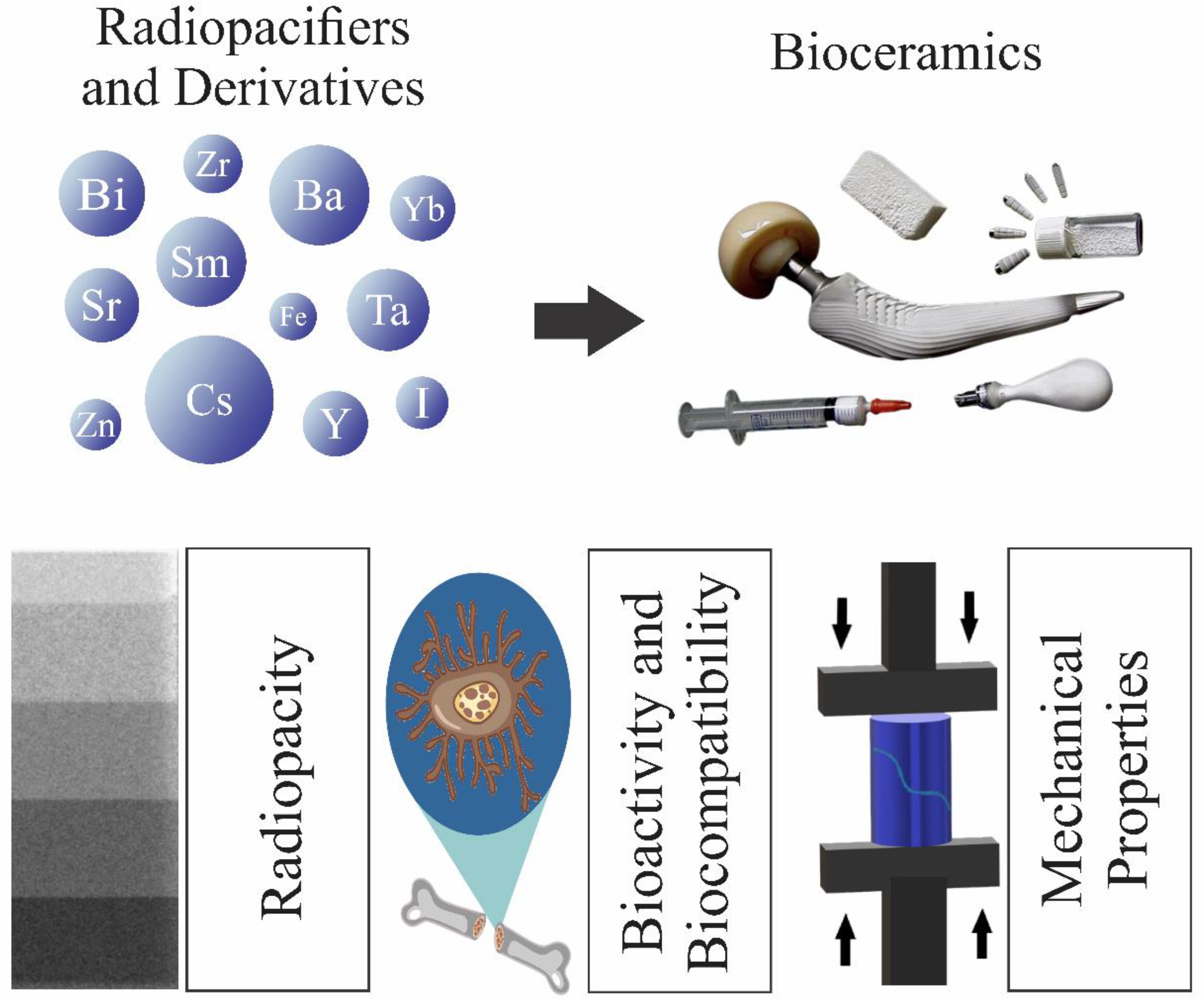
Radiopaque Crystalline, Non-Crystalline and Nanostructured Bioceramics
Abstract
1. Introduction
2. Principle and Physics of Radiopacity
2.1. Photoelectric, Compton, and Rayleigh Effects
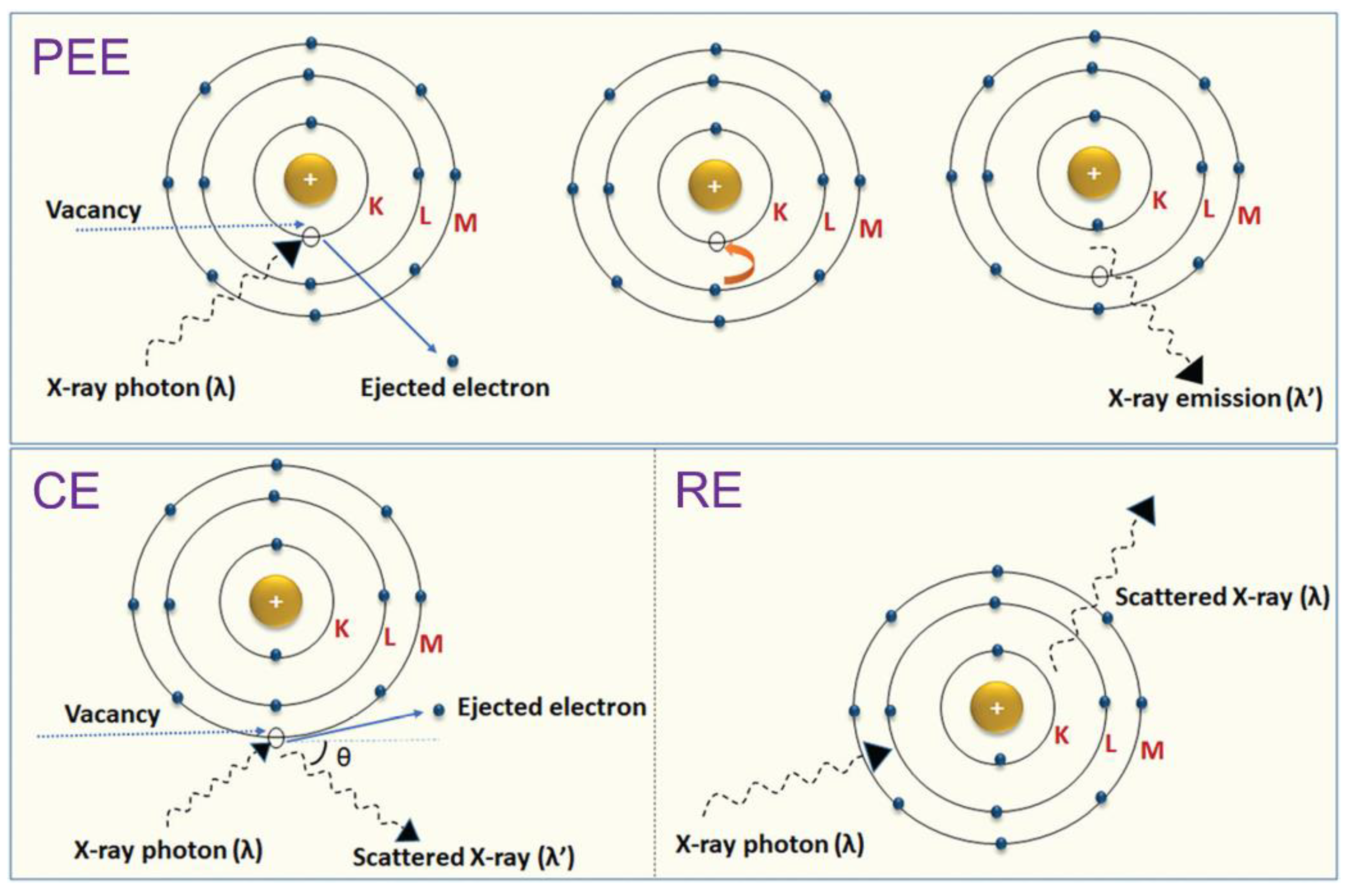
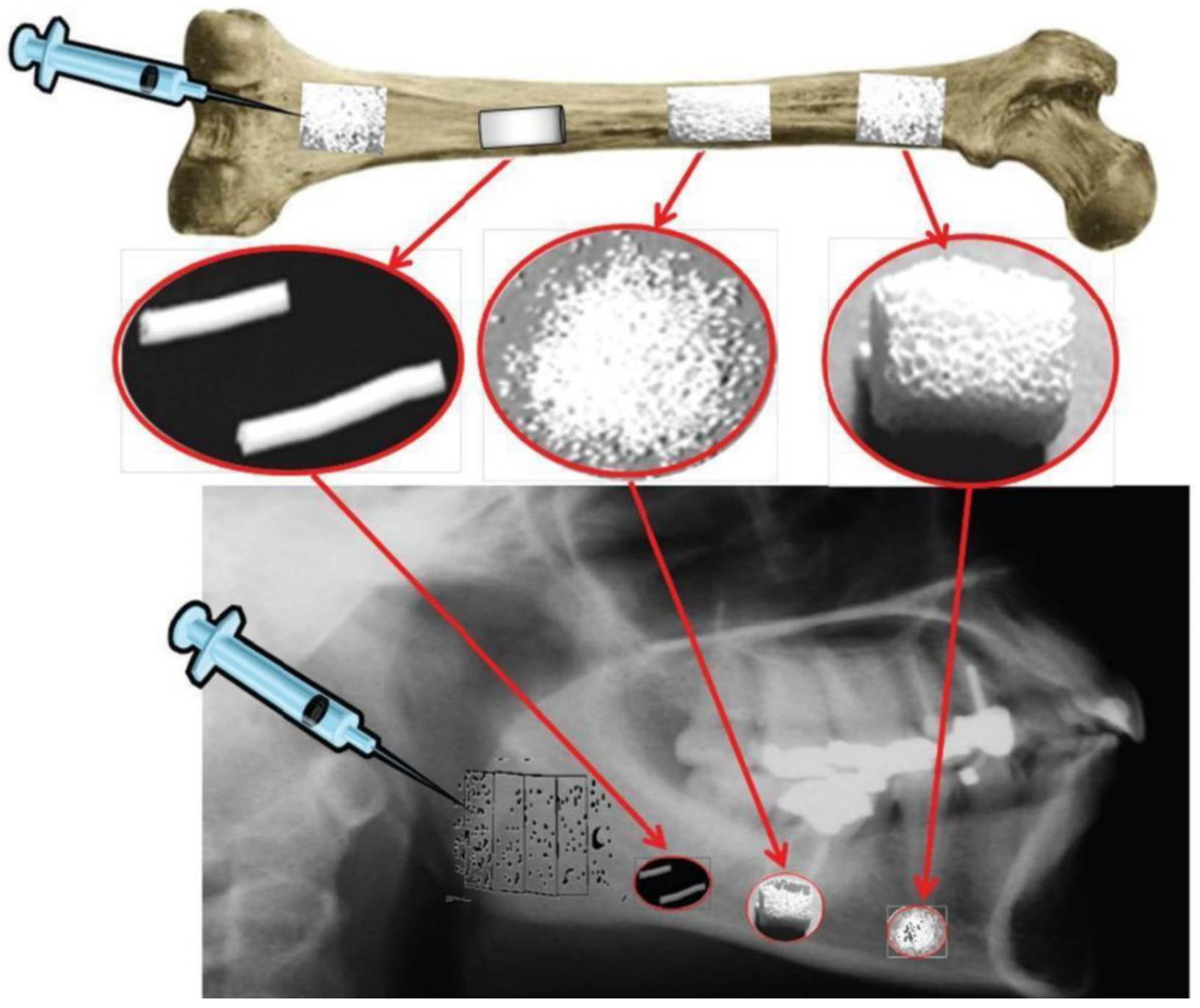
2.2. Radiopacity Measurement
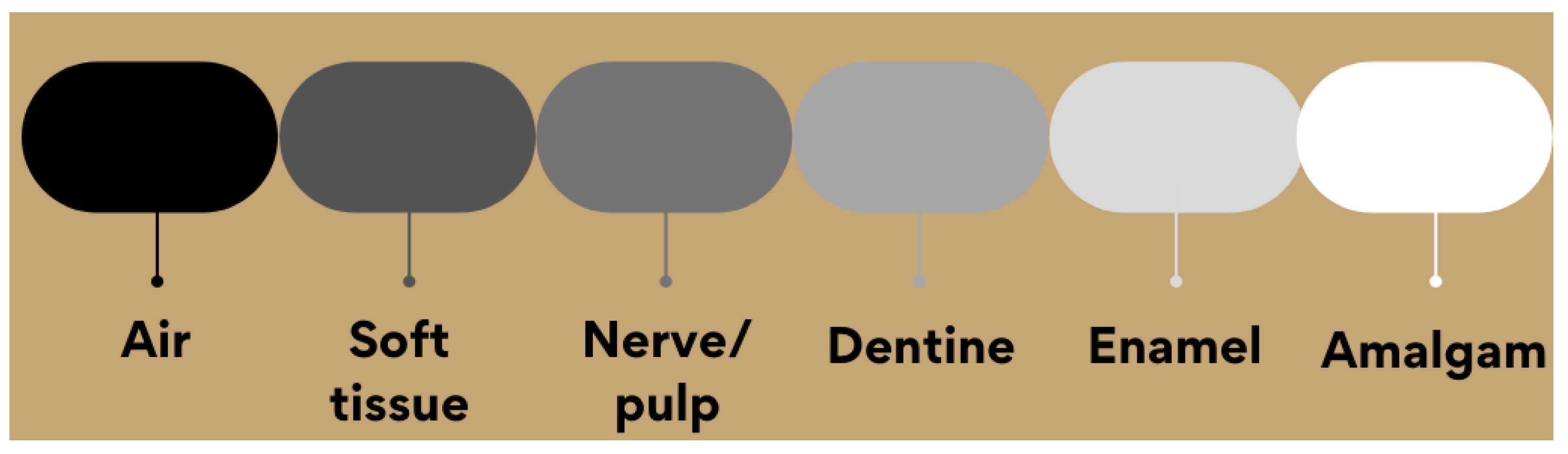
3. Applications: A Short Overview
3.1. Dentistry
3.2. Ceramic Bone Cements
3.3. Bone Grafts and Scaffolds
3.4. Composites
4. Radiopacifiers in Crystalline Bioceramics
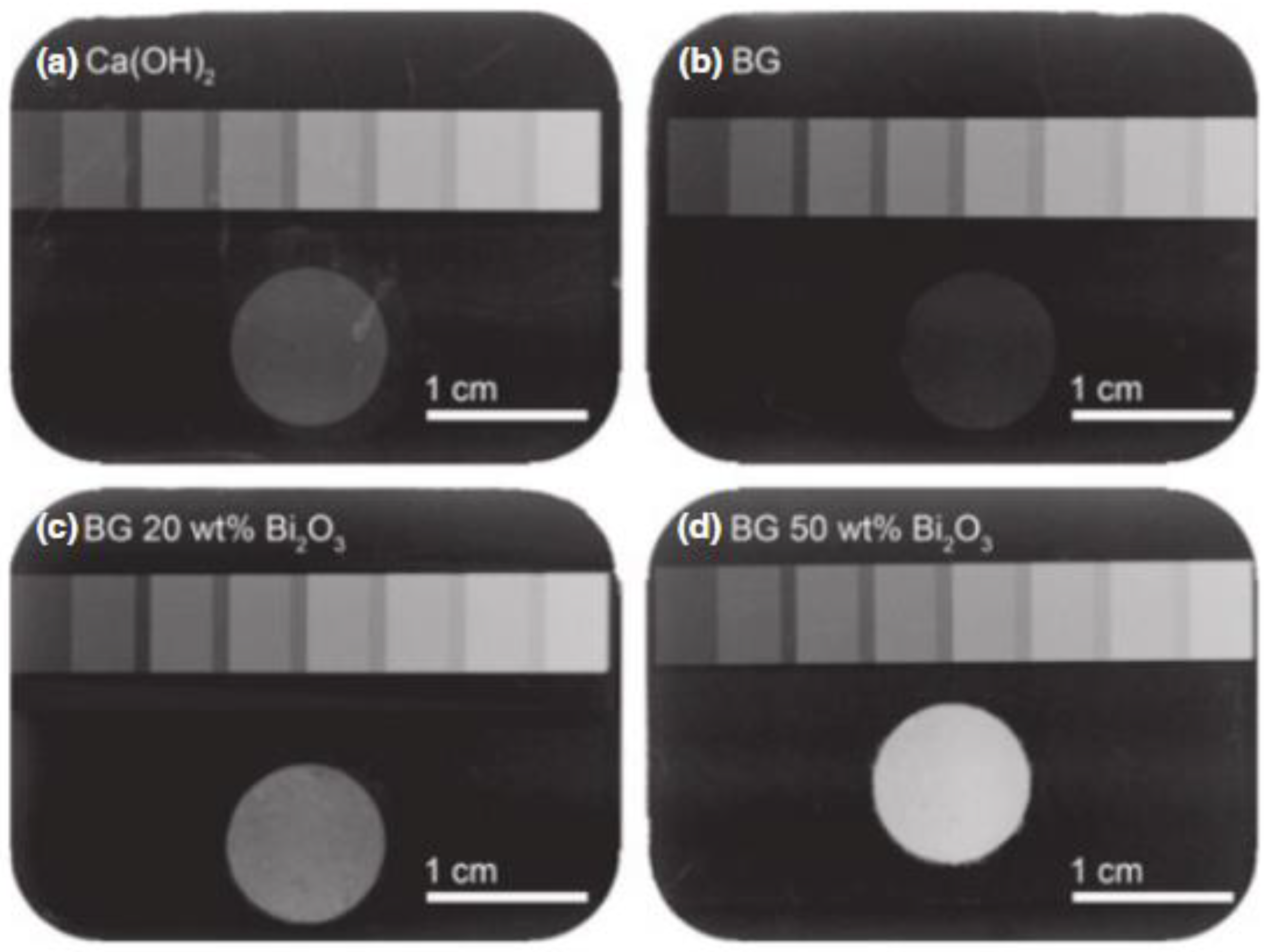
4.1. Bismuth (Bi)
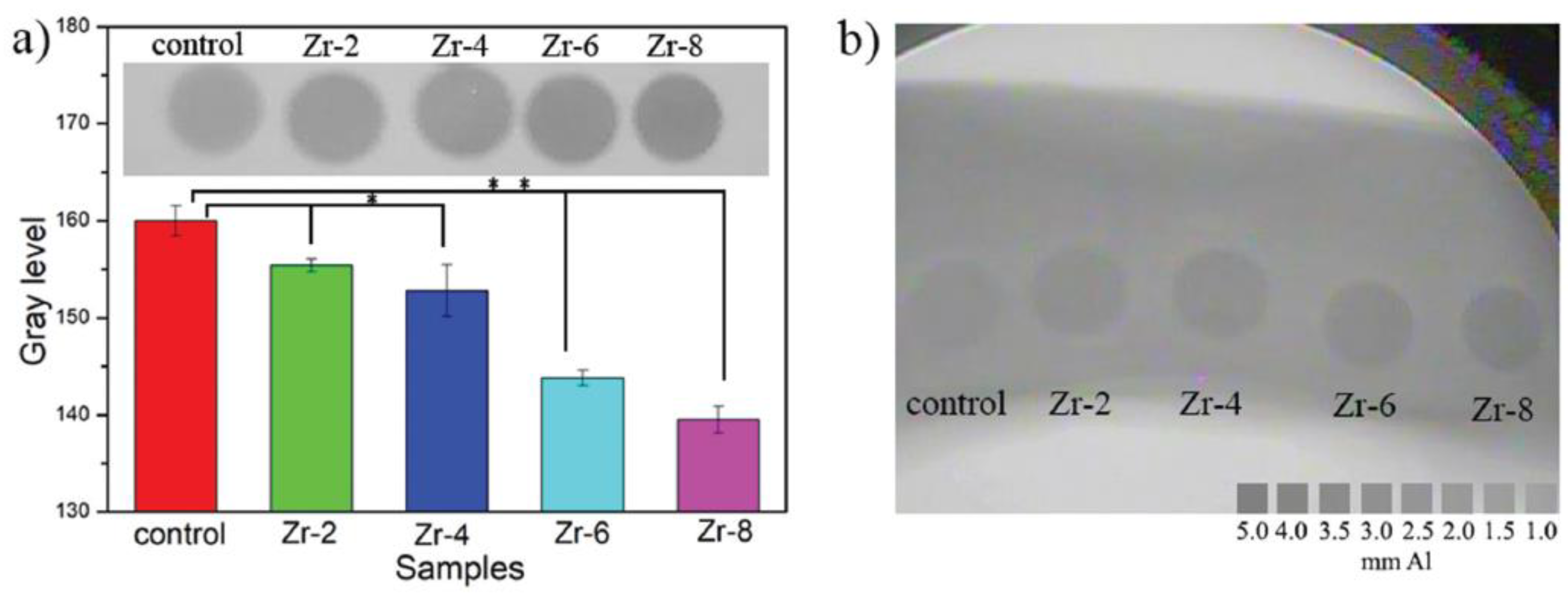
4.2. Zirconium (Zr)
4.3. Strontium (Sr)
4.4. Barium (Ba)
4.5. Other Elements
5. Radiopacifiers in Glasses and Glass-Ceramics
5.1. Strontium (Sr)
5.2. Bismuth (Bi)
5.3. Zirconium (Zr)
5.4. Barium (Ba)
5.5. Magnesium (Mg)
5.6. Zinc (Zn)
5.7. Yttrium (Y)
5.8. Other Elements
6. Polymer-Based Composites/Hybrids
| Filler | Base Polymer | Filler Concentration (wt.%) | Application | Radiopacity (mmAl) | Ref. |
|---|---|---|---|---|---|
| Ta2O5 | Bisphenol-A-glycidyl methacrylate (Bis-GMA), trimethylene glycol dimethacrylate (TEGDMA), 2-hydroxyethyl methacrylate (HEMA) | 1–10 | Dental adhesive | <1 mm | [190] |
| Fiber glass and zirconia | Ethoxylated bisphenol-A-dimethacrylate (bis-EMA), TEGDMA, diurethane dimethacrylate (UDMA) | 0–25 | Dental Composite resin | 4.6 | [191] |
| Sr-doped HAp | Bis-GMA, HEMA | 10 | Dental adhesive | 1.1 | [106] |
| Bi2O3, SiO2, YbF3 | HEMA, UDMA, TEGDMA, Bis-GMA, glycerol dimethacrylate (GDMA) | 10 | Dental adhesives | >1 mm | [192] |
| CeO2 | TEGDMA, Bis-GMA, HEMA | 0.36–5.76 vol% | Dental adhesives | >1 mm | [193] |
| CaWO, YbF3, BaSO4 | Bisphenol-A | 20–120 | Dental root canal sealer | 2.6 | [194] |
| Nb2O5 | Bis-GMA, HEMA, camphorquinone (CQ) and ethyl 4-(dimethylamino)benzoat e (EDAB) | 10 and 20 | Dental adhesives | ∼1.1 | [195] |
| Sr-doped BG | poly(vinyl phosphonic-co-acrylic acid) | 2:1 glass to polymer weight ratio | Bone cement | 2.2 | [196] |
| Sr-doped BG | PMMA | 20–40 | Injectable bone cement | 1–2.25 | [197] |
| ZrO2 and BaSO4 | PMMA | 10 | Bone cement | Contrast reported | [198] |
| Sr-dopes HAp | PMMA | 20 | Injectable bone Cement | Qualitatively studied | [199] |
7. Nanostructured Bioceramics
8. Conclusion and Perspective
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Hench, L.L. Bioceramics: From concept to clinic. J. Am. Ceram. Soc. 1991, 74, 1487–1510. [Google Scholar] [CrossRef]
- Mala, R.; Celsia, A.R. Bioceramics in Orthopaedics: A Review. Fundamental Biomaterials: Ceramics Woodhead Publishing Series in Biomaterials; Woodhead Publishing: Cambridge, UK, 2018; pp. 195–221. [Google Scholar]
- Sequeira, S.; Fernandes, M.; Neves, N.; Almeida, M. Development and characterization of zirconia–alumina composites for orthopedic implants. Ceram. Int. 2017, 43, 693–703. [Google Scholar] [CrossRef]
- Hench, L.L.; Xynos, I.D.; Polak, J.M. Bioactive glasses for in situ tissue regeneration. J. Biomater. Sci. Polym. Ed. 2004, 15, 543–562. [Google Scholar] [CrossRef] [PubMed]
- Oonishi, H. Orthopaedic applications of hydroxyapatite. Biomaterials 1991, 12, 171–178. [Google Scholar] [CrossRef]
- Tariq, U.; Hussain, R.; Tufail, K.; Haider, Z.; Tariq, R.; Ali, J. Injectable dicalcium phosphate bone cement prepared from biphasic calcium phosphate extracted from lamb bone. Mater. Sci. Eng. C 2019, 103, 109863. [Google Scholar] [CrossRef]
- Zhao, R.; Shang, T.; Yuan, B.; Zhu, X.; Zhang, X.; Yang, X. Osteoporotic bone recovery by a bamboo-structured bioceramic with controlled release of hydroxyapatite nanoparticles. Bioact. Mater. 2022, 17, 379–393. [Google Scholar] [CrossRef]
- Bohner, M. Bioresorbable ceramics. In Degradation Rate of Bioresorbable Materials; Woodhead Publishing: Sawstone, UK, 2008; pp. 95–114. [Google Scholar]
- Manzano, M.; Vallet-Regí, M. Revisiting bioceramics: Bone regenerative and local drug delivery systems. Prog. Solid State Chem. 2012, 40, 17–30. [Google Scholar] [CrossRef]
- Zhao, C.; Liu, W.; Zhu, M.; Wu, C.; Zhu, Y. Bioceramic-based scaffolds with antibacterial function for bone tissue engineering: A review. Bioact. Mater. 2022, 18, 383–398. [Google Scholar] [CrossRef]
- Baino, F.; Potestio, I. Orbital implants: State-of-the-art review with emphasis on biomaterials and recent advances. Mater. Sci. Eng. C 2016, 69, 1410–1428. [Google Scholar] [CrossRef]
- Beutner, D.; Hüttenbrink, K.-B. Passive and active middle ear implants. GMS Curr. Top. Otorhinolaryngol. Head Neck Surg. 2009, 8, Doc09. [Google Scholar]
- Gautam, S.; Bhatnagar, D.; Bansal, D.; Batra, H.; Goyal, N. Recent advancements in nanomaterials for biomedical implants. Biomed. Eng. Adv. 2022, 3, 100029. [Google Scholar] [CrossRef]
- Montazerian, M.; Hosseinzadeh, F.; Migneco, C.; Fook, M.V.; Baino, F. Bioceramic coatings on metallic implants: An overview. Ceram. Int. 2022, 48, 8987–9005. [Google Scholar] [CrossRef]
- Santos-Coquillat, A.; Esteban-Lucia, M.; Martinez-Campos, E.; Mohedano, M.; Arrabal, R.; Blawert, C.; Zheludkevich, M.; Matykina, E. PEO coatings design for Mg-Ca alloy for cardiovascular stent and bone regeneration applications. Mater. Sci. Eng. C 2019, 105, 110026. [Google Scholar] [CrossRef]
- Shekhawat, D.; Singh, A.; Banerjee, M.; Singh, T.; Patnaik, A. Bioceramic composites for orthopaedic applications: A comprehensive review of mechanical, biological, and microstructural properties. Ceram. Int. 2021, 47, 3013–3030. [Google Scholar] [CrossRef]
- Davaie, S.; Hooshmand, T.; Ansarifard, S. Different types of bioceramics as dental pulp capping materials: A systematic review. Ceram. Int. 2021, 47, 20781–20792. [Google Scholar] [CrossRef]
- Lewis, G. Injectable bone cements for use in vertebroplasty and kyphoplasty: State-of-the-art review. J. Biomed. Mater. Res. Part B Appl. Biomater. Off. J. Soc. Biomater. Jpn. Soc. Biomater. Aust. Soc. Biomater. Korean Soc. Biomater. 2006, 76, 456–468. [Google Scholar] [CrossRef]
- Galante, R.; Figueiredo-Pina, C.G.; Serro, A.P. Additive manufacturing of ceramics for dental applications: A review. Dent. Mater. 2019, 35, 825–846. [Google Scholar] [CrossRef]
- Cieza, A.; Causey, K.; Kamenov, K.; Hanson, S.W.; Chatterji, S.; Vos, T. Global estimates of the need for rehabilitation based on the Global Burden of Disease study 2019: A systematic analysis for the Global Burden of Disease Study 2019. Lancet 2020, 396, 2006–2017. [Google Scholar] [CrossRef]
- Luo, X.; Barbieri, D.; Zhang, Y.; Yan, Y.; Bruijn, J.D.d.; Yuan, H. Strontium-Containing Apatite/Poly Lactide Composites Favoring Osteogenic Differentiation and in Vivo Bone Formation. ACS Biomater. Sci. Eng. 2015, 1, 85–93. [Google Scholar] [CrossRef]
- Bose, S.; Tarafder, S. Calcium phosphate ceramic systems in growth factor and drug delivery for bone tissue engineering: A review. Acta Biomater. 2012, 8, 1401–1421. [Google Scholar] [CrossRef]
- Hasirci, V.; Hasirci, N. Fundamentals of Biomaterials; Springer: Berlin/Heidelberg, Germany, 2018. [Google Scholar]
- Barbosa, W.T.; Garcia-Carrodeguas, R.; Fook, M.V.; Rodriguez, M.A. New cement based on calcium and strontium aluminates for endodontics. Ceram. Int. 2019, 45, 19784–19792. [Google Scholar] [CrossRef]
- Dorozhkin, S.V. Bioceramics of calcium orthophosphates. Biomaterials 2010, 31, 1465–1485. [Google Scholar] [CrossRef] [PubMed]
- Rae, N.A.; Chantler, C.T.; Barnea, Z.; de Jonge, M.D.; Tran, C.Q.; Hester, J.R. X-ray mass attenuation coefficients and imaginary components of the atomic form factor of zinc over the energy range of 7.2–15.2 keV. Phys. Rev. A 2010, 81, 022904. [Google Scholar] [CrossRef]
- Carmignato, S.; Dewulf, W.; Leach, R. Industrial X-ray Computed Tomography; Springer: Berlin/Heidelberg, Germany, 2018. [Google Scholar]
- Aichinger, H.; Dierker, J.; Joite-Barfuß, S.; Säbel, M. Radiation Exposure and Image Quality in X-ray Diagnostic Radiology: Physical Principles and Clinical Applications; Springer: Berlin/Heidelberg, Germany, 2004. [Google Scholar]
- Bushberg, J.T.; Boone, J.M. The Essential Physics of Medical Imaging; Lippincott Williams & Wilkins: Philadelphia, PA, USA, 2011. [Google Scholar]
- Hubbell, J.; Seltzer, S. NIST Standard Reference Database 126; National Institute of Standards and Technology: Gaithersburg, MD, USA, 1996. [Google Scholar]
- Smith, N.B.; Webb, A. Introduction to Medical Imaging: Physics, Engineering and Clinical Applications; Cambridge University Press: Cambridge, UK, 2010. [Google Scholar]
- Poludniowski, G.; Evans, P.; Webb, S. Rayleigh scatter in kilovoltage x-ray imaging: Is the independent atom approximation good enough? Phys. Med. Biol. 2009, 54, 6931. [Google Scholar] [CrossRef] [PubMed]
- Sneha, K.; Sailaja, G. Intrinsically radiopaque biomaterial assortments: A short review on the physical principles, X-ray imageability and state-of-the-art developments. J. Mater. Chem. B 2021, 9, 8569–8593. [Google Scholar] [CrossRef]
- Nóbrega, N.F.S.; Puchnick, A.; Cerqueira, L.K.M.; Costa, C.; Ajzen, S. In vitro study on radiographic gray levels of biomaterials using two digital image methods. Rev. Odonto Ciência 2012, 27, 218–222. [Google Scholar] [CrossRef]
- Kursun-Çakmak, E.S.; Akbulut, N.; Öztas, D. Comparative evaluation of the radiopacity of bone graft materials used in dentistry. J. Contemp. Dent. 2017, 7, 150–155. [Google Scholar] [CrossRef]
- Atala, M.H.; Atala, N.; Yeğin, E.; Bayrak, S. Comparison of radiopacity of current restorative CAD/CAM blocks with digital radiography. J. Esthet. Restor. Dent. 2019, 31, 88–92. [Google Scholar] [CrossRef]
- Cunha, A.P.D. Desenvolvimento de dente molar artifical em impressora 3D para utilização em simuladores antropomórficos. Ph.D. Thesis, Instituto Federal de Educação, Ciência e Tecnologia de Santa Catarina, Florianópolis, Brasil, 2019. [Google Scholar]
- Dionysopoulos, D.; Tolidis, K.; Gerasimou, P.; Koliniotou-Koumpia, E. Effects of shade and composition on radiopacity of dental composite restorative materials. Oral Radiol. 2017, 33, 178–186. [Google Scholar] [CrossRef]
- Cooper, D.; Chapman, L.; Carter, Y.; Wu, Y.; Panahifar, A.; Britz, H.; Bewer, B.; Zhouping, W.; Duke, M.; Doschak, M. Three dimensional mapping of strontium in bone by dual energy K-edge subtraction imaging. Phys. Med. Biol. 2012, 57, 5777. [Google Scholar] [CrossRef]
- Stout, D.E.; Cortes, M.X.; Aiyagari, V.; Olson, D.M. Management of external ventricular drains during intrahospital transport for radiographic imaging. J. Radiol. Nurs. 2019, 38, 92–97. [Google Scholar] [CrossRef]
- Lührs, A.-K.; Geurtsen, W. The application of silicon and silicates in dentistry: A review. In Biosilica in Evolution, Morphogenesis, and Nanobiotechnology; Springer: Berlin/Heidelberg, Germany, 2009; pp. 359–380. [Google Scholar]
- Brzęcka, D.M.; Staniowski, T. Novel bioceramic root repair materials: Review of the literature. Dent. Med. Probl. 2016, 53, 551–558. [Google Scholar]
- Cervino, G.; Laino, L.; D’Amico, C.; Russo, D.; Nucci, L.; Amoroso, G.; Gorassini, F.; Tepedino, M.; Terranova, A.; Gambino, D. Mineral trioxide aggregate applications in endodontics: A review. Eur. J. Dent. 2020, 14, 683–691. [Google Scholar] [CrossRef]
- Kunjan, A.P.; Ballal, N.V. Calcium silicate based cements in endodontics. J. Int. Dent. Med. Res. 2020, 13, 1183–1190. [Google Scholar]
- Kunert, M.; Lukomska-Szymanska, M. Bio-inductive materials in direct and indirect pulp capping—A review article. Materials 2020, 13, 1204. [Google Scholar] [CrossRef]
- Croll, T.P.; Nicholson, J.W. Glass ionomer cements in pediatric dentistry: Review of the literature. Pediatr. Dent. 2002, 24, 423–429. [Google Scholar]
- Mount, G.J. Clinical performance of glass-ionomers. Biomaterials 1998, 19, 573–579. [Google Scholar] [CrossRef]
- Souza, L.C.D.; Yadlapati, M.; Dorn, S.O.; Silva, R.; Letra, A. Analysis of radiopacity, pH and cytotoxicity of a new bioceramic material. J. Appl. Oral Sci. 2015, 23, 383–389. [Google Scholar] [CrossRef]
- Coaguila-Llerena, H.; Ochoa-Rodriguez, V.M.; Castro-Núñez, G.M.; Faria, G.; Guerreiro-Tanomaru, J.M.; Tanomaru-Filho, M. Physicochemical properties of a bioceramic repair material-BioMTA. Braz. Dent. J. 2020, 31, 511–515. [Google Scholar] [CrossRef]
- De Miranda Candeiro, G.T.; Correia, F.C.; Duarte, M.A.H.; Ribeiro-Siqueira, D.C.; Gavini, G. Evaluation of radiopacity, pH, release of calcium ions, and flow of a bioceramic root canal sealer. J. Endod. 2012, 38, 842–845. [Google Scholar] [CrossRef]
- Hrab, D.; Chisnoiu, A.M.; Badea, M.E.; Moldovan, M.; Chisnoiu, R.M. Comparative radiographic assessment of a new bioceramic-based root canal sealer. Clujul Med. 2017, 90, 226. [Google Scholar] [CrossRef] [PubMed]
- Rathke, A.; Pfefferkorn, F.; McGuire, M.K.; Heard, R.H.; Seemann, R. One-year clinical results of restorations using a novel self-adhesive resin-based bulk-fill restorative. Sci. Rep. 2022, 12, 3934. [Google Scholar] [CrossRef] [PubMed]
- Omidi, B.R.; Naeini, F.F.; Dehghan, H.; Tamiz, P.; Savadroodbari, M.M.; Jabbarian, R. Microleakage of an enhanced resin-modified glass ionomer restorative material in primary molars. J. Dent. 2018, 15, 205. [Google Scholar]
- Soliman, T.A.; Othman, M.S. Mechanical properties of the new ketac™ universal glass ionomer restorative material: Effect of resin coating. Egypt. Dent. J. 2017, 63, 1027–1035. [Google Scholar] [CrossRef][Green Version]
- Ambard, A.J.; Mueninghoff, L. Calcium phosphate cement: Review of mechanical and biological properties. J. Prosthodont. 2006, 15, 321–328. [Google Scholar] [CrossRef]
- Kenny, S.; Buggy, M. Bone cements and fillers: A review. J. Mater. Sci. Mater. Med. 2003, 14, 923–938. [Google Scholar] [CrossRef]
- Nabiyouni, M.; Brückner, T.; Zhou, H.; Gbureck, U.; Bhaduri, S.B. Magnesium-based bioceramics in orthopedic applications. Acta Biomater. 2018, 66, 23–43. [Google Scholar] [CrossRef]
- Baino, F.; Fiume, E.; Barberi, J.; Kargozar, S.; Marchi, J.; Massera, J.; Verné, E. Processing methods for making porous bioactive glass-based scaffolds—A state-of-the-art review. J. Appl. Ceram. Technol. 2019, 16, 1762–1796. [Google Scholar] [CrossRef]
- Bohner, M. Design of ceramic-based cements and putties for bone graft substitution. Eur. Cell Mater. 2010, 20, 1–12. [Google Scholar] [CrossRef]
- Sohn, H.-S.; Oh, J.-K. Review of bone graft and bone substitutes with an emphasis on fracture surgeries. Biomater. Res. 2019, 23, 9. [Google Scholar] [CrossRef]
- Salinas, A.J.; Esbrit, P.; Vallet-Regí, M. A tissue engineering approach based on the use of bioceramics for bone repair. Biomater. Sci. 2013, 1, 40–51. [Google Scholar] [CrossRef]
- De Arenaza, I.M.; Sadaba, N.; Larrañaga, A.; Zuza, E.; Sarasua, J. High toughness biodegradable radiopaque composites based on polylactide and barium sulphate. Eur. Polym. J. 2015, 73, 88–93. [Google Scholar] [CrossRef]
- Ramesh, N.; Moratti, S.C.; Dias, G.J. Hydroxyapatite–polymer biocomposites for bone regeneration: A review of current trends. J. Biomed. Mater. Res. B Appl. Biomater. 2018, 106, 2046–2057. [Google Scholar] [CrossRef]
- James, R.; Deng, M.; Laurencin, C.T.; Kumbar, S.G. Nanocomposites and bone regeneration. Front. Mater. Sci. 2011, 5, 342–357. [Google Scholar] [CrossRef]
- Mozumder, M.S.; Mairpady, A.; Mourad, A.H.I. Polymeric nanobiocomposites for biomedical applications. J. Biomed. Mater. Res. Part B Appl. Biomater. 2017, 105, 1241–1259. [Google Scholar] [CrossRef]
- Huang, J.; Best, S.M. 1—Ceramic biomaterials. In Tissue Engineering Using Ceramics and Polymers; Boccaccini, A.R., Gough, J.E., Eds.; Woodhead Publishing: Sawston, UK, 2007; pp. 3–31. [Google Scholar]
- Baino, F.; Novajra, G.; Vitale-Brovarone, C. Bioceramics and Scaffolds: A Winning Combination for Tissue Engineering. Front. Bioeng. Biotechnol. 2015, 3, 202. [Google Scholar] [CrossRef]
- Farid, S.B.H. Bioceramics: For Materials Science and Engineering; Woodhead Publishing: Sawston, UK, 2018. [Google Scholar]
- Tavoni, M.; Dapporto, M.; Tampieri, A.; Sprio, S. Bioactive Calcium Phosphate-Based Composites for Bone Regeneration. J. Compos. Sci. 2021, 5, 227. [Google Scholar] [CrossRef]
- Roy, M.; Bandyopadhyay, A.; Bose, S. Chapter 6—Ceramics in bone grafts and coated implants. In Materials for Bone Disorders; Bose, S., Bandyopadhyay, A., Eds.; Academic Press: Cambridge, MA, USA, 2017; pp. 265–314. [Google Scholar]
- Hursh, K.A.; Kirkpatrick, T.C.; Cardon, J.W.; Brewster, J.A.; Black, S.W.; Himel, V.T.; Sabey, K.A. Shear Bond Comparison between 4 Bioceramic Materials and Dual-cure Composite Resin. J. Endod. 2019, 45, 1378–1383. [Google Scholar] [CrossRef]
- Meng, Y.; Qiang, W.; Pang, J. Fabrication and Microstructure of Laminated HAP—45S5 Bioglass Ceramics by Spark Plasma Sintering. Materials 2019, 12, 484. [Google Scholar] [CrossRef]
- Deubener, J.; Allix, M.; Davis, M.J.; Duran, A.; Höche, T.; Honma, T.; Komatsu, T.; Krüger, S.; Mitra, I.; Müller, R.; et al. Updated definition of glass-ceramics. J. Non-Cryst. Solids 2018, 501, 3–10. [Google Scholar] [CrossRef]
- Neacşu, I.A.; Nicoară, A.I.; Vasile, O.R.; Vasile, B.Ş. Chapter 9—Inorganic micro- and nanostructured implants for tissue engineering. In Nanobiomaterials in Hard Tissue Engineering; Grumezescu, A.M., Ed.; William Andrew Publishing: Norwich, NY, USA, 2016; pp. 271–295. [Google Scholar]
- Zhou, C.; Deng, C.; Chen, X.; Zhao, X.; Chen, Y.; Fan, Y.; Zhang, X. Mechanical and biological properties of the micro-/nano-grain functionally graded hydroxyapatite bioceramics for bone tissue engineering. J. Mech. Behav. Biomed. Mater. 2015, 48, 1–11. [Google Scholar] [CrossRef] [PubMed]
- Kumar, P.; Dehiya, B.S.; Sindhu, A. Bioceramics for hard tissue engineering applications: A review. Int. J. Appl. Eng. Res. 2018, 13, 2744–2752. [Google Scholar]
- Koons, G.L.; Diba, M.; Mikos, A.G. Materials design for bone-tissue engineering. Nat. Rev. Mater. 2020, 5, 584–603. [Google Scholar] [CrossRef]
- Jodati, H.; Yılmaz, B.; Evis, Z. A review of bioceramic porous scaffolds for hard tissue applications: Effects of structural features. Ceram. Int. 2020, 46, 15725–15739. [Google Scholar] [CrossRef]
- Kargozar, S.; Baino, F.; Hamzehlou, S.; Hill, R.G.; Mozafari, M. Bioactive glasses: Sprouting angiogenesis in tissue engineering. Trends Biotechnol. 2018, 36, 430–444. [Google Scholar] [CrossRef]
- Bortoluzzi, E.; Guerreiro-Tanomaru, J.; Tanomaru-Filho, M.; Duarte, M. Radiographic effect of different radiopacifiers on a potential retrograde filling material. Oral Surg. Oral Med. Oral Pathol. Oral Radiol. Endod. 2009, 108, 628–632. [Google Scholar] [CrossRef]
- Cornélio, A.L.G.; Salles, L.P.; Paz, M.C.d.; Cirelli, J.A.; Guerreiro-Tanomaru, J.M.; Filho, M.T. Cytotoxicity of Portland cement with different radiopacifying agents: A cell death study. J. Endod. 2011, 37, 203–210. [Google Scholar] [CrossRef]
- Collares, F.; FA, O.; Lima, G.; Fontanella, V.; Piva, E.; Samuel, S. Ytterbium trifluoride as a radiopaque agent for dental cements. Int. Endod. J. 2010, 43, 792–797. [Google Scholar] [CrossRef]
- Bosso-Martelo, R.; Guerreiro-Tanomaru, J.; Viapiana, R.; Berbert, F.; Duarte, M.; Tanomaru-Filho, M. Physicochemical properties of calcium silicate cements associated with microparticulate and nanoparticulate radiopacifiers. Clin. Oral Investig. 2015, 20, 83–90. [Google Scholar] [CrossRef]
- Chen, C.; SC, H.; Teng, N.; Kao, C.; Lee, S.; Lin, C.; Yang, J. Radiopacity and cytotoxicity of Portland cement containing zirconia doped bismuth oxide radiopacifiers. J. Endod. 2014, 40, 251–254. [Google Scholar] [CrossRef]
- No, Y.J.; Nguyen, T.; Lu, Z.; Mirkhalaf, M.; Fei, F.; Foley, M.; Zreiqat, H. Development of a bioactive and radiopaque bismuth doped baghdadite ceramic for bone tissue engineering | Request PDF. Bone 2021, 153, 116147. [Google Scholar] [CrossRef]
- Ajeesh, M.; Francis, B.F.; Annie, J.; Harikrishna Varma, P.R. Nano iron oxide–hydroxyapatite composite ceramics with enhanced radiopacity. J. Mater. Sci. Mater. Med. 2010, 21, 1427–1434. [Google Scholar] [CrossRef]
- Balbinot, G.d.S.; Leitune, V.C.B.; Nunes, J.S.; Visioli, F.; Mezzomo, C.F. Synthesis of sol-gel derived calcium silicate particles and development of a bioactive endodontic cement. Dent. Mater. Off. Publ. Acad. Dent. Mater. 2020, 36, 135–144. [Google Scholar] [CrossRef]
- Antonijević, D.; Ilić, D.; Medić, V.; Dodić, S.; Obradović-Djuriĉić, K.; Rakoĉević, Z. Evaluation of conventional and digital radiography capacities for distinguishing dental materials on radiograms depending on the present radiopacifying agent. Vojnosanit. Pregl. 2014, 71, 1006–1012. [Google Scholar] [CrossRef]
- Costa, B.C.; Guerreiro-Tanomaru, J.M.; Bosso-Martelo, R.; Rodrigues, E.M.; Bonetti-Filho, I.; Tanomaru-Filho, M. Ytterbium Oxide as Radiopacifier of Calcium Silicate-Based Cements. Physicochemical and Biological Properties. Braz. Dent. J. 2018, 29, 452–458. [Google Scholar] [CrossRef]
- Koju, N.; Sikder, P.; Gaihre, B.; Bhaduri, S.B. Smart Injectable Self-Setting Monetite Based Bioceramics for Orthopedic Applications. Materials 2018, 11, 1258. [Google Scholar] [CrossRef]
- Flores-Ledesma, A.; Gutiérrez-Estrada, K.; Bucio-Galindo, L. Estimación de la cantidad de trióxido de bismuto como agente radiopacificador en dos cementos minerales trióxido agregado mediante una prueba de radiopacidad. Rev. Odontológica Mex. 2019, 23, 139–148. [Google Scholar] [CrossRef]
- Wu, T.; Yang, S.; Shi, H.; Ye, J. Preparation and cytocompatibility of a novel bismuth aluminate/calcium phosphate cement with high radiopacity. J. Mater. Sci. Mater. Med. 2018, 29, 149. [Google Scholar] [CrossRef]
- Åberg, J.; Pankotai, E.; Hulsart Billström, G.; Weszl, M.; Larsson, S.; Forster-Horváth, C.; Lacza, Z.; Engqvist, H. In Vivo Evaluation of an Injectable Premixed Radiopaque Calcium Phosphate Cement. Int. J. Biomater. 2011, 7, 232574. [Google Scholar] [CrossRef]
- Zhao, S.; Pei, Z.; Zhang, K.; Li, G.; Liu, P.; Chang, J.; Zhang, K.; Mi, L. Injectable calcium phosphate containing zirconia short fiber with enhanced radiopacity. Mater. Technol. 2020, 36, 816–821. [Google Scholar] [CrossRef]
- Chen, M.-S.; Yang, J.-C.; Lai, F.-C.; Chen, Y.-C.; Hsieh, M.-Y.; Fang, A.; Chen, S.-H.; Lin, C.-K. Radiopacity performances of precipitated ZrO 2 -doped Bi 2 O 3 powders and the influences of dopant concentrations and sintering temperatures | Request PDF. Ceram. Int. 2017, 43, 14008–14014. [Google Scholar] [CrossRef]
- Meininger, S.; Moseke, C.; Spatz, K.; März, E.; Blum, C.; Ewald, A.; Vorndran, E. Effect of strontium substitution on the material properties and osteogenic potential of 3D powder printed magnesium phosphate scaffolds. Mater. Sci. Eng. C 2019, 98, 1145–1158. [Google Scholar] [CrossRef] [PubMed]
- Schumacher, M.; Henß, A.; Rohnke, M.; Gelinsky, M. A novel and easy-to-prepare strontium(II) modified calcium phosphate bone cement with enhanced mechanical properties. Acta Biomater. 2013, 9, 7536–7544. [Google Scholar] [CrossRef] [PubMed]
- Camilleri, J. Evaluation of the physical properties of an endodontic Portland cement incorporating alternative radiopacifiers used as root-end filling material. Int. Endod. J. 2010, 43, 231–240. [Google Scholar] [CrossRef] [PubMed]
- Griffith, D.M.; Li, H.; Werrett, M.V.; Andrews, P.C.; Sun, H. Medicinal chemistry and biomedical applications of bismuth-based compounds and nanoparticles. Chem. Soc. Rev. 2021, 50, 12037–12069. [Google Scholar] [CrossRef] [PubMed]
- No, Y.J.; Li, J.J.; Zreiqat, H. Doped Calcium Silicate Ceramics: A New Class of Candidates for Synthetic Bone Substitutes. Materials 2017, 10, 153. [Google Scholar] [CrossRef]
- Parirokh, M.; Torabinejad, M. Mineral trioxide aggregate: Properties and clinical applications. In Pocket Dentistry; John Wiley & Sons: Hoboken, NJ, USA, 2015; pp. 284–295. [Google Scholar]
- Jin, H.; Li, Y.; Wang, Q.; Dong, M.; Yang, M.; Chen, W.; Wang, S.; Zhang, H.; Zheng, S.; Cao, C.Y.; et al. A strontium and amorphous calcium phosphate dipped premixed injectable calcium silicate-based ceramic for dental root canal sealing. Ceram. Int. 2021, 47, 33738–33750. [Google Scholar] [CrossRef]
- Ciobanu, G.; Harja, M. Bismuth-doped nanohydroxyapatite coatings on titanium implants for improved radiopacity and antimicrobial activity. Nanomaterials 2019, 9, 1696. [Google Scholar] [CrossRef]
- Khaskhoussi, A.; Calabrese, L.; Currò, M.; Lentile, R.; Bouaziz, J.; Proverbio, E. Effect of the Compositions on the Biocompatibility of New Alumina–Zirconia–Titania Dental Ceramic Composites. Materials 2020, 13, 1374. [Google Scholar] [CrossRef]
- Mohan, B.G.; Suresh Babu, S.; Varma, H.K.; John, A. In vitro evaluation of bioactive strontium-based ceramic with rabbit adipose-derived stem cells for bone tissue regeneration. J. Mater. Sci. Mater. Med. 2013, 24, 2831–2844. [Google Scholar] [CrossRef]
- Carvalho, E.V.; de Paula, D.M.; Neto, D.A.; Costa, L.; Dias, D.F.; Feitosa, V.P.; Fechine, P.B.A. Radiopacity and mechanical properties of dental adhesives with strontium hydroxyapatite nanofillers. J. Mech. Behav. Biomed. Mater. 2019, 101, 103447. [Google Scholar] [CrossRef]
- You, J.; Yoo, J.-S.; Kum, K.-Y.; Hong, S.-H. Hydration behavior and radiopacity of strontium substituted Ca3SiO5 cement. J. Korean Ceram. Soc. 2021, 58, 330–336. [Google Scholar] [CrossRef]
- Souza, M.K.; Lima, E.P.; Nascimento, I.V.; Montazerian, M.; Baino, F.; Fook, M.V. Development, characterization and optimization of a new bone cement based on calcium–Strontium aluminates and chitosan-glycerin solution. Ceram. Int. 2022, 48, 31866–31879. [Google Scholar] [CrossRef]
- Li, Q.; Coleman, N.J. Impact of Bi2O3 and ZrO2 Radiopacifiers on the Early Hydration and C–S–H Gel Structure of White Portland Cement. J. Funct. Biomater. 2019, 10, 46. [Google Scholar] [CrossRef]
- Myat-Htun, M.; Noor, A.-F.; Kawashita, M.; Ismail, Y.M.B. Enhanced sinterability and in vitro bioactivity of barium-doped akermanite ceramic. Ceram. Int. 2020, 46, 19062–19068. [Google Scholar] [CrossRef]
- Cathers, S.; Kaminski, E.; Osetek, E. The cellular response to Hydron within the rat peritoneal cavity. J. Endod. 1984, 10, 173–181. [Google Scholar] [CrossRef]
- Smith, J.W.; Leeb, I.; Torney, D.L. A comparison of calcium hydroxide and barium hydroxide as agents for inducing apical closure. J. Endod. 1984, 10, 64–70. [Google Scholar] [CrossRef]
- Liu, H.; Zhang, Z.; Gao, C.; Bai, Y.; Liu, B.; Wang, W.; Ma, Y.; Yang, H.; Li, Y.; Chan, A. Enhancing effects of radiopaque agent BaSO4 on mechanical and biocompatibility properties of injectable calcium phosphate composite cement. Mater. Sci. Eng. C 2020, 116, 110904. [Google Scholar] [CrossRef]
- Alshemary, A.Z.; Akram, M.; Goh, Y.F.; Kadir, M.R.A.; Abdolahi, A.; Hussain, R. Structural characterization, optical properties and in vitro bioactivity of mesoporous erbium-doped hydroxyapatite. J. Alloys Compd. 2015, 645, 478–486. [Google Scholar] [CrossRef]
- Hench, L.L.; Polak, J.M. Third-generation biomedical materials. J. Sci. 2002, 295, 1014–1017. [Google Scholar] [CrossRef]
- Hench, L.L. The story of Bioglass®. J. Mater. Sci. Mater. Med. 2006, 17, 967–978. [Google Scholar] [CrossRef] [PubMed]
- Montazerian, M.; Zanotto, E.D. A guided walk through Larry Hench’s monumental discoveries. J. Mater. Sci. 2017, 52, 8695–8732. [Google Scholar] [CrossRef]
- Montazerian, M.; Zanotto, E.D. Bioactive glass-ceramics: Processing, properties and applications. In Bioactive Glasses; Royal Society of Chemistry: London, UK, 2016; pp. 27–33. [Google Scholar]
- Montazerian, M.; Dutra Zanotto, E. History and trends of bioactive glass-ceramics. J. Biomed. Mater. Res. Part A 2016, 104, 1231–1249. [Google Scholar] [CrossRef] [PubMed]
- Boccaccini, A.R.; Brauer, D.S.; Hupa, L. Bioactive Glasses: Fundamentals, Technology and Applications; Royal Society of Chemistry: London, UK, 2016. [Google Scholar]
- Baino, F.; Fiume, E.; Miola, M.; Verné, E. Bioactive sol-gel glasses: Processing, properties, and applications. Int. J. Appl. Ceram. Technol. 2018, 15, 841–860. [Google Scholar] [CrossRef]
- Jones, J.R. Review of bioactive glass: From Hench to hybrids. Acta Biomater. 2013, 9, 4457–4486. [Google Scholar] [CrossRef]
- Arcos, D.; Vallet-Regí, M. Sol–gel silica-based biomaterials and bone tissue regeneration. Acta Biomater. 2010, 6, 2874–2888. [Google Scholar] [CrossRef]
- Erol-Taygun, M.; Zheng, K.; Boccaccini, A.R. Nanoscale bioactive glasses in medical applications. Int. J. Appl. Glass Sci. 2013, 4, 136–148. [Google Scholar] [CrossRef]
- Izquierdo-Barba, I.; Salinas, A.J.; Vallet-Regí, M. Bioactive glasses: From macro to nano. Int. J. Appl. Glass Sci. 2013, 4, 149–161. [Google Scholar] [CrossRef]
- Yan, X.; Yu, C.; Zhou, X.; Tang, J.; Zhao, D. Highly ordered mesoporous bioactive glasses with superior in vitro bone-forming bioactivities. Angew. Chem. Int. Ed. 2004, 43, 5980–5984. [Google Scholar] [CrossRef]
- Salman, S.; Salama, S.; Abo-Mosallam, H. The role of strontium and potassium on crystallization and bioactivity of Na2O–CaO–P2O5–SiO2 glasses. Ceram. Int. 2012, 38, 55–63. [Google Scholar] [CrossRef]
- Mosaddad, S.A.; Yazdanian, M.; Tebyanian, H.; Tahmasebi, E.; Yazdanian, A.; Seifalian, A.; Tavakolizadeh, M. Fabrication and properties of developed collagen/strontium-doped Bioglass scaffolds for bone tissue engineering. J. Mater. Res. Technol. 2020, 9, 14799–14817. [Google Scholar] [CrossRef]
- Sharifianjazi, F.; Moradi, M.; Abouchenari, A.; Pakseresht, A.H.; Esmaeilkhanian, A.; Shokouhimehr, M.; Asl, M.S. Effects of Sr and Mg dopants on biological and mechanical properties of SiO2–CaO–P2O5 bioactive glass. Ceram. Int. 2020, 46, 22674–22682. [Google Scholar] [CrossRef]
- O’Brien, D.; Boyd, D.; Madigan, S.; Murphy, S. Evaluation of a novel radiopacifiying agent on the physical properties of surgical spineplex®. J. Mater. Sci. Mater. Med. 2010, 21, 53–58. [Google Scholar] [CrossRef]
- Doucet, J.; Tonkopi, E.; Nuschke, A.; Tremblay, M.; Brewer, K.; Beyea, S.; Filiaggi, M.; Abraham, R.; Werner-Zwanziger, U.; Boyd, D. Multi-modal imageability and degradation characteristics of high-borate glass systems for transient embolization. J. Non-Cryst. Solids 2019, 510, 26–35. [Google Scholar] [CrossRef]
- Watts, D. Radiopacity vs. composition of some barium and strontium glass composites. J. Dent. 1987, 15, 38–43. [Google Scholar] [CrossRef]
- Draghici, D.-A.; Mihai, A.-A.; Aioanei, M.-O.; Negru, N.-E.; Nicoara, A.-I.; Jinga, S.-I.; Miu, D.; Bacalum, M.; Busuioc, C. Strontium-substituted bioactive glass-ceramic films for tissue engineering. Bol. Soc. Esp. Ceram. Vidr. 2020, 61, 184–190. [Google Scholar] [CrossRef]
- Maciel, P.P.; Pessôa, J.A.M.; Medeiros, E.L.G.d.; Batista, A.U.D.; Figueiredo, L.R.F.; Medeiros, E.S.d.; Oliveira Duarte, D.F.d.; Alves, A.F.; Sousa, F.B.d.; Vieira, B.R. Use of strontium doping glass-ceramic material for bone regeneration in critical defect: In vitro and in vivo analyses. Ceram. Int. 2020, 46, 24940–24954. [Google Scholar] [CrossRef]
- Zhao, S.; Zhang, J.; Zhu, M.; Zhang, Y.; Liu, Z.; Tao, C.; Zhu, Y.; Zhang, C. Three-dimensional printed strontium-containing mesoporous bioactive glass scaffolds for repairing rat critical-sized calvarial defects. Acta Biomater. 2015, 12, 270–280. [Google Scholar] [CrossRef]
- Zhang, Y.; Wei, L.; Wu, C.; Miron, R.J. Periodontal regeneration using strontium-loaded mesoporous bioactive glass scaffolds in osteoporotic rats. PLoS ONE 2014, 9, e104527. [Google Scholar] [CrossRef]
- Höland, W.; Schweiger, M.; Dittmer, M.; Ritzberger, C. Biotechnology. Radiopaque strontium fluoroapatite glass-ceramics. Front. Bioeng. Biotechnol. 2015, 3, 149. [Google Scholar] [CrossRef]
- Montazerian, M.; Baino, F.; Fiume, E.; Migneco, C.; Alaghmandfard, A.; Sedighi, O.; DeCeanne, A.V.; Wilkinson, C.J.; Mauro, J.C. Glass-ceramics in dentistry: Fundamentals, technologies, experimental techniques, applications, and open issues. Prog. Mater. Sci. 2022, 132, 101023. [Google Scholar] [CrossRef]
- Ahmad, M.; Aly, K.; Saddeek, Y.B.; Dahshan, A. Glass transition and crystallization kinetics of Na2O–B2O3–Nb2O5–Bi2O3 ceramic glasses. J. Non-Cryst. Solids 2020, 546, 120260. [Google Scholar] [CrossRef]
- Jiménez, J.A. Spectroscopic and dilatometric analysis of low-melting bismuth borate glasses in the Bi2O3–BaO–Li2O–B2O3 quaternary. Mater. Chem. Phys. 2020, 255, 123635. [Google Scholar] [CrossRef]
- Bahgat, A.; Moustafa, M.; Shaisha, E. Enhancement of electric conductivity in transparent glass–ceramic nanocomposites of Bi2O3–BaTiO3 glasses. J. Mater. Sci. Technol. 2013, 29, 1166–1176. [Google Scholar] [CrossRef]
- Abdel-Khalek, E.; Mohamed, E.; Salem, S.M.; Kashif, I. Structural and dielectric properties of (100− x) B2O3-(x/2) Bi2O3–(x/2) Fe2O3 glasses and glass-ceramic containing BiFeO3 phase. J. Non-Cryst. Solids 2018, 492, 41–49. [Google Scholar] [CrossRef]
- Pazarçeviren, A.E.; Tahmasebifar, A.; Tezcaner, A.; Keskin, D.; Evis, Z. Investigation of bismuth doped bioglass/graphene oxide nanocomposites for bone tissue engineering. Ceram. Int. 2018, 44, 3791–3799. [Google Scholar] [CrossRef]
- Heid, S.; Stoessel, P.R.; Tauböck, T.T.; Stark, W.J.; Zehnder, M.; Mohn, D. Incorporation of particulate bioactive glasses into a dental root canal sealer. Biomed. Glasses 2016, 2, 29–37. [Google Scholar] [CrossRef]
- Mohn, D.; Zehnder, M.; Imfeld, T.; Stark, W.J. Radio-opaque nanosized bioactive glass for potential root canal application: Evaluation of radiopacity, bioactivity and alkaline capacity. Int. Endod. J. 2010, 43, 210–217. [Google Scholar] [CrossRef]
- Tallia, F.; Gallo, M.; Pontiroli, L.; Baino, F.; Fiorilli, S.; Onida, B.; Anselmetti, G.C.; Manca, A.; Vitale-Brovarone, C. Zirconia-containing radiopaque mesoporous bioactive glasses. Mater. Lett. 2014, 130, 281–284. [Google Scholar] [CrossRef]
- Montazerian, M.; Schneider, J.F.; Yekta, B.E.; Marghussian, V.K.; Rodrigues, A.M.; Zanotto, E.D. Sol–gel synthesis, structure, sintering and properties of bioactive and inert nano-apatite–zirconia glass–ceramics. Ceram. Int. 2015, 41, 11024–11045. [Google Scholar] [CrossRef]
- Taira, M.; Toyooka, H.; Miyawaki, H.; Yamaki, M. Studies on radiopaque composites containing ZrO2 SiO2 fillers prepared by the sol-gel process. Dent. Mater. 1993, 9, 167–171. [Google Scholar] [CrossRef]
- Yin, P.; Yuan, J.-W.; Liu, L.-H.; Xiao, T.; Lei, T. Effect of ZrO2 on the bioactivity properties of gel-derived CaO-P2O5-SiO2-SrO glasses. Ceram. Int. 2017, 43, 9691–9698. [Google Scholar] [CrossRef]
- Prentice, L.H.; Tyas, M.J.; Burrow, M.F. The effect of ytterbium fluoride and barium sulphate nanoparticles on the reactivity and strength of a glass-ionomer cement. Dent. Mater. 2006, 22, 746–751. [Google Scholar] [CrossRef]
- Oka, Y.; Sasaki, J.-I.; Wakabayashi, K.; Nakano, Y.; Okamura, S.-Y.; Nakamura, T.; Imazato, S.; Yatani, H. Fabrication of a radiopaque fit-testing material to evaluate the three-dimensional accuracy of dental prostheses. Dent. Mater. 2016, 32, 921–928. [Google Scholar] [CrossRef]
- Madanat, R.; Moritz, N.; Vedel, E.; Svedström, E.; Aro, H.T. Radio-opaque bioactive glass markers for radiostereometric analysis. Acta Biomater. 2009, 5, 3497–3505. [Google Scholar] [CrossRef]
- Nogueira, L.; Campos, T. Nuclear characterization and investigation of radioactive bioglass seed surfaces for brachytherapy via scanning electron microscopy. J. Sol-Gel Sci. Technol. 2011, 58, 251–258. [Google Scholar] [CrossRef]
- Khoeini, M.; Hesaraki, S.; Kolahi, A. Effect of BaO substitution for CaO on the structural and thermal properties of SiO2–B2O3–Al2O3–CaO–Na2O–P2O5 bioactive glass system used for implant coating applications. Ceram. Int. 2021, 47, 31666–31680. [Google Scholar] [CrossRef]
- Tamura, J.; Kawanabe, K.; Kobayashi, M.; Nakamura, T.; Kokubo, T.; Yoshihara, S.; Shibuya, T. Mechanical and biological properties of two types of bioactive bone cements containing MgO-CaO-SiO2-P2O5-CaF2 glass and glass—Ceramic powder. J. Biomed. Mater. Res. Off. J. Soc. Biomater. Jpn. Soc. Biomater. 1996, 30, 85–94. [Google Scholar] [CrossRef]
- Mahato, A.; De, M.; Bhattacharjee, P.; Kumar, V.; Mukherjee, P.; Singh, G.; Kundu, B.; Balla, V.K.; Nandi, S.K. Role of calcium phosphate and bioactive glass coating on in vivo bone healing of new Mg–Zn–Ca implant. J. Mater. Sci. Mater. Med. 2021, 32, 55. [Google Scholar] [CrossRef]
- Hasan, M.; Kehoe, S.; Boyd, D. Temporal analysis of dissolution by-products and genotoxic potential of spherical zinc–silicate bioglass:“Imageable beads” for transarterial embolization. J. Biomater. Appl. 2014, 29, 566–581. [Google Scholar] [CrossRef]
- Kehoe, S.; Looney, M.; Kilcup, N.; Tonkopi, E.; Daly, C.; Abraham, R.; Boyd, D. Effects of γ-irradiation and accelerated aging on composition-structure–property relationships for radiopaque embolic microspheres. J. Non-Cryst. Solids 2014, 402, 84–90. [Google Scholar] [CrossRef]
- Kehoe, S.; Tonkopi, E.; Abraham, R.; Boyd, D. Predicting the thermal responses and radiopacity of multicomponent zinc–silicate bioglasses: A focus on ZnO, La2O3, SiO2 and TiO2. J. Non-Cryst. Solids 2012, 358, 3388–3395. [Google Scholar] [CrossRef]
- Hench, L.L.; Day, D.E.; Höland, W.; Rheinberger, V.M. Glass and medicine. Int. J. Appl. Glass Sci. 2010, 1, 104–117. [Google Scholar] [CrossRef]
- Bakina, O.; Ivanova, L.Y.; Toropkov, N.; Senkina, E.; Lerner, M.; Glazkova, E.; Krinitsyn, M. Core–Shell Fe-Fe3O4 Nanoparticles for Synthesizing PLA Composites with Low Toxicity and High Radiopacity. Phys. Mesomech. 2022, 25, 270–278. [Google Scholar] [CrossRef]
- Carr, B.I. Hepatic arterial 90Yttrium glass microspheres (Therasphere) for unresectable hepatocellular carcinoma: Interim safety and survival data on 65 patients. Liver Transplant. 2004, 10, S107–S110. [Google Scholar] [CrossRef]
- White, J.E.; Day, D.E. Rare earth aluminosilicate glasses for in vivo radiation delivery. Key Eng. Mater. 1994, 94–95, 181–208. [Google Scholar] [CrossRef]
- Bretcanu, O.; Evans, I. Glasses for treatment of liver cancer by radioembolization. In Biocompatible Glasses; Springer: Berlin/Heidelberg, Germany, 2016; pp. 267–283. [Google Scholar]
- Henry, E.C.; Mawko, G.; Tonkopi, E.; Frampton, J.; Kehoe, S.; Boyd, D.; Abraham, R.; Gregoire, M.; O’Connell, K.; Kappadath, S.C. Quantification of the inherent radiopacity of glass microspheres for precision dosimetry in yttrium-90 radioembolization. Biomed. Phys. Eng. Express 2019, 5, 055011. [Google Scholar] [CrossRef]
- Henry, E.C.; Strugari, M.; Mawko, G.; Brewer, K.D.; Abraham, R.; Kappadath, S.C.; Syme, A. Post-administration dosimetry in yttrium-90 radioembolization through micro-CT imaging of radiopaque microspheres in a porcine renal model. Phys. Med. Biol. 2021, 66, 095011. [Google Scholar] [CrossRef]
- Henry, E.C.; Strugari, M.; Mawko, G.; Brewer, K.; Liu, D.; Gordon, A.C.; Bryan, J.N.; Maitz, C.; Abraham, R.; Kappadath, S.C. Precision Dosimetry in Yttrium-90 Radioembolization through CT Imaging of Radiopaque Microspheres in a Rabbit Liver Model. Res. Sq. 2021, 9, 21. [Google Scholar] [CrossRef]
- Moeini, A.; Chinijani, T.H.; Khachatourian, A.M.; Fook, M.V.L.; Baino, F.; Montazerian, M. A Critical Review of Bioactive Glasses and Glass-Ceramics in Cancer Therapy. Int. J. Appl. Glas. Sci. 2022. In Print. [Google Scholar] [CrossRef]
- Boston Scientific. A Patient’s Guide to TheraSphere™ Important Information for People with Liver Cancer; Boston Scientific: Marlborough, MA, USA, 2020. [Google Scholar]
- Dubok, O.; Shynkaruk, O.; Buzaneva, E. Lanthanides oxides usage to increase radiopaque of bioactive ceramics. Funct. Mater. 2013, 20, 172–179. [Google Scholar] [CrossRef][Green Version]
- Bauer, J.; Carvalho, E.M.; Carvalho, C.N.; Meier, M.M.; de Souza, J.P.; de Carvalho, R.M.; Loguercio, A.D. Development of a simplified etch-and-rinse adhesive containing niobiophosphate bioactive glass. Int. J. Adhes. Adhes. 2016, 69, 110–114. [Google Scholar] [CrossRef]
- Alhalawani, A.M.; Mehrvar, C.; Stone, W.; Waldman, S.D.; Towler, M.R. A novel tantalum-containing bioglass. Part II. Development of a bioadhesive for sternal fixation and repair. Mater. Sci. Eng. C 2017, 71, 401–411. [Google Scholar] [CrossRef]
- Grishchenko, D.; Slobodyuk, A.; Kuryavyi, V.; Medkov, M. Tantalum-Containing Bioactive Glass-Ceramics: A Mechanism of Suppression of the Biological Activity of the 45S5 Bioglass by Doping with Ta2O5. Russ. J. Inorg. Chem. 2020, 65, 1606–1613. [Google Scholar] [CrossRef]
- Fellows, B.; Natarajan, S.; Monroe, E.; Rausch, J. Acrylic-ceramic composite for alveolar ridge augmentation. In Biomedical Engineering II; Elsevier: Amsterdam, The Netherlands, 1983; pp. 11–16. [Google Scholar]
- Schmitt, J.M.; Buck, D.; Bennett, S.; Skalla, W.; Christoforou, C.; Buechter, D.; Gruskin, E.; Hollinger, J. Assessment of an experimental bone wax polymer plus TGF-β1 implanted into calvarial defects. J. Biomed. Mater. Res. Off. J. Soc. Biomater. Jpn. Soc. Biomater. Aust. Soc. Biomater. 1998, 41, 584–592. [Google Scholar]
- Shikinami, Y.; Okuno, M. Bioresorbable devices made of forged composites of hydroxyapatite (HA) particles and poly-L-lactide (PLLA): Part I. Basic characteristics. Biomaterials 1999, 20, 859–877. [Google Scholar] [CrossRef]
- Krämer, N.; Lohbauer, U.; Frankenberger, R. Adhesive luting of indirect restorations. Am. J. Dent. 2000, 13, 60D–76D. [Google Scholar]
- Abalymov, A.; Parakhonskiy, B.; Skirtach, A.G. Polymer-and hybrid-based biomaterials for interstitial, connective, vascular, nerve, visceral and musculoskeletal tissue engineering. Polymers 2020, 12, 620. [Google Scholar] [CrossRef]
- Fu, D.-Y.; Liu, X.; Zheng, X.; Zhou, M.; Wang, W.; Su, G.; Liu, T.; Wang, L.; Xie, Z. Polymer-metal-organic framework hybrids for bioimaging and cancer therapy. Coord. Chem. Rev. 2022, 456, 214393. [Google Scholar] [CrossRef]
- Jung, Y.; Kim, D. Recent advances in hybrid system of porous silicon nanoparticles and biocompatible polymers for biomedical applications. Biomed. Eng. Lett. 2021, 11, 171–181. [Google Scholar] [CrossRef]
- Abboud, M.; Casaubieilh, L.; Morvan, F.; Fontanille, M.; Duguet, E. PMMA-based composite materials with reactive ceramic fillers: IV. Radiopacifying particles embedded in PMMA beads for acrylic bone cements. J. Biomed. Mater. Res. Off. J. Soc. Biomater. Jpn. Soc. Biomater. Aust. Soc. Biomater. Korean Soc. Biomater. 2000, 53, 728–736. [Google Scholar] [CrossRef]
- Heini, P.; Wälchli, B.; Berlemann, U. Percutaneous transpedicular vertebroplasty with PMMA: Operative technique and early results. Eur. Spine J. 2000, 9, 445–450. [Google Scholar] [CrossRef] [PubMed]
- Lewis, G. Alternative acrylic bone cement formulations for cemented arthroplasties: Present status, key issues, and future prospects. J. Biomed. Mater. Res. Part B Appl. Biomater. Off. J. Soc. Biomater. Jpn. Soc. Biomater. Aust. Soc. Biomater. Korean Soc. Biomater. 2008, 84, 301–319. [Google Scholar] [CrossRef] [PubMed]
- Breusch, S.; Kühn, K. Bone cements based on polymethylmethacrylate. Orthopade 2003, 32, 41–50. [Google Scholar] [CrossRef] [PubMed]
- Nussbaum, D.A.; Gailloud, P.; Murphy, K. The chemistry of acrylic bone cements and implications for clinical use in image-guided therapy. J. Vasc. Interv. Radiol. 2004, 15, 121–126. [Google Scholar] [CrossRef]
- Monzón, R.A.; Coury, J.G.; Disse, G.D.; Lum, Z.C. Bone cement in total hip and knee arthroplasty. JBJS Rev. 2019, 7, e6. [Google Scholar] [CrossRef]
- Beckmann, J.; Ferguson, S.J.; Gebauer, M.; Luering, C.; Gasser, B.; Heini, P. Femoroplasty–augmentation of the proximal femur with a composite bone cement–feasibility, biomechanical properties and osteosynthesis potential. Med. Eng. Phys. 2007, 29, 755–764. [Google Scholar] [CrossRef]
- Boyd, D.; Towler, M.; Wren, A.; Clarkin, O. Comparison of an experimental bone cement with surgical Simplex® P, Spineplex® and Cortoss®. J. Mater. Sci. Mater. Med. 2008, 19, 1745–1752. [Google Scholar] [CrossRef]
- Pomrink, G.; DiCicco, M.; Clineff, T.; Erbe, E. Evaluation of the reaction kinetics of CORTOSSTM, a thermoset cortical bone void filler. Biomaterials 2003, 24, 1023–1031. [Google Scholar] [CrossRef]
- Garcia, I.M.; Leitune, V.C.B.; Ferreira, C.J.; Collares, F.M. Tantalum oxide as filler for dental adhesive resin. Dent. Mater. J. 2018, 37, 897–903. [Google Scholar] [CrossRef]
- Garoushi, S.; Vallittu, P.; Lassila, L. Mechanical properties and radiopacity of flowable fiber-reinforced composite. Dent. Mater. J. 2019, 38, 196–202. [Google Scholar] [CrossRef]
- Cocco, A.R.; Lima, G.S.; Leal, F.B.; Munchow, E.A.; Ogliari, F.A.; Piva, E. Addition of nanoparticles for development of radiopaque dental adhesives. Int. J. Adhes. Adhes. 2018, 80, 122–127. [Google Scholar] [CrossRef]
- Garcia, I.M.; Leitune, V.C.B.; Takimi, A.S.; Bergmann, C.P.; Samuel, S.M.W.; Melo, M.A.; Collares, F.M. Cerium dioxide particles to tune radiopacity of dental adhesives: Microstructural and physico-chemical evaluation. J. Funct. Biomater. 2020, 11, 7. [Google Scholar] [CrossRef]
- Collares, F.M.; Klein, M.; Santos, P.D.; Portella, F.F.; Ogliari, F.; Leitune, V.C.B.; Samuel, S.M.W. Influence of radiopaque fillers on physicochemical properties of a model epoxy resin-based root canal sealer. J. Appl. Oral Sci. 2013, 21, 533–539. [Google Scholar] [CrossRef]
- Marins, N.H.; Meereis, C.T.; Silva, R.M.; Ruas, C.P.; Takimi, A.S.; Carreño, N.L.; Ogliari, F.A. Radiopaque dental adhesive with addition of niobium pentoxide nanoparticles. Polym. Bull. 2018, 75, 2301–2314. [Google Scholar] [CrossRef]
- Fuchs, M.; Gentleman, E.; Shahid, S.; Hill, R.G.; Brauer, D.S. Therapeutic ion-releasing bioactive glass ionomer cements with improved mechanical strength and radiopacity. Front. Mater. 2015, 2, 63. [Google Scholar] [CrossRef]
- Goñi, I.; Rodríguez, R.; García-Arnáez, I.; Parra, J.; Gurruchaga, M. Preparation and characterization of injectable PMMA-strontium-substituted bioactive glass bone cement composites. J. Biomed. Mater. Res. Part B Appl. Biomater. 2018, 106, 1245–1257. [Google Scholar] [CrossRef]
- Kjellson, F.; Almén, T.; Tanner, K.; McCarthy, I.; Lidgren, L. Bone cement X-ray contrast media: A clinically relevant method of measuring their efficacy. J. Biomed. Mater. Res. Part B Appl. Biomater. Off. J. Soc. Biomater. Jpn. Soc. Biomater. Aust. Soc. Biomater. Korean Soc. Biomater. 2004, 70, 354–361. [Google Scholar] [CrossRef]
- Hernández, L.; Parra, J.; Vázquez, B.; Bravo, A.L.; Collía, F.; Goñi, I.; Gurruchaga, M.; San Román, J. Injectable acrylic bone cements for vertebroplasty based on a radiopaque hydroxyapatite. Bioactivity and biocompatibility. J. Biomed. Mater. Res. Part B Appl. Biomater. Off. J. Soc. Biomater. Jpn. Soc. Biomater. Aust. Soc. Biomater. Korean Soc. Biomater. 2009, 88, 103–114. [Google Scholar] [CrossRef]
- Liu-Snyder, P.; Webster, T.J. Developing a new generation of bone cements with nanotechnology. Curr. Nanosci. 2008, 4, 111–118. [Google Scholar] [CrossRef]
- Bhambri, S.K.; Gilbertson, L.N. Micromechanisms of fatigue crack initiation and propagation in bone cements. J. Biomed. Mater. Res. 1995, 29, 233–237. [Google Scholar] [CrossRef] [PubMed]
- Sabokbar, A.; Fujikawa, Y.; Murray, D.; Athanasou, N. Radio-opaque agents in bone cement increase bone resorption. J. Bone Jt. Surg. Br. Vol. 1997, 79, 129–134. [Google Scholar] [CrossRef]
- Aminoroaya, A.; Neisiany, R.E.; Khorasani, S.N.; Panahi, P.; Das, O.; Madry, H.; Cucchiarini, M.; Ramakrishna, S. A review of dental composites: Challenges, chemistry aspects, filler influences, and future insights. Compos. Part B Eng. 2021, 216, 108852. [Google Scholar] [CrossRef]
- Vallet-Regi, M.; Arcos, D. Nanostructured hybrid materials for bone tissue regeneration. Curr. Nanosci. 2006, 2, 179–189. [Google Scholar] [CrossRef]
- Martín, A.; Salinas, A.; Vallet-Regí, M. Bioactive and degradable organic–inorganic hybrids. J. Eur. Ceram. Soc. 2005, 25, 3533–3538. [Google Scholar] [CrossRef]
- Salarian, M.; Xu, W.Z.; Bohay, R.; Lui, E.M.; Charpentier, P.A. Angiogenic Rg1/Sr-Doped TiO2 Nanowire/Poly (Propylene Fumarate) Bone Cement Composites. Macromol. Biosci. 2017, 17, 1600156. [Google Scholar] [CrossRef]
- Sun, C.; Xu, D.; Hou, C.; Zhang, H.; Li, Y.; Zhang, Q.; Wang, H.; Zhu, M. Core-shell structured SiO2@ ZrO2@ SiO2 filler for radiopacity and ultra-low shrinkage dental composite resins. J. Mech. Behav. Biomed. Mater. 2021, 121, 104593. [Google Scholar] [CrossRef]
- Guan, Q.; He, B.; Huang, J.; Lu, H.H.; Wang, M. Hybrid ceramics-based cancer theranostics. J. Korean Ceram. Soc. 2022, 59, 401–426. [Google Scholar] [CrossRef]
- Tchounwou, C.; Sinha, S.S.; Viraka Nellore, B.P.; Pramanik, A.; Kanchanapally, R.; Jones, S.; Chavva, S.R.; Ray, P.C. Hybrid theranostic platform for second near-IR window light triggered selective two-photon imaging and photothermal killing of targeted melanoma cells. ACS Appl. Mater. Interfaces 2015, 7, 20649–20656. [Google Scholar] [CrossRef]
- Cao, Y.; Dong, H.; Yang, Z.; Zhong, X.; Chen, Y.; Dai, W.; Zhang, X. Aptamer-conjugated graphene quantum dots/porphyrin derivative theranostic agent for intracellular cancer-related microRNA detection and fluorescence-guided photothermal/photodynamic synergetic therapy. ACS Appl. Mater. Interfaces 2017, 9, 159–166. [Google Scholar] [CrossRef]
- Ren, S.; Yang, J.; Ma, L.; Li, X.; Wu, W.; Liu, C.; He, J.; Miao, L. Ternary-responsive drug delivery with activatable dual mode contrast-enhanced in vivo imaging. ACS Appl. Mater. Interfaces 2018, 10, 31947–31958. [Google Scholar] [CrossRef]
- Sneha, K.; Sreeja, S.; Sailaja, G. Radiopacity endowed magnetic nanocomposite with hyperthermia and in vitro mineralization potential: A combinatorial therapeutic system for osteosarcoma. Biomed. Mater. 2021, 16, 045029. [Google Scholar] [CrossRef]
- Sneha, K.; Benny, N.; Nair, B.N.; Sailaja, G. Natural rubber latex assisted shape-attuned synthesis of intrinsically radiopaque and magnetic bioceramic nanocomposite with hyperthermia potential for cancer therapeutics. New J. Chem. 2021, 45, 9892–9903. [Google Scholar] [CrossRef]
- Sharifi, E.; Bigham, A.; Yousefiasl, S.; Trovato, M.; Ghomi, M.; Esmaeili, Y.; Samadi, P.; Zarrabi, A.; Ashrafizadeh, M.; Sharifi, S. Mesoporous bioactive glasses in cancer diagnosis and therapy: Stimuli-responsive, toxicity, immunogenicity, and clinical translation. Adv. Sci. 2022, 9, 2102678. [Google Scholar] [CrossRef]
- Niu, W.; Guo, Y.; Xue, Y.; Wang, M.; Chen, M.; Winston, D.D.; Cheng, W.; Lei, B. Biodegradable multifunctional bioactive Eu-Gd-Si-Ca glass nanoplatform for integrative imaging-targeted tumor therapy-recurrence inhibition-tissue repair. Nano Today 2021, 38, 101137. [Google Scholar] [CrossRef]
- Vitale-Brovarone, C.; Verné, E.; Bergui, M.; Onida, B.; Ferraris, S.; Miola, M.; Baino, F.; Tallia, F.J.P.N.E. Injectable Osteoinductive Bone Cements. International Patent Application No. PCT/IB2011/052094, 12 May 2015. [Google Scholar]
- Vitale-Brovarone, C.; Pontiroli, L.; Novajra, G.; Tcacencu, I.; Reis, J.; Manca, A. Spine-Ghost: A New Bioactive Cement for Vertebroplasty. Key Eng. Mater. 2014, 631, 43–47. [Google Scholar] [CrossRef]
- Dadkhah, M.; Pontiroli, L.; Fiorilli, S.; Manca, A.; Tallia, F.; Tcacencu, I.; Vitale-Brovarone, C. Preparation and characterisation of an innovative injectable calcium sulphate based bone cement for vertebroplasty application. J. Mater. Chem. B 2017, 5, 102–115. [Google Scholar] [CrossRef]
- Zhu, Y.; Zhang, Y.; Wu, C.; Fang, Y.; Yang, J.; Wang, S.J.M.; Materials, M. The effect of zirconium incorporation on the physiochemical and biological properties of mesoporous bioactive glasses scaffolds. Microporous Mesoporous Mater. 2011, 143, 311–319. [Google Scholar] [CrossRef]
- Zhu, Y.; Li, X.; Yang, J.; Wang, S.; Gao, H.; Hanagata, N. Composition–structure–property relationships of the CaO–M x O y–SiO 2–P 2 O 5 (M = Zr, Mg, Sr) mesoporous bioactive glass (MBG) scaffolds. J. Mater. Chem. 2011, 21, 9208–9218. [Google Scholar] [CrossRef]
- Miola, M.; Gerbaldo, R.; Laviano, F.; Bruno, M.; Vernè, E. Multifunctional ferrimagnetic glass–ceramic for the treatment of bone tumor and associated complications. J. Mater. Sci. 2017, 52, 9192–9201. [Google Scholar] [CrossRef]
- Kargozar, S.; Montazerian, M.; Hamzehlou, S.; Kim, H.-W.; Baino, F.J.A.B. Mesoporous bioactive glasses: Promising platforms for antibacterial strategies. Acta Biomater. 2018, 81, 1–19. [Google Scholar] [CrossRef] [PubMed]
- Migneco, C.; Fiume, E.; Verné, E.; Baino, F.J.N. A guided walk through the world of mesoporous bioactive glasses (MBGs): Fundamentals, processing, and applications. Nanomaterials 2020, 10, 2571. [Google Scholar] [CrossRef] [PubMed]
- Hernández-Rivera, M.; Kumar, I.; Cho, S.Y.; Cheong, B.Y.; Pulikkathara, M.X.; Moghaddam, S.E.; Whitmire, K.H.; Wilson, L.J. High-performance hybrid bismuth–carbon nanotube based contrast agent for X-ray CT imaging. ACS Appl. Mater. Interfaces 2017, 9, 5709–5716. [Google Scholar] [CrossRef] [PubMed]
- Robison, L.; Zhang, L.; Drout, R.J.; Li, P.; Haney, C.R.; Brikha, A.; Noh, H.; Mehdi, B.L.; Browning, N.D.; Dravid, V.P. A bismuth metal–organic framework as a contrast agent for X-ray computed tomography. ACS Appl. Bio Mater. 2019, 2, 1197–1203. [Google Scholar] [CrossRef]
- Montazerian, M.; Zanotto, E.D.; Mauro, J.C. Model-driven design of bioactive glasses: From molecular dynamics through machine learning. Int. Mater. Rev. 2020, 65, 297–321. [Google Scholar] [CrossRef]





| Material | Composition | Radiopacifying | Company | Ref. |
|---|---|---|---|---|
| ProRoot MTA | Portland cement 75% Calcium sulfate dihydrate 5% Bismuth oxide 20% | Bismuth oxide | Dentsply Tulsa Dental, Tulsa, OK, USA | [48] |
| RetroMTA | Calcium Carbonate 60–80% Silicon dioxide 5–15% Aluminum oxide 5–10% Calcium zirconia complex 20–30% | Zirconia complex | BioMTA, Seoul, Korea | [48] |
| BioMTA | Powder: Calcium carbonate, silicon dioxide, aluminum oxide, and calcium zirconia complex. Liquid: Distilled water | Zirconia complex | Intradent, Belém, PA, Brazil | [49] |
| MTA Angelus | Powder: silicon dioxide, potassium oxide, aluminum oxide, sodium oxide, ferric oxide, sulfur trioxide, calcium oxide, bismuth oxide, magnesium oxide. Insoluble residues of calcium oxide, potassium sulfate, sodium sulfate, and crystalline silica. Liquid: distilled water. | Bismuth oxide | Angelus, Londrina, PR, Brazil | [49] |
| Endosequence BC Sealer | Zirconium oxide, calcium silicates, calcium phosphate monobasic, calcium hydroxide, filler, and thickening agents | Zirconium oxide | Brasseler, Savannah, GA, USA | [50] |
| Total Fill BC sealer | Zirconium oxide (35–45%), tricalcium silicate (20–35%), dicalcium silicate (7–15%), and calcium hydroxide (1–4%) | Zirconium oxide | FKG Dentaire, Switzerland | [51] |
| AH Plus | Epoxy paste: diepoxy, calcium tungstate, zirconium oxide, aerosol, and dye. Amine paste: 1-adamantane amine, N.N’dibenzy l-5 oxanonandiamine-1,9, TCD-diamine, calcium tungstate, zirconium oxide, aerosol, and silicon oil. | Zirconium oxide | Dentsply De Trey Gmbh, Konstanz, Germany | [50] |
| Ceramir® Bioceramic Implant Cement QuikCap | Polyacrylic acid (<10%) Strontium fluoride (<5%) Tartaric acid (<5%) | Strontium fluoride | Doxa Dental AB, Sweden | [24] |
| Surefil one | Aluminum-phosphor-strontium-sodium-fluoro-silicate glass, water, highly dispersed silicon dioxide, acrylic acid, polycarboxylic acid (MOPOS), ytterbium fluoride, bifunctional acrylate (BADEP), self-cure initiator, iron oxide pigments, barium sulfate pigment, manganese pigment, camphorquinone, stabilizer | Glass Ytterbium fluoride Barium sulfate | Dentsply Sirona, Konstanz, Germany | [52] |
| Fuji IX GP Fast | Aluminofluorosilicate glass, polyacrylic acid, distilled water, poly carboxylic acid | Glass | GC Corporation, Tokyo, Japan | [53] |
| Ketac™ Molar Quick Aplicap™ | Al-Ca-La fluorosilicate glass, 5% copolymer acid (acrylic and maleic acid), Polyalkenoic acid, tartaric acid, water | Glass | 3M ESPE, Deutschland, Germany | [54] |
| Radiopacifying Agents (Element/Compounds) | Proportion Used | Host Bioceramic | Radiopacity | Ref. | |
|---|---|---|---|---|---|
| Bi | Bi2O3 | 20% | Calcium silicate cement | 5.78 ± 0.5 mmAl | [83] |
| Bi-doped | 0.1 mol | Baghdadite | Increased by 33% | [85] | |
| Bi2O3 | 15–25% | MTA cement | 4.3 to 6.0 mmAl | [91] | |
| Bi2(Al2O4)3 | 9–15% | Calcium phosphate cement | 1.86 to 2.88 mmAl | [92] | |
| Zr | ZrO2 | 30% | Calcium silicate cement | 5.94 ± 0.9 mmAl | [83] |
| 20–40% | Calcium phosphate cement | 1.5 to 2.5 mmAl | [93] | ||
| ZrO2 short fiber | 2–8% | Calcium phosphate cement | Increased by 12% | [94] | |
| Bi1.8Zr0.2O3.1 | 0.2 mol | MTA | 5.57 ± 0.28 mmAl | [95] | |
| Sr | Sr-doped | 8.2–24.6% | Magnesium phosphate scaffolds | 1.2 to 2.0 mmAl | [96] |
| 1.10–2.21% | Tricalcium phosphate cement | 2.0 to 3.0 mmAl | [97] | ||
| 10% | Tricalcium silicate cement | Increased by 25% | [85] | ||
| Ba | BaSO4 | 20% | Portland cement | 2.35 ± 0.08 mmAl | [80] |
| 25% | 3.5 mmAl | [98] | |||
| W | CaWO4 | 30% | Calcium silicate cement | 5.67 ± 0.5 mmAl | [83] |
| 10–30% | Calcium silicate particles | 3.24 to 3.85 mmAl | [87] | ||
| Fe | Fe2O3 | 20–60% | HAp | Increased up to 38% | [86] |
| Yb | Yb2O3 | 30% | Calcium silicate cement | 5.02 ± 0.43 mmAl | [89] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Montazerian, M.; Gonçalves, G.V.S.; Barreto, M.E.V.; Lima, E.P.N.; Cerqueira, G.R.C.; Sousa, J.A.; Malek Khachatourian, A.; Souza, M.K.S.; Silva, S.M.L.; Fook, M.V.L.; et al. Radiopaque Crystalline, Non-Crystalline and Nanostructured Bioceramics. Materials 2022, 15, 7477. https://doi.org/10.3390/ma15217477
Montazerian M, Gonçalves GVS, Barreto MEV, Lima EPN, Cerqueira GRC, Sousa JA, Malek Khachatourian A, Souza MKS, Silva SML, Fook MVL, et al. Radiopaque Crystalline, Non-Crystalline and Nanostructured Bioceramics. Materials. 2022; 15(21):7477. https://doi.org/10.3390/ma15217477
Chicago/Turabian StyleMontazerian, Maziar, Geovanna V. S. Gonçalves, Maria E. V. Barreto, Eunice P. N. Lima, Glauber R. C. Cerqueira, Julyana A. Sousa, Adrine Malek Khachatourian, Mairly K. S. Souza, Suédina M. L. Silva, Marcus V. L. Fook, and et al. 2022. "Radiopaque Crystalline, Non-Crystalline and Nanostructured Bioceramics" Materials 15, no. 21: 7477. https://doi.org/10.3390/ma15217477
APA StyleMontazerian, M., Gonçalves, G. V. S., Barreto, M. E. V., Lima, E. P. N., Cerqueira, G. R. C., Sousa, J. A., Malek Khachatourian, A., Souza, M. K. S., Silva, S. M. L., Fook, M. V. L., & Baino, F. (2022). Radiopaque Crystalline, Non-Crystalline and Nanostructured Bioceramics. Materials, 15(21), 7477. https://doi.org/10.3390/ma15217477










