Influence of Quaternary Ammonium Salt Functionalized Chitosan Additive as Sustainable Filler for High-Density Polyethylene Composites
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Preparation of CTAB Functionalized CHITOSAN
2.3. Preparation of HDPE/Chitosan Composites
2.4. Characterization
3. Results and Discussion
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Acknowledgments
Conflicts of Interest
References
- Kumar, S.; Panda, A.K.; Singh, R.K. A review on tertiary recycling of high-density polyethylene to fuel. Resour. Conserv. Recycl. 2011, 55, 893–910. [Google Scholar] [CrossRef]
- Tyagi, P.; Salem, K.S.; Hubbe, M.A.; Pal, L. Advances in barrier coatings and film technologies for achieving sustainable packaging of food products—A review. Trends Food Sci. Technol. 2021, 115, 461–485. [Google Scholar] [CrossRef]
- Licciardello, F.; Piergiovanni, L. Packaging and food sustainability. In The Interaction of Food Industry and Environment; Elsevier: Amsterdam, The Netherlands, 2020; pp. 191–222. [Google Scholar]
- Fabiyi, J.S.; McDonald, A.G.; McIlroy, D. Wood modification effects on weathering of HDPE-based wood plastic composites. J. Polym. Environ. 2009, 17, 34–48. [Google Scholar] [CrossRef]
- Mulinari, D.R.; Voorwald, H.J.; Cioffi, M.O.H.; Da Silva, M.L.C.; da Cruz, T.G.; Saron, C. Sugarcane bagasse cellulose/HDPE composites obtained by extrusion. Compos. Sci. Technol. 2009, 69, 214–219. [Google Scholar] [CrossRef]
- Campos, A.D.; Marconato, J.C.; Franchetti, S.M. Biodegradação de Filmes de PP/PCL em Solo e Solo com Chorume. Polímeros 2010, 20, 295–300. [Google Scholar] [CrossRef] [Green Version]
- Faria, A.U.d.; Martins-Franchetti, S.M. Biodegradação de filmes de polipropileno (PP), poli (3-hidroxibutirato)(PHB) e blenda de PP/PHB por microrganismos das águas do Rio Atibaia. Polímeros 2010, 20, 141–147. [Google Scholar] [CrossRef] [Green Version]
- Mulinari, D.R.; Voorwald, H.J.; Cioffi, M.O.H.; Rocha, G.J.; Da Silva, M.L.C.P. Surface modification of sugarcane bagasse cellulose and its effect on mechanical and water absorption properties of sugarcane bagasse cellulose/HDPE composites. BioResources 2010, 5, 661–671. [Google Scholar]
- Imre, B.; Pukánszky, B. Compatibilization in bio-based and biodegradable polymer blends. Eur. Polym. J. 2013, 49, 1215–1233. [Google Scholar] [CrossRef] [Green Version]
- Vasile, C.; Darie, R.N.; Cheaburu-Yilmaz, C.N.; Pricope, G.-M.; Bračič, M.; Pamfil, D.; Hitruc, G.E.; Duraccio, D. Low density polyethylene–chitosan composites. Compos. Part B Eng. 2013, 55, 314–323. [Google Scholar] [CrossRef]
- Reesha, K.; Panda, S.K.; Bindu, J.; Varghese, T. Development and characterization of an LDPE/chitosan composite antimicrobial film for chilled fish storage. Int. J. Biol. Macromol. 2015, 79, 934–942. [Google Scholar] [CrossRef]
- Siracusa, V.; Blanco, I. Bio-Polyethylene (Bio-PE), Bio-Polypropylene (Bio-PP) and Bio-Poly (ethylene terephthalate)(Bio-PET): Recent developments in bio-based polymers analogous to petroleum-derived ones for packaging and engineering applications. Polymers 2020, 12, 1641. [Google Scholar] [CrossRef] [PubMed]
- Sunilkumar, M.; Francis, T.; Thachil, E.T.; Sujith, A. Low density polyethylene–chitosan composites: A study based on biodegradation. Chem. Eng. J. 2012, 204, 114–124. [Google Scholar] [CrossRef]
- Hasan, K.F.; Wang, H.; Mahmud, S.; Jahid, M.A.; Islam, M.; Jin, W.; Genyang, C. Colorful and antibacterial nylon fabric via in-situ biosynthesis of chitosan mediated nanosilver. J. Mater. Res. Technol. 2020, 9, 16135–16145. [Google Scholar] [CrossRef]
- Jou, C.-H. Antibacterial activity and cytocompatibility of chitosan-N-hydroxy-2,3-propyl-N methyl-N,N-diallylammonium methyl sulfate. Colloids Surf. B Biointerfaces 2011, 88, 448–454. [Google Scholar] [CrossRef]
- Zhao, L.; Luo, F.; Wasikiewicz, J.M.; Mitomo, H.; Nagasawa, N.; Yagi, T.; Tamada, M.; Yoshii, F. Adsorption of humic acid from aqueous solution onto irradiation-crosslinked carboxymethylchitosan. Bioresour. Technol. 2008, 99, 1911–1917. [Google Scholar] [CrossRef]
- Chao, A.-C.; Shyu, S.-S.; Lin, Y.-C.; Mi, F.-L. Enzymatic grafting of carboxyl groups on to chitosan––to confer on chitosan the property of a cationic dye adsorbent. Bioresour. Technol. 2004, 91, 157–162. [Google Scholar] [CrossRef]
- Sudarshan, N.; Hoover, D.; Knorr, D. Antibacterial action of chitosan. Food Biotechnol. 1992, 6, 257–272. [Google Scholar] [CrossRef]
- Gutha, Y.; Pathak, J.L.; Zhang, W.; Zhang, Y.; Jiao, X. Antibacterial and wound healing properties of chitosan/poly(vinyl alcohol)/zinc oxide beads (CS/PVA/ZnO). Int. J. Biol. Macromol. 2017, 103, 234–241. [Google Scholar] [CrossRef]
- Shin, Y.; Yoo, D.; Jang, J. Molecular weight effect on antimicrobial activity of chitosan treated cotton fabrics. J. Appl. Polym. Sci. 2001, 80, 2495–2501. [Google Scholar] [CrossRef]
- Fei Liu, X.; Lin Guan, Y.; Zhi Yang, D.; Li, Z.; De Yao, K. Antibacterial action of chitosan and carboxymethylated chitosan. J. Appl. Polym. Sci. 2001, 79, 1324–1335. [Google Scholar] [CrossRef]
- Tsai, G.-J.; Su, W.-H. Antibacterial activity of shrimp chitosan against Escherichia coli. J. Food Prot. 1999, 62, 239–243. [Google Scholar] [CrossRef] [PubMed]
- Kong, M.; Chen, X.G.; Xing, K.; Park, H.J. Antimicrobial properties of chitosan and mode of action: A state of the art review. Int. J. Food Microbiol. 2010, 144, 51–63. [Google Scholar] [CrossRef] [PubMed]
- Younes, I.; Sellimi, S.; Rinaudo, M.; Jellouli, K.; Nasri, M. Influence of acetylation degree and molecular weight of homogeneous chitosans on antibacterial and antifungal activities. Int. J. Food Microbiol. 2014, 185, 57–63. [Google Scholar] [CrossRef] [PubMed]
- Vasile, C.; Pascu, M. Practical Guide to Polyethylene; iSmithers Rapra Publishing: Shawbury, UK, 2005. [Google Scholar]
- Malpass, D.B. Introduction to Industrial Polyethylene: Properties, Catalysts, and Processes; John Wiley & Sons: New York, NY, USA, 2010. [Google Scholar]
- Husseinsyah, S.; Azmin, A.N.; Ismail, H. Effect of maleic anhydride-grafted-polyethylene (MAPE) and silane on properties of recycled polyethylene/chitosan biocomposites. Polym.-Plast. Technol. Eng. 2013, 52, 168–174. [Google Scholar] [CrossRef]
- Tolinski, M. Additives for Polyolefins: Getting the Most Out of Polypropylene, Polyethylene and TPO.; William Andrew: Norwich, NY, USA, 2015. [Google Scholar]
- Kissin, Y.V. Polyethylene; Carl Hanser: Munich, Germany, 2021. [Google Scholar]
- Lipponen, S.; Saarikoski, E.; Rissanen, M.; Seppälä, J. Preparation and properties of cellulose/PE-co-AA blends. Eur. Polym. J. 2012, 48, 1439–1445. [Google Scholar] [CrossRef]
- Shelly, M.; Mathew, M.; Pradyumnan, P.; Francis, T. Dielectric and thermal stability studies on high density polyethylene–Chitosan composites plasticized with palm oil. Mater. Today Proc. 2021, 46, 2742–2746. [Google Scholar] [CrossRef]
- Daramola, O.; Taiwo, A.; Oladele, I.; Olajide, J.; Adeleke, S.; Adewuyi, B.; Sadiku, E. Mechanical properties of high density polyethylene matrix composites reinforced with chitosan particles. Mater. Today Proc. 2021, 38, 682–687. [Google Scholar] [CrossRef]
- Sagoo, S.; Board, R.; Roller, S. Chitosan inhibits growth of spoilage micro-organisms in chilled pork products. Food Microbiol. 2002, 19, 175–182. [Google Scholar] [CrossRef]
- Martínez-Camacho, A.; Cortez-Rocha, M.; Graciano-Verdugo, A.; Rodríguez-Félix, F.; Castillo-Ortega, M.; Burgos-Hernández, A.; Ezquerra-Brauer, J.; Plascencia-Jatomea, M. Extruded films of blended chitosan, low density polyethylene and ethylene acrylic acid. Carbohydr. Polym. 2013, 91, 666–674. [Google Scholar] [CrossRef] [PubMed]
- Mir, S.; Yasin, T.; Halley, P.J.; Siddiqi, H.M.; Nicholson, T. Thermal, rheological, mechanical and morphological behavior of HDPE/chitosan blend. Carbohydr. Polym. 2011, 83, 414–421. [Google Scholar] [CrossRef]
- Kurek, M.; Brachais, C.-H.; Nguimjeu, C.M.; Bonnotte, A.; Voilley, A.; Couvercelle, J.-P.; Debeaufort, F. Structure and thermal properties of a chitosan coated polyethylene bilayer film. Polym. Degrad. Stab. 2012, 97, 1232–1240. [Google Scholar] [CrossRef]
- Lima, P.S.; Brito, R.S.; Santos, B.F.; Tavares, A.A.; Agrawal, P.; Andrade, D.L.; Wellen, R.M.; Canedo, E.L.; Silva, S.M. Rheological properties of HDPE/chitosan composites modified with PE-g-MA. J. Mater. Res. 2017, 32, 775–787. [Google Scholar] [CrossRef]
- De Araújo, M.J.G.; Barbosa, R.C.; Fook, M.V.L.; Canedo, E.L.; Silva, S.M.; Medeiros, E.S.; Leite, I.F. HDPE/chitosan blends modified with organobentonite synthesized with quaternary ammonium salt impregnated chitosan. Materials 2018, 11, 291. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lima, P.S.; Trocolli, R.; Wellen, R.M.; Rojo, L.; Lopez-Manchado, M.A.; Fook, M.V.; Silva, S.M. HDPE/chitosan composites modified with PE-g-MA. thermal, morphological and antibacterial analysis. Polymers 2019, 11, 1559. [Google Scholar]
- Di Maro, M.; Faga, M.; Malucelli, G.; Mussano, F.; Genova, T.; Morsi, R.; Hamdy, A.; Duraccio, D. Influence of chitosan on the mechanical and biological properties of HDPE for biomedical applications. Polym. Test. 2020, 91, 106610. [Google Scholar] [CrossRef]
- de Britto, D.; de Assis, O.B. Synthesis and mechanical properties of quaternary salts of chitosan-based films for food application. Int. J. Biol. Macromol. 2007, 41, 198–203. [Google Scholar] [CrossRef]
- Rúnarsson, Ö.V.; Holappa, J.; Malainer, C.; Steinsson, H.; Hjálmarsdóttir, M.; Nevalainen, T.; Másson, M. Antibacterial activity of N-quaternary chitosan derivatives: Synthesis, characterization and structure activity relationship (SAR) investigations. Eur. Polym. J. 2010, 46, 1251–1267. [Google Scholar] [CrossRef]
- He, G.; Ke, W.; Chen, X.; Kong, Y.; Zheng, H.; Yin, Y.; Cai, W. Preparation and properties of quaternary ammonium chitosan-g-poly (acrylic acid-co-acrylamide) superabsorbent hydrogels. React. Funct. Polym. 2017, 111, 14–21. [Google Scholar] [CrossRef]
- Oyervides-Muñoz, E.; Pollet, E.; Ulrich, G.; de Jesús Sosa-Santillán, G.; Avérous, L. Original method for synthesis of chitosan-based antimicrobial agent by quaternary ammonium grafting. Carbohydr. Polym. 2017, 157, 1922–1932. [Google Scholar] [CrossRef]
- Wei, L.; Tan, W.; Wang, G.; Li, Q.; Dong, F.; Guo, Z. The antioxidant and antifungal activity of chitosan derivatives bearing Schiff bases and quaternary ammonium salts. Carbohydr. Polym. 2019, 226, 115256. [Google Scholar] [CrossRef]
- Andreica, B.-I.; Cheng, X.; Marin, L. Quaternary ammonium salts of chitosan. A critical overview on the synthesis and properties generated by quaternization. Eur. Polym. J. 2020, 139, 110016. [Google Scholar]
- Khonakdar, H.; Jafari, S.; Taheri, M.; Wagenknecht, U.; Jehnichen, D.; Häussler, L. Thermal and wide angle X-ray analysis of chemically and radiation-crosslinked low and high density polyethylenes. J. Appl. Polym. Sci. 2006, 100, 3264–3271. [Google Scholar] [CrossRef]
- Wunderlich, B. Macromolecular Physics: Crystal Melting; Academic Press: New York, NY, USA, 2013. [Google Scholar]
- Brandrup, J.; Immergut, E. Polymer Handbook, 3rd ed.; Wiley: New York, NY, USA, 1989. [Google Scholar]
- Jis, Z. 2801: 2000 Antimicrobial Products—Test for Antimicrobial Activity and Efficacy; Japanese Standards Association: Tokyo, Japan, 2000. [Google Scholar]
- Stelescu, D.M.; Airinei, A.; Homocianu, M.; Fifere, N.; Timpu, D.; Aflori, M. Structural characteristics of some high density polyethylene/EPDM blends. Polym. Test. 2013, 32, 187–196. [Google Scholar] [CrossRef]
- Gu, J.; Xu, H.; Wu, C. Thermal and crystallization properties of HDPE and HDPE/PP blends modified with DCP. Adv. Polym. Technol. 2014, 33. [Google Scholar] [CrossRef]
- Kusumastuti, Y.; Putri, N.R.E.; Timotius, D.; Syabani, M.W. Effect of chitosan addition on the properties of low-density polyethylene blend as potential bioplastic. Heliyon 2020, 6, e05280. [Google Scholar] [CrossRef]
- Kahar, A.; Ismail, H.; Abdul Hamid, A. The correlation between crosslink density and thermal properties of high-density polyethylene/natural rubber/thermoplastic tapioca starch blends prepared via dynamic vulcanisation approach. J. Therm. Anal. Calorim. 2016, 123, 301–308. [Google Scholar] [CrossRef]
- Bengtsson, M.; Gatenholm, P.; Oksman, K. The effect of crosslinking on the properties of polyethylene/wood flour composites. Compos. Sci. Technol. 2005, 65, 1468–1479. [Google Scholar] [CrossRef]
- Bengtsson, M.; Oksman, K. The use of silane technology in crosslinking polyethylene/wood flour composites. Compos. Part A Appl. Sci. Manuf. 2006, 37, 752–765. [Google Scholar] [CrossRef]
- Santos, J.E.D.; Soares, J.D.P.; Dockal, E.R.; Campana Filho, S.P.; Cavalheiro, É.T. Caracterização de quitosanas comerciais de diferentes origens. Polímeros 2003, 13, 242–249. [Google Scholar] [CrossRef]
- Lima, M.D.S.P.D. Preparo e Caracterização de Membranas de Quitosana Modificadas com poli (Ácido Acrílico). Master’s Thesis, Universidade Federal do Rio Grande do Norte, Natal, Brazil, 2006. [Google Scholar]
- Leite, I.; Soares, A.; Carvalho, L.; Raposo, C.; Malta, O.; Silva, S. Characterization of pristine and purified organobentonites. J. Therm. Anal. Calorim. 2010, 100, 563–569. [Google Scholar] [CrossRef]
- Marras, S.; Tsimpliaraki, A.; Zuburtikudis, I.; Panayiotou, C. Thermal and colloidal behavior of amine-treated clays: The role of amphiphilic organic cation concentration. J. Colloid Interface Sci. 2007, 315, 520–527. [Google Scholar] [CrossRef] [PubMed]
- Prasanna, K.; Sailaja, R. Blends of LDPE/chitosan using epoxy-functionalized LDPE as compatibilizer. J. Appl. Polym. Sci. 2012, 124, 3264–3275. [Google Scholar] [CrossRef]
- Dean, K.; Sangwan, P.; Way, C.; Zhang, X.; Martino, V.P.; Xie, F.; Halley, P.J.; Pollet, E.; Avérous, L. Glycerol plasticised chitosan: A study of biodegradation via carbon dioxide evolution and nuclear magnetic resonance. Polym. Degrad. Stab. 2013, 98, 1236–1246. [Google Scholar] [CrossRef]
- Agrawal, P.; Araújo, E.M.; Mélo, T.J. Reometria de torque, propriedades mecânicas e morfologia de blendas compatibilizadas de PA6/PEAD. Polímeros 2008, 18, 152–157. [Google Scholar] [CrossRef] [Green Version]
- Ferreira, K.R.; Leite, I.F.; Siqueira, A.d.S.; Raposo, C.M.; Carvalho, L.H.; Silva, S.M. Uso de argila organofílica na compatibilização de misturas PP/EPDM. Polímeros 2011, 21, 421–428. [Google Scholar] [CrossRef] [Green Version]
- Sloan, J.M.; Maghochetti, M.J.; Zukas, W.X. Effect of resin components on the reversion process of sulfur-vulcanized guayule rubber. Rubber Chem. Technol. 1986, 59, 800–808. [Google Scholar] [CrossRef]
- Filippone, G.; Dintcheva, N.T.; La Mantia, F.; Acierno, D. Using organoclay to promote morphology refinement and co-continuity in high-density polyethylene/polyamide 6 blends–Effect of filler content and polymer matrix composition. Polymer 2010, 51, 3956–3965. [Google Scholar] [CrossRef]
- Georgieva, V.; Zvezdova, D.; Vlaev, L. Non-isothermal kinetics of thermal degradation of chitosan. Chem. Cent. J. 2012, 6, 81. [Google Scholar] [CrossRef] [Green Version]
- Goy, R.C.; Britto, D.D.; Assis, O.B. A review of the antimicrobial activity of chitosan. Polímeros 2009, 19, 241–247. [Google Scholar] [CrossRef]
- Coma, V.; Martial-Gros, A.; Garreau, S.; Copinet, A.; Salin, F.; Deschamps, A. Edible antimicrobial films based on chitosan matrix. J. Food Sci. 2002, 67, 1162–1169. [Google Scholar] [CrossRef]
- Raafat, D.; Von Bargen, K.; Haas, A.; Sahl, H.-G. Insights into the mode of action of chitosan as an antibacterial compound. Appl. Environ. Microbiol. 2008, 74, 3764–3773. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tan, H.; Ma, R.; Lin, C.; Liu, Z.; Tang, T. Quaternized chitosan as an antimicrobial agent: Antimicrobial activity, mechanism of action and biomedical applications in orthopedics. Int. J. Mol. Sci. 2013, 14, 1854–1869. [Google Scholar] [CrossRef] [PubMed]
- Young, D.H.; Köhle, H.; Kauss, H. Effect of chitosan on membrane permeability of suspension-cultured Glycine max and Phaseolus vulgaris cells. Plant Physiol. 1982, 70, 1449–1454. [Google Scholar] [CrossRef]
- Jeon, Y.-J.; Park, P.-J.; Kim, S.-K. Antimicrobial effect of chitooligosaccharides produced by bioreactor. Carbohydr. Polym. 2001, 44, 71–76. [Google Scholar] [CrossRef]
- No, H.K.; Park, N.Y.; Lee, S.H.; Meyers, S.P. Antibacterial activity of chitosans and chitosan oligomers with different molecular weights. Int. J. Food Microbiol. 2002, 74, 65–72. [Google Scholar] [CrossRef]
- Coma, V.; Deschamps, A.; Martial-Gros, A. Bioactive packaging materials from edible chitosan polymer—antimicrobial activity assessment on dairy-related contaminants. J. Food Sci. 2003, 68, 2788–2792. [Google Scholar] [CrossRef]
- Dutta, P.; Tripathi, S.; Mehrotra, G.; Dutta, J. Perspectives for chitosan based antimicrobial films in food applications. Food Chem. 2009, 114, 1173–1182. [Google Scholar] [CrossRef]
- Chung, Y.-C.; Su, Y.P.; Chen, C.-C.; Jia, G.; Wang, H.L.; Wu, J.G.; Lin, J.G. Relationship between antibacterial activity of chitosan and surface characteristics of cell wall. Acta Pharmacol. Sin. 2004, 25, 932–936. [Google Scholar]
- Helander, I.; Nurmiaho-Lassila, E.-L.; Ahvenainen, R.; Rhoades, J.; Roller, S. Chitosan disrupts the barrier properties of the outer membrane of Gram-negative bacteria. Int. J. Food Microbiol. 2001, 71, 235–244. [Google Scholar]
- Chen, Y.-M.; Chung, Y.-C.; Woan Wang, L.; Chen, K.-T.; Li, S.-Y. Antibacterial properties of chitosan in waterborne pathogen. J. Environ. Sci. Health Part A 2002, 37, 1379–1390. [Google Scholar] [CrossRef]
- Šimůnek, J.; Tishchenko, G.; Hodrová, B.; Bartoňová, H. Effect of chitosan on the growth of human colonic bacteria. Folia Microbiol. 2006, 51, 306–308. [Google Scholar] [CrossRef]
- Eaton, P.; Fernandes, J.C.; Pereira, E.; Pintado, M.E.; Malcata, F.X. Atomic force microscopy study of the antibacterial effects of chitosans on Escherichia coli and Staphylococcus aureus. Ultramicroscopy 2008, 108, 1128–1134. [Google Scholar] [CrossRef] [PubMed]
- Eldin, M.M.; Soliman, E.; Hashem, A.; Tamer, T. Antibacterial activity of chitosan chemically modified with new technique. Trends Biomater. Artif. Organs 2008, 22, 125–137. [Google Scholar]
- Raafat, D.; Sahl, H.G. Chitosan and its antimicrobial potential–a critical literature survey. Microb. Biotechnol. 2009, 2, 186–201. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Vallapa, N.; Wiarachai, O.; Thongchul, N.; Pan, J.; Tangpasuthadol, V.; Kiatkamjornwong, S.; Hoven, V.P. Enhancing antibacterial activity of chitosan surface by heterogeneous quaternization. Carbohydr. Polym. 2011, 83, 868–875. [Google Scholar] [CrossRef]
- Mohamed, N.A.; Sabaa, M.W.; El-Ghandour, A.H.; Abdel-Aziz, M.M.; Abdel-Gawad, O.F. Quaternized N-substituted carboxymethyl chitosan derivatives as antimicrobial agents. Int. J. Biol. Macromol. 2013, 60, 156–164. [Google Scholar] [CrossRef] [PubMed]
- Sajomsang, W.; Tantayanon, S.; Tangpasuthadol, V.; Daly, W.H. Quaternization of N-aryl chitosan derivatives: Synthesis, characterization, and antibacterial activity. Carbohydr. Res. 2009, 344, 2502–2511. [Google Scholar] [CrossRef]
- Li, Z.; Yang, F.; Yang, R. Synthesis and characterization of chitosan derivatives with dual-antibacterial functional groups. Int. J. Biol. Macromol. 2015, 75, 378–387. [Google Scholar] [CrossRef]


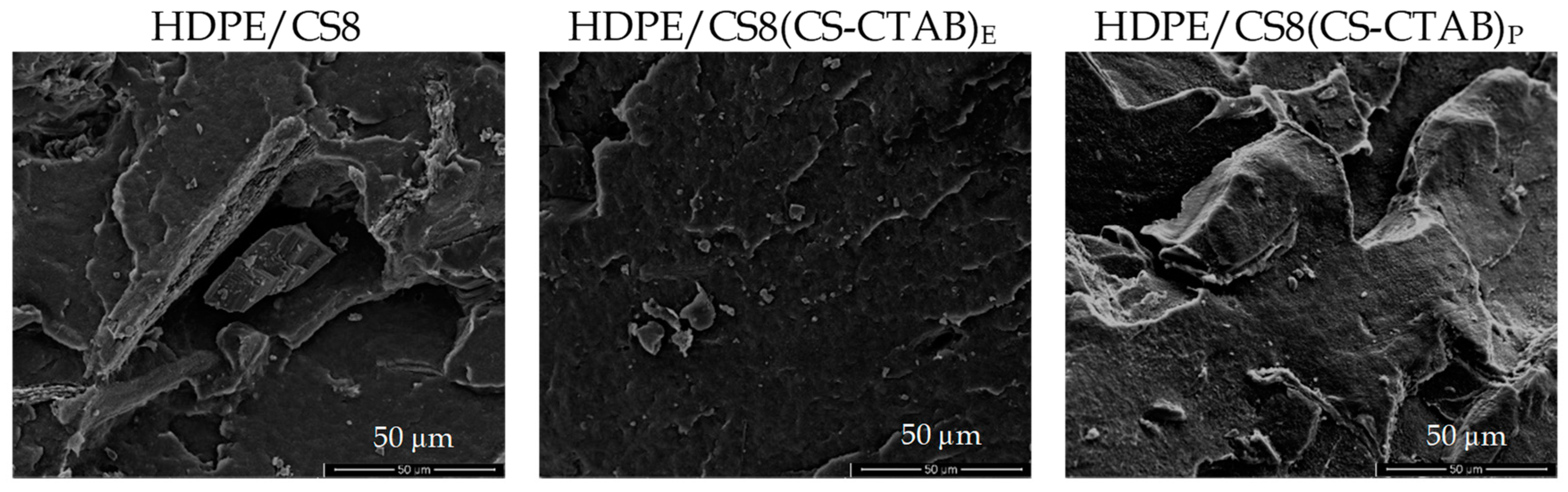
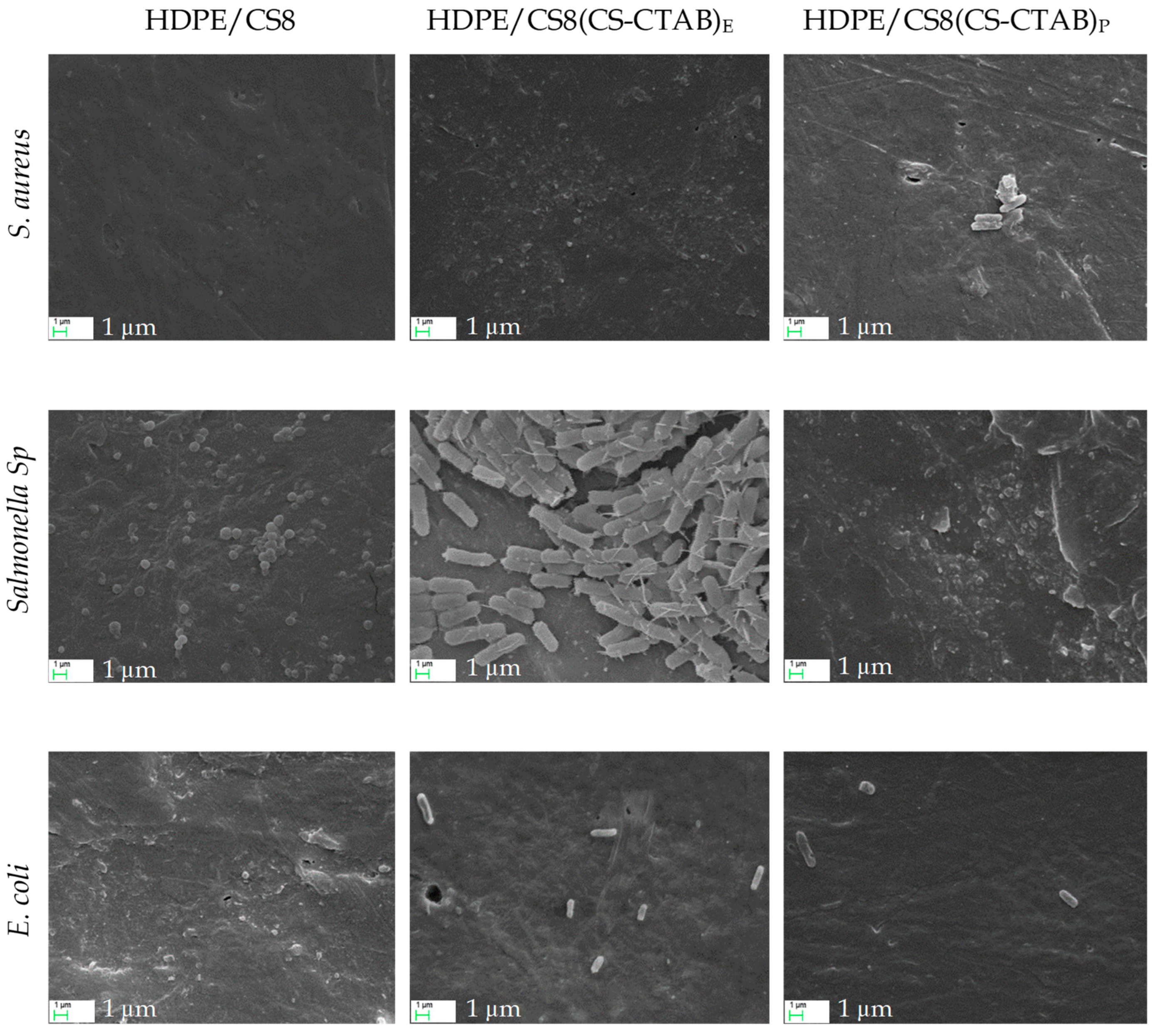
| Code | Composition | HDPE (g) | CS (g) | CS-CTAB (g) |
|---|---|---|---|---|
| HDPE | 100 | 50 | 0 | 0 |
| HDPE/CS9 | 90/10 | 45 | 5 | 0 |
| HDPE/CS8 | 80/20 | 40 | 10 | 0 |
| HDPE/CS7 | 70/30 | 35 | 15 | 0 |
| HDPE/CS6 | 60/40 | 30 | 20 | 0 |
| HDPE/CS9/(CS-CTAB)E | 90/10/0.1 | 45 | 5 | 0.05 |
| HDPE/CS8/(CS-CTAB)E | 80/20/0.2 | 40 | 10 | 0.10 |
| HDPE/CS7/(CS-CTAB)E | 70/30/0.3 | 35 | 15 | 0.15 |
| HDPE/CS6/(CS-CTAB)E | 60/40/0.4 | 30 | 20 | 0.20 |
| HDPE/CS9/(CS-CTAB)P | 90/10/0.1 | 45 | 5 | 0.05 |
| HDPE/CS8/(CS-CTAB)P | 80/20/0.2 | 40 | 10 | 0.10 |
| HDPE/CS7/(CS-CTAB)P | 70/30/0.3 | 35 | 15 | 0.15 |
| HDPE/CS6/(CS-CTAB)P | 60/40/0.4 | 30 | 20 | 0.20 |
| Sample | Xc (%) |
|---|---|
| HDPE | 54 |
| HDPE/CS9 | 45 |
| HDPE/CS9/(CS-CTAB)E | 45 |
| HDPE/CS9/(CS-CTAB)P | 50 |
| HDPE/CS8 | 43 |
| HDPE/CS8/(CS-CTAB)E | 41 |
| HDPE/CS8/(CS-CTAB)P | 39 |
| HDPE/CS7 | 38 |
| HDPE/CS7/(CS-CTAB)E | 34 |
| HDPE/CS7/(CS-CTAB)P | 40 |
| HDPE/CS6 | 39 |
| HDPE/CS6/(CS-CTAB)E | 32 |
| HDPE/CS6/(CS-CTAB)P | 36 |
| Sample | Tc (°C) | ∆Hc (J/g) | Tm (°C) | ∆Hm (J/g) | Xc (%) |
|---|---|---|---|---|---|
| HDPE | 115 | 155 | 130 | 184 | 64 |
| HDPE/CS9 | 115 | 110 | 130 | 141 | 53 |
| HDPE/CS9/(CS-CTAB)E | 111 | 69 | 125 | 97 | 37 |
| HDPE/CS9/(CS-CTAB)P | 115 | 63 | 129 | 96 | 36 |
| HDPE/CS8 | 114 | 40 | 129 | 99 | 42 |
| HDPE/CS8/(CS-CTAB)E | 110 | 51 | 124 | 82 | 35 |
| HDPE/CS8/(CS-CTAB)P | 110 | 53 | 124 | 84 | 36 |
| HDPE/CS7 | 113 | 45 | 128 | 56 | 27 |
| HDPE/CS7/(CS-CTAB)E | 114 | 42 | 128 | 61 | 30 |
| HDPE/CS7/(CS-CTAB)P | 116 | 57 | 129 | 77 | 37 |
| HDPE/CS6 | 114 | 50 | 127 | 63 | 36 |
| HDPE/CS6/(CS-CTAB)E | 115 | 41 | 128 | 53 | 30 |
| HDPE/CS6/(CS-CTAB)P | 113 | 44 | 128 | 52 | 30 |
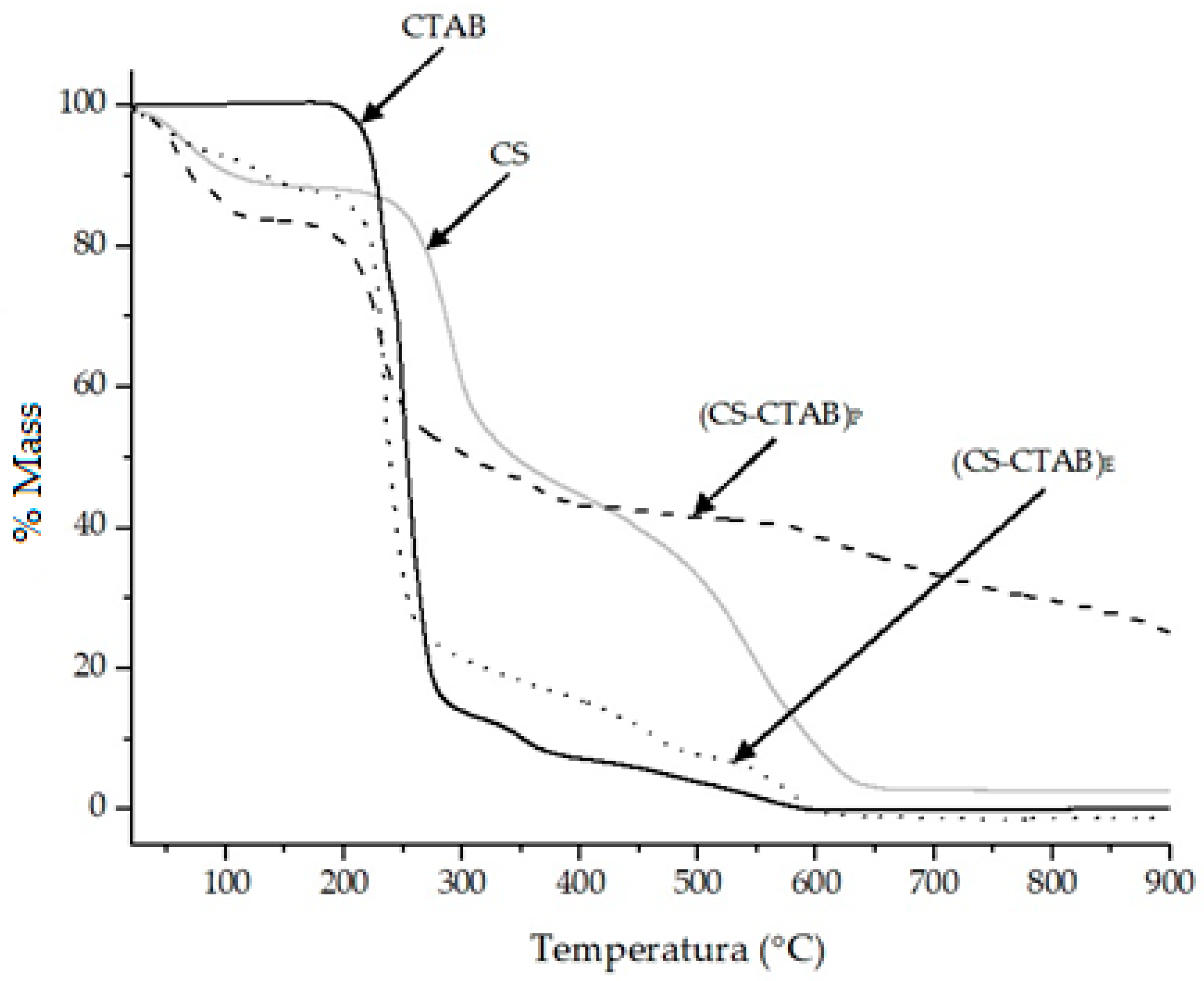
| Sample | Ti–Tf (°C) | |||
|---|---|---|---|---|
| I | II | III | T20 (°C) | |
| HDPE | 463–489 | 460 | ||
| CS | 62–98 | 263–309 | 496–592 | 266 |
| HDPE/CS9 | 40–78 | 263–311 | 459–490 | 440 |
| HDPE/CS9/(CS-CTAB)E | 37–76 | 270–312 | 460–491 | 321 |
| HDPE/CS9/(CS-CTAB)P | 36–73 | 271–310 | 456–488 | 332 |
| HDPE/CS8 | 38–77 | 268–312 | 458–489 | 302 |
| HDPE/CS8/(CS-CTAB)E | 37–76 | 269–312 | 469–490 | 302 |
| HDPE/CS8/(CS-CTAB)P | 30–81 | 266–310 | 456–487 | 312 |
| HDPE/CS7 | 36–78 | 265–310 | 451–486 | 302 |
| HDPE/CS7/(CS-CTAB)E | 38–77 | 269–311 | 454–486 | 293 |
| HDPE/CS7/(CS-CTAB)P | 39–78 | 267–312 | 456–488 | 291 |
| HDPE/CS6 | 50–93 | 267–309 | 406–488 | 290 |
| HDPE/CS6/(CS-CTAB)E | 42–82 | 267–314 | 456–487 | 293 |
| HDPE/CS6/(CS-CTAB)P | 37–82 | 268–311 | 455–488 | 289 |
| Sample | Tensile Strength (MPa) | Elastic Modulus (MPa) | Elongation at Break (%) |
|---|---|---|---|
| HDPE | 16.38 ± 2.11 | 400.72 ± 52.49 | 67.14 ± 12.33 |
| HDPE/CS9 | 16.70 ± 0.58 | 443.50 ± 25.59 | 15.99 ± 0.65 |
| HDPE/CS9/(CS-CTAB)E | 14.49 ± 0.31 | 399.28 ± 11.43 | 12.79 ± 0.09 |
| HDPE/CS9/(CS-CTAB)P | 16.61 ± 0.39 | 421.18 ± 12.61 | 12.10 ± 0.22 |
| HDPE/CS8 | 18.35 ± 0.98 | 538.28 ± 21.47 | 12.01 ± 3.92 |
| HDPE/CS8/(CS-CTAB)E | 16.94 ± 0.39 | 435.20 ± 16.41 | 9.63 ± 1.11 |
| HDPE/CS8/(CS-CTAB)P | 15.15 ± 0.63 | 467.57 ± 13.29 | 9.18 ± 1.31 |
| HDPE/CS7 | 17.26 ± 0.45 | 537.47 ± 16.41 | 11.88 ± 0.88 |
| HDPE/CS7/(CS-CTAB)E | 17.99 ± 0.67 | 538.97 ± 19.37 | 6.75 ± 1.20 |
| HDPE/CS7/(CS-CTAB)P | 17.08 ± 0.71 | 571.40 ± 22.68 | 7.21 ± 0.49 |
| HDPE/CS6 | 17.52 ± 0.60 | 597.84 ± 22.78 | 9.17 ± 1.35 |
| HDPE/CS6/(CS-CTAB)E | 15.41 ± 0.57 | 576.45 ± 43.41 | 5.93 ± 0.53 |
| HDPE/CS6/(CS-CTAB)P | 14.34 ± 0.54 | 563.34 ± 18.61 | 5.61 ± 0.46 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
de Araújo, M.J.G.; Barbosa, F.C.; Fook, M.V.L.; Silva, S.M.L.; Leite, I.F. Influence of Quaternary Ammonium Salt Functionalized Chitosan Additive as Sustainable Filler for High-Density Polyethylene Composites. Materials 2022, 15, 7418. https://doi.org/10.3390/ma15217418
de Araújo MJG, Barbosa FC, Fook MVL, Silva SML, Leite IF. Influence of Quaternary Ammonium Salt Functionalized Chitosan Additive as Sustainable Filler for High-Density Polyethylene Composites. Materials. 2022; 15(21):7418. https://doi.org/10.3390/ma15217418
Chicago/Turabian Stylede Araújo, Maria José G., Francivandi C. Barbosa, Marcus Vinícius L. Fook, Suédina Maria L. Silva, and Itamara F. Leite. 2022. "Influence of Quaternary Ammonium Salt Functionalized Chitosan Additive as Sustainable Filler for High-Density Polyethylene Composites" Materials 15, no. 21: 7418. https://doi.org/10.3390/ma15217418
APA Stylede Araújo, M. J. G., Barbosa, F. C., Fook, M. V. L., Silva, S. M. L., & Leite, I. F. (2022). Influence of Quaternary Ammonium Salt Functionalized Chitosan Additive as Sustainable Filler for High-Density Polyethylene Composites. Materials, 15(21), 7418. https://doi.org/10.3390/ma15217418










