The Influence of Air Nanobubbles on Controlling the Synthesis of Calcium Carbonate Crystals
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Methods
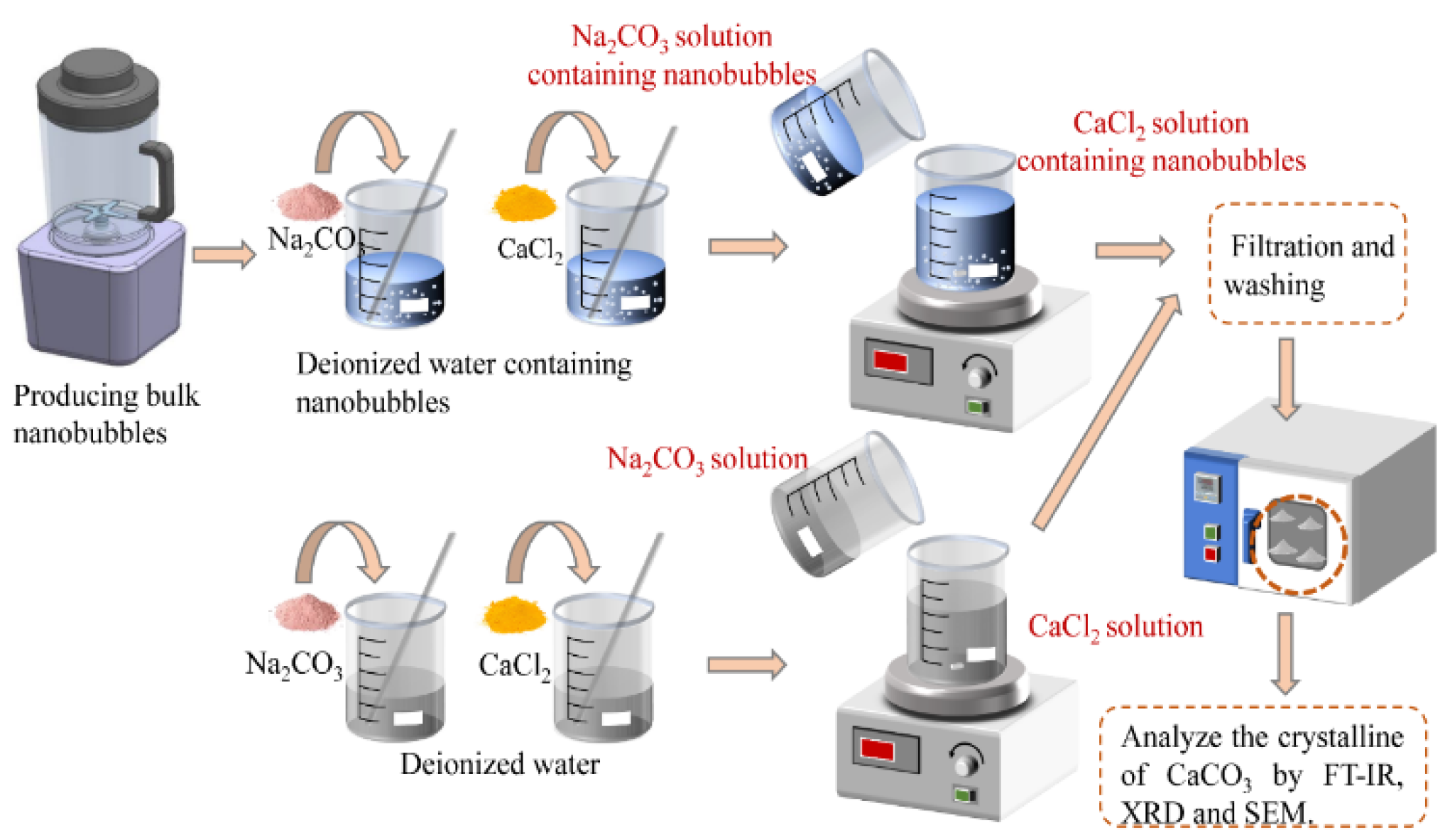
2.2.1. Preparation of Nanobubble Suspension
2.2.2. Calcium Carbonate Synthesis
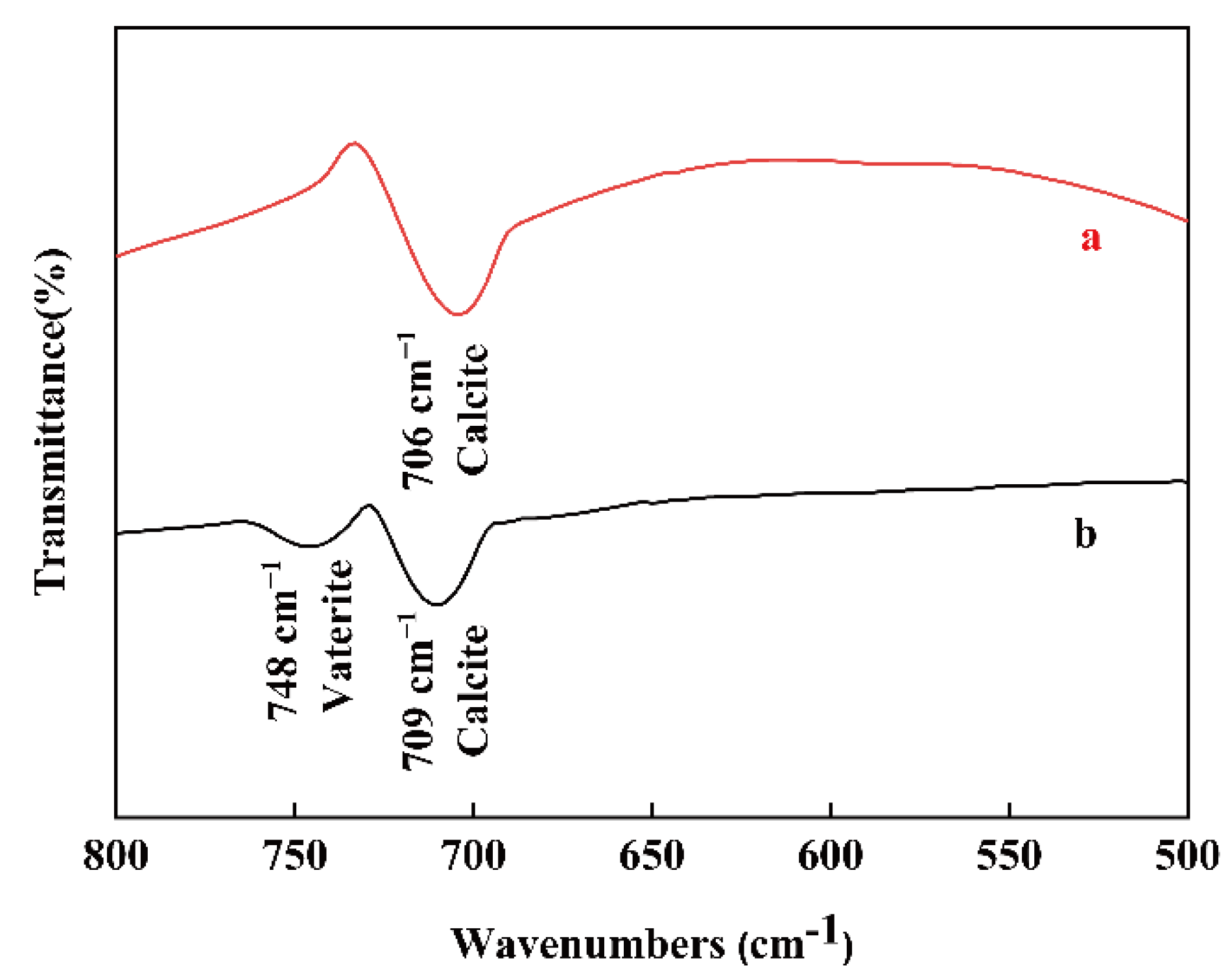
2.2.3. Characterization
3. Results
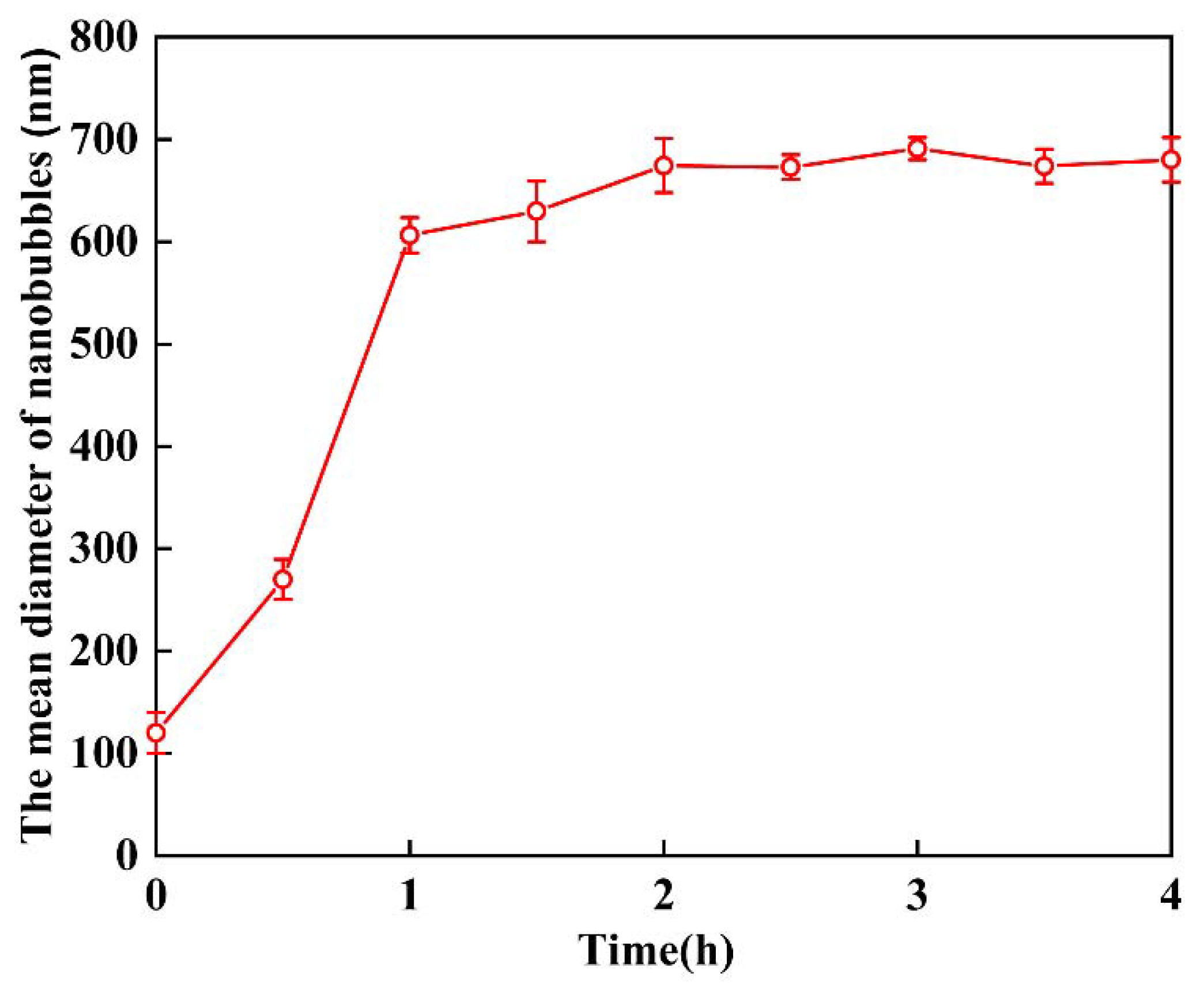
3.1. Generation of Air Nanobubbles
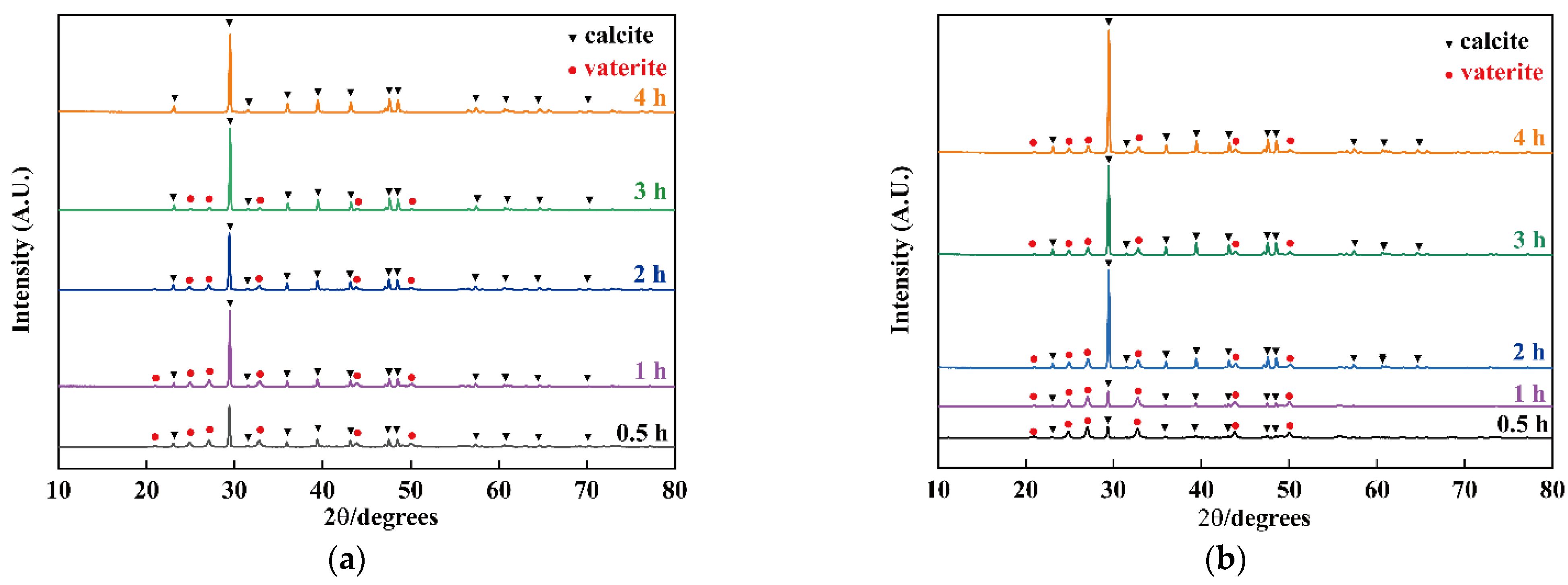
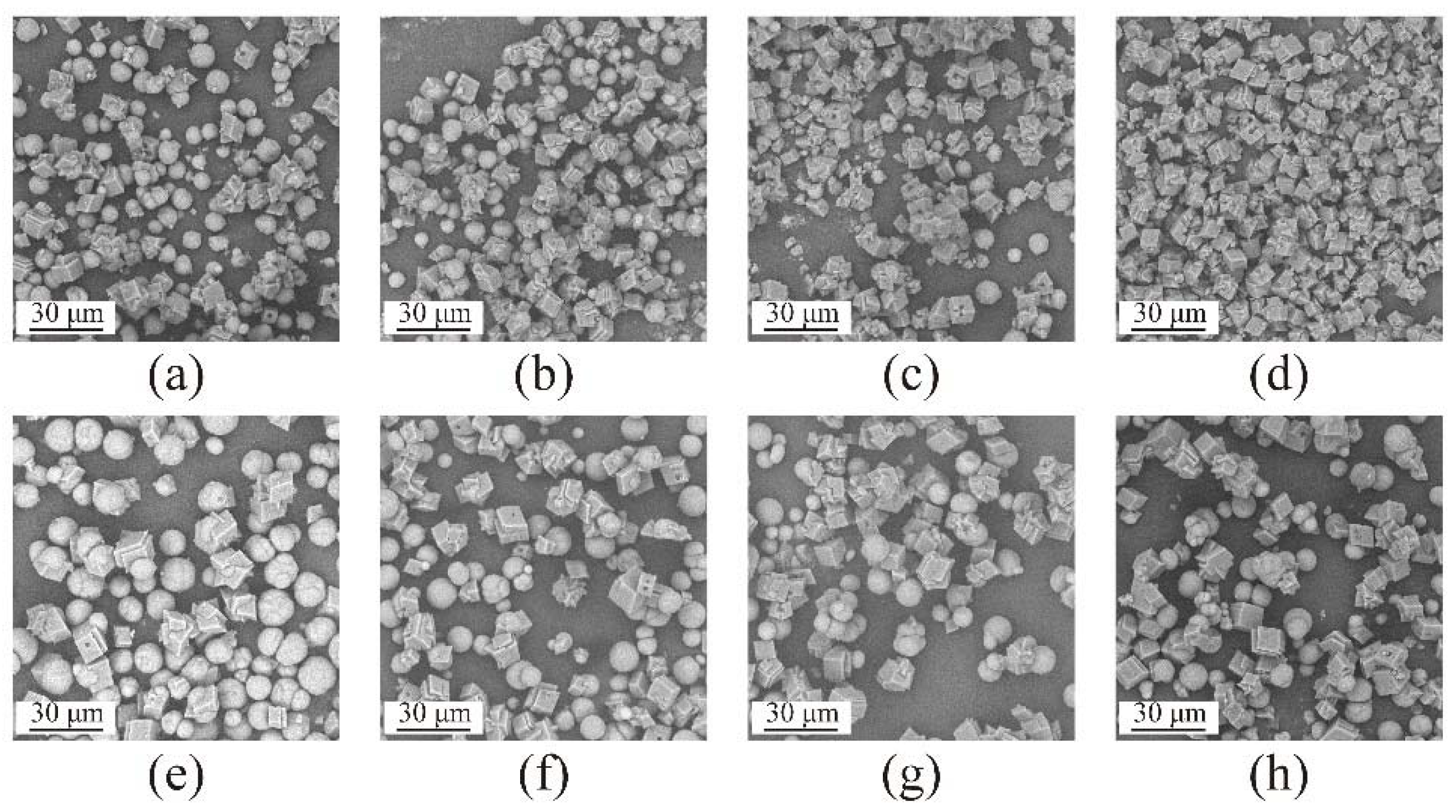
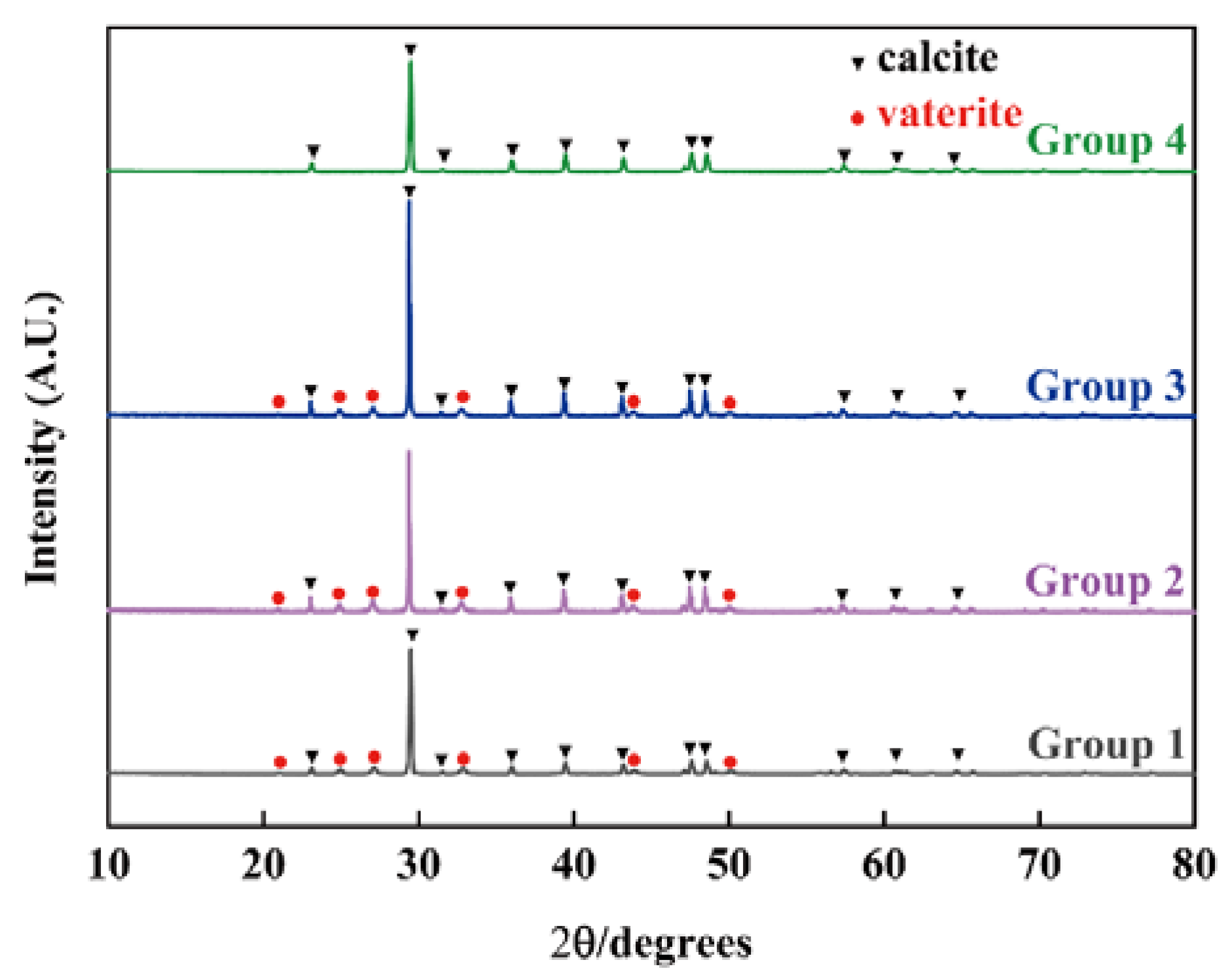
3.2. The Influence of Nanobubbles on the Transformation from Vaterite to Calcite
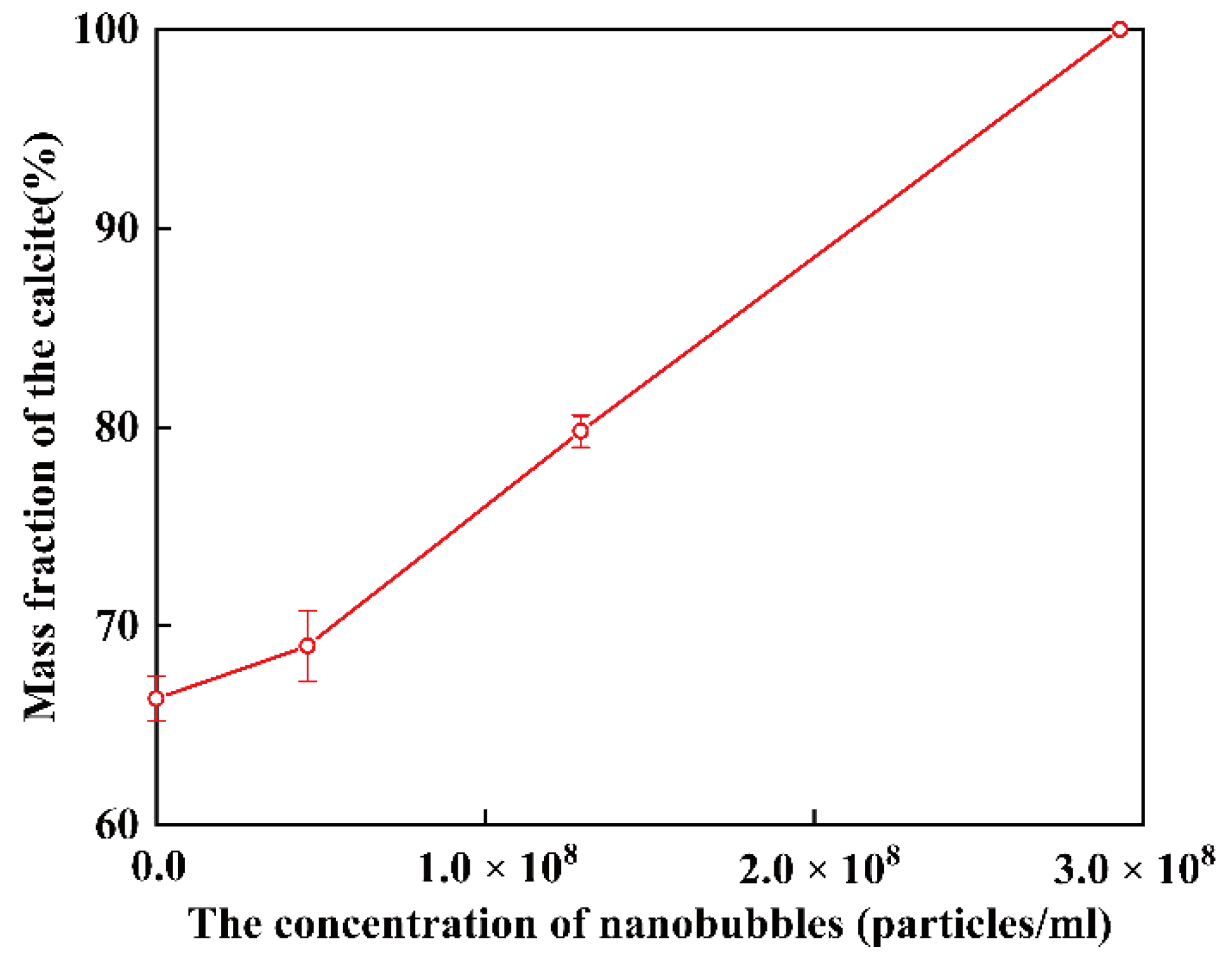
3.3. The Influence of Concentration of Nanobubbles on the Transformation from Vaterite to Calcite
4. Discussion
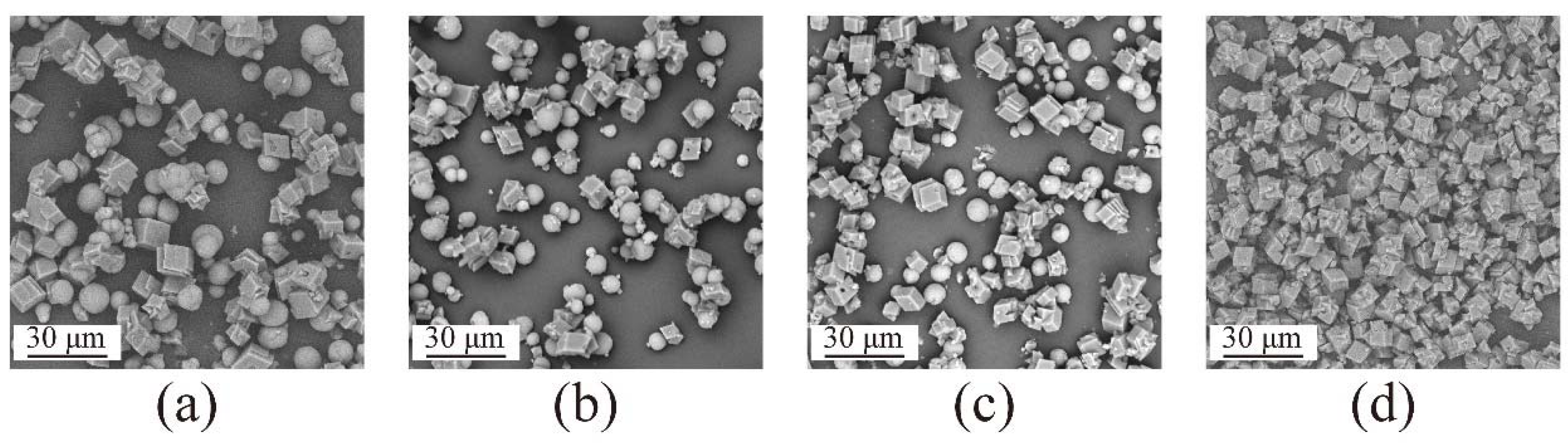
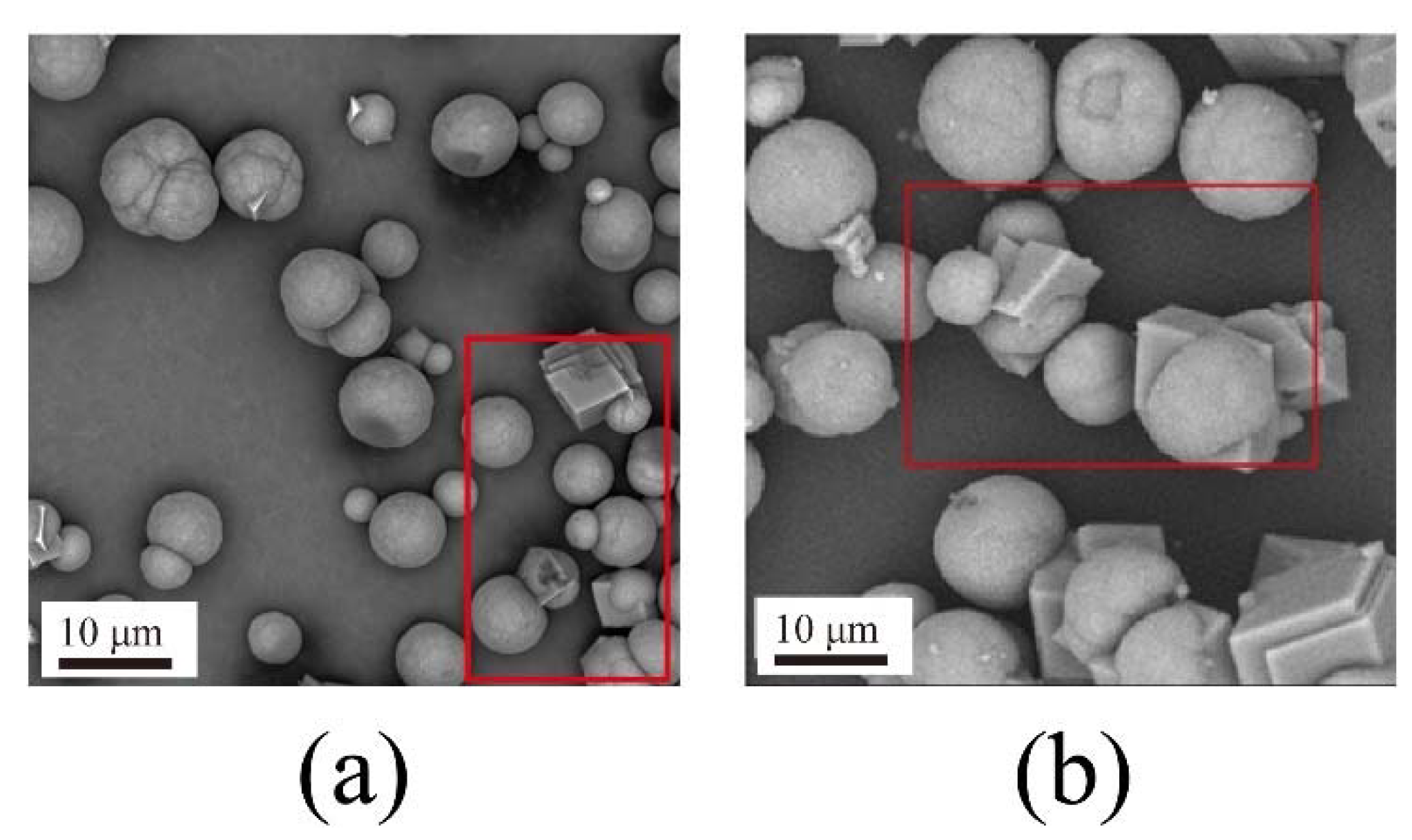
4.1. CaCO3 Particle Synthesis with Different Nanobubble Concentrations
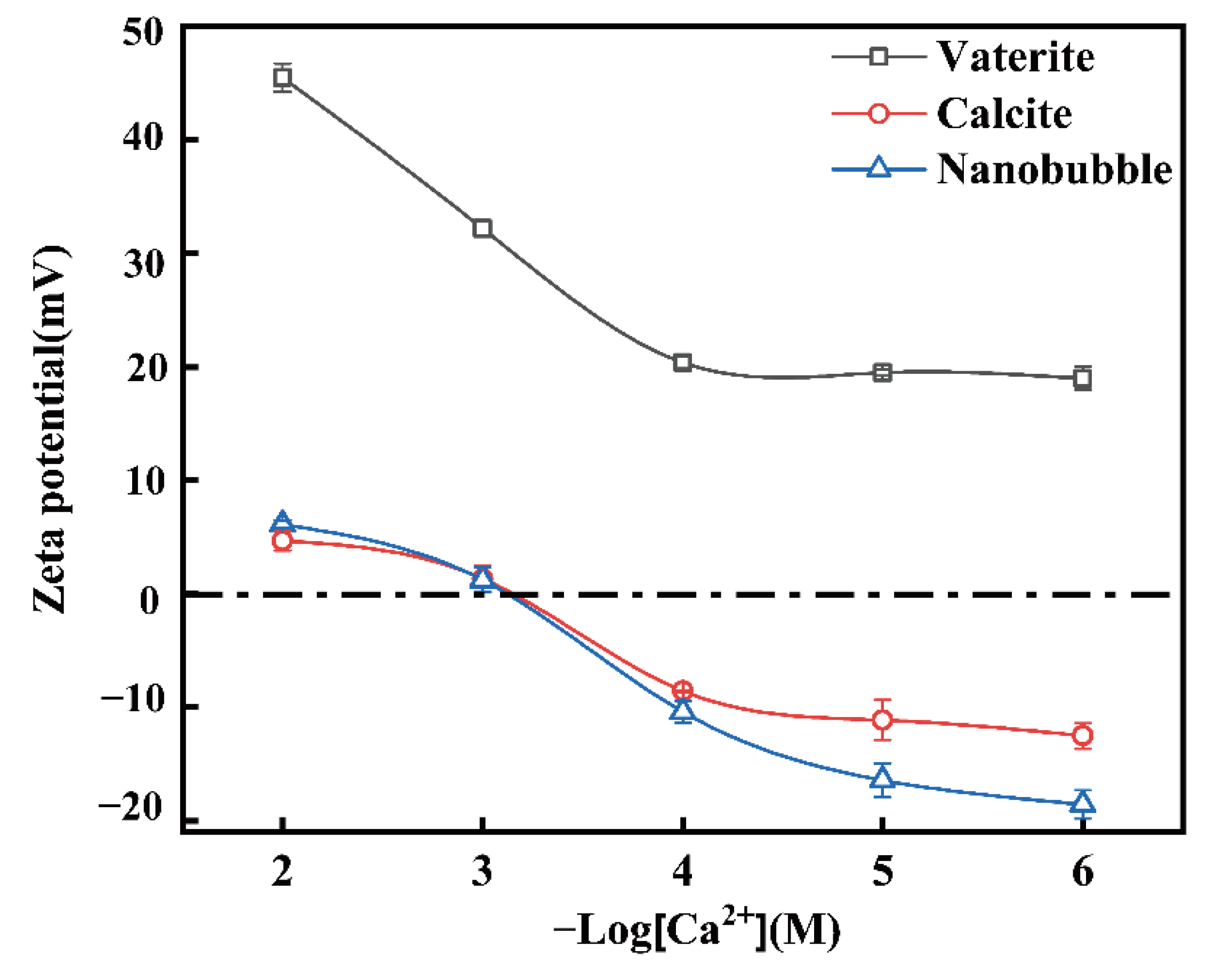
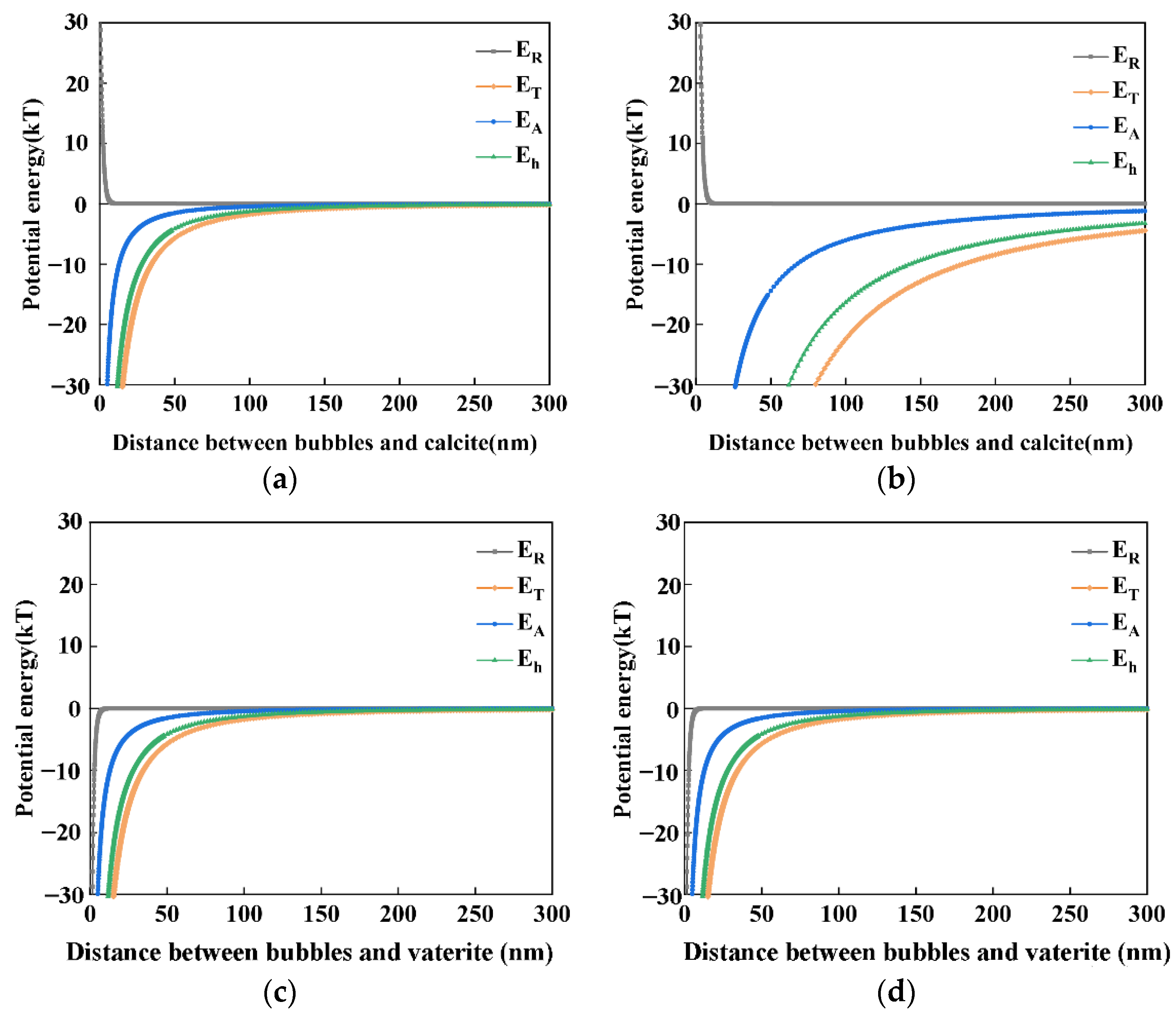
4.2. The Interaction between Nanobubbles, CaCO3 Particles and Nanobubble-CaCO3 Particles
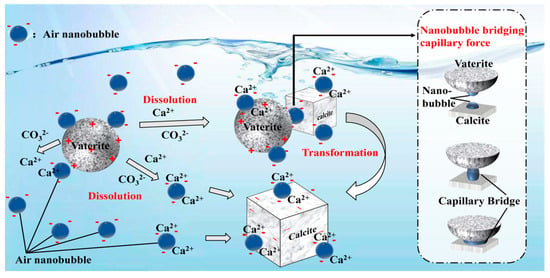
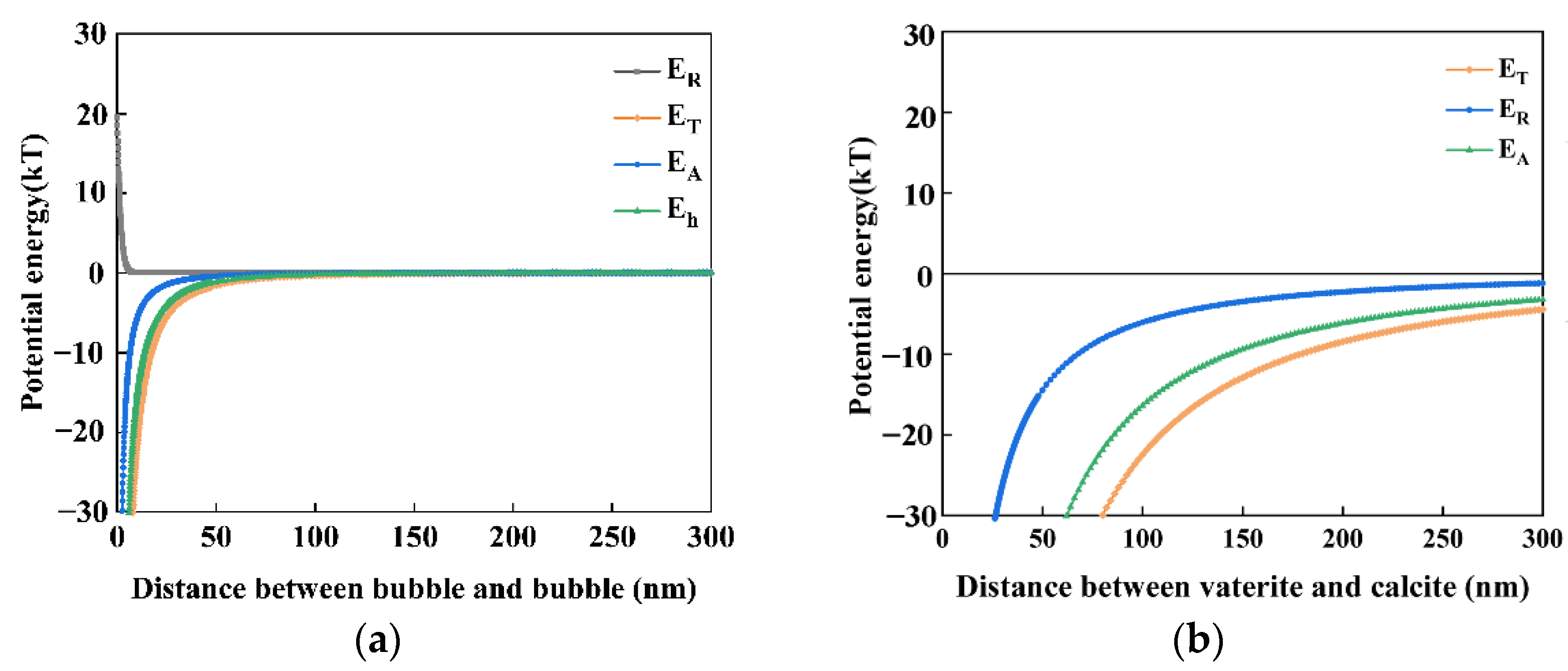
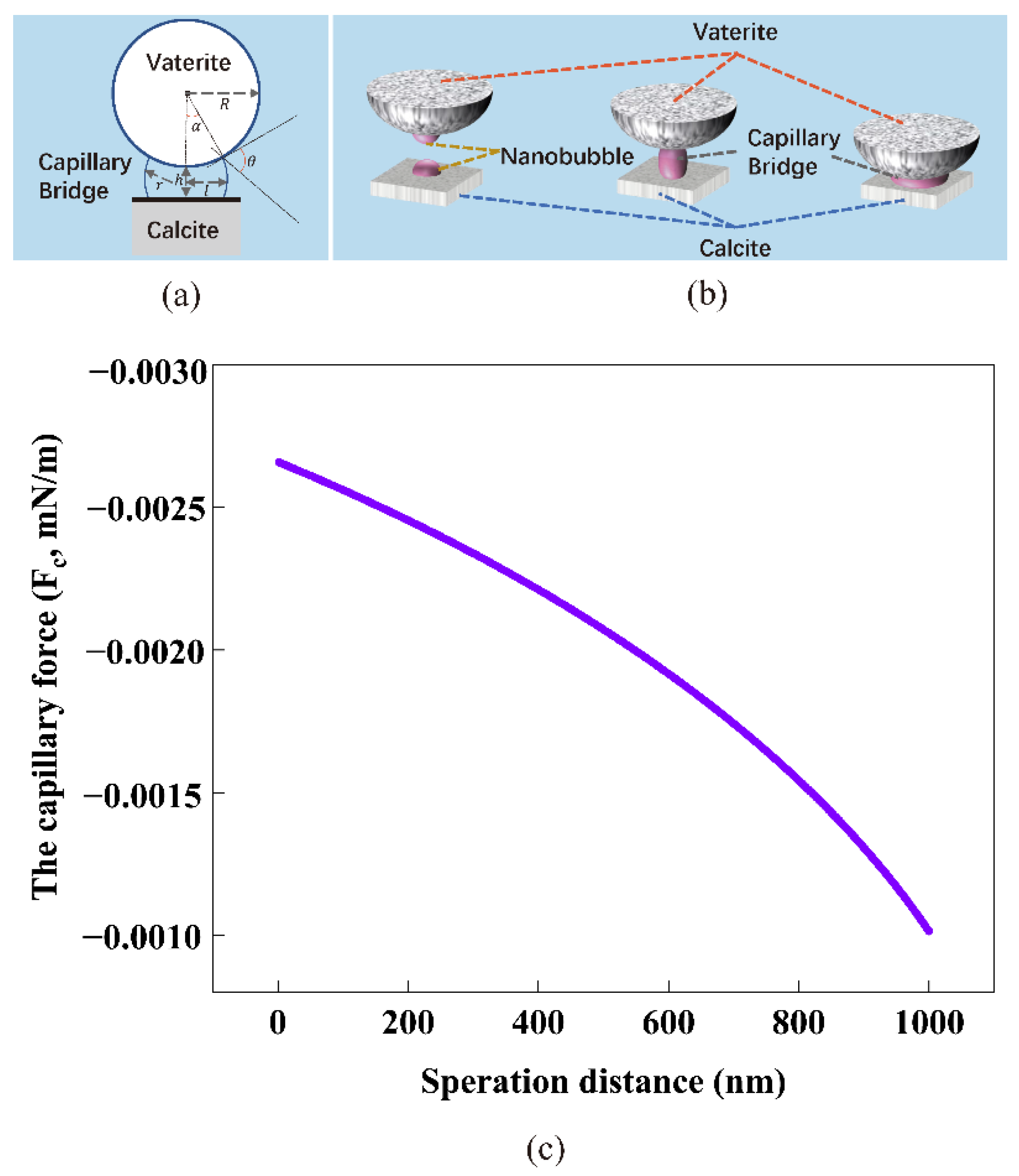
4.3. The Capillary Force Model between Calcite and Vaterite
4.4. Formation Mechanism of the Crystallization Transformation of CaCO3
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Gurung, A.; Dahl, O.; Jansson, K. The fundamental phenomena of nanobubbles and their behavior in wastewater treatment technologies. Geosystem. Eng. 2016, 19, 133–142. [Google Scholar] [CrossRef]
- Michailidi, E.D.; Bomis, G.; Varoutoglou, A.; Kyzas, G.Z.; Mitrikas, G.; Mitropoulos, A.C.; Efthimiadou, E.K.; Favvas, E.P. Bulk nanobubbles: Production and investigation of their formation/stability mechanism. J. Colloid. Interf. Sci. 2020, 564, 371–380. [Google Scholar] [CrossRef] [PubMed]
- Nirmalkar, N.; Pacek, A.W.; Barigou, M. On the Existence and Stability of Bulk Nanobubbles. Langmuir 2018, 34, 10964–10973. [Google Scholar] [CrossRef] [PubMed]
- Ulatowski, K.; Sobieszuk, P.; Mróz, A.; Ciach, T. Stability of nanobubbles generated in water using porous membrane system. Chem. Eng. Process. 2019, 136, 62–71. [Google Scholar] [CrossRef]
- Ducker, W.A. Contact angle and stability of interfacial nanobubbles. Langmuir 2009, 25, 8907–8910. [Google Scholar] [CrossRef]
- Ma, X.; Li, M.; Pfeiffer, P.; Eisener, J.; Ohl, C.D.; Sun, C. Ion adsorption stabilizes bulk nanobubbles. J. Colloid. Interf. Sci. 2022, 606 Pt 2, 1380–1394. [Google Scholar] [CrossRef]
- Zhang, H.; Guo, Z.; Zhang, X. Surface enrichment of ions leads to the stability of bulk nanobubbles. Soft. Matter. 2020, 16, 5470–5477. [Google Scholar] [CrossRef]
- Zhou, Y.; Han, Z.; He, C.; Feng, Q.; Wang, K.; Wang, Y.; Luo, N.; Dodbiba, G.; Wei, Y.; Otsuki, A.; et al. Long-Term Stability of Different Kinds of Gas Nanobubbles in Deionized and Salt Water. Materials 2021, 14, 1808. [Google Scholar] [CrossRef] [PubMed]
- Agarwal, A.; Ng, W.J.; Liu, Y. Principle and applications of microbubble and nanobubble technology for water treatment. Chemosphere 2011, 84, 1175–1180. [Google Scholar] [CrossRef]
- Mai, L.; Yao, A.; Li, J.; Wei, Q.; Yuchi, M.; He, X.; Ding, M.; Zhou, Q. Cyanine 5.5 conjugated nanobubbles as a tumor selective contrast agent for dual ultrasound-fluorescence imaging in a mouse model. PLoS ONE 2013, 8, e61224. [Google Scholar] [CrossRef] [PubMed]
- Cavalli, R.; Bisazza, A.; Giustetto, P.; Civra, A.; Lembo, D.; Trotta, G.; Guiot, C.; Trotta, M. Preparation and characterization of dextran nanobubbles for oxygen delivery. Int. J. Pharm. 2009, 381, 160–165. [Google Scholar] [CrossRef]
- Batagoda, J.H.; Hewage, S.A.; Meegoda, J.N. Remediation of heavy-metal-contaminated sediments in USA using ultrasound and ozone nanobubbles. J. Environ. Eng. Sci. 2019, 14, 130–138. [Google Scholar] [CrossRef]
- Wang, Y.; Pan, Z.; Luo, X.; Qin, W.; Jiao, F. Effect of nanobubbles on adsorption of sodium oleate on calcite surface. Miner. Eng. 2019, 133, 127–137. [Google Scholar] [CrossRef]
- Calgaroto, S.; Wilberg, K.Q.; Rubio, J. On the nanobubbles interfacial properties and future applications in flotation. Miner. Eng. 2014, 60, 33–40. [Google Scholar] [CrossRef]
- Khoshroo, M.; Javid, A.; Katebi, A. Effects of micro-nano bubble water and binary mineral admixtures on the mechanical and durability properties of concrete. Constr. Build. Mater. 2018, 164, 371–385. [Google Scholar] [CrossRef]
- Bull, D.S.; Kienle, D.F.; Sosa, A.F.C.; Nelson, N.; Roy, S.; Cha, J.N.; Schwartz, D.K.; Kaar, J.L.; Goodwin, A.P. Surface-Templated Nanobubbles Protect Proteins from Surface-Mediated Denaturation. J. Phys. Chem. Lett. 2019, 10, 2641–2647. [Google Scholar] [CrossRef] [PubMed]
- Hernandez, C.; Abenojar, E.C.; Hadley, J.; de Leon, A.C.; Coyne, R.; Perera, R.; Gopalakrishnan, R.; Basilion, J.P.; Kolios, M.C.; Exner, A.A. Sink or float? Characterization of shell-stabilized bulk nanobubbles using a resonant mass measurement technique. Nanoscale 2019, 11, 851–855. [Google Scholar] [CrossRef]
- Kurokawa, H.; Matsui, H.; Ito, H.; Taninaka, A.; Shigekawa, H.; Dodbiba, G.; Wei, Y.; Fujita, T. Antioxidant Effect of Hydrogen Nanobubble Contributes to Suppression of Tumor Cell Growth. Biomed. J. Sci. Tech. Res. 2019, 19, 14592–14594. [Google Scholar] [CrossRef]
- Etchepare, R.; Azevedo, A.; Calgaroto, S.; Rubio, J. Removal of ferric hydroxide by flotation with micro and nanobubbles. Sep. Purif. Technol. 2017, 184, 347–353. [Google Scholar] [CrossRef]
- Temesgen, T.; Bui, T.; Han, M.; Kim, T.; Park, H. Micro and nanobubble technologies as a new horizon for water-treatment techniques: A review. Adv. Colloid. Interf. Sci. 2017, 246, 40–51. [Google Scholar] [CrossRef]
- Calgaroto, S.; Azevedo, A.; Rubio, J. Separation of amine-insoluble species by flotation with nano and microbubbles. Miner. Eng. 2016, 89, 24–29. [Google Scholar] [CrossRef]
- Rodriguez-Blanco, J.D.; Shaw, S.; Benning, L.G. The kinetics and mechanisms of amorphous calcium carbonate (ACC) crystallization to calcite, via vaterite. Nanoscale 2011, 3, 265–271. [Google Scholar] [CrossRef] [PubMed]
- Sawada, K. The mechanisms of crystallization and transformation of calcium carbonates. Pure. Appl. Chem. 1997, 69, 921–928. [Google Scholar] [CrossRef]
- Kim, G.; Kim, S.; Kim, M. Effect of sucrose on CO2 storage, vaterite content, and CaCO3 particle size in indirect carbonation using seawater. J. CO2 Util. 2022, 57, 101894. [Google Scholar] [CrossRef]
- Beck, R.; Andreassen, J. The onset of spherulitic growth in crystallization of calcium carbonate. J. Cryst. Growth 2010, 312, 2226–2238. [Google Scholar] [CrossRef]
- Wang, W.; Wang, G.; Liu, Y.; Zheng, C.; Zhan, Y. Synthesis and characterization of aragonite whiskers by a novel and simple route. J. Mater. Chem. 2001, 11, 1752–1754. [Google Scholar] [CrossRef]
- Han, J.; Jung, S.; Kang, D.; Seo, Y. Development of Flexible Calcium Carbonate for Papermaking Filler. ACS Sustain. Chem. Eng. 2020, 8, 8994–9001. [Google Scholar] [CrossRef]
- Lizandara-Pueyo, C.; Fan, X.; Ayats, C.; Pericàs, M.A. Calcium carbonate as heterogeneous support for recyclable organocatalysts. J. Catal. 2021, 393, 107–115. [Google Scholar] [CrossRef]
- Dou, J.; Zhao, F.; Fan, W.; Chen, Z.; Guo, X. Preparation of non-spherical vaterite CaCO3 particles by flash nano precipitation technique for targeted and extended drug delivery. J. Drug. Deliv. Sci. Tec. 2020, 57, 101768. [Google Scholar] [CrossRef]
- Lin, P.; Wu, H.; Hsieh, S.; Li, J.; Dong, C.; Chen, C.; Hsieh, S. Preparation of vaterite calcium carbonate granules from discarded oyster shells as an adsorbent for heavy metal ions removal. Chemosphere 2020, 254, 126903. [Google Scholar] [CrossRef]
- Nakamura, J.; Kasuga, T.; Sakka, Y. Preparation of carbamate-containing vaterite particles for strontium removal in wastewater treatment. J. Asian Ceram. Soc. 2018, 5, 364–369. [Google Scholar] [CrossRef]
- Song, X.; Cao, Y.; Bu, X.; Luo, X. Porous vaterite and cubic calcite aggregated calcium carbonate obtained from steamed ammonia liquid waste for Cu2+ heavy metal ions removal by adsorption process. Appl. Surf. Sci. 2021, 536, 147958. [Google Scholar] [CrossRef]
- Sasamoto, R.; Kanda, Y.; Yamanaka, S. Difference in cadmium chemisorption on calcite and vaterite porous particles. Chemosphere 2022, 297, 134057. [Google Scholar] [CrossRef] [PubMed]
- Hadiko, G.; Han, Y.; Fuji, M.; Takahashi, M. Synthesis of hollow calcium carbonate particles by the bubble templating method. Mater. Lett. 2005, 59, 2519–2522. [Google Scholar] [CrossRef]
- Sha, F.; Zhu, N.; Bai, Y.; Li, Q.; Guo, B.; Zhao, T.; Zhang, F.; Zhang, J. Controllable Synthesis of Various CaCO3 Morphologies Based on a CCUS Idea. Chem. Eng. 2016, 4, 3032–3044. [Google Scholar] [CrossRef]
- Yang, L.; Chu, D.; Sun, H.; Ge, G. Room temperature synthesis of flower-like CaCO3 architectures. New. J. Chem. 2016, 40, 571–577. [Google Scholar] [CrossRef]
- Jiang, J.; Ye, J.; Zhang, G.; Gong, X.; Nie, L.; Liu, J. Polymorph and Morphology Control of CaCO3 via Temperature and PEG During the Decomposition of Ca(HCO3)2. J. Am. Ceram. Soc. 2012, 95, 3735–3738. [Google Scholar] [CrossRef]
- Zheng, T.; Zhang, X.; Yi, H. Spherical vaterite microspheres of calcium carbonate synthesized with poly (acrylic acid) and sodium dodecyl benzene sulfonate. J. Cryst. Growth. 2019, 528, 125275. [Google Scholar] [CrossRef]
- Qi, R.; Zhu, Y. Microwave-Assisted Synthesis of Calcium Carbonate (Vaterite) of Various Morphologies. J. Phys. Chem. B 2006, 110, 8302–8306. [Google Scholar] [CrossRef]
- Nan, Z.; Chen, X.; Yang, Q.; Wang, X.; Shi, Z.; Hou, W. Structure transition from aragonite to vaterite and calcite by the assistance of SDBS. J. Colloid. Interf. Sci. 2008, 325, 331–336. [Google Scholar] [CrossRef]
- Ramesh, T.N.; Inchara, S.A.; Pallavi, K. Para-amino benzoic acid-mediated synthesis of vaterite phase of calcium carbonate. J. Chem. Sci. 2015, 127, 843–848. [Google Scholar] [CrossRef]
- Brennen, C.E. Cavitation and Bubble Dynamics; Cambridge University Press: Cambridge, UK, 1995. [Google Scholar] [CrossRef]
- Azevedo, A.; Oliveira, H.; Rubio, J. Bulk nanobubbles in the mineral and environmental areas: Updating research and applications. Adv. Colloid. Interf. Sci. 2019, 271, 101992. [Google Scholar] [CrossRef] [PubMed]
- Verwey, E.J.W.; Overbeek, J.T.G. Theory of the Stability of Lyophobic Colloids. J. Colloid. Sci. 1955, 10, 224–225. [Google Scholar] [CrossRef]
- Derjaguin, B.; Landau, L. Theory of the Stability of Strongly Charged Lyophobic Sols and of the Adhesion of Strongly Charged Particles in Solutions of Electrolytes. Prog. Surf. Sci. 1993, 43, 30–53. [Google Scholar] [CrossRef]
- Gogate, P.R.; Pandit, A.B. Engineering Design Methods for Cavitation Reactors II: Hydrodynamic Cavitation. AIChE J. 2000, 46, 1641–1649. [Google Scholar] [CrossRef]
- Kontoyannis, C.G.; Vagenas, N.V. Calcium carbonate phase analysis using XRD and FT-Raman spectroscopy. Analyst. 2000, 25, 251–255. [Google Scholar] [CrossRef]
- Proto, S.P.S.; Giordmaine, J.A.; Damen, T.C. Depolarization of Raman Scattering in Calcite. Phys. Rev. 1966, 147, 608–611. [Google Scholar] [CrossRef]
- Nagabhushana, H.; Nagabhushana, B.M.; Lakshminarasappa, B.N.; Singhd, F.; Chakradhar, R.P.S. Swift heavy ion irradiation induced phase transformation in calcite single crystals. Solid State Commun. 2009, 149, 1905–1908. [Google Scholar] [CrossRef][Green Version]
- Elfil, H.; Roques, H. Role of hydrate phases of calcium carbonate on the scaling phenomenon. Desalination 2001, 137, 177–186. [Google Scholar] [CrossRef]
- Zhang, F.; Yang, X.; Tian, F. Calcium carbonate growth in the presence of water soluble cellulose ethers. Mater. Sci. Eng. C 2009, 29, 2530–2538. [Google Scholar] [CrossRef]
- Rumble, J.R. CRC Handbook of Chemistry and Physics, 98th ed.; CRC Press LLC.: Boca Raton, FL, USA, 2017. [Google Scholar]
- Yoon, R.; Aksoy, B.S. Hydrophobic Forces in Thin Water Films Stabilized by Dodecylammonium Chloride. J. Colloid. Interf. Sci. 1999, 211, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Wang, L.; Yoon, R. Hydrophobic Forces in the Foam Films Stabilized by Sodium Dodecyl Sulfate Effect of Electrolyte. Langmuir 2004, 20, 11457–11464. [Google Scholar] [CrossRef] [PubMed]
- Tao, D.; Wu, Z.; Sobhy, A. Investigation of nanobubble enhanced reverse anionic flotation of hematite and associated mechanisms. Powder Technol. 2021, 379, 12–25. [Google Scholar] [CrossRef]
- Hampton, M.A.; Nguyen, A.V. Nanobubbles and the nanobubble bridging capillary force. Adv. Colloid. Interf. Sci. 2010, 154, 30–55. [Google Scholar] [CrossRef] [PubMed]
- Tselishchev, Y.G.; Val’tsifer, V.A. Influence of the Type of Contact between Particles Joined by a Liquid Bridge on the Capillary Cohesive Forces. Colloid. J. 2003, 65, 385–389. [Google Scholar] [CrossRef]
- Sirghi, L.; Szoszkiewicz, R.; Riedo, E. Volume of a Nanoscale Water Bridge. Langmuir 2006, 22, 1093–1098. [Google Scholar] [CrossRef]
- Wang, X.; Yuan, S.; Liu, J.; Zhu, Y.; Han, Y. Nanobubble-enhanced flotation of ultrafine molybdenite and the associated mechanism. J. Mol. Liq. 2022, 346, 118312. [Google Scholar] [CrossRef]
- Fan, M.; Tao, D.; Honaker, R.; Luo, Z. Nanobubble generation and its applications in froth flotation (part II): Fundamental study and theoretical analysis. MST 2010, 20, 159–177. [Google Scholar] [CrossRef]
- Wang, D.; Liu, Q. Hydrodynamics of froth flotation and its effects on fine and ultrafine mineral particle flotation: A literature review. Miner. Eng. 2021, 173, 107220. [Google Scholar] [CrossRef]
- Sobhy, A.; Tao, D. Nanobubble column flotation of fine coal particles and associated fundamentals. Int. J. Miner. Process. 2013, 124, 109–116. [Google Scholar] [CrossRef]
- Li, B.; Zhang, H. A review of bulk nanobubbles and their roles in flotation of fine particles. Powder Technol. 2022, 395, 618–633. [Google Scholar] [CrossRef]
- Priezjev, N.V.; Darhuber, A.A.; Troian, S.M. Slip behavior in liquid films on surfaces of patterned wettability: Comparison between continuum and molecular dynamics simulations. Phys. Rev. E. Stat. Nonlin. Soft. Matter. Phys. 2005, 71, 041608. [Google Scholar] [CrossRef] [PubMed]
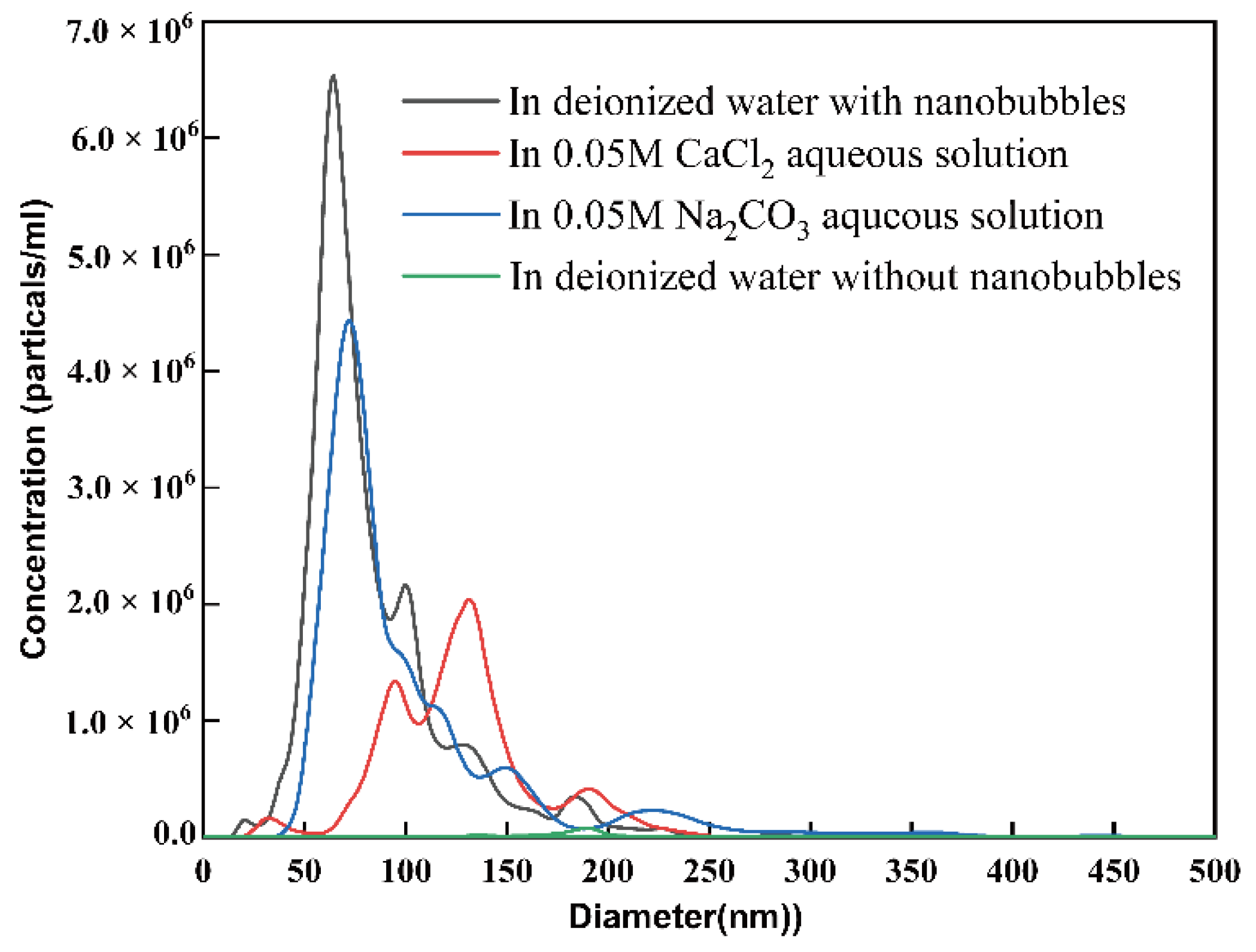


| Mean Diameter (nm) | Zeta Potential(mV) | Concentration (bubbles/mL) | pH | |
|---|---|---|---|---|
| In deionized water | 83.6 | −23.0 | 2.96 × 108 | 6.3 |
| In 0.05 M CaCl2 aqueous solution | 126.2 | 7.3 | 1.48 × 108 | 5.6 |
| In 0.05 M Na2CO3 aqueous solution | 101.8 | −17.8 | 2.49 × 108 | 11.7 |
| The Volume Ratio of Pure Water to Nanobubbles (Vdeionized water containing nanobubble: Vdeionized water) | The Concentration of Nanobubbles (Bubbles/mL) | |
|---|---|---|
| Group1 | Deionized water | 0 |
| Group2 | 1:3 | 4.59 × 107 |
| Group3 | 1:1 | 1.29 × 108 |
| Group4 | Initial deionized water containing nanobubbles | 2.96 × 108 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wu, Y.; Huang, M.; He, C.; Wang, K.; Nhung, N.T.H.; Lu, S.; Dodbiba, G.; Otsuki, A.; Fujita, T. The Influence of Air Nanobubbles on Controlling the Synthesis of Calcium Carbonate Crystals. Materials 2022, 15, 7437. https://doi.org/10.3390/ma15217437
Wu Y, Huang M, He C, Wang K, Nhung NTH, Lu S, Dodbiba G, Otsuki A, Fujita T. The Influence of Air Nanobubbles on Controlling the Synthesis of Calcium Carbonate Crystals. Materials. 2022; 15(21):7437. https://doi.org/10.3390/ma15217437
Chicago/Turabian StyleWu, Yongxiang, Minyi Huang, Chunlin He, Kaituo Wang, Nguyen Thi Hong Nhung, Siming Lu, Gjergj Dodbiba, Akira Otsuki, and Toyohisa Fujita. 2022. "The Influence of Air Nanobubbles on Controlling the Synthesis of Calcium Carbonate Crystals" Materials 15, no. 21: 7437. https://doi.org/10.3390/ma15217437
APA StyleWu, Y., Huang, M., He, C., Wang, K., Nhung, N. T. H., Lu, S., Dodbiba, G., Otsuki, A., & Fujita, T. (2022). The Influence of Air Nanobubbles on Controlling the Synthesis of Calcium Carbonate Crystals. Materials, 15(21), 7437. https://doi.org/10.3390/ma15217437