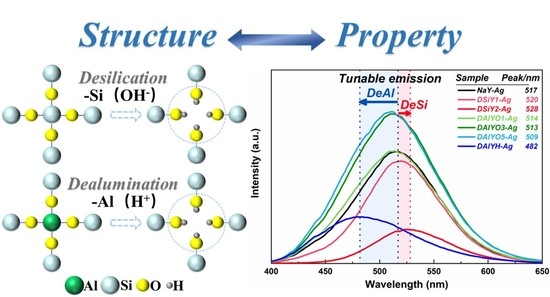
Tailoring the Luminescence Properties of Silver Clusters Confined in Faujasite Zeolite through Framework Modification
Abstract
:1. Introduction
2. Materials and Methods
2.1. Reagents
2.2. Desilication on FAU Zeolite
2.3. Dealumination on FAU Zeolite
2.4. Preparing of Ag+-Exchanged Zeolites
2.5. Measurements
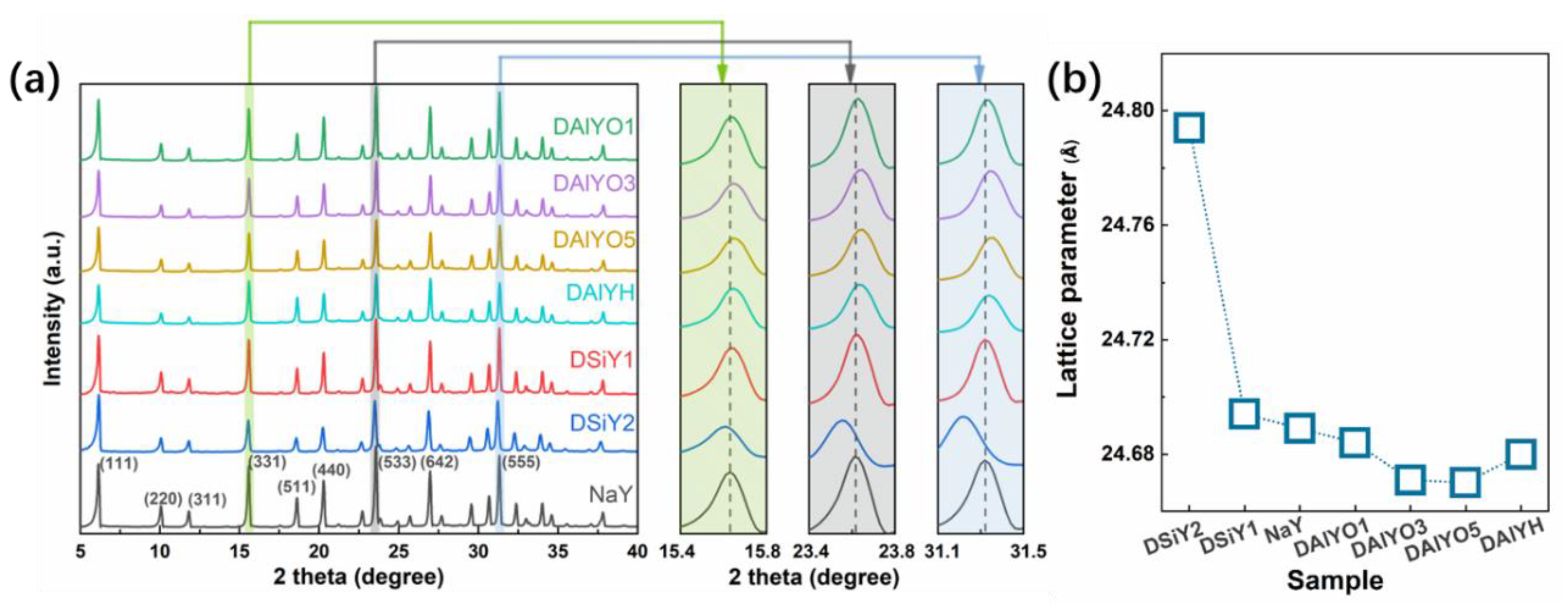
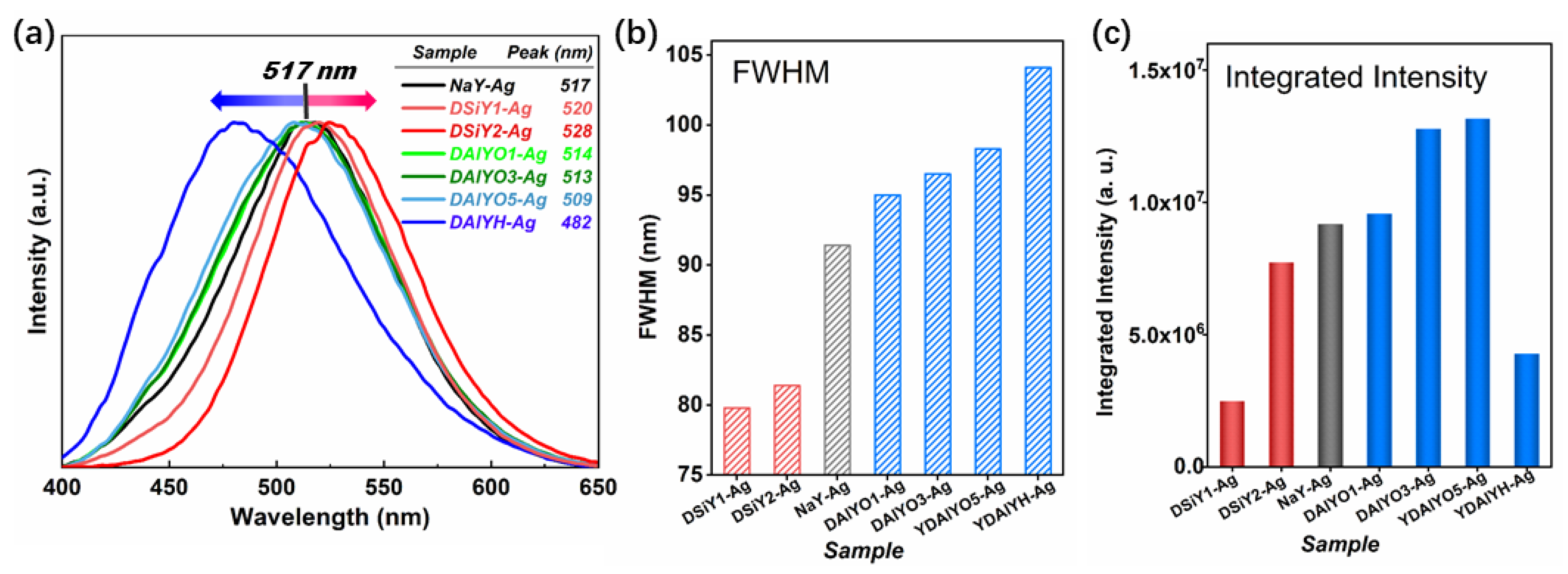
3. Results and Discussion
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- Coutino-Gonzalez, E.; Baekelant, W.; Steele, J.A.; Kim, C.W.; Roeffaers, M.B.J.; Hofkens, J. Silver clusters in zeolites: From self-assembly to ground-breaking luminescent properties. Acc. Chem. Res. 2017, 50, 2353–2361. [Google Scholar] [CrossRef] [PubMed]
- Zhao, Y.; Zhou, H.; Zhang, S.; Xu, J. The synthesis of metal nanoclusters and their applications in bio-sensing and imaging. Methods Appl. Fluoresc. 2019, 8, 012001. [Google Scholar] [CrossRef] [PubMed]
- Gomez, R.S.; Li, X.; Yson, R.L.; Patterson, H.H. Zeolite-supported silver and silver–iron nanoclusters and their activities as photodecomposition catalysts. Res. Chem. Intermed. 2011, 37, 729–745. [Google Scholar] [CrossRef]
- Chai, Y.; Shang, W.; Li, W.; Wu, G.; Dai, W.; Guan, N.; Li, L. Noble metal particles confined in zeolites: Synthesis, characterization, and applications. Adv. Sci. 2019, 6, 1900299. [Google Scholar] [CrossRef] [Green Version]
- Yu, J.; Ye, S.; Shi, Y.; Liao, H.; Wang, D. Thermally formation and luminescent performance of silver nanoclusters confined within LTA zeolites. J. Alloys Compd. 2021, 857, 157614. [Google Scholar] [CrossRef]
- Diez, I.; Ras, R.H. Fluorescent silver nanoclusters. Nanoscale 2011, 3, 1963–1970. [Google Scholar] [CrossRef] [PubMed]
- Ye, S.; Guo, Z.; Wang, H.; Li, S.; Liu, T.; Wang, D. Evolution of Ag species and molecular-like Ag cluster sensitized Eu3+ emission in oxyfluoride glass for tunable light emitting. J. Alloys Compd. 2016, 685, 891–895. [Google Scholar] [CrossRef]
- Li, X.; Zhong, H.; Chen, B.; Sui, G.; Sun, J.; Xu, S.; Cheng, L.; Zhang, J. Highly stable and tunable white luminescence from Ag-Eu3+ co-doped fluoroborate glass phosphors combined with violet LED. Opt. Express 2018, 26, 1870–1881. [Google Scholar] [CrossRef]
- Kennes, K.; Coutino-Gonzalez, E.; Martin, C.; Baekelant, W.; Roeffaers, M.B.J.; Van der Auweraer, M. Silver zeolite composites-based LEDs: A novel solid-state lighting approach. Adv. Funct. Mater. 2017, 27, 1606411. [Google Scholar] [CrossRef]
- Grandjean, D.; Coutino-Gonzalez, E.; Cuong, N.T.; Fron, E.; Baekelant, W.; Aghakhani, S.; Schlexer, P.; D’Acapito, F.; Banerjee, D.; Roeffaers, M.B.J.; et al. Origin of the bright photoluminescence of few-atom silver clusters confined in LTA zeolites. Science 2018, 361, 686–689. [Google Scholar] [CrossRef]
- Baekelant, W.; Aghakhani, S.; Coutino-Gonzalez, E.; Kennes, K.; D’Acapito, F.; Grandjean, D.; Van der Auweraer, M.; Lievens, P.; Roeffaers, M.B.J.; Hofkens, J.; et al. Shaping the optical properties of silver clusters inside zeolite a via guest-host-guest interactions. J. Phys. Chem. Lett. 2018, 9, 5344–5350. [Google Scholar] [CrossRef] [PubMed]
- Romolini, G.; Steele, J.A.; Hofkens, J.; Roeffaers, M.B.J.; Coutino-Gonzalez, E. Tunable luminescence from stable silver nanoclusters confined in microporous zeolites. Adv. Opt. Mater. 2021, 9, 2100526. [Google Scholar] [CrossRef]
- Fenwick, O.; Coutino-Gonzalez, E.; Grandjean, D.; Baekelant, W.; Richard, F.; Bonacchi, S.; De Vos, D.; Lievens, P.; Roeffaers, M.; Hofkens, J.; et al. Tuning the energetics and tailoring the optical properties of silver clusters confined in zeolites. Nat. Mater. 2016, 15, 1017–1022. [Google Scholar] [CrossRef] [PubMed]
- Yao, D.; Xu, S.; Wang, Y.; Li, H. White-emitting phosphors with high color-rendering index based on silver cluster-loaded zeolites and their application to near-UV LED-based white LEDs. Mater. Chem. Front. 2019, 3, 1080–1084. [Google Scholar] [CrossRef]
- De Cremer, G.; Coutino-Gonzalez, E.; Roeffaers, M.B.J.; Moens, B.; Ollevier, J.; Van der Auweraer, M.; Schoonheydt, R.; Jacobs, P.A.; De Schryver, F.C.; Hofkens, J.; et al. Characterization of fluorescence in heat-treated silver-exchanged zeolites. J. Am. Chem. Soc. 2009, 131, 3049–3056. [Google Scholar] [CrossRef]
- Altantzis, T.; Coutino-Gonzalez, E.; Baekelant, W.; Martinez, G.T.; Abakumov, A.M.; Tendeloo, G.V.; Roeffaers, M.B.; Bals, S.; Hofkens, J. Direct observation of luminescent silver clusters confined in faujasite zeolites. ACS Nano 2016, 10, 7604–7611. [Google Scholar] [CrossRef]
- Yao, D.; Wang, Y.; Li, P.; Li, H. Luminescent Ag+ exchanged SOD zeolites with their potential applications in white LEDs. Dalton Trans. 2020, 49, 8179–8185. [Google Scholar] [CrossRef]
- Johan, E.; Kanda, Y.; Matsue, N.; Itagaki, Y.; Aono, H. Whitish fluorescence of partially Ag-exchanged zeolite Y affected by coexisting cations. J. Lumin. 2019, 213, 482–488. [Google Scholar] [CrossRef]
- Yu, J.; Ye, S.; Liao, H.; Xv, X.; Wang, D. Luminescence control of silver nanoclusters by tailoring extra-framework cations in FAU-Y zeolite: Implications for tunable emission. ACS Appl. Nano Mater. 2021, 4, 13692–13699. [Google Scholar] [CrossRef]
- Tian, X.L.; Li, Q.R.; Yao, D.C.; Li, P.L.; Li, H.R.; Wang, Y.G. Tunable luminescence of silver-exchanged SOD zeolite thermally treated under mild conditions. J. Mater. Chem. C 2022, 10, 1666–1671. [Google Scholar] [CrossRef]
- Aono, H.; Yahara, K.; Johan, E.; Itagaki, Y. Effect of coexisting lithium content on fluorescent properties of silver ion-exchanged LTA zeolite. J. Ceram. Soc. Jpn. 2020, 128, 670–676. [Google Scholar] [CrossRef]
- Shi, Y.; Ye, S.; Liao, H.; Liu, J.; Wang, D. Formation of luminescent silver-clusters and efficient energy transfer to Eu3+ in faujasite NaX zeolite. J. Solid State Chem. 2020, 285, 121227. [Google Scholar] [CrossRef]
- Yi, X.; Sun, J.; Jiang, X.-F.; Li, Y.; Xu, Q.-H.; Zhang, Q.; Ye, S. Variations in the 5D0 → 7F0–4 transitions of Eu3+ and white light emissions in Ag–Eu exchanged zeolite-Y. RSC Adv. 2016, 6, 95925–95935. [Google Scholar] [CrossRef]
- Fenwick, O.; Coutino-Gonzalez, E.; Richard, F.; Bonacchi, S.; Baekelant, W.; De Vos, D.; Roeffaers, M.B.J.; Hofkens, J.; Samori, P. X-ray-induced growth dynamics of luminescent silver clusters in zeolites. Small 2020, 16, e2002063. [Google Scholar] [CrossRef] [PubMed]
- De Cremer, G.; Coutino-Gonzalez, E.; Roeffaers, M.B.; De Vos, D.E.; Hofkens, J.; Vosch, T.; Sels, B.F. In situ observation of the emission characteristics of zeolite-hosted silver species during heat treatment. Chemphyschem 2010, 11, 1627–1631. [Google Scholar] [CrossRef] [PubMed]
- Suzuki, Y.; Miyanaga, T.; Yamauchi, K.; Mori, N.; Nakamura, R. Photoluminescence of Ag-loaded A, X, and Y type zeolites heat-treated in atmosphere. Adv. Appl. Phys. 2019, 7, 19–28. [Google Scholar] [CrossRef]
- Fron, E.; Aghakhani, S.; Baekelant, W.; Grandjean, D.; Coutino-Gonzalez, E.; Van der Auweraer, M.; Roeffaers, M.B.J.; Lievens, P.; Hofkens, J. Structural and photophysical characterization of Ag clusters in LTA zeolites. J. Phys. Chem. C 2019, 123, 10630–10638. [Google Scholar] [CrossRef]
- Algarra, M.; Reis, A.; Jiménez-Jiménez, J.; Moreno-Tost, R.; Esteves da Silva, J.C.G. CdS quantum dots nanoparticles dispersed in zeolites. Optical study. J. Dispers. Sci. Technol. 2012, 33, 786–791. [Google Scholar] [CrossRef]
- Liao, H.; Ye, S.; Ding, J.; Yu, J.; Xv, X.; Pan, L.; Lin, P.; Wang, D. Ship-in-a-bottle growth of NaYF4: Yb3+/Tm3+ upconversion nanocrystals in desilicated ZSM-5 zeolite for drug release monitoring. Mater. Res. Bull. 2022, 154, 111926. [Google Scholar] [CrossRef]
- Zhang, R.X.; Raja, D.; Zhang, Y.; Yan, Y.; Garforth, A.A.; Jiao, Y.L.; Fan, X.L. Sequential microwave-assisted dealumination and hydrothermal alkaline treatments of Y zeolite for preparing hierarchical mesoporous zeolite catalysts. Top. Catal. 2020, 63, 340–350. [Google Scholar] [CrossRef]
- Lee, E.F.T.; Rees, L.V.C. Dealumination of sodium-Y zeolite with hydrochloric acid. J. Chem. Soc. Faraday Trans. I 1987, 83, 1531–1537. [Google Scholar] [CrossRef]
- Srivastava, R.; Iwasa, N.; Fujita, S.-I.; Arai, M. Dealumination of zeolite beta catalyst under controlled conditions for enhancing its activity in acylation and esterification. Catal. Lett. 2009, 130, 655–663. [Google Scholar] [CrossRef] [Green Version]
- Possato, L.G.; Chaves, T.F.; Cassinelli, W.H.; Pulcinelli, S.H.; Santilli, C.V.; Martins, L. The multiple benefits of glycerol conversion to acrolein and acrylic acid catalyzed by vanadium oxides supported on micro-mesoporous MFI zeolites. Catal. Today 2017, 289, 20–28. [Google Scholar] [CrossRef] [Green Version]
- Giudici, R.; Kouwenhoven, H.W.; Prins, R. Comparison of nitric and oxalic acid in the dealumination of mordenite. Appl. Catal. A-Gen. 2000, 203, 101–110. [Google Scholar] [CrossRef]
- Apelian, M.R.; Fung, A.S.; Kennedy, G.J.; Degnan, T.F. Dealumination of zeolite beta via dicarboxylic acid treatment. J. Phys. Chem. 1996, 100, 16577–16583. [Google Scholar] [CrossRef]
- Barr, T.L.; Lishka, M.A. ESCA studies of the surface-chemistry of zeolites. J. Am. Chem. Soc. 1986, 108, 3178–3186. [Google Scholar] [CrossRef]
- Remy, M.J.; Genet, M.J.; Poncelet, G.; Lardinois, P.F.; Notte, P.P. Investigation of dealuminated mordenites by X-ray photoelectron-spectroscopy. J. Phys. Chem. 1992, 96, 2614–2617. [Google Scholar] [CrossRef]
- Wang, X.; Wang, K.; Plackowski, C.A.; Nguyen, A.V. Sulfuric acid dissolution of 4A and Na-Y synthetic zeolites and effects on Na-Y surface and particle properties. Appl. Surf. Sci. 2016, 367, 281–290. [Google Scholar] [CrossRef] [Green Version]
- Grunert, W.; Muhler, M.; Schroder, K.P.; Sauer, J.; Schlogl, R. Investigations of zeolites by photoelectron and ion-scattering spectroscopy. 2. A new interpretation of XPS binding-energy shifts in zeolites. J. Phys. Chem. 1994, 98, 10920–10929. [Google Scholar] [CrossRef]
- Huang, M.; Adnot, A.; Kaliaguine, S. Cation-framework interaction in alkali-cation-exchanged zeolites: An XPS study. J. Am. Chem. Soc. 2002, 114, 10005–10010. [Google Scholar] [CrossRef]
- Merlen, E.; Lynch, J.; Bisiaux, M.; Raatz, F. Surface modifications duringg Y zeolite dealumination. Surf. Interface Anal. 1990, 16, 364–368. [Google Scholar] [CrossRef]
- Zhao, Z.; Yu, R.; Zhao, R.; Shi, C.; Gies, H.; Xiao, F.-S.; De Vos, D.; Yokoi, T.; Bao, X.; Kolb, U.; et al. Cu-exchanged Al-rich SSZ-13 zeolite from organotemplate-free synthesis as NH3-SCR catalyst: Effects of Na+ ions on the activity and hydrothermal stability. Appl. Catal. B Environ. 2017, 217, 421–428. [Google Scholar] [CrossRef]
- Dong, S.T.; Li, X.W.; Li, D.D.; Shi, Y.H.; Nie, H.; Kang, X.H. Study on the formation of mesopore during hydrothermal dealumination of Y zeolite. Acta Phys. Chim. Sin. 2002, 18, 201–206. [Google Scholar]
- Yu, J.Z.; Ye, S.; Shi, Y.L.; Liao, H.Z.; Xv, X.L.; Wang, D.P. Confinement of highly luminescent silver nanoclusters in FAUY Zeolite: A study on the formation effects of silver nanoclusters and the sensitization of Tb3+. Acs Appl. Nano Mater. 2021, 4, 6290–6298. [Google Scholar] [CrossRef]
- Chebbi, M.; Azambre, B.; Cantrel, L.; Koch, A. A combined DRIFTS and DR-UV-Vis spectroscopic in situ study on the trapping of CH3I by silver-exchanged faujasite zeolite. J. Phys. Chem. C 2016, 120, 18694–18706. [Google Scholar] [CrossRef]
- Chebbi, M.; Azambre, B.; Cantrel, L.; Hove, M.; Albiol, T. Influence of structural, textural and chemical parameters of silver zeolites on the retention of methyl iodide. Microporous Mesoporous Mater. 2017, 244, 137–150. [Google Scholar] [CrossRef]
- Silaghi, M.-C.; Chizallet, C.; Raybaud, P. Challenges on molecular aspects of dealumination and desilication of zeolites. Microporous Mesoporous Mater. 2014, 191, 82–96. [Google Scholar] [CrossRef]
- Groen, J.C.; Moulijn, J.A.; Pérez-Ramírez, J. Decoupling mesoporosity formation and acidity modification in ZSM-5 zeolites by sequential desilication–dealumination. Microporous Mesoporous Mater. 2005, 87, 153–161. [Google Scholar] [CrossRef]
- Shin, H.S.; Choi, H.C.; Jung, Y.; Kim, S.B.; Song, H.J.; Shin, H.J. Chemical and size effects of nanocomposites of silver and polyvinyl pyrrolidone determined by X-ray photoemission spectroscopy. Chem. Phys. Lett. 2004, 383, 418–422. [Google Scholar] [CrossRef]
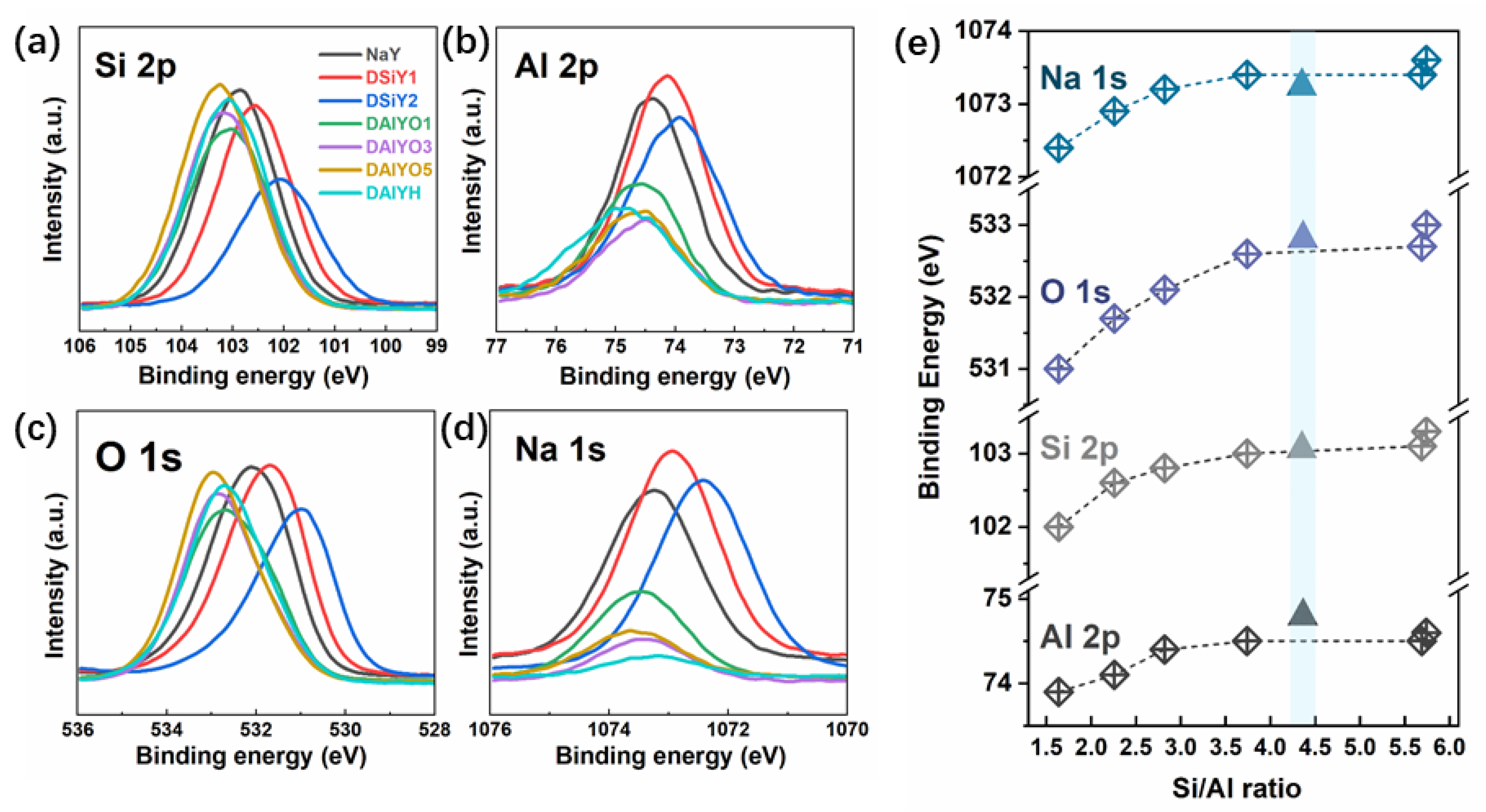



| Sample | XPS Atomic (%) | Si/Al | |||
|---|---|---|---|---|---|
| Si | Al | O | Na | ||
| NaY | 21.15 | 7.50 | 61.85 | 9.50 | 2.82 |
| DSiY1 | 19.33 | 8.57 | 60.86 | 11.24 | 2.26 |
| DSiY2 | 16.70 | 10.16 | 59.61 | 13.53 | 1.64 |
| DAlYO1 | 23.13 | 6.18 | 64.89 | 5.80 | 3.74 |
| DAlYO3 | 25.33 | 4.45 | 67.33 | 2.89 | 5.69 |
| DAlYO5 | 25.65 | 4.47 | 66.97 | 2.91 | 5.74 |
| DAlYH | 24.92 | 5.66 | 68.09 | 1.33 | 4.40 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Xv, X.; Ye, S.; Pan, L.; Lin, P.; Liao, H.; Wang, D. Tailoring the Luminescence Properties of Silver Clusters Confined in Faujasite Zeolite through Framework Modification. Materials 2022, 15, 7431. https://doi.org/10.3390/ma15217431
Xv X, Ye S, Pan L, Lin P, Liao H, Wang D. Tailoring the Luminescence Properties of Silver Clusters Confined in Faujasite Zeolite through Framework Modification. Materials. 2022; 15(21):7431. https://doi.org/10.3390/ma15217431
Chicago/Turabian StyleXv, Xinling, Song Ye, Ling Pan, Peixuan Lin, Huazhen Liao, and Deping Wang. 2022. "Tailoring the Luminescence Properties of Silver Clusters Confined in Faujasite Zeolite through Framework Modification" Materials 15, no. 21: 7431. https://doi.org/10.3390/ma15217431