Utilization of Lead Slag as In Situ Iron Source for Arsenic Removal by Forming Iron Arsenate
Abstract
1. Introduction
2. Methods and Materials
2.1. Materials Characterization
2.2. Experiment Procedures and Methods
2.3. Chemical Analysis of Solid Phase
3. Results and discussion
3.1. Characterization of Lead Slag
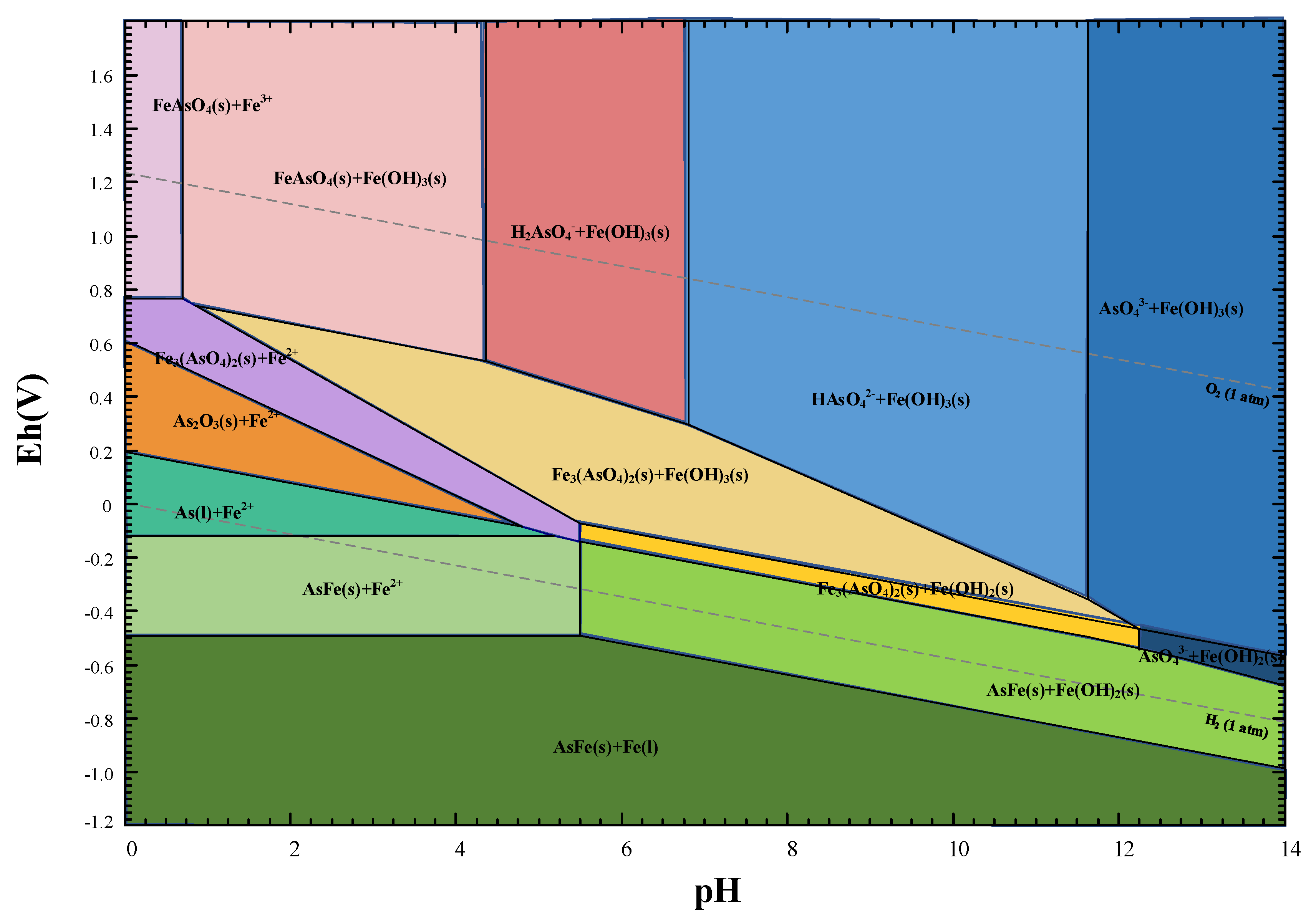
3.2. Thermodynamic Analysis
3.3. Removal of Arsenic from Wastewater
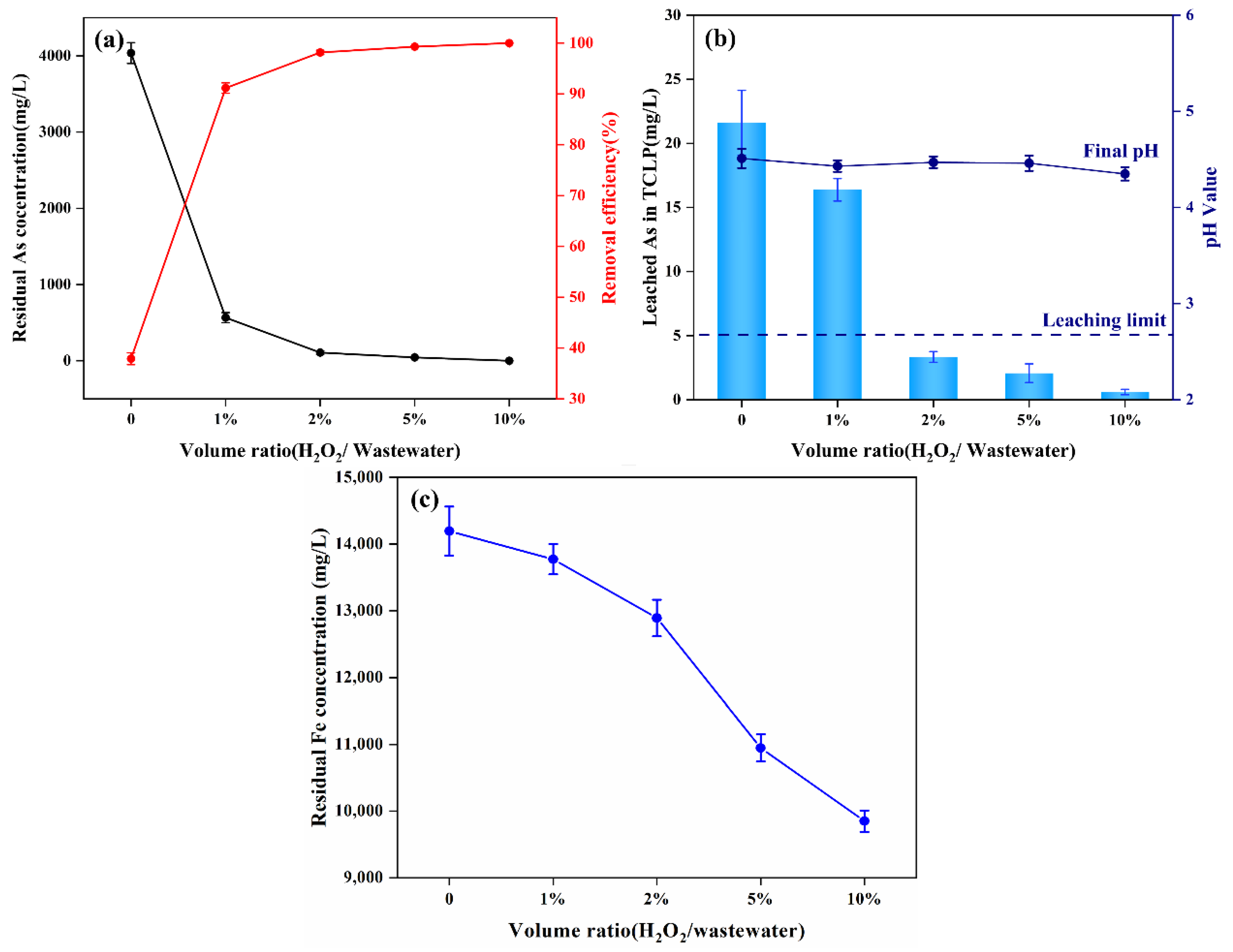
3.3.1. Effect of Dosage of H2O2
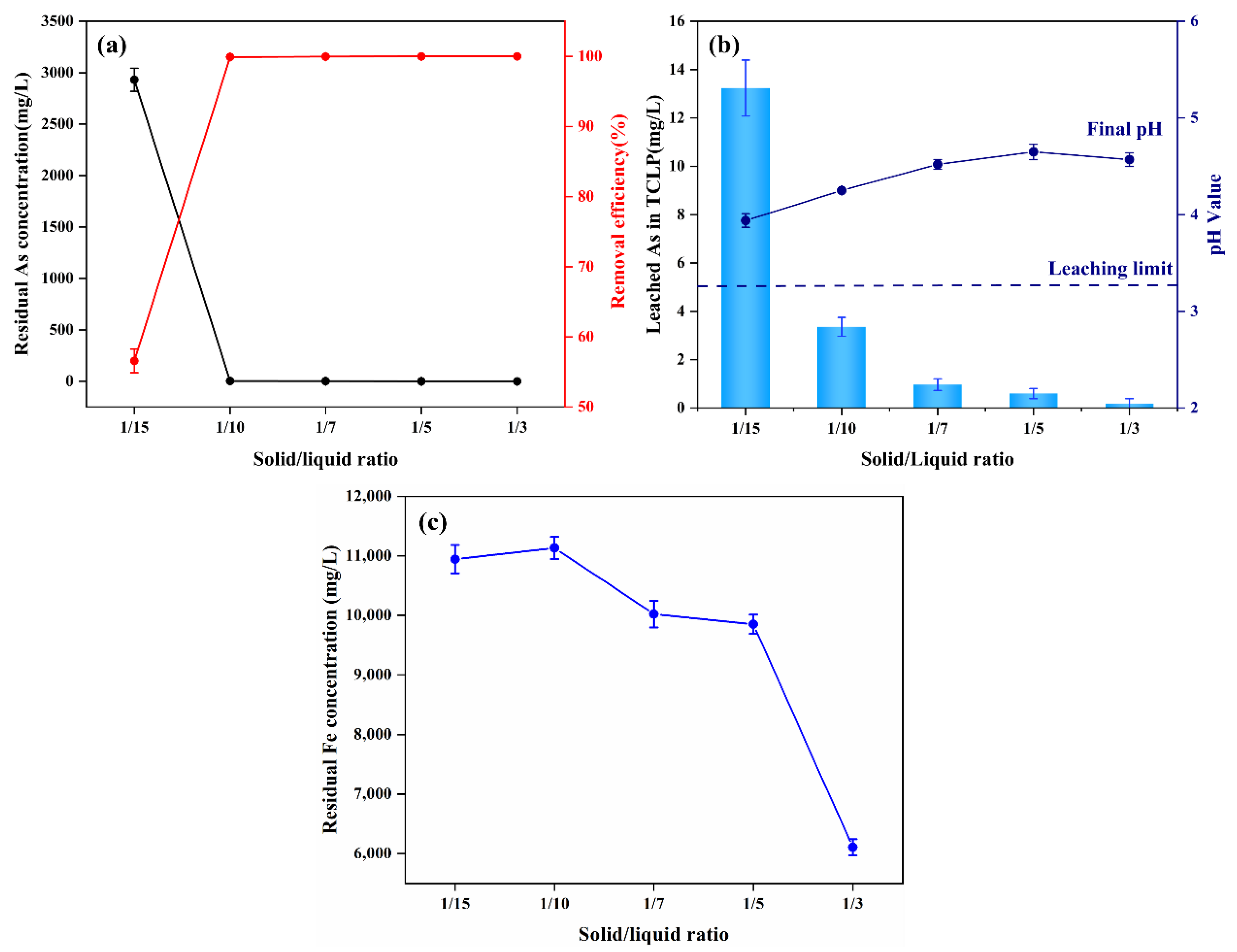
3.3.2. Effect of the Solid–Liquid Ratio
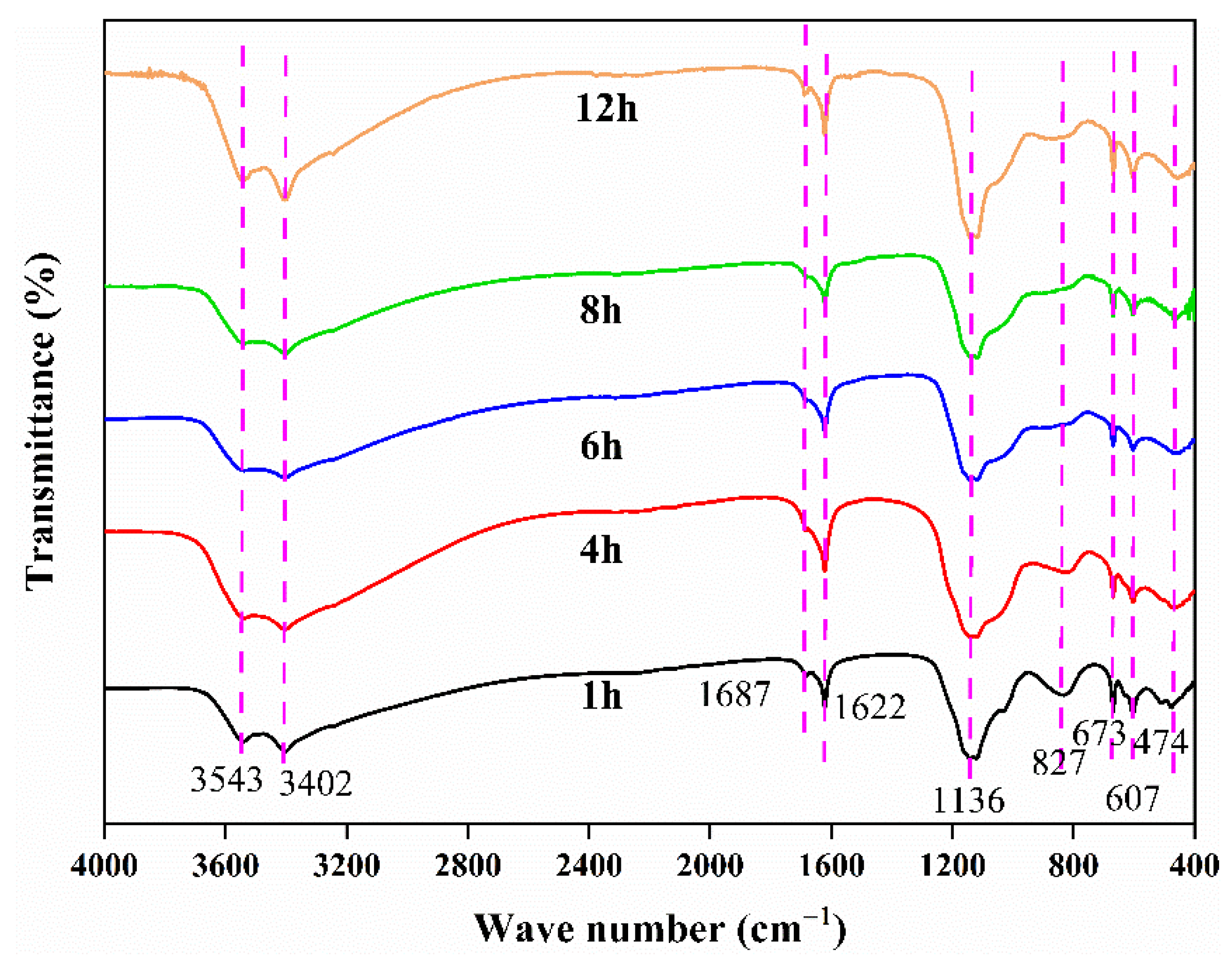
3.3.3. Effect of Reaction Time
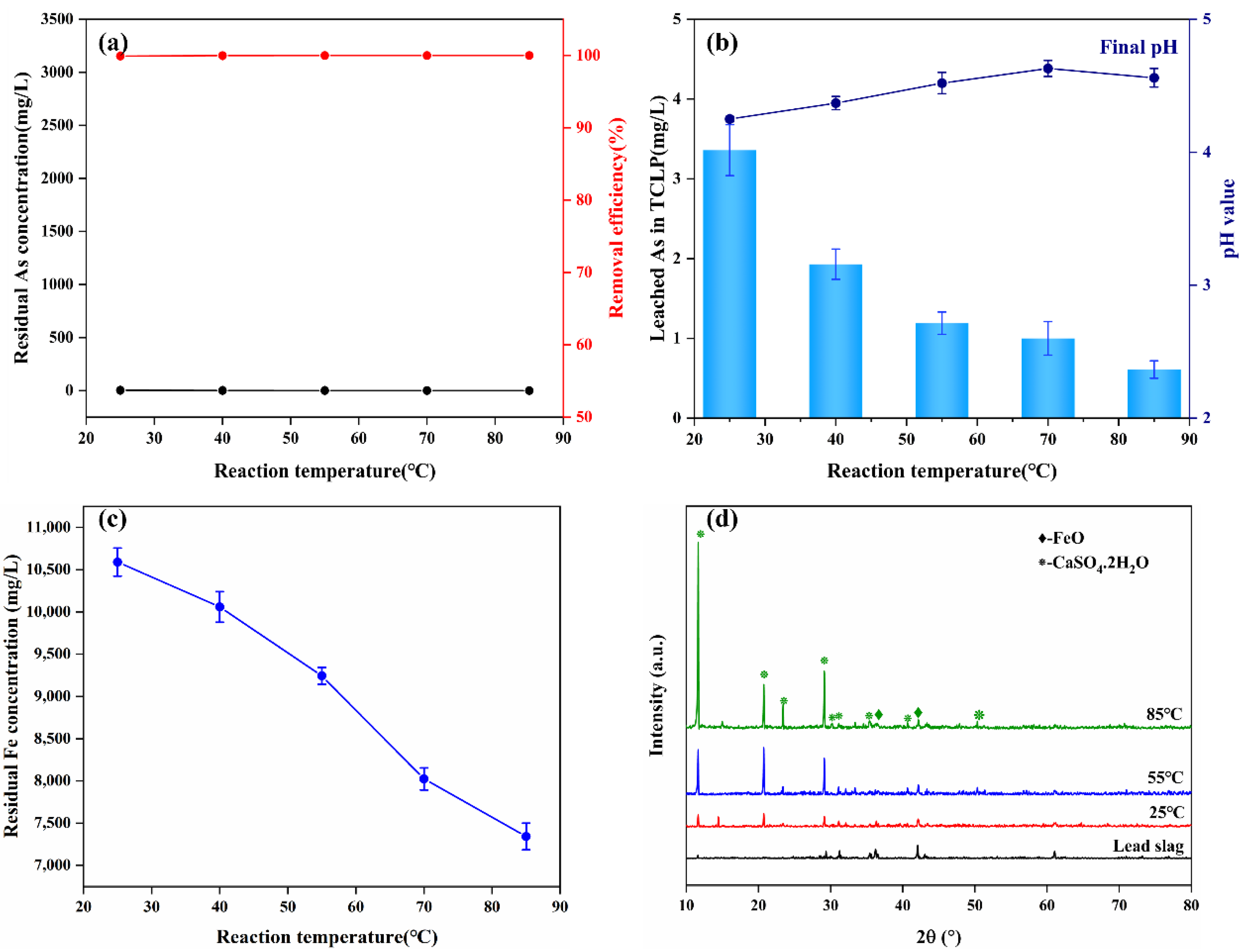
3.3.4. Effect of Reaction Temperature
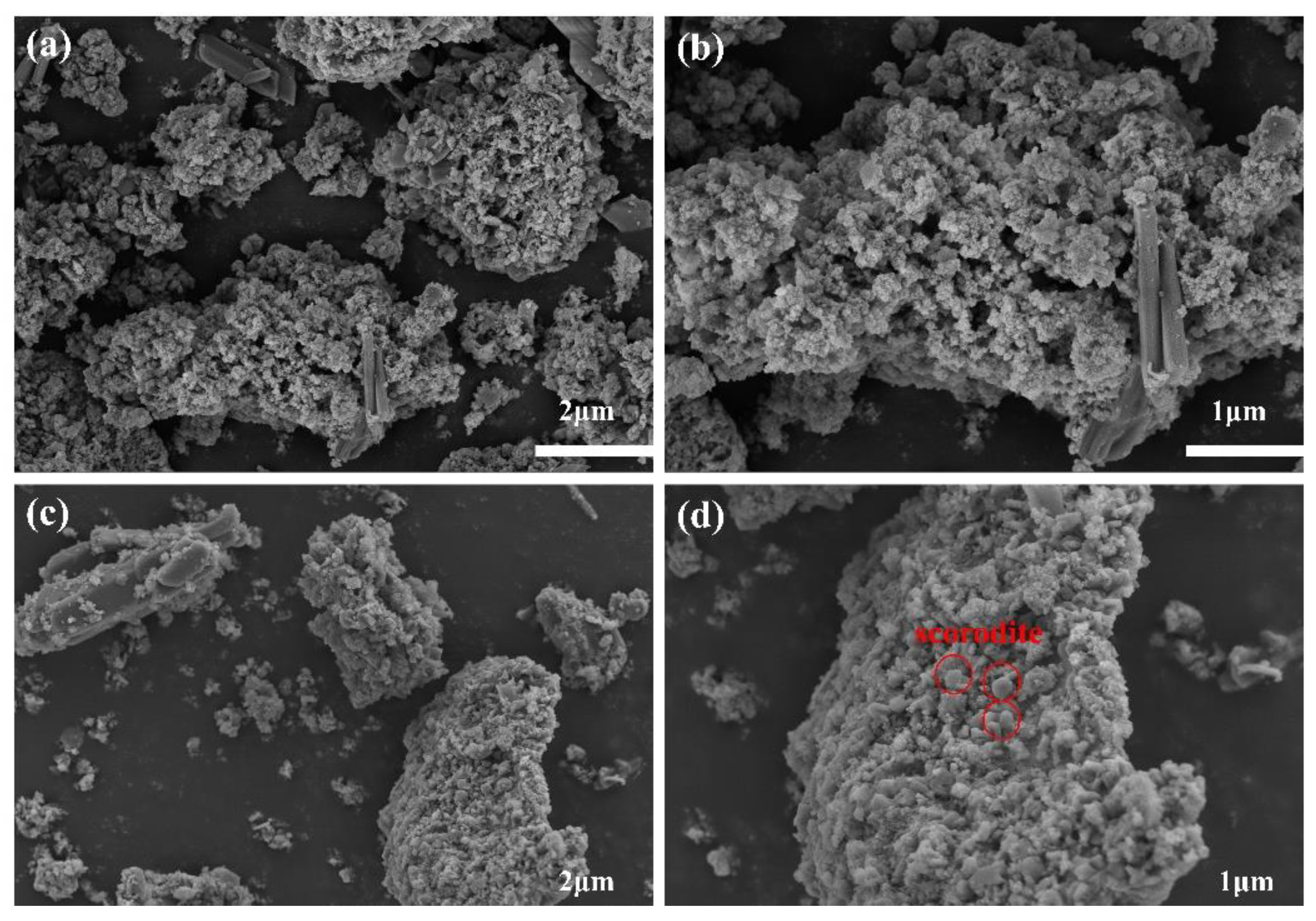
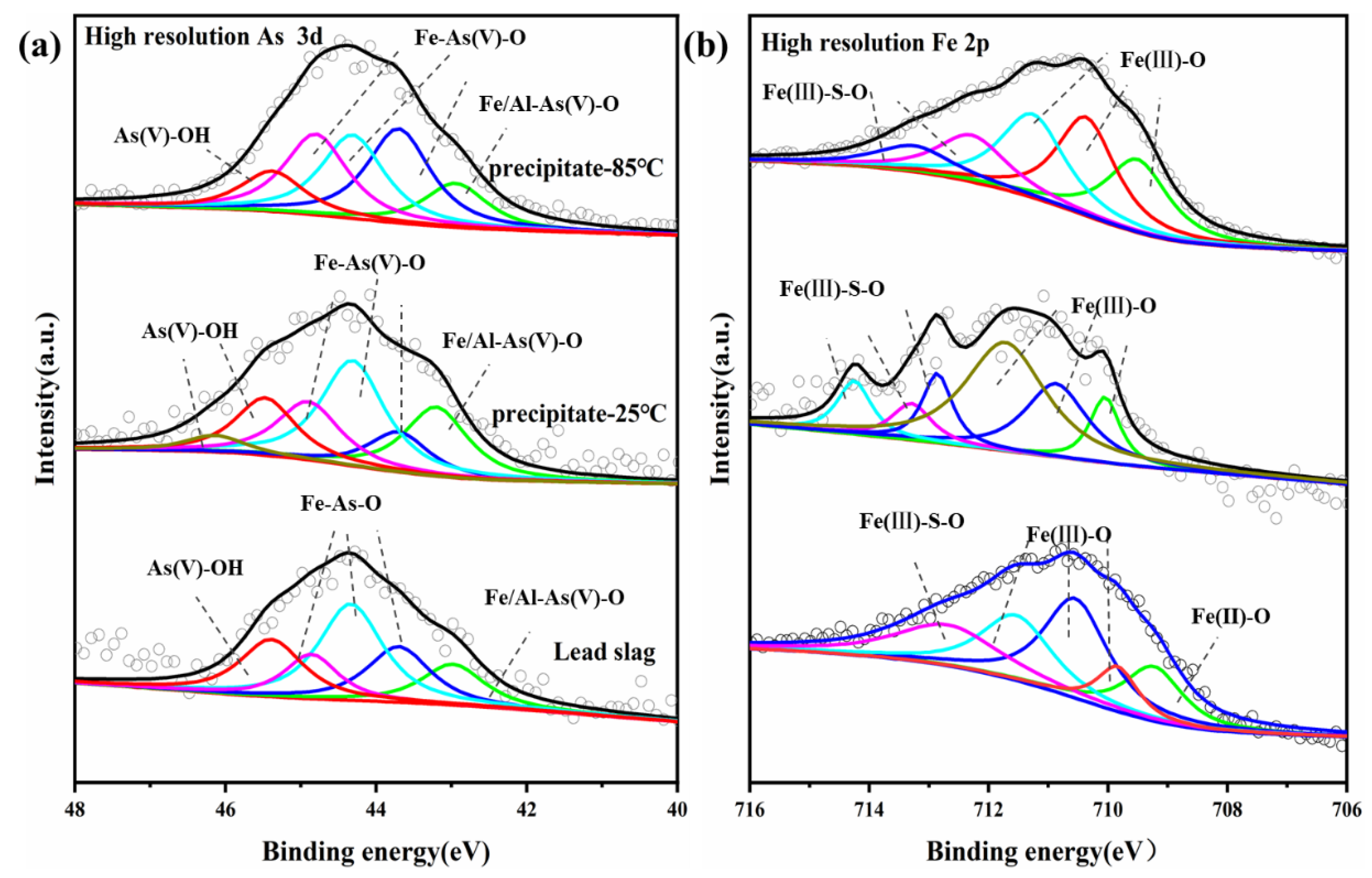
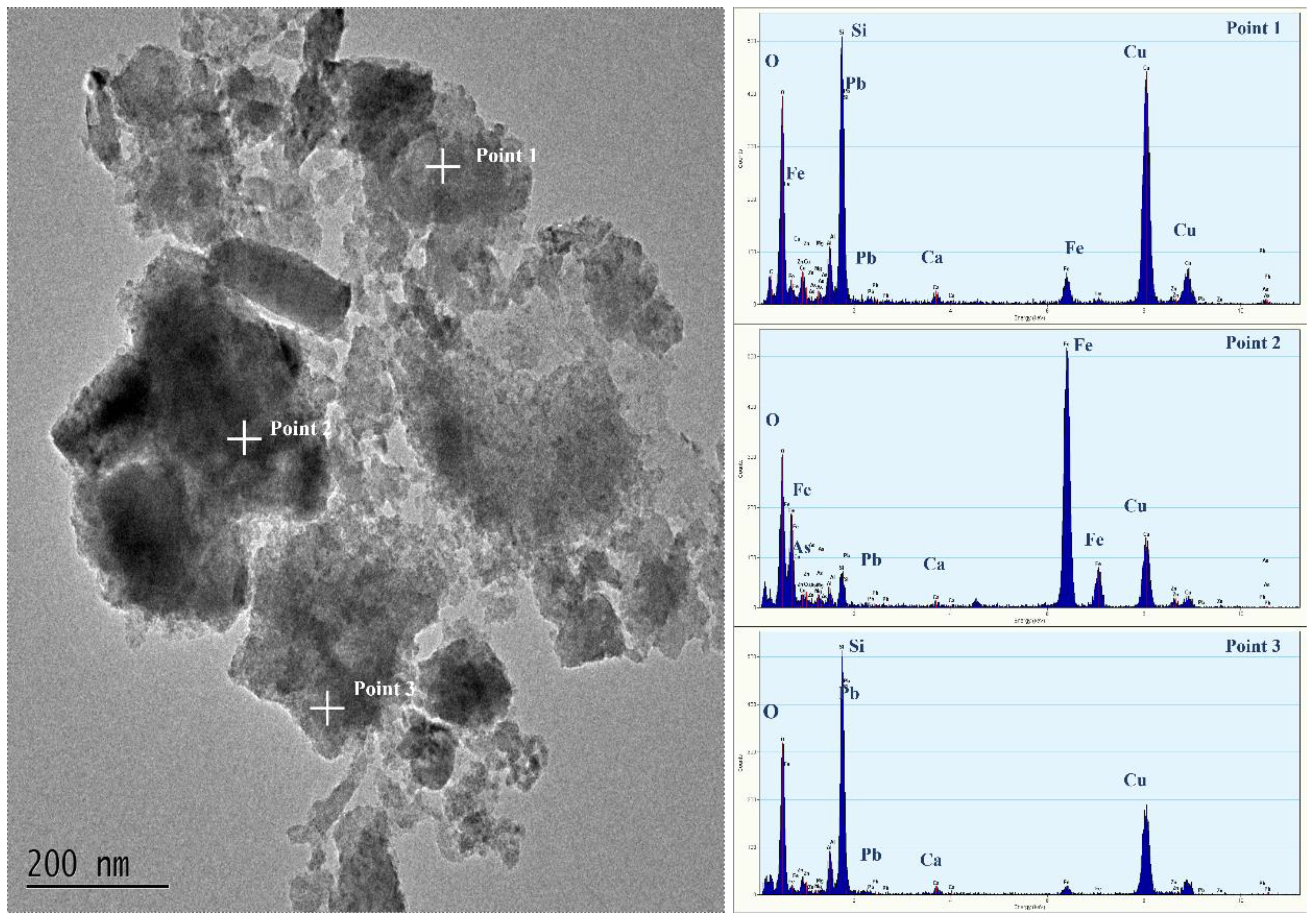
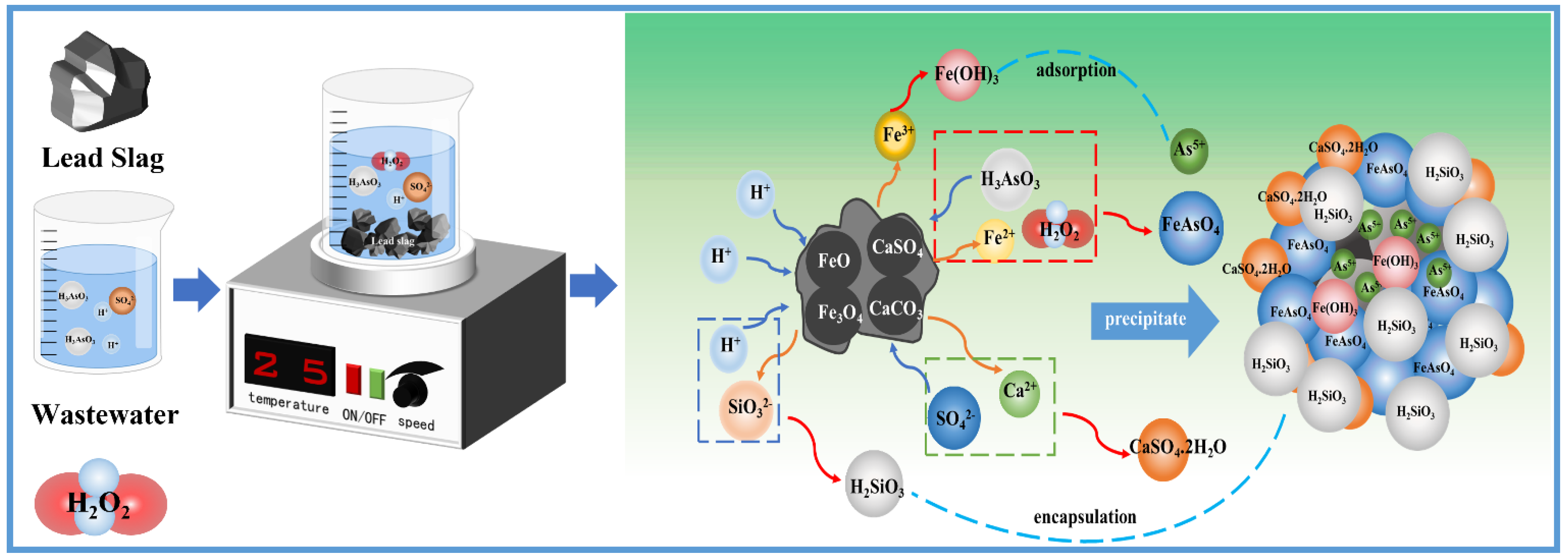
3.3.5. Arsenic Removal Mechanism by Lead Slag
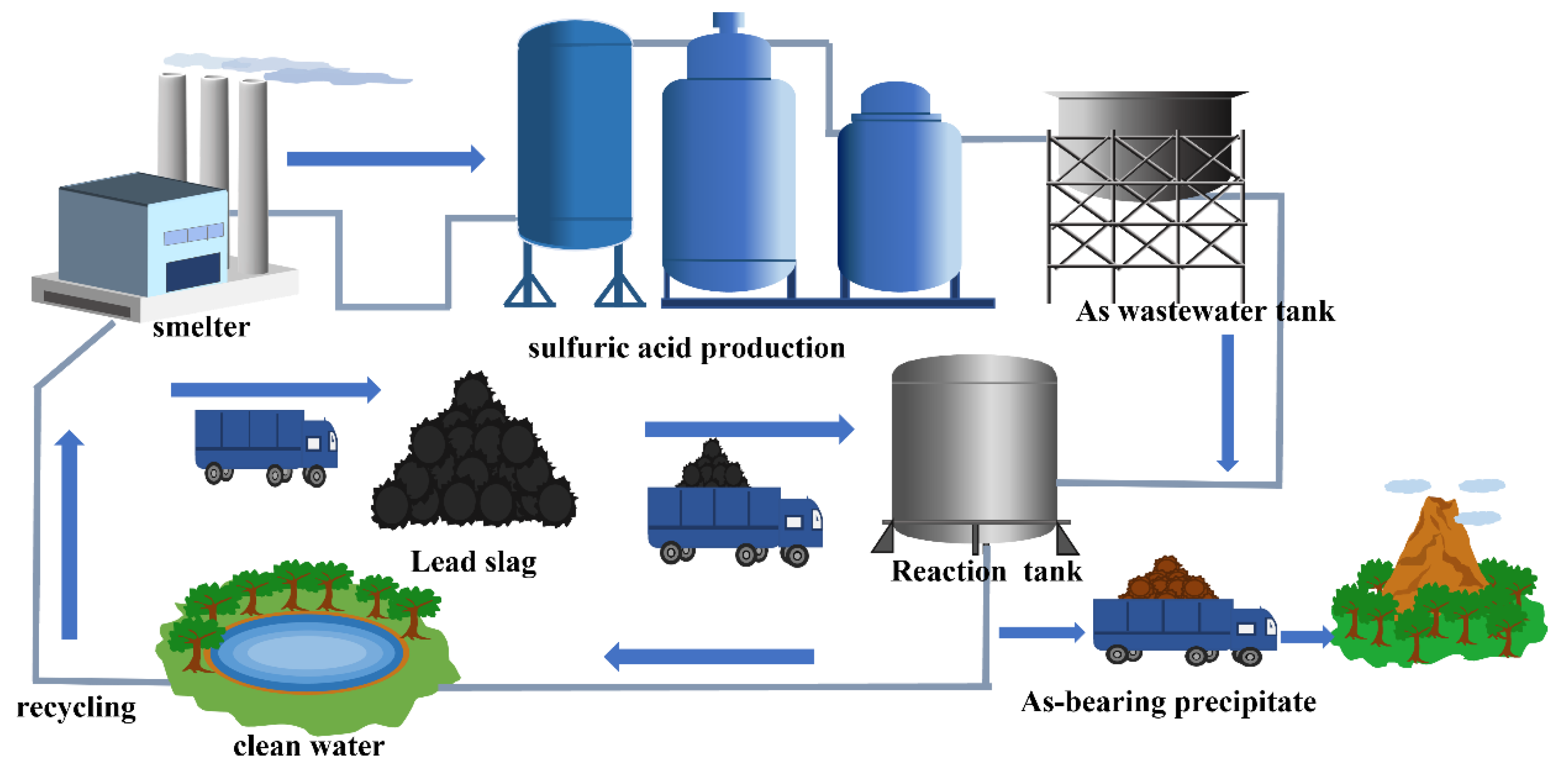
3.3.6. Prospects of Treating Wastewater Using Lead Slag
4. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Alonso, D.L.; Perez, R.; Okio, C.K.Y.A.; Castillo, E. Assessment of mining activity on arsenic contamination in surface water and sediments in southwestern area of Santurban paramo, Colombia. J. Environ. Manag. 2020, 264, 110478. [Google Scholar] [CrossRef]
- Fan, J.; Chen, X.; Xu, Z.B.; Xu, X.Y.; Zhao, L.; Qiu, H.; Cao, X.D. One-pot synthesis of nZVI-embedded biochar for remediation of two mining arsenic-contaminated soils: Arsenic immobilization associated with iron transformation. J. Hazard. Mater. 2020, 398, 122901. [Google Scholar] [CrossRef] [PubMed]
- Yuan, Y.Q.; Xiang, M.; Liu, C.Q.; Theng, B.K.G. Geochemical characteristics of heavy metal contamination induced by a sudden wastewater discharge from a smelter. J. Geochem. Explor. 2017, 176, 33–41. [Google Scholar] [CrossRef]
- Li, Y.C.; Min, X.B.; Chai, L.Y.; Shi, M.Q.; Tang, C.J.; Wang, Q.W.; Liang, Y.J.; Lei, J.; Liyang, W.J. Co-treatment of gypsum sludge and Pb/Zn smelting slag for the solidification of sludge containing arsenic and heavy metals. J. Environ. Manag. 2016, 181, 756–761. [Google Scholar] [CrossRef] [PubMed]
- Min, X.B.; Liao, Y.P.; Chai, L.Y.; Yang, Z.H.; Xiong, S.; Liu, L.; Li, Q.Z. Removal and stabilization of arsenic from anode slime by forming crystal scorodite. Trans. Nonferrous Met. Soc. 2015, 25, 1298–1306. [Google Scholar] [CrossRef]
- Li, Y.K.; Zhu, X.; Qi, X.J.; Shu, B.; Zhang, X.; Li, K.Z.; Wei, Y.G.; Wang, H. Removal and immobilization of arsenic from copper smelting wastewater using copper slag by in situ encapsulation with silica gel. Chem. Eng. J. 2020, 394, 124833. [Google Scholar] [CrossRef]
- Chai, L.Y.; Shi, M.Q.; Liang, Y.J.; Tang, J.W.; Li, Q.Z. Behavior, distribution and environmental influence of arsenic in a typical lead smelter. J. Cent. South Univ. 2015, 22, 1276–1286. [Google Scholar] [CrossRef]
- Lin, Y.F.; Chen, J.L.; Xu, C.Y.; Chung, T.W. One-pot synthesis of paramagnetic iron(III) hydroxide nanoplates and ferrimagnetic magnetite nanoparticles for the removal of arsenic ions. Chem. Eng. J. 2014, 250, 409–415. [Google Scholar] [CrossRef]
- Du, Y.; Qiu, S.J.; Zhang, X.L.; Nie, G.Z. Nanoconfined hydrous titanium oxides with excellent acid stability for selective and efficient removal of As(V) from acidic wastewater. Chem. Eng. J. 2020, 400, 125907. [Google Scholar] [CrossRef]
- Ortega, A.; Oliva, I.; Contreras, K.E.; Gonzalez, I.; Cruz-Diaz, M.R.; Rivero, E.P. Arsenic removal from water by hybrid electro-regenerated anion exchange resin/electrodialysis process. Sep. Purif. Technol. 2017, 184, 319–326. [Google Scholar] [CrossRef]
- Battaglia-Brunet, F.; Crouzet, C.; Burnol, A.; Coulon, S.; Morin, D.; Joulian, C. Precipitation of arsenic sulphide from acidic water in a fixed-film bioreactor. Water Res. 2012, 46, 3923–3933. [Google Scholar] [CrossRef]
- Nguyen, V.T.; Vigneswaran, S.; Ngo, H.H.; Shon, H.K.; Kandasamy, J. Arsenic removal by a membrane hybrid filtration system. Desalination 2009, 236, 363–369. [Google Scholar] [CrossRef]
- Peng, X.J.; Chen, J.Y.; Kong, L.H.; Hu, X.Y. Removal of Arsenic from Strongly Acidic Wastewater Using Phosphorus Pentasulfide As Precipitant: UV-Light Promoted Sulfuration Reaction and Particle Aggregation. Environ. Sci. Technol. 2018, 52, 4794–4801. [Google Scholar] [CrossRef] [PubMed]
- Zhu, Y.N.; Jiang, Z.N.; Zhu, Z.Q.; Liu, H.L.; Zhang, L.H.; Lin, J. Arsenic immobilization from aqueous solution by the precipitation of the pseudo-octahedral arsenate-substituted natroalunite solid solutions. Sci. Total Environ. 2019, 669, 754–766. [Google Scholar] [CrossRef] [PubMed]
- Kong, L.H.; Hu, X.Y.; Peng, X.J.; Wang, X.L. Specific H2S Release from Thiosulfate Promoted by UV Irradiation for Removal of Arsenic and Heavy Metals from Strongly Acidic Wastewater. Environ. Sci. Technol. 2020, 54, 14076–14084. [Google Scholar] [CrossRef] [PubMed]
- Kong, L.H.; Zhao, J.M.; Hu, X.Y.; Zhu, F.; Peng, X.J. Reductive Removal and Recovery of As(V) and As(III) from Strongly Acidic Wastewater by a UV/Formic Acid Process. Environ. Sci. Technol. 2022, 56, 9732–9743. [Google Scholar] [CrossRef]
- Cai, G.; Zhu, X.; Li, K.; Qi, X.; Wei, Y.; Wang, H.; Hao, F. Self-enhanced and efficient removal of arsenic from waste acid using magnetite as an in situ iron donator. Water Res. 2019, 157, 269–280. [Google Scholar] [CrossRef]
- Li, X.; Cai, G.; Li, Y.; Zhu, X.; Qi, X.; Zhang, X.; Shu, B.; Li, K.; Wei, Y.; Wang, H. Limonite as a source of solid iron in the crystallization of scorodite aiming at arsenic removal from smelting wastewater. J. Clean. Prod. 2021, 278, 123552. [Google Scholar] [CrossRef]
- Iizuka, A.; Shinoda, K.; Shibata, E. Scorodite Synthesis in As(V)-Containing Fe(II) Solution in the Presence of Hematite as a Fe(III) Source. Mater. Trans. 2018, 59, 843–849. [Google Scholar] [CrossRef]
- Tabelin, C.B.; Corpuz, R.D.; Iarashi, T.; Villacorte-Tabelin, M.; Ito, M.; Hiroyoshi, N. Hematite-catalysed scorodite formation as a novel arsenic immobilisation strategy under ambient conditions. Chemosphere 2019, 233, 946–953. [Google Scholar] [CrossRef]
- Kitamura, Y.; Okawa, H.; Kato, T.; Sugawara, K. Effect of reaction temperature on the size and morphology of scorodite synthesized using ultrasound irradiation. Ultrason. Sonochem. 2017, 35, 598–604. [Google Scholar] [CrossRef] [PubMed]
- Paktunc, D.; Bruggeman, K. Solubility of nanocrystalline scorodite and amorphous ferric arsenate: Implications for stabilization of arsenic in mine wastes. Appl. Geochem. 2010, 25, 674–683. [Google Scholar] [CrossRef]
- Dutrizac, J.E.; Jambor, J.L. The Synthesis of Crystalline Scorodite, FeAsO4.2H2O. Hydrometallurgy 1988, 19, 377–384. [Google Scholar] [CrossRef]
- Paktunc, D.; Dutrizac, J.; Gertsman, V. Synthesis and phase transformations involving scorodite, ferric arsenate and arsenical ferrihydrite: Implications for arsenic mobility. Geochim. Cosmochim. Acta 2008, 72, 2649–2672. [Google Scholar] [CrossRef]
- Caetano, M.L.; Ciminelli, V.S.T.; Rocha, S.D.F.; Spitale, M.C.; Caldeira, C.L. Batch and continuous precipitation of scorodite from dilute industrial solutions. Hydrometallurgy 2009, 95, 44–52. [Google Scholar] [CrossRef]
- Li, Y.; Qi, X.; Li, G.; Duan, X.; Yang, N. Removal of arsenic in acidic wastewater using Lead-Zinc smelting slag: From waste solid to As-stabilized mineral. Chemosphere 2022, 301, 134736. [Google Scholar] [CrossRef]
- Lu, C.-C.; Hsu, M.H.; Lin, Y.-P. Evaluation of heavy metal leachability of incinerating recycled aggregate and solidification/stabilization products for construction reuse using TCLP, multi-final pH and EDTA-mediated TCLP leaching tests. J. Hazard. Mater. 2019, 368, 336–344. [Google Scholar] [CrossRef]
- Nieva, N.E.; Borgnino, L.; Garcia, M.G. Long term metal release and acid generation in abandoned mine wastes containing metal-sulphides. Environ. Pollut. 2018, 242, 264–276. [Google Scholar] [CrossRef]
- Sharma, V.K.; Sohn, M. Aquatic arsenic: Toxicity, speciation, transformations, and remediation. Environ. Int. 2009, 35, 743–759. [Google Scholar] [CrossRef]
- Zhu, X.; Nordstrom, D.K.; McCleskey, R.B.; Wang, R.; Lu, X.; Li, S.; Teng, H.H. On the thermodynamics and kinetics of scorodite dissolution. Geochim. Cosmochim. Acta 2019, 265, 468–477. [Google Scholar] [CrossRef]
- Yang, J.Q.; Chai, L.Y.; Li, Q.Z.; Shu, Y.D. Redox behavior and chemical species of arsenic in acidic aqueous system. Trans. Nonferrous Met. Soc. 2017, 27, 2063–2072. [Google Scholar] [CrossRef]
- Chai, L.Y.; Chen, Y.N.; Yang, Z.H. Kinetics and Thermodynamics of Arsenate and Arsenite Biosorption by Pretreated Spent Grains. Water Environ. Res. 2009, 81, 843–848. [Google Scholar] [CrossRef] [PubMed]
- Ilic, N.I.; Lazarevic, S.S.; Rajakovic-Ognjanovic, V.N.; Rajakovic, L.V.; Janackovic, D.T.; Petrovic, R.D. The sorption of inorganic arsenic on modified sepiolite: The effect of hydrated iron(III) oxide. J. Serb. Chem. Soc. 2014, 79, 815–828. [Google Scholar] [CrossRef]
- Gomez, M.A.; Becze, L.; Cutler, J.N.; Demopoulos, G.P. Hydrothermal reaction chemistry and characterization of ferric arsenate phases precipitated from Fe2(SO4)3–As2O5–H2SO4 solutions. Hydrometallurgy 2011, 107, 74–90. [Google Scholar] [CrossRef]
- Yue, T.; Niu, Z.; Hu, Y.; Han, H.; Sun, W.; Tian, J.; Xu, Z.; Wang, L.; Yang, Y. Arsenic(V) adsorption on ferric oxyhydroxide gel at high alkalinity for securely recycling of arsenic-bearing copper slag. Appl. Surf. Sci. 2019, 478, 213–220. [Google Scholar] [CrossRef]
- Fujita, T.; Taguchi, R.; Abumiya, M.; Matsumoto, M.; Shibata, E.; Nakamura, T. Effects of zinc, copper and sodium ions on ferric arsenate precipitation in a novel atmospheric scorodite process. Hydrometallurgy 2008, 93, 30–38. [Google Scholar] [CrossRef]
- GB 5085.3-2007; Identification Standards for Hazardous Wastes. Identification for Extraction Toxicity. Ministry of Environmental Protection (MEP): Beijing, China, 2007.
- Jia, Y.F.; Demopoulos, G.P. Coprecipitation of arsenate with iron(III) in aqueous sulfate media: Effect of time, lime as base and co-ions on arsenic retention. Water Res. 2008, 42, 661–668. [Google Scholar] [CrossRef]
- Jia, Y.F.; Xu, L.Y.; Wang, X.; Demopoulos, G.P. Infrared spectroscopic and X-ray diffraction characterization of the nature of adsorbed arsenate on ferrihydrite. Geochim. Cosmochim. Acta 2007, 71, 1643–1654. [Google Scholar] [CrossRef]
- Paktunc, D. Phase transformations in the system Fe-AsO4-SO4 and the structure of amorphous ferric arsenate: Implications for arsenic stabilization in mine drainage and industrial effluents. Can. Miner. 2015, 53, 921–936. [Google Scholar] [CrossRef]
- Le Berre, J.F.; Gauvin, R.; Demopoulos, G.P. A study of the crystallization kinetics of scorodite via the transformation of poorly crystalline ferric arsenate in weakly acidic solution. Colloid Surf. A 2008, 315, 117–129. [Google Scholar] [CrossRef]
- Otgon, N.; Zhang, G.J.; Zhang, K.L.; Yang, C. Removal and fixation of arsenic by forming a complex precipitate containing scorodite and ferrihydrite. Hydrometallurgy 2019, 186, 58–65. [Google Scholar] [CrossRef]
- Duan, X.X.; Li, X.Z.; Li, Y.K.; Qi, X.J.; Li, G.H.; Lu, Z.X.; Yang, N.N. Separation and stabilization of arsenic in copper smelting wastewater by zinc slag. J. Clean. Prod. 2021, 312, 127797. [Google Scholar] [CrossRef]
- Yuan, Z.; Zhang, D.; Wang, S.; Xu, L.; Wang, K.; Song, Y.; Xiao, F.; Jia, Y. Effect of hydroquinone-induced iron reduction on the stability of scorodite and arsenic mobilization. Hydrometallurgy 2016, 164, 228–237. [Google Scholar] [CrossRef]
- Fujita, T.; Taguchi, R.; Abumiya, M.; Matsumoto, M.; Shibata, E.; Nakamura, T. Novel atmospheric scorodite synthesis by oxidation of ferrous sulfate solution. Part I. Hydrometallurgy 2008, 90, 92–102. [Google Scholar] [CrossRef]
- Kitamura, Y.; Okawa, H.; Kato, T.; Sugawara, K. Effect of ultrasound intensity on the size and morphology of synthesized scorodite particles. Adv. Powder Technol. 2016, 27, 891–897. [Google Scholar] [CrossRef]
- Ye, C.J.; Ariya, P.A.; Fu, F.L.; Yu, G.D.; Tang, B. Influence of Al(III) and Sb(V) on the transformation of ferrihydrite nanoparticles: Interaction among ferrihydrite, coprecipitated Al(III) and Sb (V). J. Hazard. Mater. 2021, 408, 124423. [Google Scholar] [CrossRef]
- Han, X.; Song, J.; Li, Y.L.; Jia, S.Y.; Wang, W.H.; Huang, F.G.; Wu, S.H. As(III) removal and speciation of Fe (Oxyhydr)oxides during simultaneous oxidation of As(III) and Fe(II). Chemosphere 2016, 147, 337–344. [Google Scholar] [CrossRef]
- Yuan, Z.D.; Zhang, G.Q.; Ma, X.; Yu, L.; Wang, X.; Wang, S.F.; Jia, Y.F. Rapid abiotic As removal from As-rich acid mine drainage: Effect of pH, Fe/As molar ratio, oxygen, temperature, initial As concentration and neutralization reagent. Chem. Eng. J. 2019, 378, 122156. [Google Scholar] [CrossRef]
- Yang, Z.L.; Zhang, N.; Sun, B.H.; Su, S.M.; Wang, Y.A.; Zhang, Y.; Wu, C.X.; Zeng, X.B. Contradictory tendency of As(V) releasing from Fe-As complexes: Influence of organic and inorganic anions. Chemosphere 2022, 286, 131469. [Google Scholar] [CrossRef]
- Tabelin, C.B.; Veerawattananun, S.; Ito, M.; Hiroyoshi, N.; Igarashi, T. Pyrite oxidation in the presence of hematite and alumina: I. Batch leaching experiments and kinetic modeling calculations. Sci. Total Environ. 2017, 580, 687–698. [Google Scholar] [CrossRef]
- Drouet, C.; Navrotsky, A. Synthesis, characterization, and thermochemistry of K-Na-H3O jarosites. Geochim. Cosmochim. Acta 2003, 67, 2063–2076. [Google Scholar] [CrossRef]
- Sunyer, A.; Vinals, J. Arsenate substitution in natroalunite: A potential medium for arsenic immobilization. Part 1: Synthesis and compositions. Hydrometallurgy 2011, 109, 54–64. [Google Scholar] [CrossRef]
- Bolanz, R.M.; Gottlicher, J.; Steininger, R.; Wieczorek, A. Structural incorporation of As5+ into rhomboclase ((H5O2)Fe3+(SO4)2. 2H2O) and (H3O)Fe(SO4)2. Chemosphere 2016, 146, 338–345. [Google Scholar] [CrossRef]
- Yang, K.; Peng, H.B.; Wen, Y.H.; Li, N. Re-examination of characteristic FTIR spectrum of secondary layer in bilayer oleic acid-coated Fe3O4 nanoparticles. Appl. Surf. Sci. 2010, 256, 3093–3097. [Google Scholar] [CrossRef]
- Kloprogge, J.T.; Wood, B.J. X-ray Photoelectron Spectroscopic and Raman microscopic investigation of the variscite group minerals: Variscite, strengite, scorodite and mansfieldite. Spectrochim. Acta Part A Mol. Biomol. Spectrosc. 2017, 185, 163–172. [Google Scholar] [CrossRef]
- Tabelin, C.B.; Corpuz, R.D.; Igarashi, T.; Villacorte-Tabelin, M.; Alorro, R.D.; Yoo, K.; Raval, S.; Ito, M.; Hiroyoshi, N. Acid mine drainage formation and arsenic mobility under strongly acidic conditions: Importance of soluble phases, iron oxyhydroxides/oxides and nature of oxidation layer on pyrite. J. Hazard. Mater. 2020, 399, 122844. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Qi, X.; Li, G.; Wang, H. Double-pathway arsenic removal and immobilization from high arsenic-bearing wastewater by using nature pyrite as in situ Fe and S donator. Chem. Eng. J. 2021, 410, 128303. [Google Scholar] [CrossRef]



| Elements | Concentration (wt. %) | Elements | Concentration (wt. %) | Elements | Concentration (wt. %) |
|---|---|---|---|---|---|
| O | 19.60 | Pb | 2.71 | As | 0.54 |
| Fe | 36.93 | S | 2.62 | Cu | 0.36 |
| Si | 12.29 | Mn | 1.56 | Cl | 0.30 |
| Ca | 11.05 | Mg | 0.87 | Na | 0.24 |
| Al | 4.96 | K | 0.77 | Ba | 0.22 |
| Zn | 3.81 | Ti | 0.64 | others | 0.53 |
| Element | As | Pb | Fe | Zn | Sb | Cd | H2SO4 |
|---|---|---|---|---|---|---|---|
| Concentration | 6500 ± 12 | 4.50 ± 0.12 | 35.20 ± 1.04 | 21.50 ± 0.72 | 15.89 ± 0.57 | 87.50 ± 1.49 | 56,000 ± 49 |
| Spectral Peak | BE (eV) | FWHM | Area (%) | Chemical States | Comment |
|---|---|---|---|---|---|
| As3d | 42.98 | 1 | 14.13% | As(III)-S | Estratite |
| As3d | 43.7 | 1 | 19.30% | Fe-As(V)-O | Amorphous iron arsenate/Scorodite |
| As3d | 44.34 | 1 | 33.53% | Fe-As(V)-O | Amorphous iron arsenate/Scorodite |
| As3d | 44.86 | 0.85 | 13.12% | Fe-As(V)-O | Amorphous iron arsenate/Scorodite |
| As3d | 45.39 | 1 | 19.92% | As-O(V)-OH | Adsorbed arsenate |
| Fe2p | 709.24 | 1.33 | 18.58% | Fe(II)-O | Ferrous oxide |
| Fe2p | 710.04 | 1.2 | 23.18% | Fe(III)-As-O | Amorphous iron arsenate/Scorodite |
| Fe2p | 710.80 | 1.18 | 22.17% | Fe(III)-O | Hematite/Magnetite |
| Fe2p | 711.70 | 1.48 | 23.41% | Fe(III)-O | Iron-oxyhydroxide |
| Fe2p | 712.81 | 1.76 | 12.66% | Fe(III)-O | Iron-oxyhydroxide |
| Spectral Peak | BE (eV) | FWHM | Area (%) | Chemical States | Comment |
|---|---|---|---|---|---|
| As3d | 43.14 | 1.36 | 17.56% | Fe/Al-As(V)-O | Fe/Al-arsenate solid solution |
| As3d | 43.77 | 1 | 11.70% | Fe-As(V)-O | Amorphous iron arsenate/Scorodite |
| As3d | 44.12 | 1.18 | 22.30% | Fe-As(V)-O | Amorphous iron arsenate/Scorodite |
| As3d | 44.75 | 1.1 | 14.87% | Fe-As(V)-O | Amorphous iron arsenate/Scorodite |
| As3d | 44.99 | 1.46 | 20.14% | Fe--As(V)-O | Amorphous iron arsenate/Scorodite |
| As3d | 45.62 | 1.25 | 13.43% | As(V)-O-OH | Adsorbed arsenate |
| Fe2p | 710.05 | 0.6 | 9.62% | Fe(III)-As-O | Amorphous iron arsenate/Scorodite |
| Fe2p | 710.87 | 1.24 | 22.11% | Fe(III)-O | Hematite/Magnetite |
| Fe2p | 711.72 | 1.6 | 42.23% | Fe(III)-O | Iron-oxyhydroxide |
| Fe2p | 712.86 | 0.6 | 10.16% | Fe(III)-As-O | Amorphous iron arsenate/Scorodite |
| Fe2p | 713.29 | 0.79 | 7.04% | Fe(III)-S-O | Fe-sulphate complex |
| Fe2p | 714.25 | 0.68 | 8.84% | Fe(III)-S-O | Fe-sulphate complex |
| Spectral Peak | BE (eV) | FWHM | Area (%) | Chemical States | Comment |
|---|---|---|---|---|---|
| As3d | 42.94 | 1 | 12.84% | Fe/Al-As(V)-O | Fe/Al-arsenate solid solution |
| As3d | 43.7 | 1 | 27.48% | Fe-As(V)-O | Amorphous iron arsenate/Scorodite |
| As3d | 44.31 | 1 | 24.38% | Fe-As(V)-O | Amorphous iron arsenate/Scorodite |
| As3d | 44.80 | 1 | 23.57% | Fe-As(V)-O | Amorphous iron arsenate/Scorodite |
| As3d | 45.38 | 1 | 11.73% | As(V)-O | Adsorbed arsenate |
| Fe2p | 709.50 | 1.23 | 21.53% | Fe(III)-As-O | Amorphous iron arsenate/Scorodite |
| Fe2p | 710.37 | 1.17 | 27.52% | Fe(III)-O | Hematite/Magnetite |
| Fe2p | 711.26 | 1.3 | 25.72% | Fe(III)-O | Iron-oxyhydroxide |
| Fe2p | 712.28 | 1.4 | 15.61% | Fe(III)-As-O | Amorphous iron arsenate/Scorodite |
| Fe2p | 713.25 | 1.57 | 9.64% | Fe(III)-S-O | Fe-sulphate complex |
| Point | O | Mg | Al | Si | S | Ca | Fe | Zn | As |
|---|---|---|---|---|---|---|---|---|---|
| 1 | 56.84 | 0.21 | 1.49 | 5.51 | 13.64 | 13.01 | 7.33 | 0.51 | 1.47 |
| 2 | 59.71 | 1.36 | 2.33 | 8.33 | 3.71 | 1.01 | 17.84 | 2.25 | 3.45 |
| 3 | 50.38 | 0.15 | 1.69 | 5.33 | 8.21 | 6.53 | 23.79 | 1 | 2.94 |
| 4 | 50.58 | 0.09 | 1.67 | 7.4 | 2.55 | 0.37 | 28.29 | 1.13 | 7.91 |
| Reaction Time (h) | 1 | 2 | 4 | 6 | 8 | 10 | 12 |
|---|---|---|---|---|---|---|---|
| Concentration (mg/L) | 30.42 ± 0.87 | 18.17 ± 0.77 | 1.68 ± 0.25 | 0.28 ± 0.05 | 0.15 ± 0.03 | 0.001 | 0.001 |
| Reaction Time (h) | 1 | 2 | 4 | 6 | 8 | 10 | 12 |
|---|---|---|---|---|---|---|---|
| Concentration (mg/L) | 2.70 ± 0.32 | 1.55 ± 0.56 | 1.90 ± 0.34 | 2.96 ± 0.24 | 2.88 ± 0.13 | 2.81 ± 0.16 | 2.93 ± 0.23 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chen, P.; Zhao, Y.; Yao, J.; Zhu, J.; Cao, J. Utilization of Lead Slag as In Situ Iron Source for Arsenic Removal by Forming Iron Arsenate. Materials 2022, 15, 7471. https://doi.org/10.3390/ma15217471
Chen P, Zhao Y, Yao J, Zhu J, Cao J. Utilization of Lead Slag as In Situ Iron Source for Arsenic Removal by Forming Iron Arsenate. Materials. 2022; 15(21):7471. https://doi.org/10.3390/ma15217471
Chicago/Turabian StyleChen, Pan, Yuxin Zhao, Jun Yao, Jianyu Zhu, and Jian Cao. 2022. "Utilization of Lead Slag as In Situ Iron Source for Arsenic Removal by Forming Iron Arsenate" Materials 15, no. 21: 7471. https://doi.org/10.3390/ma15217471
APA StyleChen, P., Zhao, Y., Yao, J., Zhu, J., & Cao, J. (2022). Utilization of Lead Slag as In Situ Iron Source for Arsenic Removal by Forming Iron Arsenate. Materials, 15(21), 7471. https://doi.org/10.3390/ma15217471








