrometallurgy 2010, 101, 141–147. [Google Scholar] [CrossRef]
- He, Y.; Huang, J.; Zhang, Y.M.; Liu, H.; Hu, P.C.; Luo, D.S. Extration of vanadium from shale leaching solutiom with mextral 984H. Nonferrous Met. (Extr. Metall.) 2019, 10, 33–38. [Google Scholar]
- Luo, D.S.; Huang, J.; Zhang, Y.M.; Liu, T.; Hu, P.C. Efficient and environment-friendly vanadium (V) extraction from vanadiumshale leachate using tri-n-octylmethylammonium chloride. Sep. Purif. Technol. 2020, 15, 237. [Google Scholar]
- Zheng, R.W.; Bao, S.X.; Zhang, Y.M.; Chen, B. Adsorption of Vanadium (IV) on the Synthesized D2EHPA-TBP Impregnated Resin. J. Chem. Appl. Chem. Eng. 2018, 2, 1–4. [Google Scholar]
- Zhu, X.B.; Li, W.; Tang, S.; Zeng, M.J.; Bai, P.Y.; Chen, L.J. Selective recovery of vanadium and scandium by ion exchange with D201 and solvent extraction using P507 from hydrochloric acid leaching solution of red mud. Chemosphere 2017, 175, 365–372. [Google Scholar] [PubMed]
- Gomes, H.I.; Jones, A.; Rogerson, M.; Burke, I.T.; Mayes, W.M. Vanadium removal and recovery from bauxite residue leachates by ion exchange. Environ. Sci. Pollut. Res. (Int.) 2016, 23, 23034–23042. [Google Scholar]
- Tang, Y.P.; Bao, S.X.; Zhang, Y.M.; Liang, L. Effect of support properties on preparation process and adsorption performances of solvent impregnated resins. React. Funct. Polym. 2017, 113, 50–57. [Google Scholar] [CrossRef]
- Lucaci, A.R.; Bulgariu, D.; Ahmad, I.; Lisă, G.; Mocanu, A.M.; Bulgariu, L. Potential Use of Biochar from Various Waste Biomass as Biosorbent in Co(II) Removal Processes. Water 2019, 11, 1565. [Google Scholar] [CrossRef]
- Praveen, S.; Jegan, J.; Bhagavathi Pushpa, T.; Gokulan, R.; Bulgariu, L. Biochar for removal of dyes in contaminated water: An overview. Biochar 2022, 4, 10. [Google Scholar] [CrossRef]
- Jayaprina, G.; Archina, B.; Abdul, A.A.R. Insight into metal-impregnated biomass based activated carbon for enhanced carbon dioxide adsorption: A review. J. Ind. Eng. Chem. 2022, 113, 72–95. [Google Scholar]
- Song, M.; Song, B.; Meng, F.Y.; Chen, D.D.; Fei, S.; Wei, Y.X. Incorporation of humic acid into biomass derived carbon for enhanced adsorption of phenol. Sci. Rep. 2019, 9, 19931. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Park, J.H.; Ok, Y.S.; Kim, S.H.; Cho, J.S.; Heo, J.S.; Delaune, R.D.; Seo, D.C. Competitive adsorption of heavy metals onto sesame straw biochar in aqueous solutions. Chemosphere 2016, 142, 77–83. [Google Scholar] [PubMed]
- Depci, T.; Kul, A.R.; Önal, Y. Competitive adsorption of lead and zinc from aqueous solution on activated carbon prepared from Van apple pulp: Study in single- and multi-solute systems. Chem. Eng. J. 2012, 200–202, 224–236. [Google Scholar] [CrossRef]
- Demirbaş, E. Adsorption of cobalt(II) ions from aqueous solution onto activated carbon prepared from hazelnut shells. Adsorpt. Sci. Technol. 2003, 21, 951–963. [Google Scholar] [CrossRef] [Green Version]
- Mohubedu, R.P.; Diagboya, P.N.E.; Abasi, C.Y.; Dikio, E.D.; Mtunzi, F. Magnetic valorization of biomass and biochar of a typical plant nuisance for toxic metals contaminated water treatment. J. Clean. Prod. 2019, 209, 1016–1024. [Google Scholar]
- Annadurai, G.; Juang, R.S.; Lee, D.J. Adsorption of heavy metals from water using banana and orange peels. Water Sci. Technol. 2003, 47, 185–190. [Google Scholar] [CrossRef]
- Sari, T.; Hanna, R.; Henrik, R.; Ulla, L.; Teija, K. Multiple heavy metal removal simultaneously by a biomass-based porous carbon. Water Environ. Fed. 2021, 93, 1303–1314. [Google Scholar]
- Roop, C.B.; Meenakshi, G. Activated Carbon Adsorption; CRC Press: Boca Raton, FL, USA, 2005. [Google Scholar]
- Pan, H.-W.; Iizuka, A.; Shibata, E. Gold recovery from dilute aqueous solution by a biosorbent derived from woody biomass. Chem. Eng. Commun. 2021, 208, 1711–1724. [Google Scholar] [CrossRef]
- Li, W.; Zhang, Y.M.; Liu, T.; Huang, J.; Wang, Y. Comparison of ion exchange and solvent extraction in recovering vanadium from sulfuric acid leach solutions of stone coal. Hydrometallurgy 2013, 131, 1–7. [Google Scholar] [CrossRef]
- Shi, Q.H.; Zhang, Y.M.; Huang, J.; Liu, T.; Liu, H.; Wang, L.Y. Synergistic solvent extraction of vanadium from leaching solution of stone coal using D2EHPA and PC88A. Sep. Purif. Technol. 2018, 18, 1–7. [Google Scholar] [CrossRef]
- Keno, D.; Kowanga, K.D.; Gatebe, E.; Mauti, G.O.; Mauti, E.M. Kinetic, sorption isotherms, pseudo-first-order model, and pseudo-second-order model studies of Cu(II) and Pb(II) using defatted Moringa oleifera seed powder. J. Phytopharm. 2016, 5, 17–78. [Google Scholar]
- Chen, W.; Mai, L.Q.; Jun, F.P.; Xu, Q.; Zhu, Q.Y. FTIR study of vanadium oxide nanotubes from lamellar structure. J. Mater. Sci. 2004, 39, 2625–2627. [Google Scholar] [CrossRef]
- Jay, C.B.; Sarawud, S.; Kerry, G.; Dominik, J.W. A Revised Pseudo-Second-Order Kinetic Model for Adsorption, Sensitive to Changes in Adsorbate and Adsorbent Concentrations. Langmuir 2021, 37, 16–37. [Google Scholar]
- Ho, Y.S.; McKay, G. Pseudo-second order model for sorption processes. Process Biochem. 1999, 34, 451–465. [Google Scholar] [CrossRef]
- Mahajan, G.; Sud, D. Nano sized carbonized waste biomass for heavy metal ion remediation. Pol. J. Chem. Technol. 2014, 16, 6–13. [Google Scholar] [CrossRef] [Green Version]
- Korovin, V.; Shestak, Y. Scandium extraction from hydrochloric acid media by Levextrel-type resins containing di-isooctyl methyl phosphonate. Hydrometallurgy 2009, 95, 346–349. [Google Scholar] [CrossRef]
- Kabay, N.; Cortina, J.L.; Trochimczuk, A.; Streat, M. Solvent-impregnated resins (SIRs)—Methods of preparation and their applications. React. Funct. Polym. 2010, 70, 484–496. [Google Scholar] [CrossRef]
- Liang, L. Research on Preparation of Solvent Impregnated Resin and Adsorption and Separation Properties for Vanadium-Bearing Solution; Wuhan University of Technology: Wuhan, China, 2016. [Google Scholar]
- Liang, L.; Bao, S.X.; Zhang, Y.M.; Tang, Y.P. Separation and recovery of V(IV) from sulfuric acid solutions containing Fe(III) and Al(III) using bis(2-ethylhexyl)phosphoric acid impregnated resin. Chem. Eng. Res. Des. 2016, 111, 109–116. [Google Scholar] [CrossRef]
- Burghoff, B.; Goetheer, E.L.; de Haan, V.A.B. Solvent impregnated resins for the removal of low concentration phenol from water. React. Funct. Polym. 2008, 68, 1314–1324. [Google Scholar] [CrossRef]
- Gbor, P.K.; Jia, C.Q. Critical evaluation of coupling particle size distribution with the shrinking core model. Chem. Eng. Sci. 2004, 59, 1979–1987. [Google Scholar] [CrossRef]
- Hori, H.; Otsu, T.; Yasukawa, T.; Morita, R.; Ishii, S.; Asai, T. Recovery of rhenium from aqueous mixed metal solutions by selective precipitation: A photochemical approach. Hydrometallurgy 2018, 183, 151–158. [Google Scholar] [CrossRef]
- Dada, A.; Olalekan, A.; Olatunya, A.; Dada, A. Langmuir, Freundlich, Temkin and Dubinin- Radushkevich isotherms studies of equilibrium sorption of Zn2+ unto phosphoric acid modified rice husk. IOSR J. Appl. Chem. 2012, 3, 38–45. [Google Scholar]

| Category | BET Surface Area m2/g | Langmuir Surface Area m2/g | t-Plot Micropore Area m2/g |
|---|---|---|---|
| Biomass | 0.76 | 0.80 | 0.76 |
| CB | 148.88 | 151.38 | 124.92 |
| Kinetic Model | Pseudo-First-Order | Pseudo-First-Order | ||||
|---|---|---|---|---|---|---|
| R2 | Q | K | R2 | Q | K2 | |
| 0.83225 | 135.114 | 0.1068 | 0.9954 | 21.990 | 0.0502 | |
| y = 2.1307 − 0.10679 x | y = 0.0456 x + 0.04114 | |||||
| Liquid Film Diffusion Fitted Curve | Particle Diffusion Fitted Curve | Chemical Reaction Control Fitted Curve | |||
|---|---|---|---|---|---|
| R2 | 0.67772 | R2 | 0.82137 | R2 | 0.95226 |
| y = 0.61261 + 0.02649 x | y = 0.18305 + 0.03013 x | y = 0.25508 + 0.05802 x | |||
| Isotherm Model | Langmuir | Freundlich | ||||
|---|---|---|---|---|---|---|
| Q | KL | R2 | n | KF | R2 | |
| 48.34 | 0.024 | 0.966 | 1.43 | 1.40 | 0.9962 | |
| y = 0.02068 + 1.16817 x | y = 0.014512 + 0.70009 x | |||||
| Category | Saturated Capacity/mg·g−1 |
|---|---|
| D2EHPA and TBP | 24.21 |
| D2EHPA and TBP impregnated resin | 29.95 [11] |
| D2EHPA and TBP impregnated biomass | 48.34 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yu, Z.; Fan, Y.; Liu, T.; Zhang, Y.; Hu, P. Surface Modification of Biomass with Di-(2-Ethylhexyl)phosphoric Acid and Its Use for Vanadium Adsorption. Materials 2022, 15, 7300. https://doi.org/10.3390/ma15207300
Yu Z, Fan Y, Liu T, Zhang Y, Hu P. Surface Modification of Biomass with Di-(2-Ethylhexyl)phosphoric Acid and Its Use for Vanadium Adsorption. Materials. 2022; 15(20):7300. https://doi.org/10.3390/ma15207300
Chicago/Turabian StyleYu, Zhekun, Yong Fan, Tao Liu, Yimin Zhang, and Pengcheng Hu. 2022. "Surface Modification of Biomass with Di-(2-Ethylhexyl)phosphoric Acid and Its Use for Vanadium Adsorption" Materials 15, no. 20: 7300. https://doi.org/10.3390/ma15207300




