Investigating the Effect of PCL Concentrations on the Characterization of PLA Polymeric Blends for Biomaterial Applications
Abstract
:1. Introduction
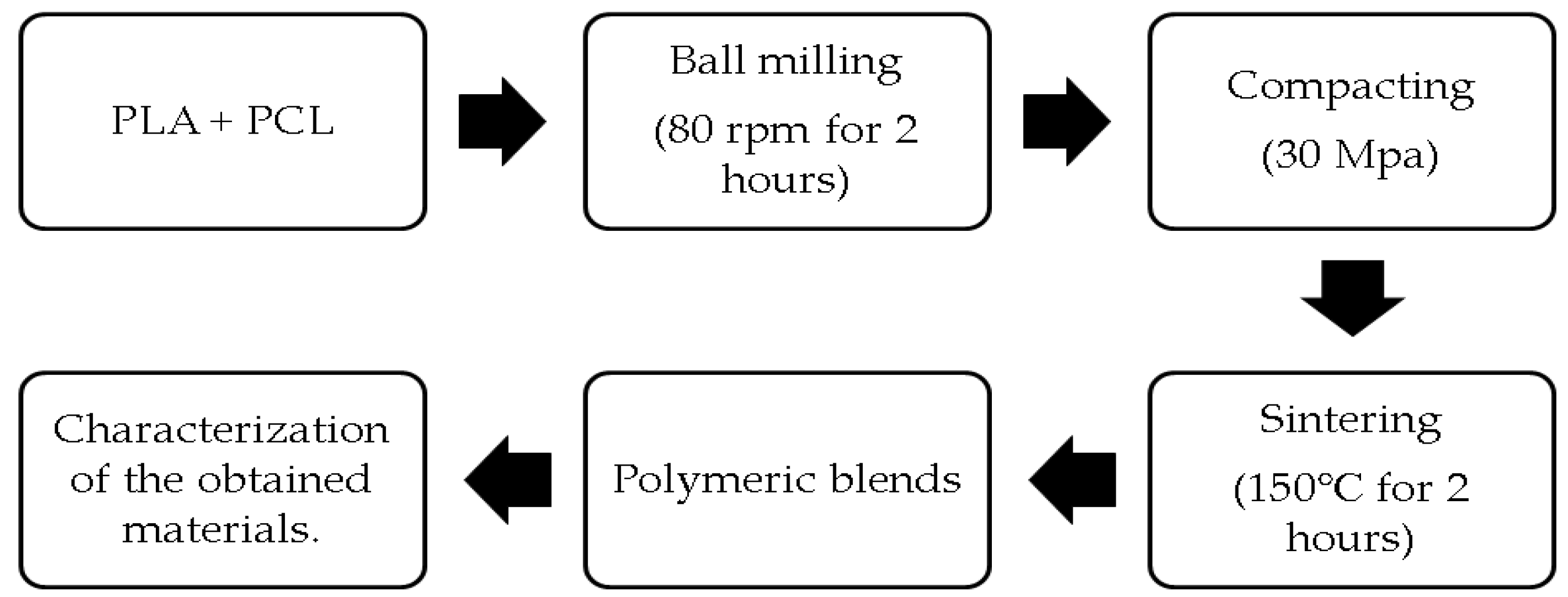
2. Materials and Methods
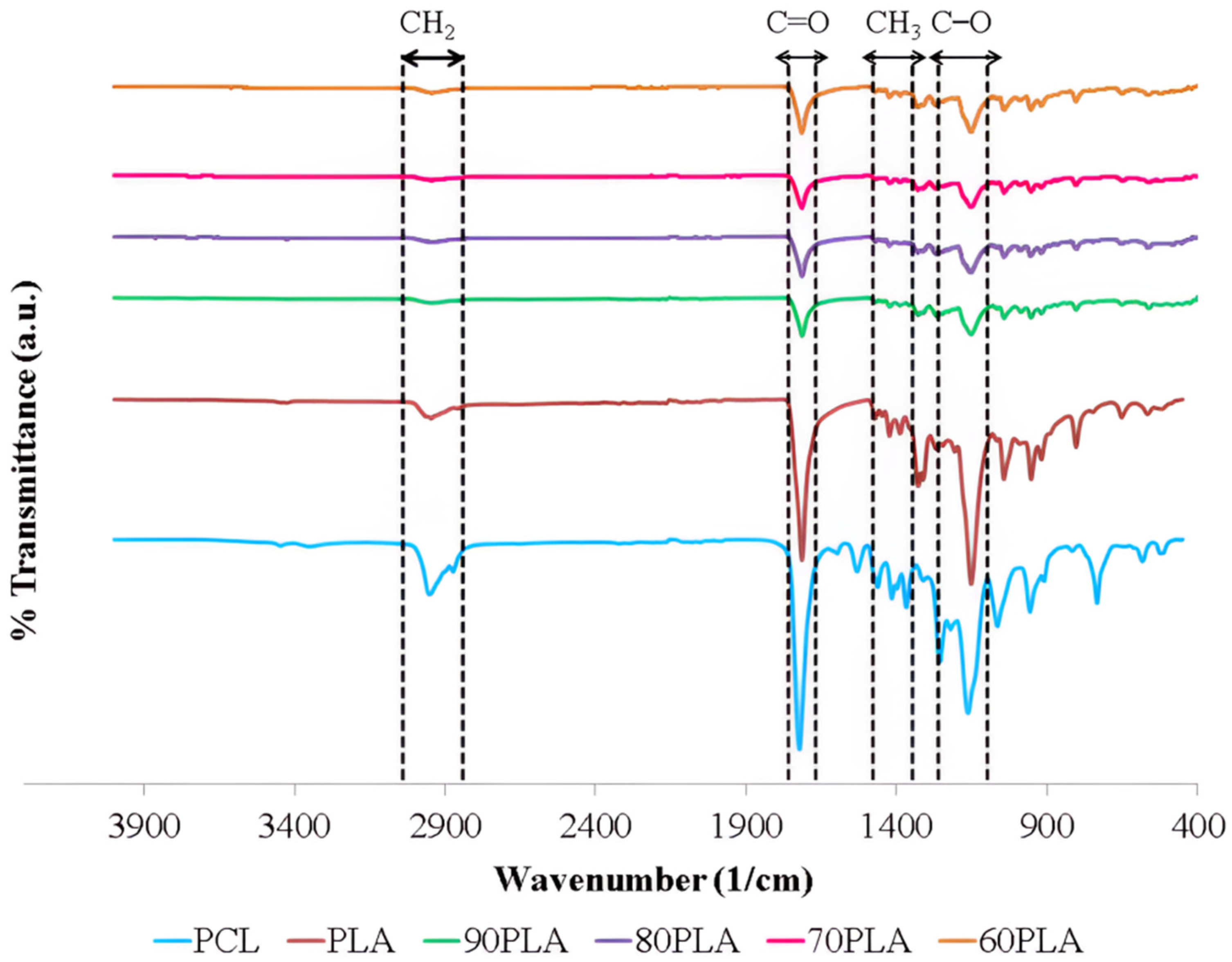
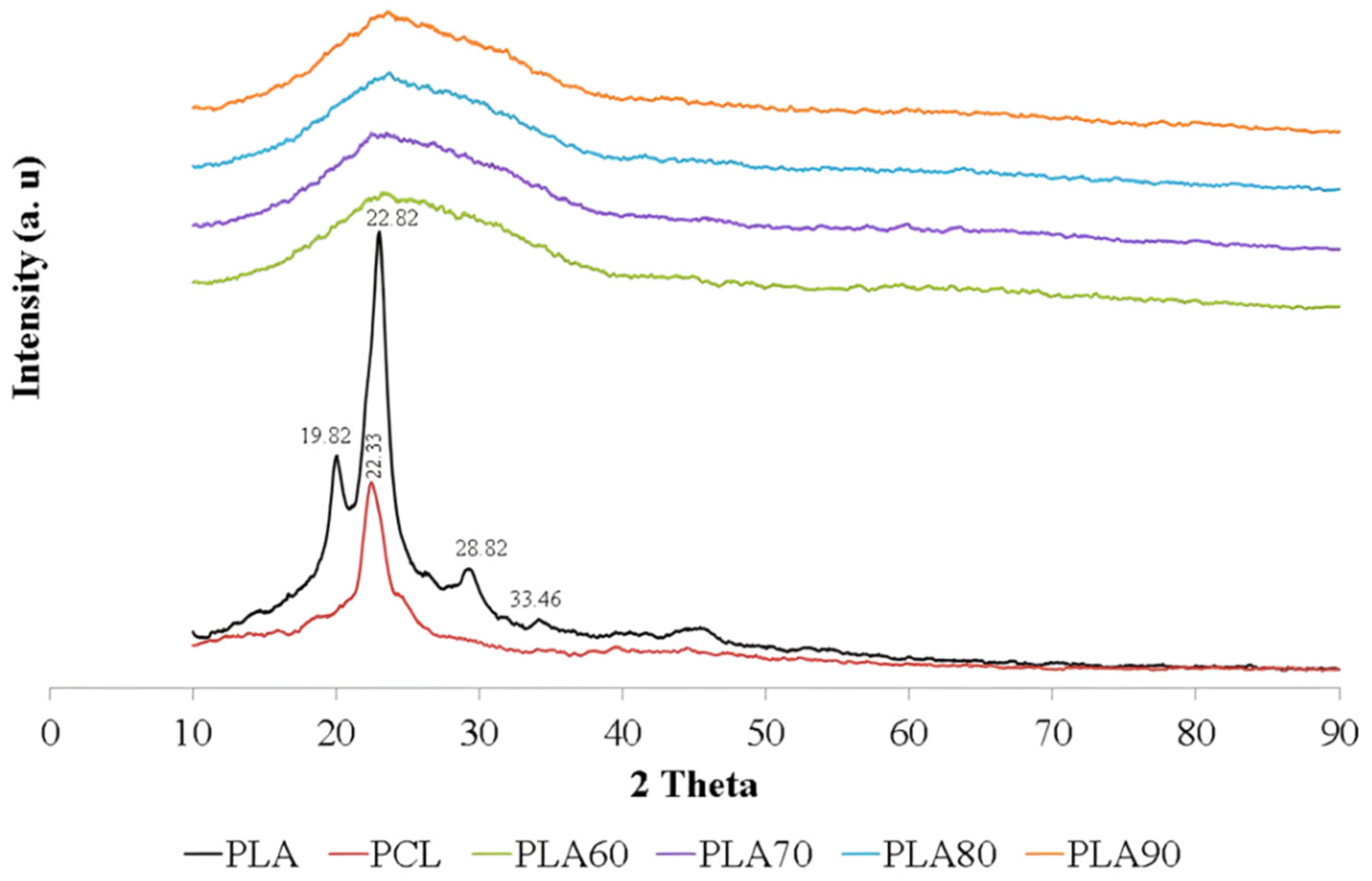
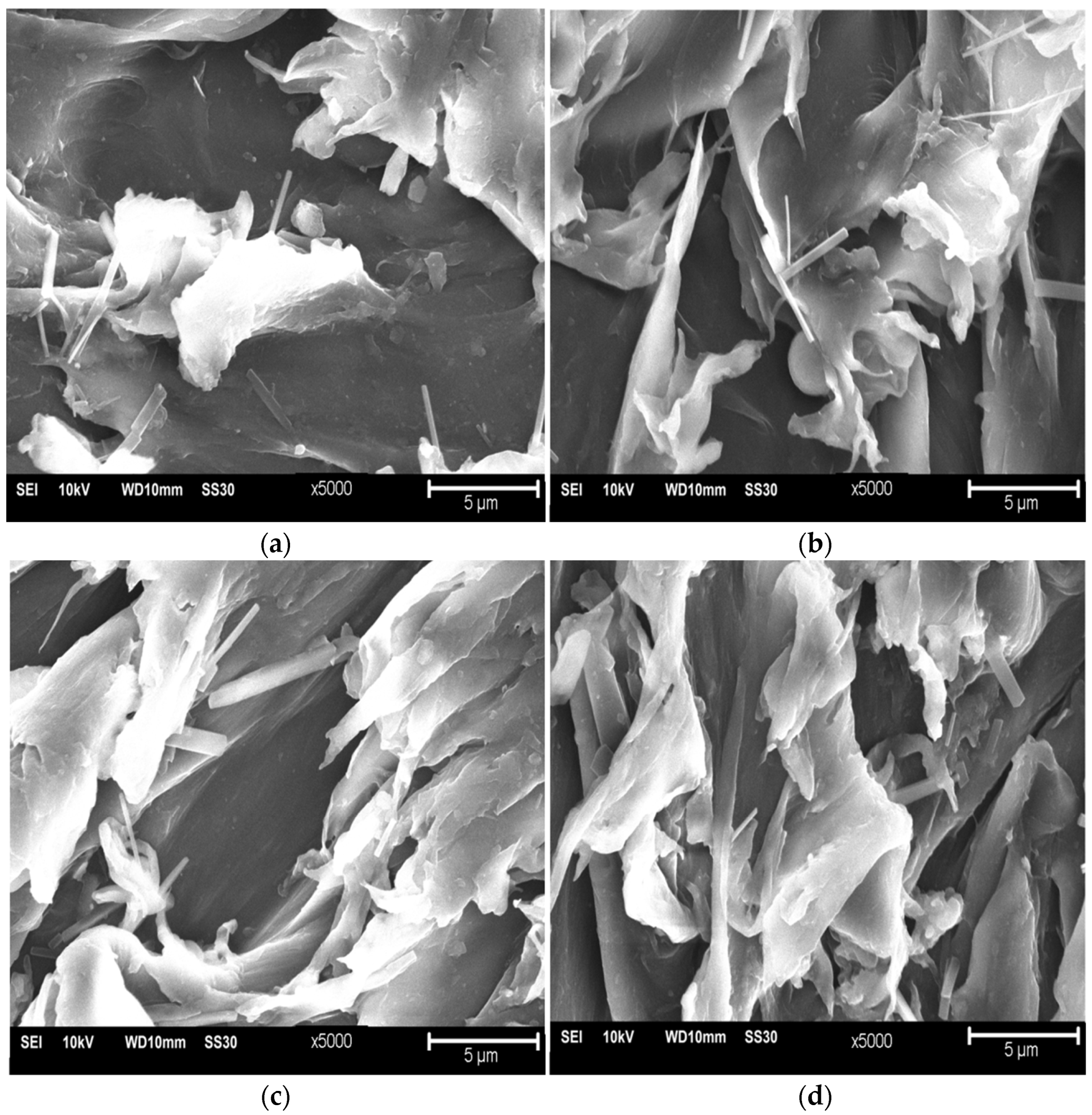
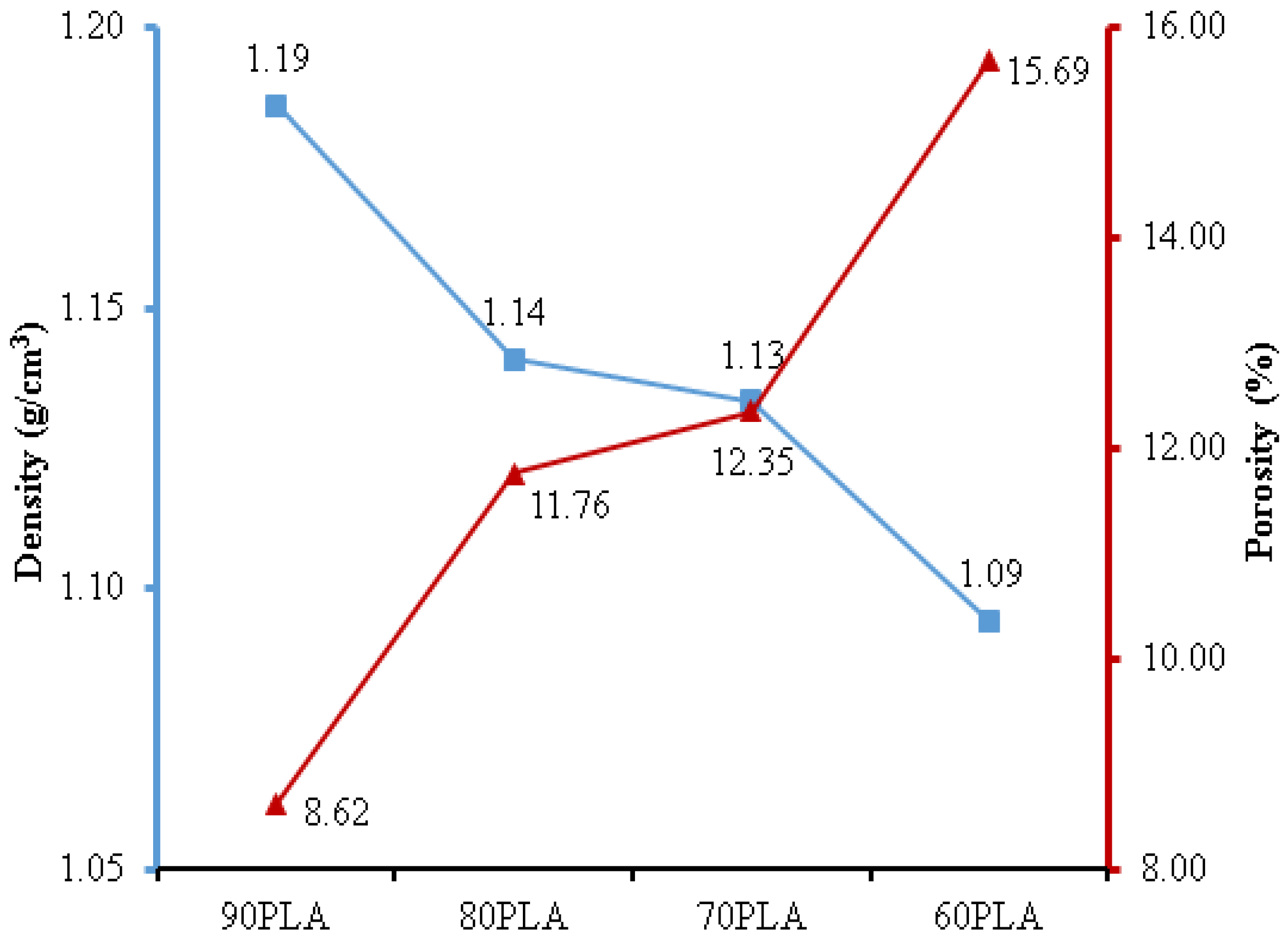
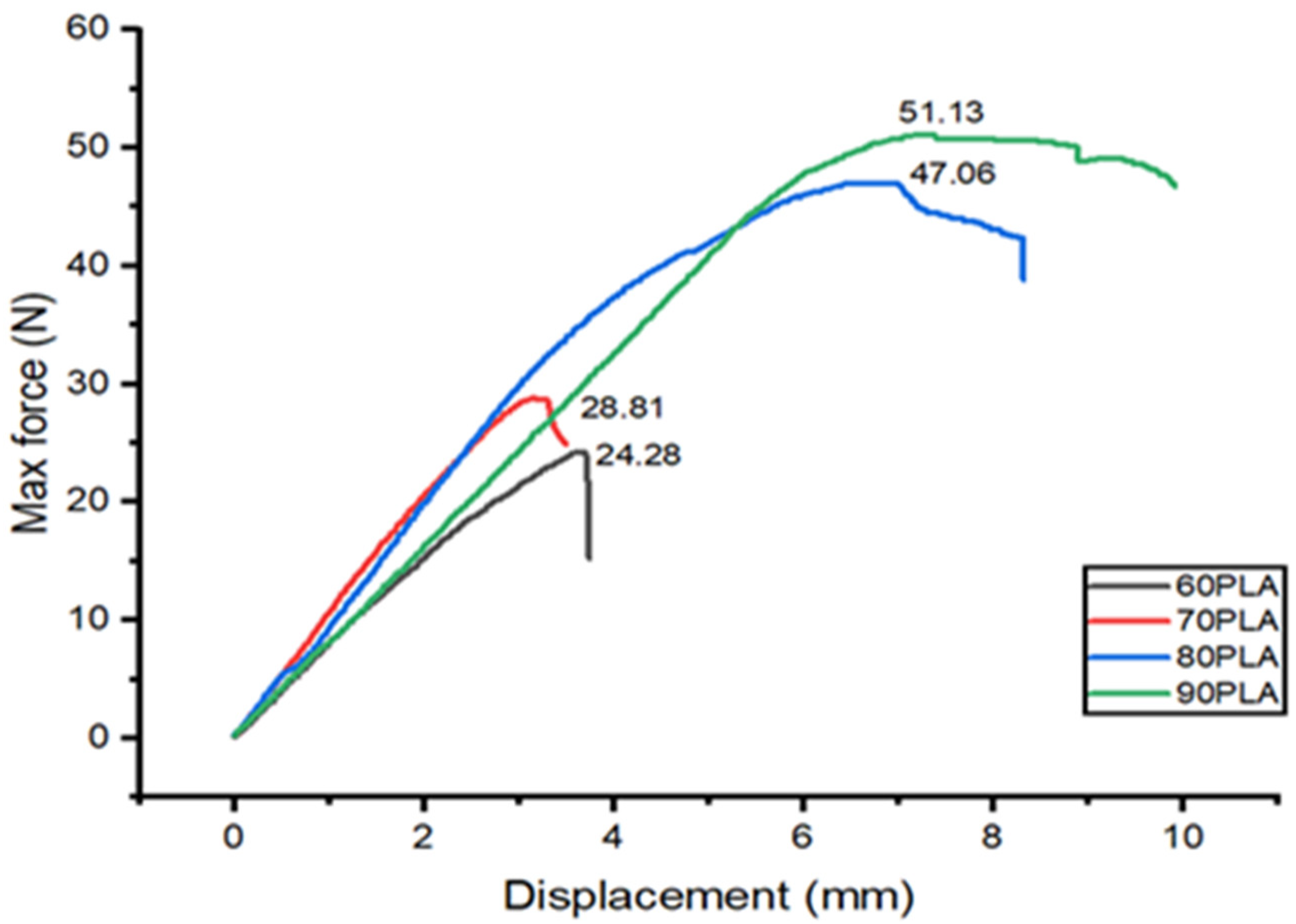
3. Results and Discussion
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Nashrullah, M.; Shinta, B.C.; Hidayat, M.I.P.; Purniawan, A.; Setiyorini, Y. Effect of Screw Diameter in Femoral Fracture Fixation Modeled by Finite Element Method. IPTEK J. Proc. Ser. 2017, 3, 173–180. [Google Scholar] [CrossRef] [Green Version]
- Marsell, R.; Einhorn, T.A. The biology of fracture healing. Injury 2011, 42, 551–555. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kim, T.; See, C.W.; Li, X.; Zhu, D. Orthopedic implants and devices for bone fractures and defects: Past, present and perspective. Eng. Regen. 2020, 1, 6–18. [Google Scholar] [CrossRef]
- Chen, F.-M.; Liu, X. Advancing biomaterials of human origin for tissue engineering. Prog. Polym. Sci. 2016, 53, 86–168. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ferrer, I.; Manresa, A.; Méndez, J.; Delgado-Aguilar, M.; Garcia-Romeu, M. Manufacturing PLA/PCL Blends by Ultrasonic Molding Technology. Polymers 2021, 13, 2412. [Google Scholar] [CrossRef] [PubMed]
- Song, R.; Murphy, M.; Li, C.; Ting, K.; Soo, C.; Zheng, Z. Current development of biodegradable polymeric materials for biomedical applications. Drug Des. Dev. Ther. 2018, 12, 3117–3145. [Google Scholar] [CrossRef] [Green Version]
- Xia, D.; Chen, J.; Zhang, Z.; Dong, M. Emerging polymeric biomaterials and manufacturing techniques in regenerative medicine. Aggregate 2022, e176. [Google Scholar] [CrossRef]
- Ulery, B.D.; Nair, L.S.; Laurencin, C.T. Biomedical applications of biodegradable polymers. J. Polym. Sci. Part B Polym. Phys. 2011, 49, 832–864. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Pradhan, S.; Rajamani, S.; Agrawal, G.; Dash, M.; Samal, S.K. NMR, FT-IR and Raman Characterization of Biomaterials. In Characterization of Polymeric Biomaterials; Tanzi, M.C., Farè, S., Eds.; Woodhead Publishing: Sawston, UK, 2017; pp. 147–173. ISBN 978-0-08-100737-2. [Google Scholar]
- Filippi, M.; Born, G.; Chaaban, M.; Scherberich, A. Natural Polymeric Scaffolds in Bone Regeneration. Front. Bioeng. Biotechnol. 2020, 8, 474. [Google Scholar] [CrossRef] [PubMed]
- Troy, E.; Tilbury, M.A.; Power, A.M.; Wall, J.G. Nature-Based Biomaterials and Their Application in Biomedicine. Polymers 2021, 13, 3321. [Google Scholar] [CrossRef] [PubMed]
- Elmowafy, E.M.; Tiboni, M.; Soliman, M.E. Biocompatibility, biodegradation and biomedical applications of poly(lactic acid)/poly(lactic-co-glycolic acid) micro and nanoparticles. J. Pharm. Investig. 2019, 49, 347–380. [Google Scholar] [CrossRef]
- Manavitehrani, I.; Fathi, A.; Badr, H.; Daly, S.; Negahi Shirazi, A.; Dehghani, F. Biomedical Applications of Biodegradable Polyesters. Polymers 2016, 8, 20. [Google Scholar] [CrossRef] [Green Version]
- Kundu, J.; Pati, F.; Jeong, Y.H.; Cho, D.-W. Biomaterials for Biofabrication of 3D Tissue Scaffolds. In Biofabrication; Forgacs, G., Sun, W.B.T.-B., Eds.; William Andrew Publishing: Boston, MA, USA, 2013; pp. 23–46. ISBN 978-1-4557-2852-7. [Google Scholar]
- Vasile, C.; Pamfil, D.; Stoleru, E.; Baican, M. New Developments in Medical Applications of Hybrid Hydrogels Containing Natural Polymers. Molecules 2020, 25, 1539. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Samir, A.; Ashour, F.H.; Hakim, A.A.A.; Bassyouni, M. Recent advances in biodegradable polymers for sustainable applications. NPJ Mater. Degrad. 2022, 6, 68. [Google Scholar] [CrossRef]
- Kasa, S.N.; Omar, M.F.; Abdullah, M.M.A.B.; Ismail, I.N.; Ting, S.S.; Vac, S.C.; Vizureanu, P. Effect of Unmodified and Modified Nanocrystalline Cellulose Reinforced Polylactic Acid (PLA) Polymer Prepared by Solvent Casting Method Morphology, mechanical and thermal properties. Mater. Plast. 2017, 54, 91–97. [Google Scholar] [CrossRef]
- Hassanajili, S.; Karami-Pour, A.; Oryan, A.; Talaei-Khozani, T. Preparation and characterization of PLA/PCL/HA composite scaffolds using indirect 3D printing for bone tissue engineering. Mater. Sci. Eng. C 2019, 104, 109960. [Google Scholar] [CrossRef]
- Ostafinska, A.; Fortelny, I.; Nevoralova, M.; Hodan, J.; Kredatusova, J.; Slouf, M. Synergistic effects in mechanical properties of PLA/PCL blends with optimized composition, processing, and morphology. RSC Adv. 2015, 5, 98971–98982. [Google Scholar] [CrossRef]
- Fortelny, I.; Ujcic, A.; Fambri, L.; Slouf, M. Phase Structure, Compatibility, and Toughness of PLA/PCL Blends: A Review. Front. Mater. 2019, 6, 206. [Google Scholar] [CrossRef] [Green Version]
- Delgado-Aguilar, M.; Puig, R.; Sazdovski, I.; Fullana-I-Palmer, P. Polylactic Acid/Polycaprolactone Blends: On the Path to Circular Economy, Substituting Single-Use Commodity Plastic Products. Materials 2020, 13, 2655. [Google Scholar] [CrossRef]
- Ferri, J.M.; Fenollar, O.; Jorda-Vilaplana, A.; García-Sanoguera, D.; Balart, R. Effect of miscibility on mechanical and thermal properties of poly(lactic acid)/polycaprolactone blends. Polym. Int. 2016, 65, 453–463. [Google Scholar] [CrossRef]
- Wachirahuttapong, S.; Thongpin, C.; Sombatsompop, N. Effect of PCL and Compatibility Contents on the Morphology, Crystallization and Mechanical Properties of PLA/PCL Blends. Energy Procedia 2016, 89, 198–206. [Google Scholar] [CrossRef] [Green Version]
- Navarro-Baena, I.; Sessini, V.; Dominici, F.; Torre, L.; Kenny, J.M.; Peponi, L. Design of biodegradable blends based on PLA and PCL: From morphological, thermal and mechanical studies to shape memory behavior. Polym. Degrad. Stab. 2016, 132, 97–108. [Google Scholar] [CrossRef]
- Hou, A.-L.; Qu, J.-P. Super-Toughened Poly(lactic Acid) with Poly(ε-caprolactone) and Ethylene-Methyl Acrylate-Glycidyl Methacrylate by Reactive Melt Blending. Polymers 2019, 11, 771. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Broz, M.E.; VanderHart, D.L.; Washburn, N.R. Structure and Mechanical Properties of Poly(D, L-Lactic Acid)/Poly(Epsilon -Caprolactone) Blends. Biomaterials 2003, 24, 4181–4190. [Google Scholar] [CrossRef]
- Zhai, W.; Ko, Y.; Zhu, W.; Wong, A.; Park, C.B. A Study of the Crystallization, Melting, and Foaming Behaviors of Polylactic Acid in Compressed CO2. Int. J. Mol. Sci. 2009, 10, 5381–5397. [Google Scholar] [CrossRef]
- López-Rodríguez, N.; Lopez-Arraiza, A.; Meaurio, E.; Sarasua, J.R. Crystallization, morphology, and mechanical behavior of polylactide/poly(ɛ-caprolactone) blends. Polym. Eng. Sci. 2006, 46, 1299–1308. [Google Scholar] [CrossRef]
- Urquijo, J.; Guerrica-Echevarria, G.; Eguiazábal, J.I. Melt processed PLA/PCL blends: Effect of processing method on phase structure, morphology, and mechanical properties. J. Appl. Polym. Sci. 2015, 132, 42641. [Google Scholar] [CrossRef]
- PLA. PLA Material Safety Data Sheet; PLA: Kowloon, Hong Kong, 2010. [Google Scholar]
- Polycaprolactone. CAPA ® 6500 Polycaprolactone Material Safety Data Sheet No 79398; PCL: Warrington, UK, 2022. [Google Scholar]
- Fitriyana, D.F.; Ismail, R.; Santosa, Y.I.; Nugroho, S.; Hakim, A.J.; Al Mulqi, M.S. Hydroxyapatite Synthesis from Clam Shell Using Hydrothermal Method: A Review. In Proceedings of the 2019 International Biomedical Instrumentation and Technology Conference (IBITeC), Special Region of Yogyakarta, Indonesia, 23–24 October 2019; Volume 1, pp. 7–11. [Google Scholar] [CrossRef]
- Ismail, R.; Laroybafih, M.B.; Fitriyana, D.F.; Nugroho, S.; Santoso, Y.I.; Hakim, A.J.; Al Mulqi, M.S.; Bayuseno, A.P. The Effect of Hydrothermal Holding Time on The Characterization of Hydroxyapatite Synthesized from Green Mussel Shells. J. Adv. Res. Fluid Mech. Therm. Sci. 2021, 80, 84–93. [Google Scholar] [CrossRef]
- Fitriyana, D.; Suhaimi, H.; Sulardjaka, S.; Noferi, R.; Caesarendra, W. Synthesis of Na-P Zeolite from Geothermal Sludge. In Proceedings in Physics; Springer: Singapore, 2020; pp. 51–59. ISBN 978-981-15-2293-2. [Google Scholar]
- Fitriyana, D.F.; Caesarendra, W.; Nugroho, S.; Haryadi, G.D.; Herawan, M.A.; Rizal, M.; Ismail, R. The Effect of Compressed Air Pressure and Stand-off Distance on the Twin Wire Arc Spray (TWAS) Coating for Pump Impeller from AISI 304 Stainless Steel. Springer Proc. Phys. 2020, 242, 119–130. [Google Scholar] [CrossRef]
- Fitriyana, D.F.; Anis, S.; Al Qudus, A.R.; Lakuy, M.A.N.; Ismail, R.; Nugroho, S.; Haryadi, G.D.; Bayuseno, A.P.; Siregar, J.P. The Effect of Post-Heat Treatment on The Mechanical Properties of FeCrBMnSi Coatings Prepared by Twin Wire Arc Spraying (TWAS) Method on Pump Impeller from 304 Stainless Steel. J. Adv. Res. Fluid Mech. Therm. Sci. 2022, 93, 138–147. [Google Scholar] [CrossRef]
- Fitriyana, D.F.; Nugraha, F.W.; Laroybafih, M.B.; Ismail, R.; Bayuseno, A.P.; Muhamadin, R.C.; Ramadan, M.B.; Qudus, A.R.; Siregar, J.P. The effect of hydroxyapatite concentration on the mechanical properties and degradation rate of biocomposite for biomedical applications. IOP Conf. Ser. Earth Environ. Sci. 2022, 969, 12045. [Google Scholar] [CrossRef]
- Taib, M.N.A.M.; Julkapli, N.M. Dimensional Stability of Natural Fiber-Based and Hybrid Composites; Elsevier Ltd.: Amsterdam, The Netherlands, 2018; ISBN 9780081022924. [Google Scholar]
- Nanda, B.P.; Satapathy, A. Processing and Characterization of Epoxy Composites Reinforced with Short Human Hair. J. Phys. Conf. Ser. 2017, 178, 012012. [Google Scholar] [CrossRef]
- Conrad, A.O.; Rodriguez-Saona, L.E.; McPherson, B.A.; Wood, D.L.; Bonello, P. Identification of Quercus agrifolia (coast live oak) resistant to the invasive pathogen Phytophthora ramorum in native stands using Fourier-transform infrared (FT-IR) spectroscopy. Front. Plant Sci. 2014, 5, 521. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Fang, J.; Zhang, L.; Sutton, D.; Wang, X.; Lin, T. Needleless Melt-Electrospinning of Polypropylene Nanofibres. J. Nanomater. 2012, 2012, 382639. [Google Scholar] [CrossRef] [Green Version]
- Shojaei, S.; Nikuei, M.; Goodarzi, V.; Hakani, M.; Khonakdar, H.A.; Saeb, M.R. Disclosing the role of surface and bulk erosion on the viscoelastic behavior of biodegradable poly(ε-caprolactone)/poly(lactic acid)/hydroxyapatite nanocomposites. J. Appl. Polym. Sci. 2018, 136, 47151. [Google Scholar] [CrossRef]
- Ferati, F. Structural Information from Ratio Bands in the FTIR Spectra of Long Chain and Branched Alkanes in Petrodiesel Samples. J. Environ. Treat. Tech. 2020, 8, 1140–1143. [Google Scholar]
- Shalumon, K.T.; Sreerekha, P.R.; Sathish, D.; Tamura, H.; Nair, S.V.; Chennazhi, K.P.; Jayakumar, R. Hierarchically designed electrospun tubular scaffolds for cardiovascular applications. J. Biomed. Nanotechnol. 2011, 7, 609–620. [Google Scholar] [CrossRef] [PubMed]
- Chomachayi, M.D.; Jalali-Arani, A.; Beltrán, F.R.; de la Orden, M.U.; Urreaga, J.M. Biodegradable Nanocomposites Developed from PLA/PCL Blends and Silk Fibroin Nanoparticles: Study on the Microstructure, Thermal Behavior, Crystallinity and Performance. J. Polym. Environ. 2020, 28, 1252–1264. [Google Scholar] [CrossRef]
- Decol, M.; Pachekoski, W.M.; Becker, D. Compatibilization and ultraviolet blocking of PLA/PCL blends via interfacial localization of titanium dioxide nanoparticles. J. Appl. Polym. Sci. 2017, 135, 44849. [Google Scholar] [CrossRef]
- Chee, W.K.; Ibrahim, N.A.; Zainuddin, N.; Rahman, M.F.A.; Chieng, B.W. Impact Toughness and Ductility Enhancement of Biodegradable Poly(lactic acid)/Poly(ε-caprolactone) Blends via Addition of Glycidyl Methacrylate. Adv. Mater. Sci. Eng. 2013, 2013, 976373. [Google Scholar] [CrossRef]
- Sundar, N.; Keerthana, P.; Kumar, S.A.; Ghosh, S. Dual purpose, bio-based polylactic acid (PLA)-polycaprolactone (PCL) blends for coated abrasive and packaging industrial coating applications. J. Polym. Res. 2020, 27, 386. [Google Scholar] [CrossRef]
- Sun, H.; Yu, B.; Han, J.; Kong, J.; Meng, L.; Zhu, F. Microstructure, Thermal Properties and Rheological Behavior of PLA/PCL Blends for Melt-blown Nonwovens. Polym. Korea 2014, 38, 477–483. [Google Scholar] [CrossRef] [Green Version]
- Hasan, A.; Soliman, S.; El Hajj, F.; Tseng, Y.-T.; Yalcin, H.C.; Marei, H. Fabrication and In Vitro Characterization of a Tissue Engineered PCL-PLLA Heart Valve. Sci. Rep. 2018, 8, 8187. [Google Scholar] [CrossRef] [PubMed]
- Liao, Y.; Liu, C.; Coppola, B.; Barra, G.; Di Maio, L.; Incarnato, L.; Lafdi, K. Effect of Porosity and Crystallinity on 3D Printed PLA Properties. Polymers 2019, 11, 1487. [Google Scholar] [CrossRef] [Green Version]
- Silverajah, V.S.G.; Ibrahim, N.A.; Yunus, W.M.Z.W.; Hassan, H.A.; Woei, C.B. A Comparative Study on the Mechanical, Thermal and Morphological Characterization of Poly(Lactic Acid)/Epoxidized Palm Oil Blend. Int. J. Mol. Sci. 2012, 13, 5878–5898. [Google Scholar] [CrossRef] [Green Version]
- Lü, Y.; Chen, Y.-C.; Zhang, P.-H. Preparation and Characterisation of Polylactic acid (PLA)/Polycaprolactone (PCL) Composite Microfibre Membranes. Fibres Text. East. Eur. 2016, 24, 17–25. [Google Scholar] [CrossRef] [Green Version]
- Kun, E.; Marossy, K. Effect of Crystallinity on PLA’s Microbiological Behaviour. Mater. Sci. Forum 2013, 752, 241–247. [Google Scholar] [CrossRef]
- Thunsiri, K.; Pitjamit, S.; Pothacharoen, P.; Pruksakorn, D.; Nakkiew, W.; Wattanutchariya, W. The 3D-Printed Bilayer’s Bioactive-Biomaterials Scaffold for Full-Thickness Articular Cartilage Defects Treatment. Materials 2020, 13, 3417. [Google Scholar] [CrossRef]
- Pantani, R.; Sorrentino, A. Influence of crystallinity on the biodegradation rate of injection-moulded poly(lactic acid) samples in controlled composting conditions. Polym. Degrad. Stab. 2013, 98, 1089–1096. [Google Scholar] [CrossRef]
- Jiao, Z.; Luo, B.; Xiang, S.; Ma, H.; Yu, Y.; Yang, W. 3D printing of HA / PCL composite tissue engineering scaffolds. Adv. Ind. Eng. Polym. Res. 2019, 2, 196–202. [Google Scholar] [CrossRef]
- Albertsson, A.C. Advances in Polymer Science: Degradable Aliphatic Polyesters; Springer: Berlin/Heidelberg, Germany, 2002; ISBN 9783540371021. [Google Scholar]
- Noroozi, N.; Schafer, L.L.; Hatzikiriakos, S.G. Thermorheological properties of poly (ε-caprolactone)/polylactide blends. Polym. Eng. Sci. 2012, 52, 2348–2359. [Google Scholar] [CrossRef]
- Molinero-Mourelle, P.; Canals, S.; Gómez-Polo, M.; Sola-Ruiz, M.; Highsmith, J.D.R.; Viñuela, A. Polylactic Acid as a Material for Three-Dimensional Printing of Provisional Restorations. Int. J. Prosthodont. 2018, 31, 349–350. [Google Scholar] [CrossRef]
- Liu, H.B.; Luo, G.B.; Wei, H.B.; Yu, H. Strength, Permeability, and Freeze-Thaw Durability of Pervious Concrete with Different Aggregate Sizes, Porosities, and Water-Binder Ratios. Appl. Sci. 2018, 8, 1217. [Google Scholar] [CrossRef] [Green Version]
- Qiao, Y.; Li, Q.; Jalali, A.; Yang, J.; Wang, X.; Zhao, N.; Jiang, Y.; Wang, S.; Hou, J.; Jiang, J. In-situ microfibrillated Poly(ε-caprolactone)/ Poly(lactic acid) composites with enhanced rheological properties, crystallization kinetics and foaming ability. Compos. Part B Eng. 2020, 208, 108594. [Google Scholar] [CrossRef]
- Jeantet, L.; Regazzi, A.; Taguet, A.; Pucci, M.F.; Caro, A.-S.; Quantin, J.-C. Biopolymer blends for mechanical property gradient 3D printed parts. Express Polym. Lett. 2021, 15, 137–152. [Google Scholar] [CrossRef]
- Li, Z.; Jiang, J.; Jin, Z.; Cai, B.; Cao, Y.; Li, Q. Preparation and Oil Absorption Performance of Biodegradable PCL/PLA Open-Cell Foam Material. CIESC J. 2020, 71, 5842–5853. [Google Scholar]
- Yeh, J.-T.; Wu, C.-J.; Tsou, C.-H.; Chai, W.-L.; Chow, J.-D.; Huang, C.-Y.; Chen, K.-N.; Wu, C.-S. Study on the Crystallization, Miscibility, Morphology, Properties of Poly(lactic acid)/Poly(ε-caprolactone) Blends. Polym. Technol. Eng. 2009, 48, 571–578. [Google Scholar] [CrossRef]
- Chen, J.; Zhang, T.-Y.; Jin, F.-L.; Park, S.-J. Fracture Toughness Improvement of Poly(lactic acid) Reinforced with Poly(ε-caprolactone) and Surface-Modified Silicon Carbide. Adv. Mater. Sci. Eng. 2018, 2018, 6537621. [Google Scholar] [CrossRef]
| Sample Codes | PLA (wt.%) | PCL (wt.%) |
|---|---|---|
| 90PLA | 90 | 10 |
| 80PLA | 80 | 20 |
| 70PLA | 70 | 30 |
| 60PLA | 60 | 40 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Solechan, S.; Suprihanto, A.; Widyanto, S.A.; Triyono, J.; Fitriyana, D.F.; Siregar, J.P.; Cionita, T. Investigating the Effect of PCL Concentrations on the Characterization of PLA Polymeric Blends for Biomaterial Applications. Materials 2022, 15, 7396. https://doi.org/10.3390/ma15207396
Solechan S, Suprihanto A, Widyanto SA, Triyono J, Fitriyana DF, Siregar JP, Cionita T. Investigating the Effect of PCL Concentrations on the Characterization of PLA Polymeric Blends for Biomaterial Applications. Materials. 2022; 15(20):7396. https://doi.org/10.3390/ma15207396
Chicago/Turabian StyleSolechan, Solechan, Agus Suprihanto, Susilo Adi Widyanto, Joko Triyono, Deni Fajar Fitriyana, Januar Parlaungan Siregar, and Tezara Cionita. 2022. "Investigating the Effect of PCL Concentrations on the Characterization of PLA Polymeric Blends for Biomaterial Applications" Materials 15, no. 20: 7396. https://doi.org/10.3390/ma15207396
APA StyleSolechan, S., Suprihanto, A., Widyanto, S. A., Triyono, J., Fitriyana, D. F., Siregar, J. P., & Cionita, T. (2022). Investigating the Effect of PCL Concentrations on the Characterization of PLA Polymeric Blends for Biomaterial Applications. Materials, 15(20), 7396. https://doi.org/10.3390/ma15207396








