Influence of Milling Time on Phase Composition and Product Structure of Mg-Zn-Ca-Ag Alloys Obtained by Mechanical Synthesis
Abstract
1. Introduction
- Zinc (Zn), which is biocompatible, when added to magnesium, creates a dispersive phase, enabling precipitation hardening.
- Calcium (Ca), which is biocompatible, improves fluidity, and refines the grain, and when added to the Mg-Zn system, increases hardness and creep resistance. The addition of Ca increases the hardness of alloys, which is related to precipitation hardening.
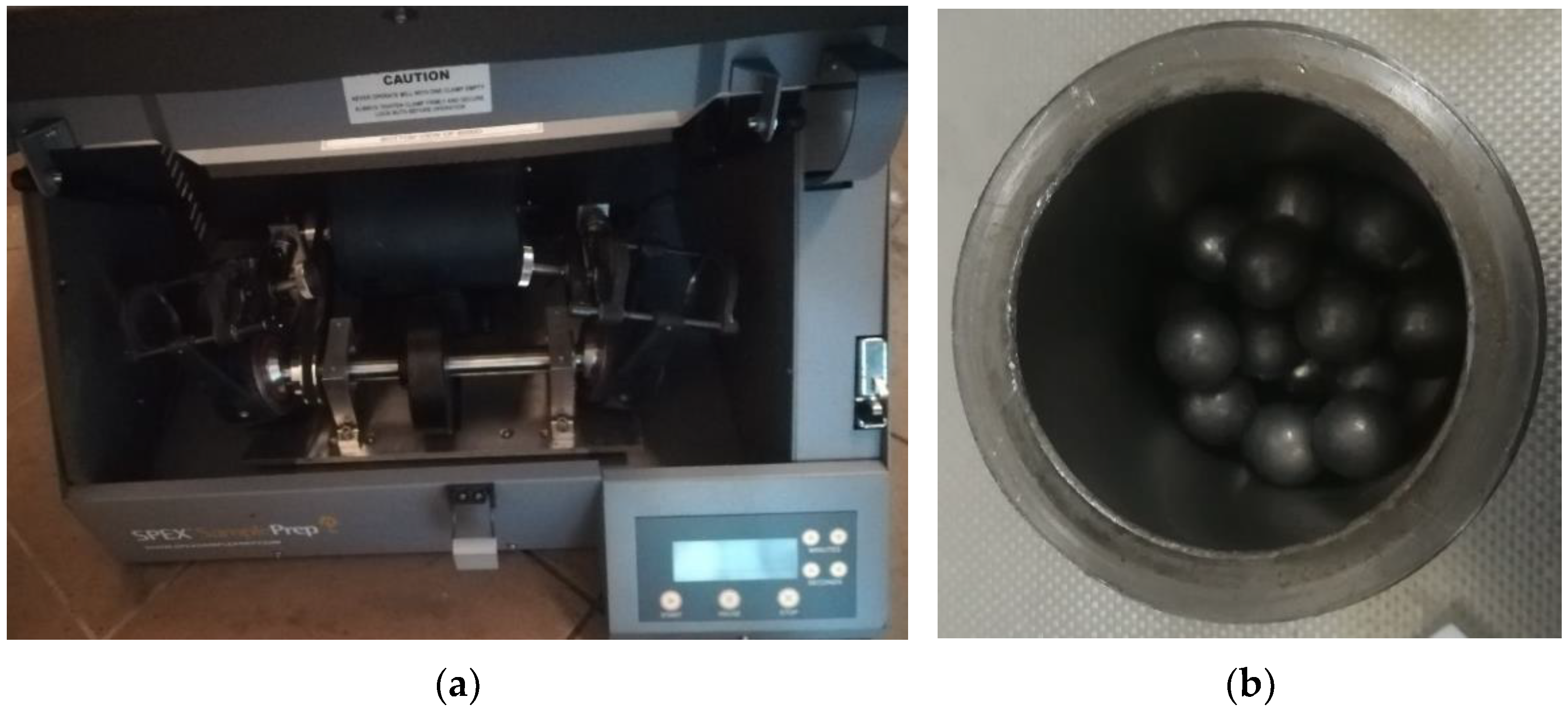
2. Materials and Methods
- Zinc (Zinc powder)—100 mesh, 99.9% (metal basis).
- Magnesium (Magnesium powder)—20 + 100 mesh, 99.8% (metal basis).
- Calcium (Calcium shot), redistilled, 1 cm & down, 99.5% (metal basis).
- Silver (Silver powder)—100 mesh, 99.95% (metal basis).
3. Results and Discussion
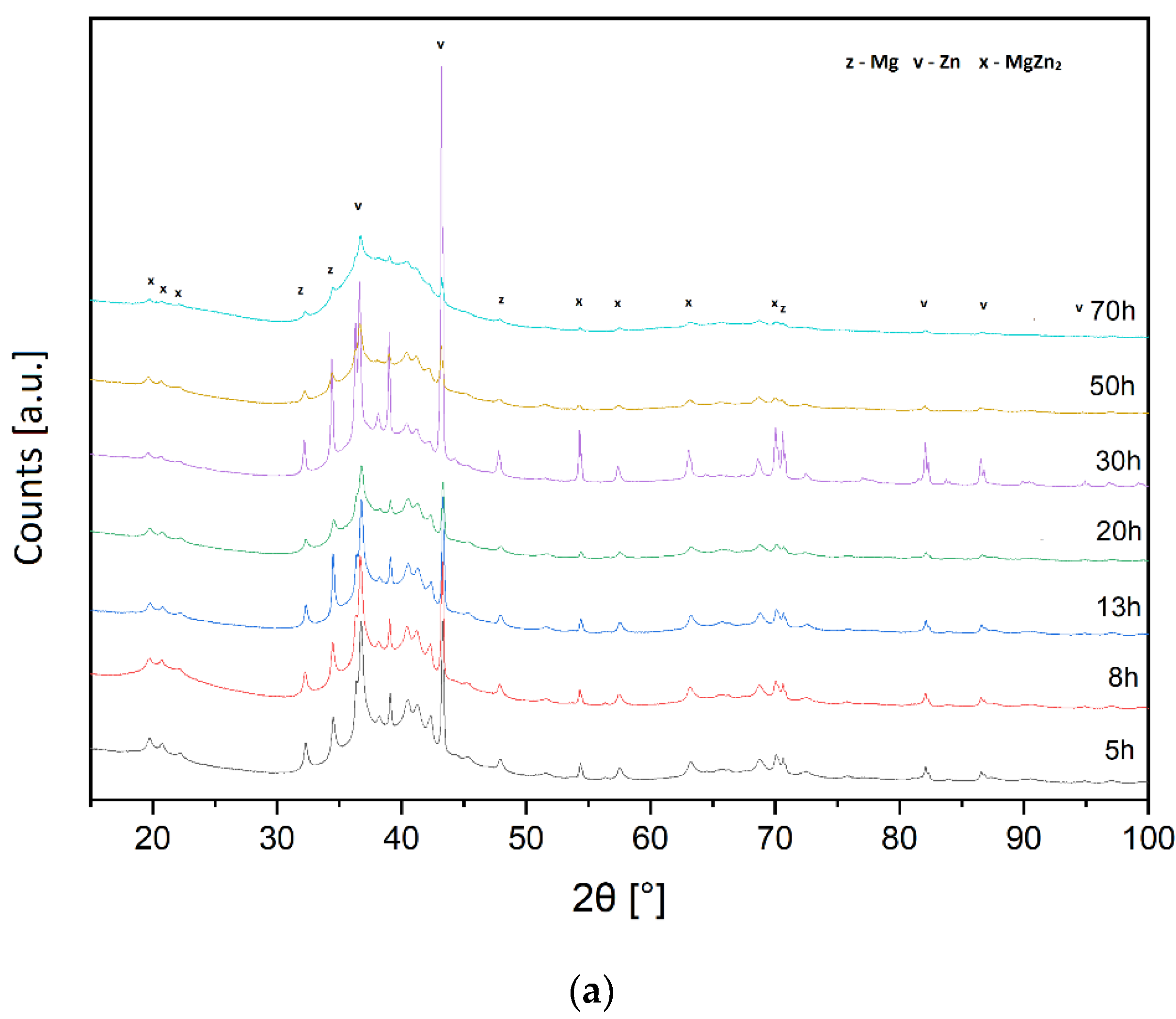
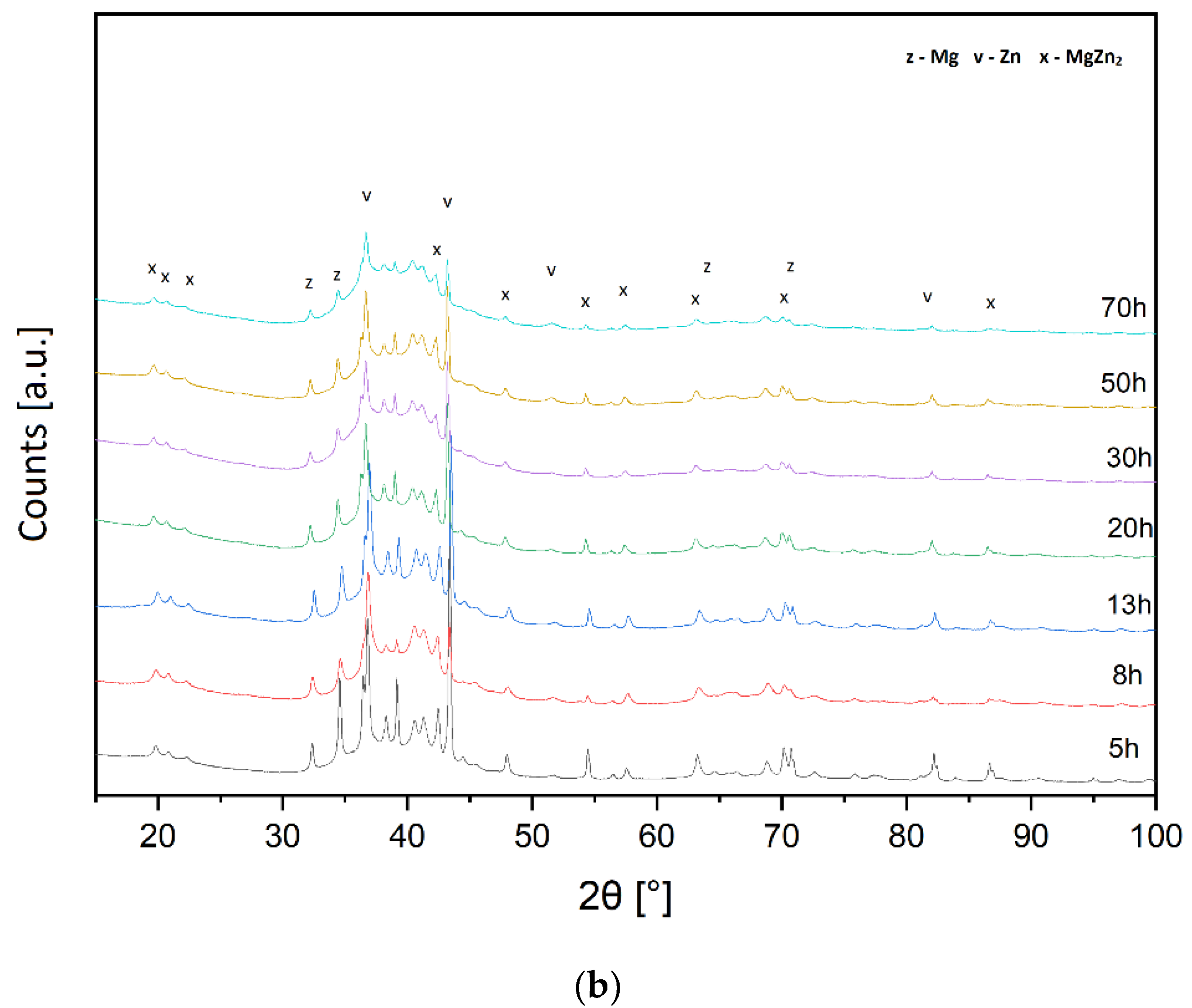
3.1. XRD Analysis
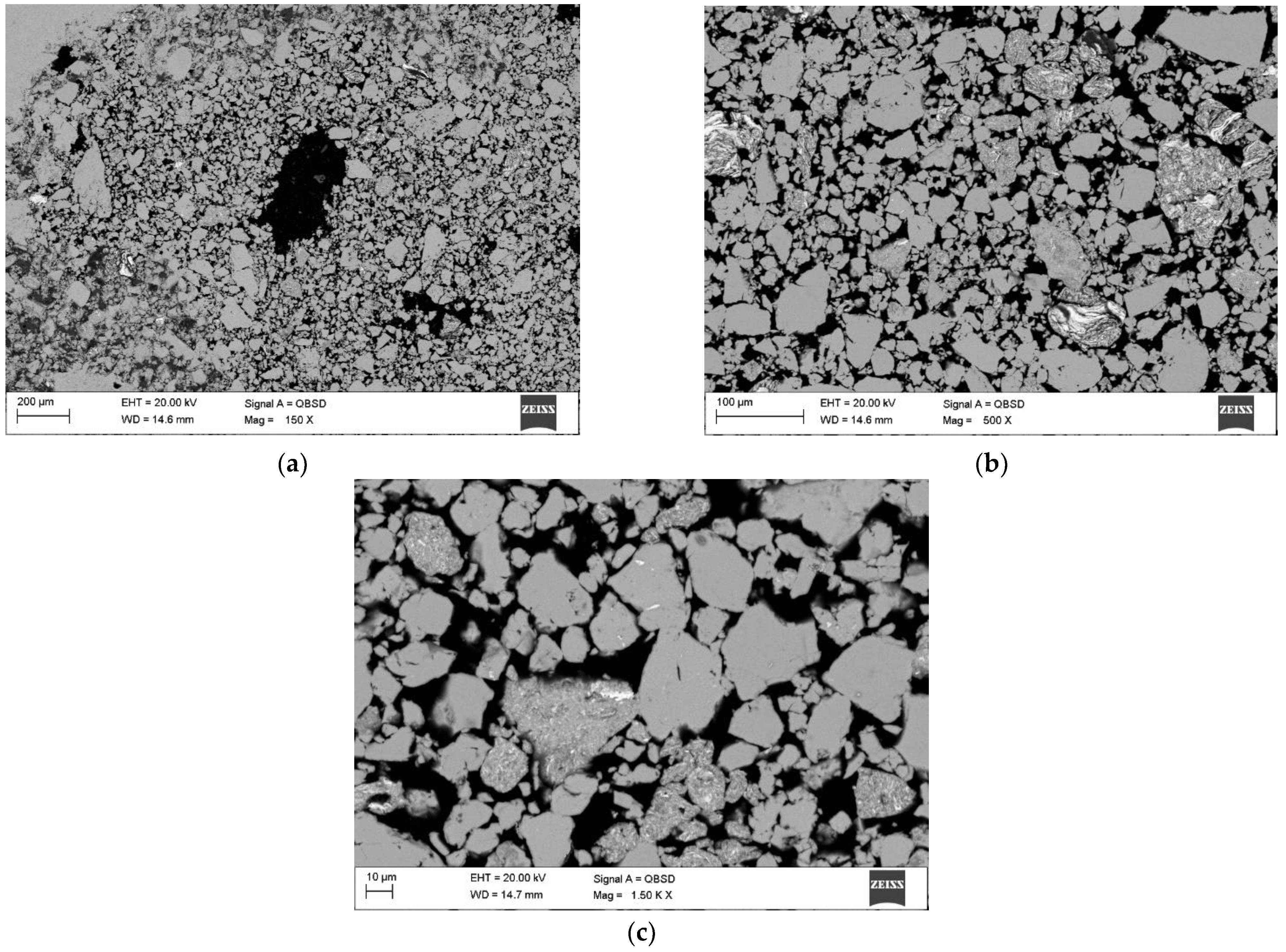
3.2. SEM Analysis
3.3. TEM Analysis
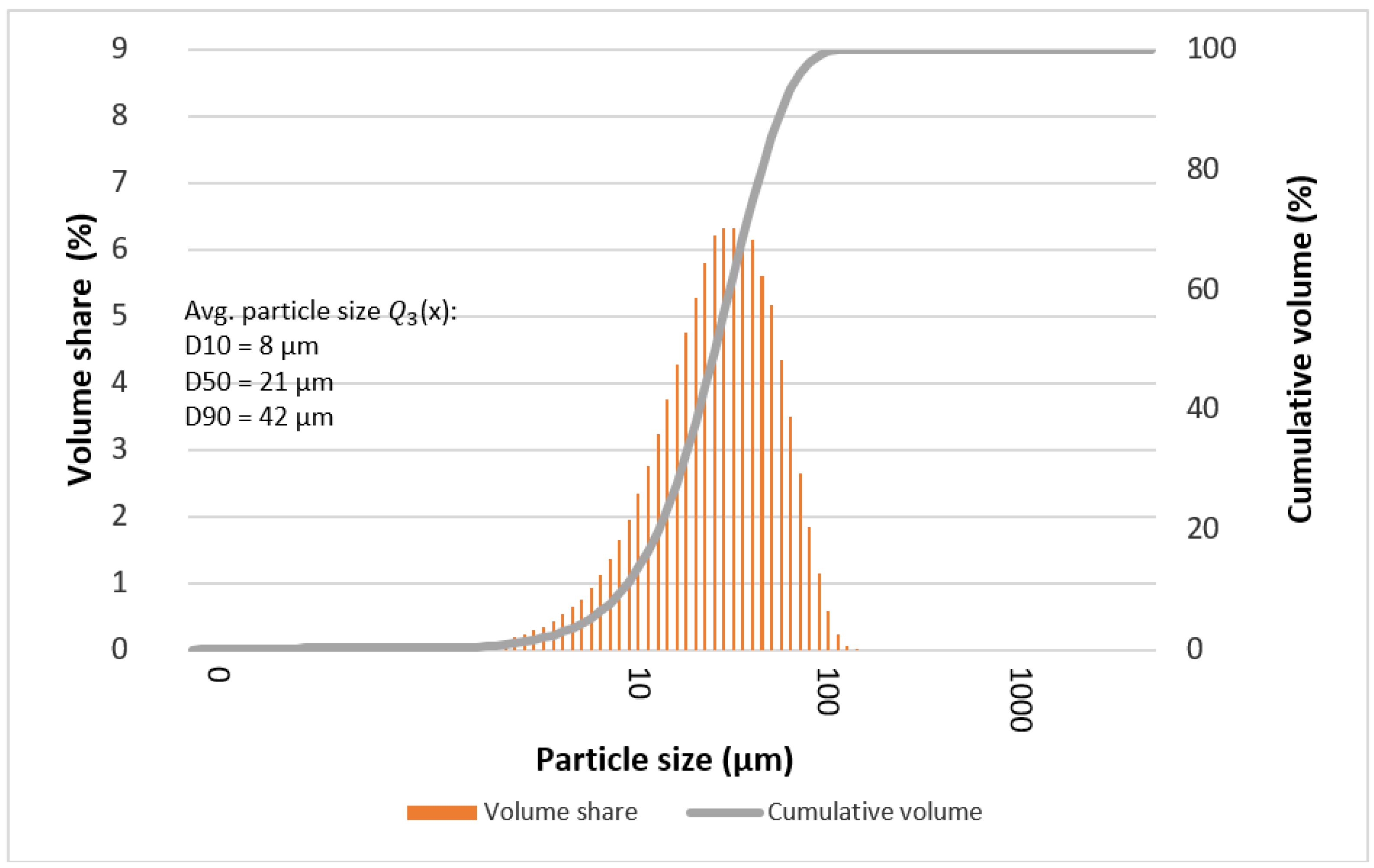
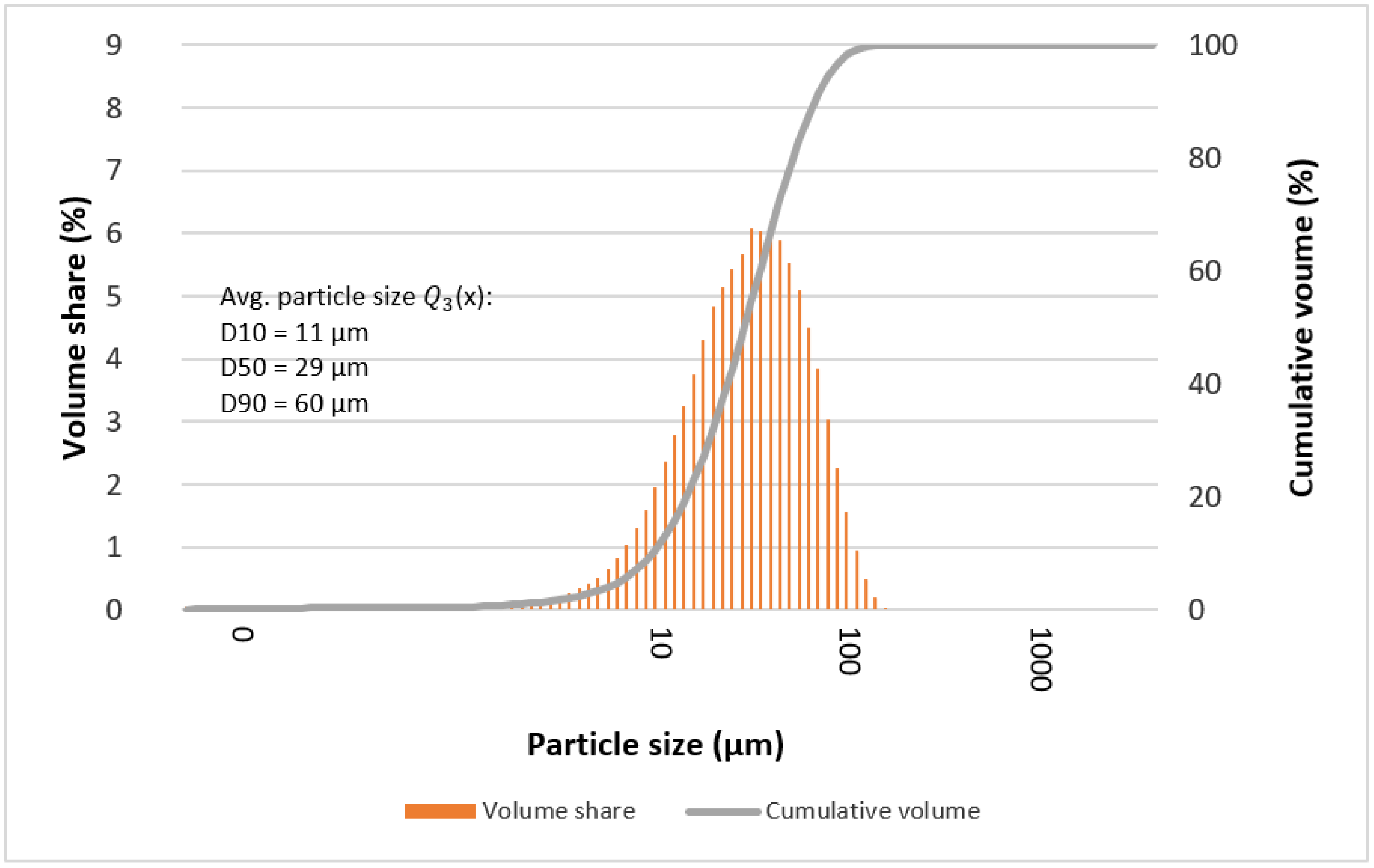
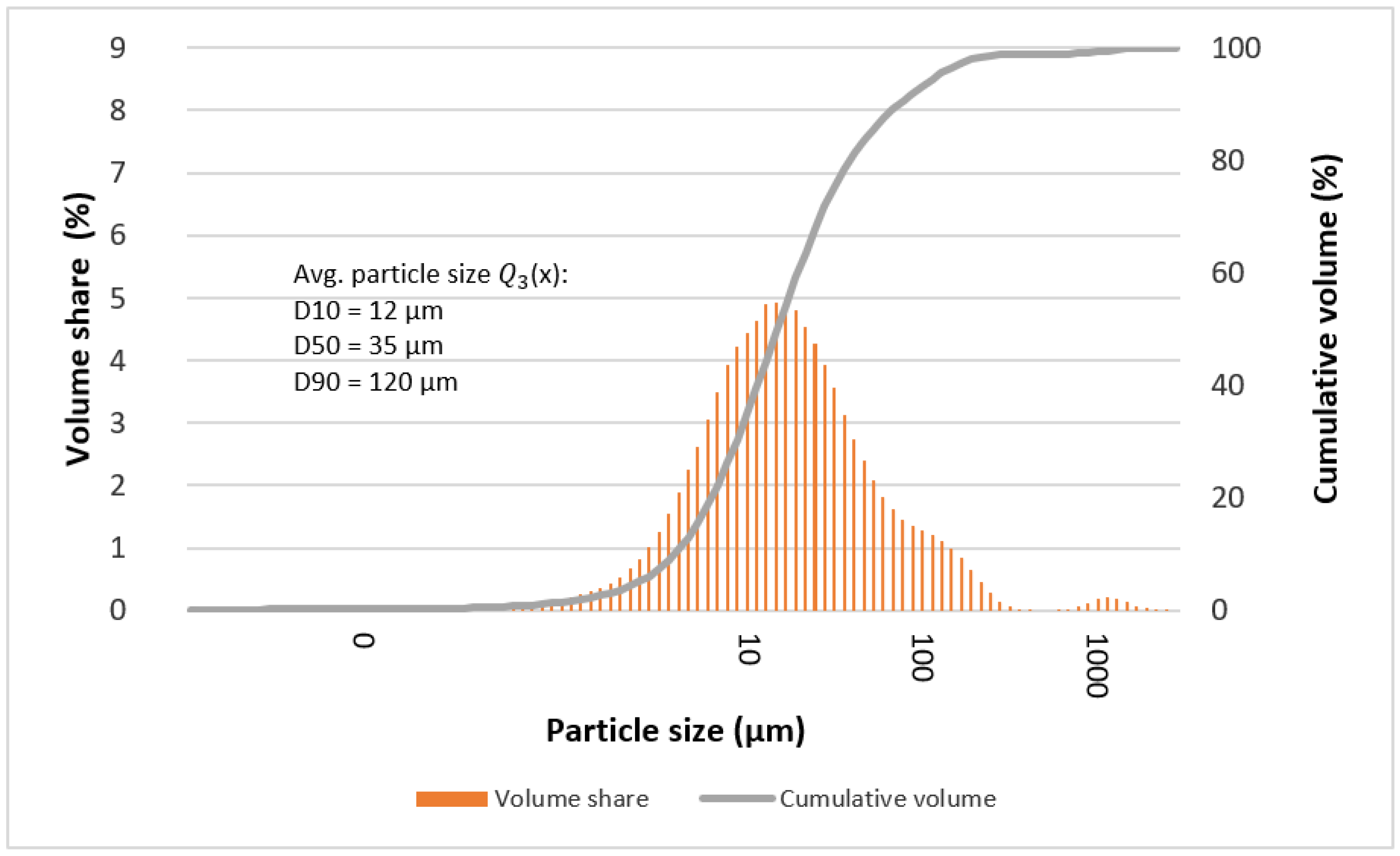
3.4. Particle Size
4. Summary
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Świeczko-Żurek, B. Biomaterials; Gdansk University of Technology Publishing House: Gdansk, Poland, 2009. [Google Scholar]
- Nałęcz, M. Biomaterials—Biocybernetics and Biomedical Engineering 2000; Academic Publishing House EXIT: Warsaw, Poland, 2003. [Google Scholar]
- Clinical Applications of Biomaterials. NIH Consens Statement. In Proceedings of the Biomaterials Consensus Conference at the National Institute of Health, Online, 1–3 November 1982; Volume 4, pp. 1–19.
- Będziński, R. Engineering Biomechanics; Selected Issues; Wroclaw University of Technology Publishing House: Wrocław, Poland, 1997. [Google Scholar]
- Marciniak, J. Biomaterials; The Publishing House of the Silesian University of Technology: Gliwice, Poland, 2002. [Google Scholar]
- Rodriguez, B.; Romero, A.; Soto, O.; de Varorna, O. Biomaterials for orthopedics. In Applications of Engineering Mechanics in Medicine; University of Puerto Rico: Mayagüez, Puerto Rico, 2004; pp. 1–26. [Google Scholar]
- Kamachi Mudali, U.; Sridhar, T.M.; Raj, B. Corrosion of bio implants. Sadhana 2003, 28, 601–637. [Google Scholar] [CrossRef]
- Łaskawiec, J.; Michalik, R. Theoretical and Application Issues in Implants; The Publishing House of the Silesian University of Technology: Gliwice, Poland, 2002. [Google Scholar]
- Williams, D.F. Definitions in biomaterials. J. Polym. Sci. Polym. Lett. Ed. 1988, 26, 414. [Google Scholar]
- Marciniak, J.; Paszenda, Z. Biotolerance of metallic biomaterials. In Advanced Spine Treatment Spondyloimplantology System DERO; Ciupik, L.F., Zarzycki, D., Eds.; Polish Group DERO, Association for Studies and Research of the Spine (Stowarzyszenie Studiów i Badań Kręgosłupa): Zielona Góra, Poland, 2004; pp. 133–142. [Google Scholar]
- Świeczko-Żurek, B.; Krzemiński, M. The degradation of metal implants. Adv. Mater. Sci. 2008, 8, 195–199. [Google Scholar] [CrossRef][Green Version]
- Drygała, A.; Dobrzański, L.A.; Szindler, M.; Prokopiuk vel Prokopowicz, M.; Pawlyta, M.; Lukaszkowicz, K. Carbon nanotubes counter electrode for dye-sensitized solar cells application. Arch. Metall. Mater. 2016, 61, 803–806. [Google Scholar] [CrossRef]
- Drygała, A. Influence of TiO2 film thickness on photovoltaic properties of dye-sensitized solar cells. IOP Conf. Ser. Earth Environ. Sci. 2021, 642, 012001. [Google Scholar] [CrossRef]
- Barzegari, M.; Mei, D.; Lamaka, S.V.; Geris, L. Computational modeling of degradation process of biodegradable magnesium biomaterials. Corros. Sci. 2021, 190, 109674. [Google Scholar] [CrossRef]
- Papierkowski, A. The importance of magnesium in medical practice. Part I. Causes and symptoms of magnesium metabolism disorders. In Borgis—Family Medicine (Medycyna Rodzinna); Termedia: Poznań, Poland, 2002; Volume 1, pp. 31–34. [Google Scholar]
- Rajkumar, K.; Ramraji, K.; Namratha, G.; Manoj, S.; Gnanavelbabu, A. A study of bio and tribo modifier on degradation of magnesium-calcium carbonate biomaterial. Mater. Today Proc. 2020, 27, 691–695. [Google Scholar] [CrossRef]
- Wypartowicz, J.; Łędzki, A.; Drożdż, P.; Stachura, R. Metallurgy of Non-Ferrous Metals; Lecture Materials; AGH University of Science and Technology: Kraków, Poland, 2018. (In Polish) [Google Scholar]
- Song, J.; She, J.; Chen, D.; Pan, F. Latest research advances on magnesium and magnesium alloys worldwide. J. Magnes. Alloy 2020, 8, 1–41. [Google Scholar] [CrossRef]
- Wei, L.Y.; Dunlop, G.L.; Westengen, H. Precipitation hardening of Mg-Zn and Mg-ZnRE alloys. Metall. Mater. Trans. A 1995, 26, 1705–1716. [Google Scholar] [CrossRef]
- Bogucka, A. Testing the Corrosion Resistance of the ZM21 Magnesium Alloy Used in Biomedical Applications after the Anodic Oxidation Process; Warsaw University of Technology: Warsaw, Poland, 2013. [Google Scholar]
- Kubok, K. Biodegradable Magnesium Alloys in Medical Applications; Institute of Metallurgy and Materials Science of Polish Academy of Sciences: Kraków, Poland, 2013. (In Polish) [Google Scholar]
- Wesołowski, K. Metallography, 3rd ed.; PWT, Polish Technical Publisher: Warsaw, Poland, 1957. (In Polish) [Google Scholar]
- Hansen, M.; Anderko, K. Constitution of Binary Alloys, 2nd ed.; McGraw-Hill: New York, NY, USA, 1958. [Google Scholar]
- Przybyłowicz, K. Metallography, 6th ed.; Scientific and Technical: Warsaw, Poland, 1999. (In Polish) [Google Scholar]
- Staub, F. Metallography; Świat Książki Publishing House, Silesian Technical Publisher: Katowice, Poland, 1994. (In Polish) [Google Scholar]
- Świdwińska-Gajewska, A.M.; Czerczak, S. Nano gold—Biological effects and acceptable levels of occupational exposure. Occup. Med. 2017, 4, 68. [Google Scholar] [CrossRef][Green Version]
- Lansdown, A.B. Silver in health care: Antimicrobial effects and safety in use. Curr. Probl. Dermatol. 2006, 33, 17–34. [Google Scholar] [CrossRef] [PubMed]
- Szyba, D.; Bajorek, A.; Babilas, D.; Temleitner, L.; Łukowiec, D.; Babilas, R. New resorbable Ca-Mg-Zn-Yb-B-Au alloys: Structural and corrosion resistance characterization. Mater. Des. 2022, 213, 110327. [Google Scholar] [CrossRef]
- Nowosielski, R. (Ed.) Resorbable Materials for Medical Implants; The Publishing House of the Silesian University of Technology: Gliwice, Poland, 2017. (In Polish) [Google Scholar]
- Zberg, B.; Uggowitzer, P.J.; Loffler, J.F. MgZnCa glasses without clinically observable hydrogen evolution for biodegradable implants. Nat. Mater. 2009, 8, 887–891. [Google Scholar] [CrossRef] [PubMed]
- Rakowska, J.; Radwan, K.; Ślosorz, Z. Problems of estimation the size and shape of grain solids, Chemical and Fire Research Laboratories. Mater. Sci. 2012, 3, 59–64. [Google Scholar]
- Kelsall, R.W.; Hamley, I.W.; Geoghegan, M. Nanotechnologies; Polish Scientific Publishers PWN: Warsaw, Poland, 2008. (In Polish) [Google Scholar]
- Lesz, S.; Tański, T.; Hrapkowicz, B.; Karolus, M.; Popis, J.; Wiechniak, K. Characterization of Mg-Zn-Ca-Y powders manufactured by mechanical milling. J. Achiev. Mater. Manuf. Eng. 2020, 103, 49–59. [Google Scholar] [CrossRef]
- Suryanarayana, C. Mechanical Alloying and Milling; Marcel Dekker: New York, NY, USA, 2004. [Google Scholar] [CrossRef]
- Jurczyk, M. Mechanical Synthesis; Publishing House of the Poznań University of Technology: Poznań, Poland, 2003. (In Polish) [Google Scholar]
- Wang, Y.P.; Li, B.S.; Ren, M.X.; Yang, C.; Fu, H.Z. Microstructure and compressive properties of AlCrFeCoNi high entropy alloy. Mater. Sci. Eng. A 2008, 491, 154–158. [Google Scholar] [CrossRef]
- Garbiec, D. Spark plasma sintering (SPS): Theory and practice. Mater. Eng. 2015, 36, 60–64. (In Polish) [Google Scholar]
- Lesz, S.; Kraczla, J.; Nowosielski, R. Synthesis of Mg-Zn-Ca alloy by the spark plasma sintering. In Materials Design and Applications II; da Silva, L.F.M., Ed.; Advanced Structured Materials; Springer: Cham, Switzerland, 2019; Volume 98, pp. 85–96. [Google Scholar]
- Lesz, S.; Kremzer, M.; Gołombek, K.; Nowosielski, R. Influence of milling time on amorphization of Mg-Zn-Ca powders synthesized by mechanical alloying technique. Arch. Metall. Mater. 2018, 63, 845–851. [Google Scholar] [CrossRef]
- Lesz, S.; Hrapkowicz, B.; Karolus, M.; Gołombek, K. Characteristics of the Mg-Zn-Ca-Gd alloy after mechanical alloying. Materials 2021, 14, 226. [Google Scholar] [CrossRef]
- Lesz, S.; Kraczla, J.; Nowosielski, R. Structure and compression strength characteristics of the sintered Mg–Zn–Ca–Gd alloy for medical applications. Arch. Civ. Mech. Eng. 2018, 18, 1288–1299. [Google Scholar] [CrossRef]
- Gabryś, A. Sintered Magnesium-Based Biomaterials with Noble Metals Additions. Ph.D. Thesis, Silesian University of Technology, Gliwice, Poland, 2022. (In Polish). [Google Scholar]
- Young, R. The Rietveld Method; Oxford University Press: Oxford, UK, 1993. [Google Scholar]
- McCusker, L.B.; Von Dreele, R.B.; Cox, D.E.; Louër, D.; Scardi, P. Rietveld refinement guidelines. J. Appl. Crystallogr. 1999, 32, 36–50. [Google Scholar] [CrossRef]
- Karolus, M.; Łągiewka, E. Crystallite size and lattice strain in nanocrystalline Ni-Mo alloys studied by Rietveld Refinement. J. Alloys Compd. 2004, 367, 235–238. [Google Scholar] [CrossRef]
- Karolus, M. Applications of Rietveld refinement in Fe–B–Nb alloy structure studies. J. Mater. Process. Technol. 2006, 175, 246–250. [Google Scholar] [CrossRef]
- Karolus, M.; Panek, J. Nanostructured N-Ti alloys obtained by mechanical synthesis and heat treatment. J. Alloys Compd. 2016, 658, 709–715. [Google Scholar] [CrossRef]
- Hrapkowicz, B.; Lesz, S.; Karolus, M.; Garbiec, D.; Wiśniewski, J.; Rubach, R.; Gołombek, K.; Kremzer, M.; Popis, J. Microstructure and Mechanical Properties of Spark Plasma Sintered Mg-Zn-Ca-Pr Alloy. Metals 2022, 12, 375. [Google Scholar] [CrossRef]
- Polmear, I.J. Magnesium alloys and applications. Mater. Sci. Technol. 1994, 10, 1–16. [Google Scholar] [CrossRef]
- Oh, J.C.; Ohkubo, T.; Mukai, T.; Hono, K. TEM and 3 DAP characterization of an agehardened Mg-Ca-Zn alloy. Scr. Mater. 2005, 53, 675–679. [Google Scholar] [CrossRef]
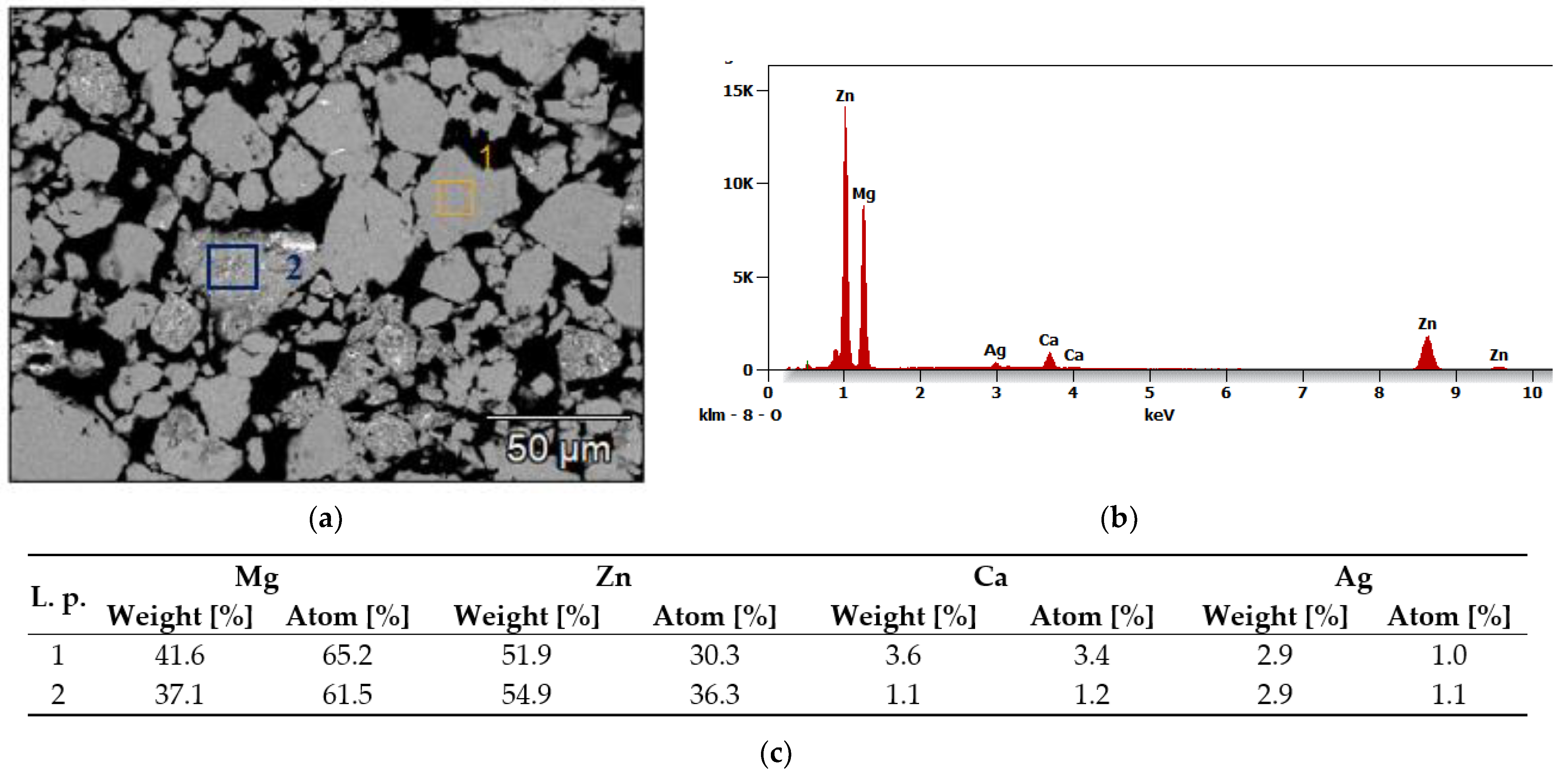
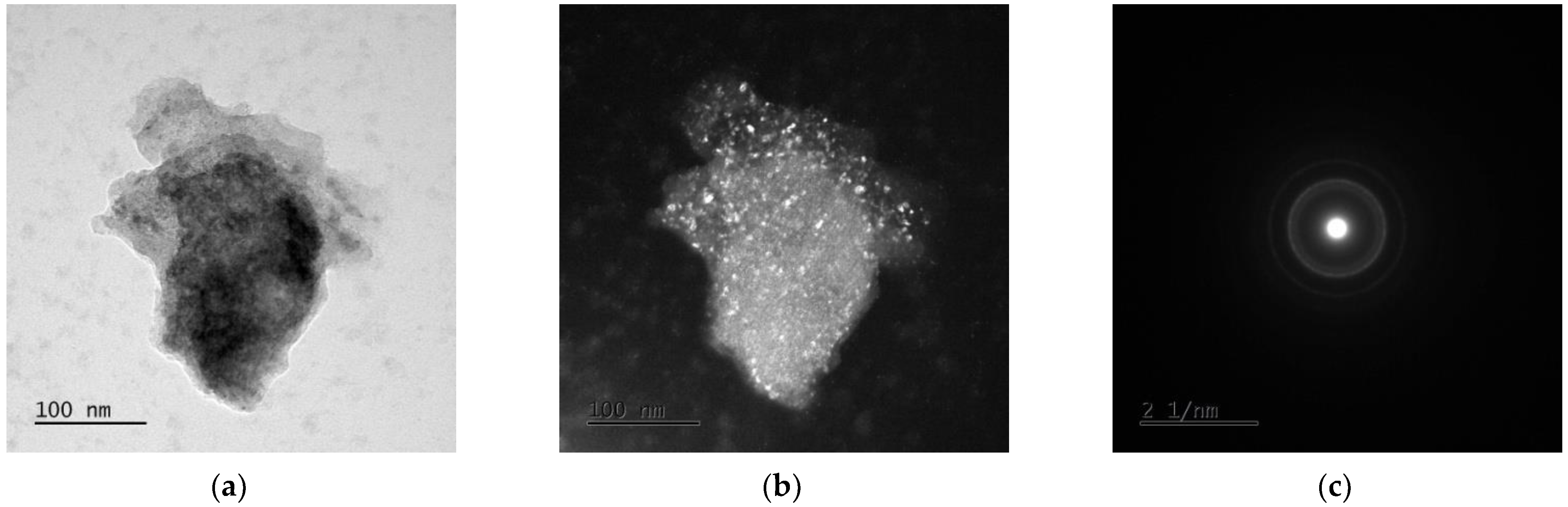
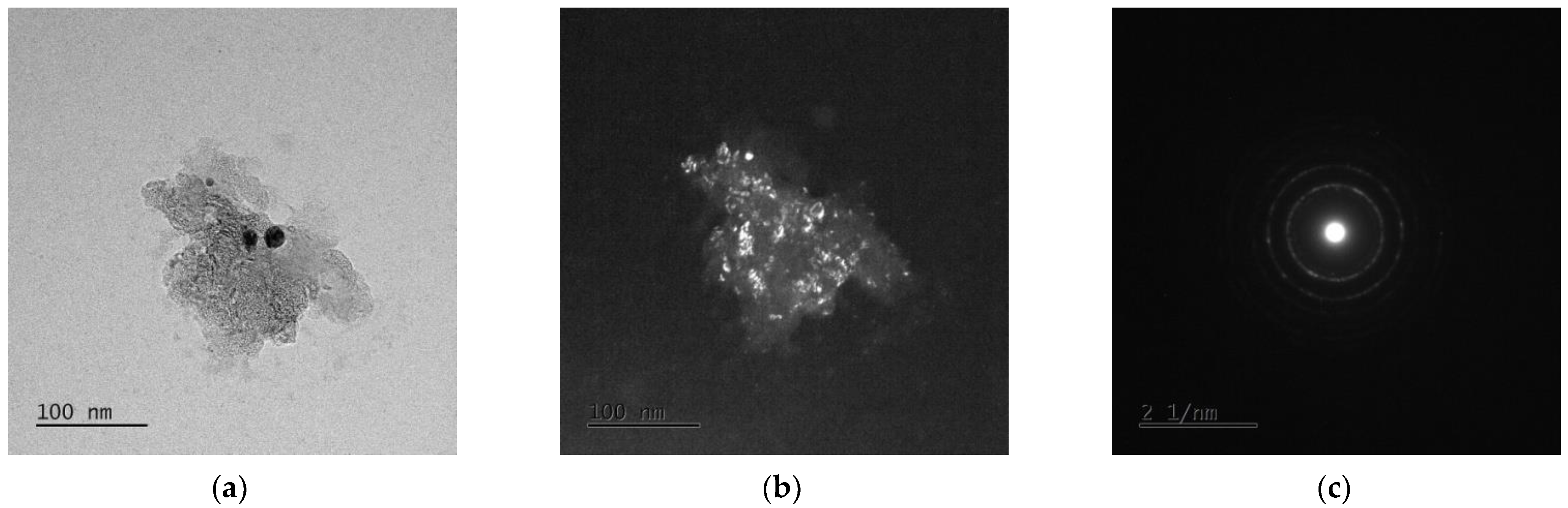
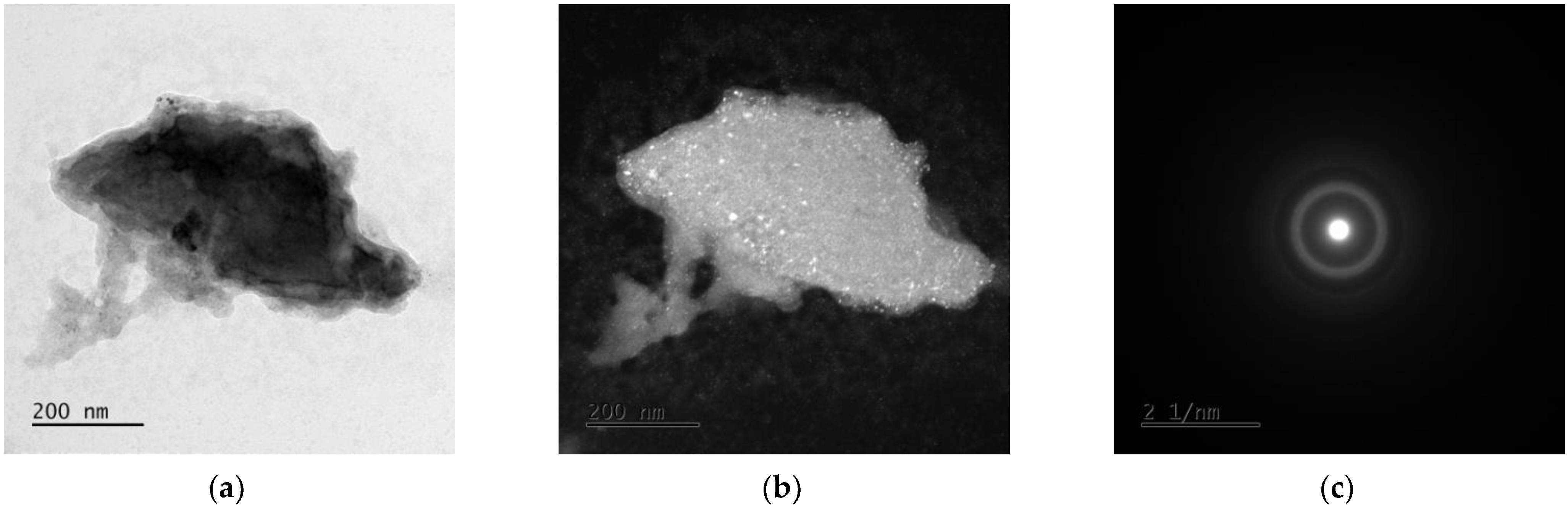


| Alloy | Mg [g] | Zn [g] | Ca [g] | Ag [g] |
|---|---|---|---|---|
| Mg65Zn30Ca4Ag1 | 4.147 | 5.149 | 0.421 | 0.283 |
| Mg64Zn30Ca4Ag2 | 3.996 | 5.038 | 0.412 | 0.554 |
| Alloy | ICDD PDF4: 00-035-0821 MgZn2 | Unit Cell Parameters after Rietveld Refinement [Å] | Crystallite Size D [Å] | Lattice Strain η [%] | ICDD PDF4: 04-008-7744 Mg (X) (Where X = Zn, Ca, Ag) | Unit Cell Parameters after Rietveld Refinement [Å] | Crystallite Size D [Å] | Lattice Strain η [%] |
|---|---|---|---|---|---|---|---|---|
| Ag1 5 h | a = 3.2050 [Å] c = 5.2150 [Å] Space Group: P63/mmc | a = 3.2040 (5) c = 5.2011 (9) | 544 | 0.47 | a = 5.2120 [Å] c = 8.6000 [Å] Space Group: P63/mmc | a = 5.2632 (9) c = 8.7770 (1) | 500 | 0.03 |
| Alloy | ICDD PDF4: 00-035-0821 MgZn2 | Unit cell Parameters after Rietveld Refinement [Å] | Crystallite Size D [Å] | Lattice Strain η [%] | ICDD PDF4: 04-008-7744 Mg(X) (Where X = Zn, Ca, Ag) | Unit cell Parameters after Rietveld Refinement [Å] | Crystallite Size D [Å] | Lattice Strain η [%] |
|---|---|---|---|---|---|---|---|---|
| Ag2 5 h | a = 3.2050 [Å] c = 5.2150 [Å] Space Group: P63/mmc | a = 3.2086 (9) c = 5.2115 (4) | 550 | 0.21 | a = 5.2120 [Å] c = 8.6000 [Å] Space Group: P63/mmc | a = 5.2399 (5) c = 9.0966 (4) | 500 | 0.03 |
| Ag2 30 h | a = 3.2050 [Å] c = 5.2150 [Å] Space Group: P63/mmc | a = 3.2021 (1) c = 5.1916 (7) | 500 | 0.82 | a = 5.2120 [Å] c = 8.6000 [Å] Space Group: P63/mmc | a = 5.3245 (1) c = 8.7726 (8) | 400 | 0.03 |
| Mg65Zn30Ca4Ag1 | |||||||
|---|---|---|---|---|---|---|---|
| Hours | 5 | 8 | 13 | 20 | 30 | 50 | 70 |
| D10 | 14 | 8 | 8 | 8 | 7 | 8 | 11 |
| D50 | 36 | 21 | 27 | 34 | 32 | 21 | 29 |
| D90 | 70 | 41 | 61 | 65 | 60 | 42 | 60 |
| Mg64Zn30Ca4Ag2 | |||||||
|---|---|---|---|---|---|---|---|
| Hours | 5 | 8 | 13 | 20 | 30 | 50 | 70 |
| D10 | 9 | 13 | 12 | 13 | 9 | 11 | 12 |
| D50 | 52 | 37 | 35 | 39 | 28 | 38 | 35 |
| D90 | 76 | 89 | 108 | 91 | 75 | 104 | 120 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lesz, S.; Karolus, M.; Gabryś, A.; Kremzer, M. Influence of Milling Time on Phase Composition and Product Structure of Mg-Zn-Ca-Ag Alloys Obtained by Mechanical Synthesis. Materials 2022, 15, 7333. https://doi.org/10.3390/ma15207333
Lesz S, Karolus M, Gabryś A, Kremzer M. Influence of Milling Time on Phase Composition and Product Structure of Mg-Zn-Ca-Ag Alloys Obtained by Mechanical Synthesis. Materials. 2022; 15(20):7333. https://doi.org/10.3390/ma15207333
Chicago/Turabian StyleLesz, Sabina, Małgorzata Karolus, Adrian Gabryś, and Marek Kremzer. 2022. "Influence of Milling Time on Phase Composition and Product Structure of Mg-Zn-Ca-Ag Alloys Obtained by Mechanical Synthesis" Materials 15, no. 20: 7333. https://doi.org/10.3390/ma15207333
APA StyleLesz, S., Karolus, M., Gabryś, A., & Kremzer, M. (2022). Influence of Milling Time on Phase Composition and Product Structure of Mg-Zn-Ca-Ag Alloys Obtained by Mechanical Synthesis. Materials, 15(20), 7333. https://doi.org/10.3390/ma15207333








