Effect of Precursors on the Electrochemical Properties of Mixed RuOx/MnOx Electrodes Prepared by Thermal Decomposition
Abstract
1. Introduction
2. Materials and Methods
2.1. Chemicals and Materials
2.2. Electrode Preparation
2.3. Electrode Characterization
3. Results
3.1. Surface Morphology
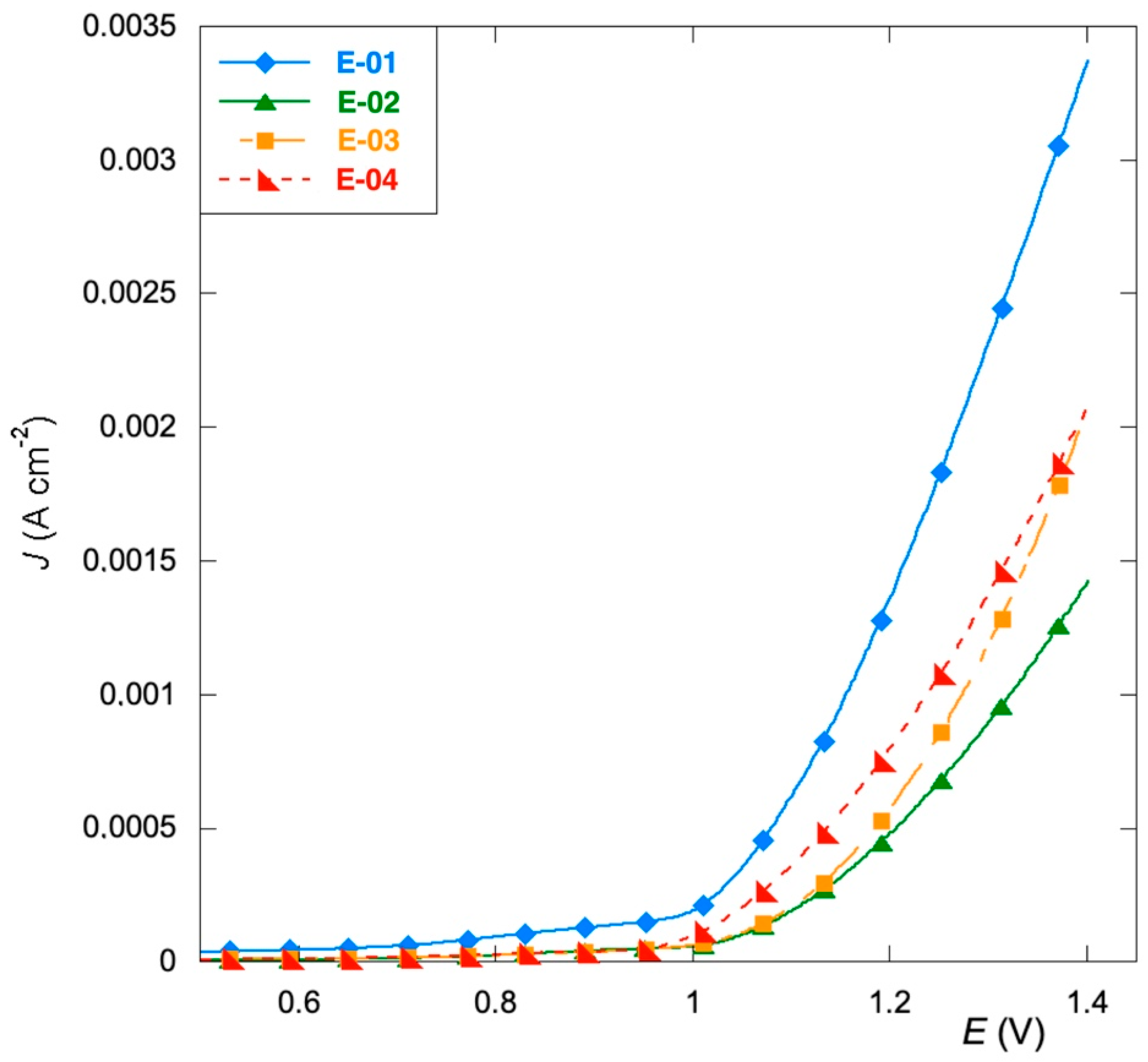
3.2. Corrosion Tests
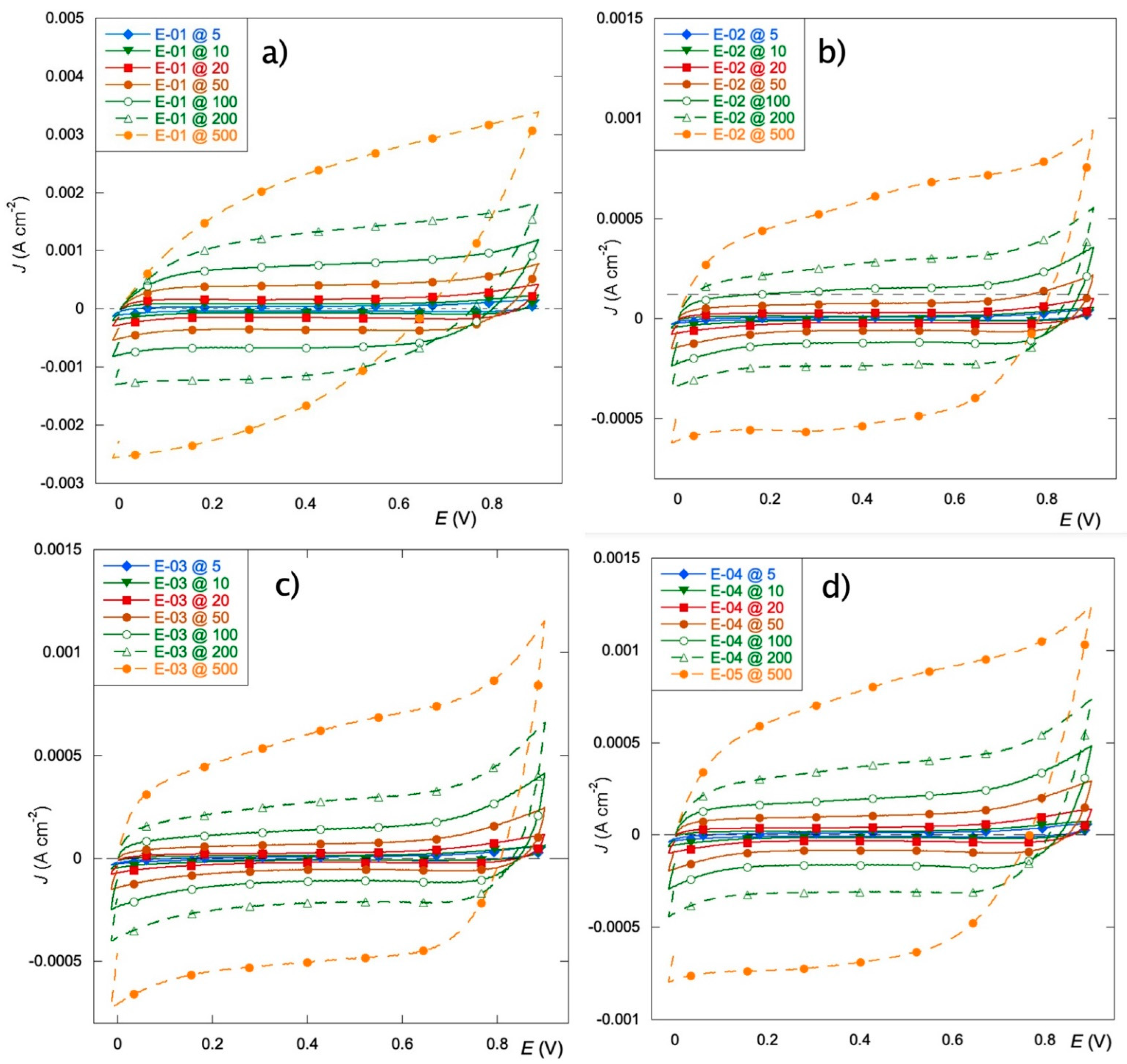
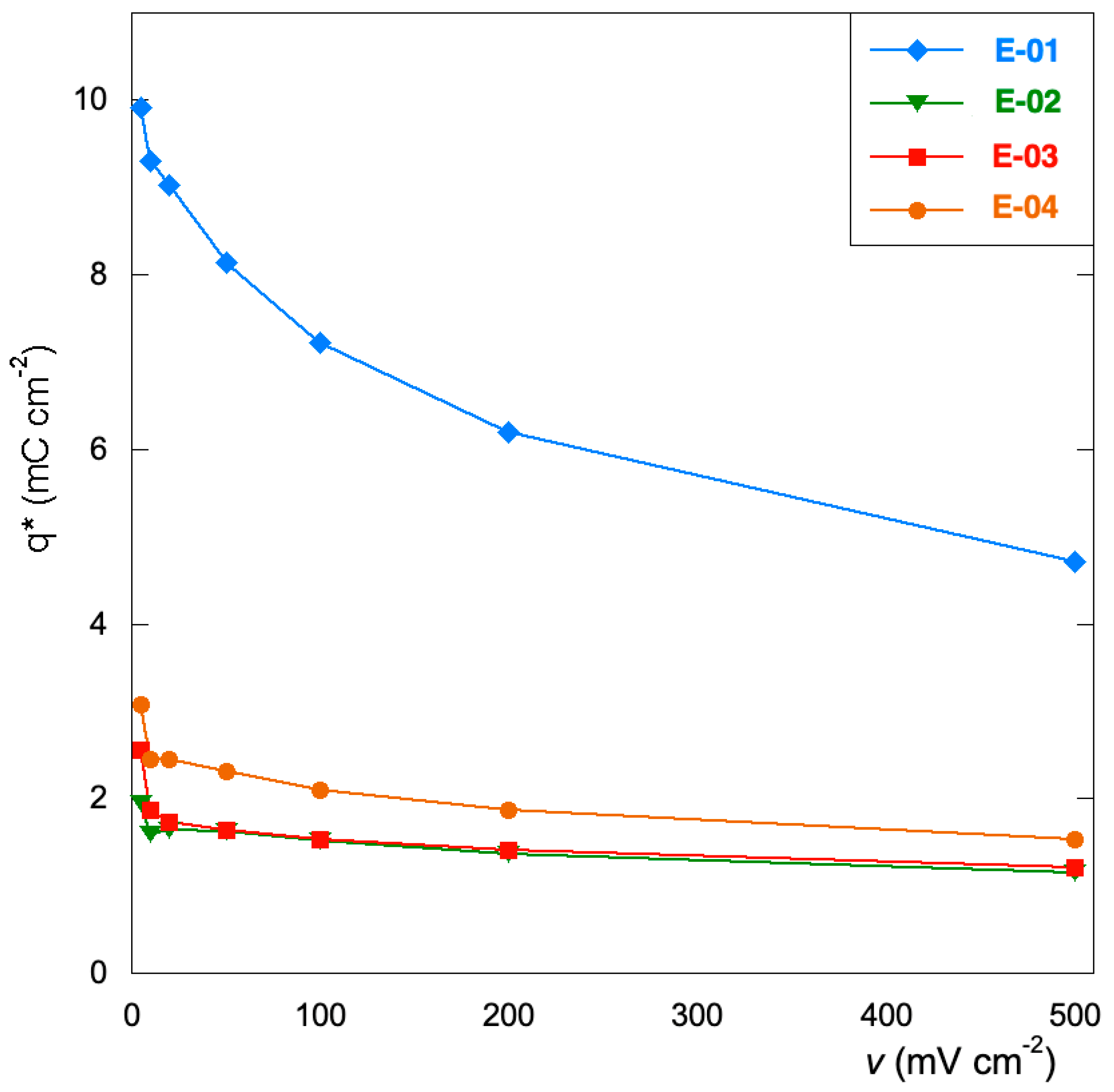
3.3. Voltammetric Tests
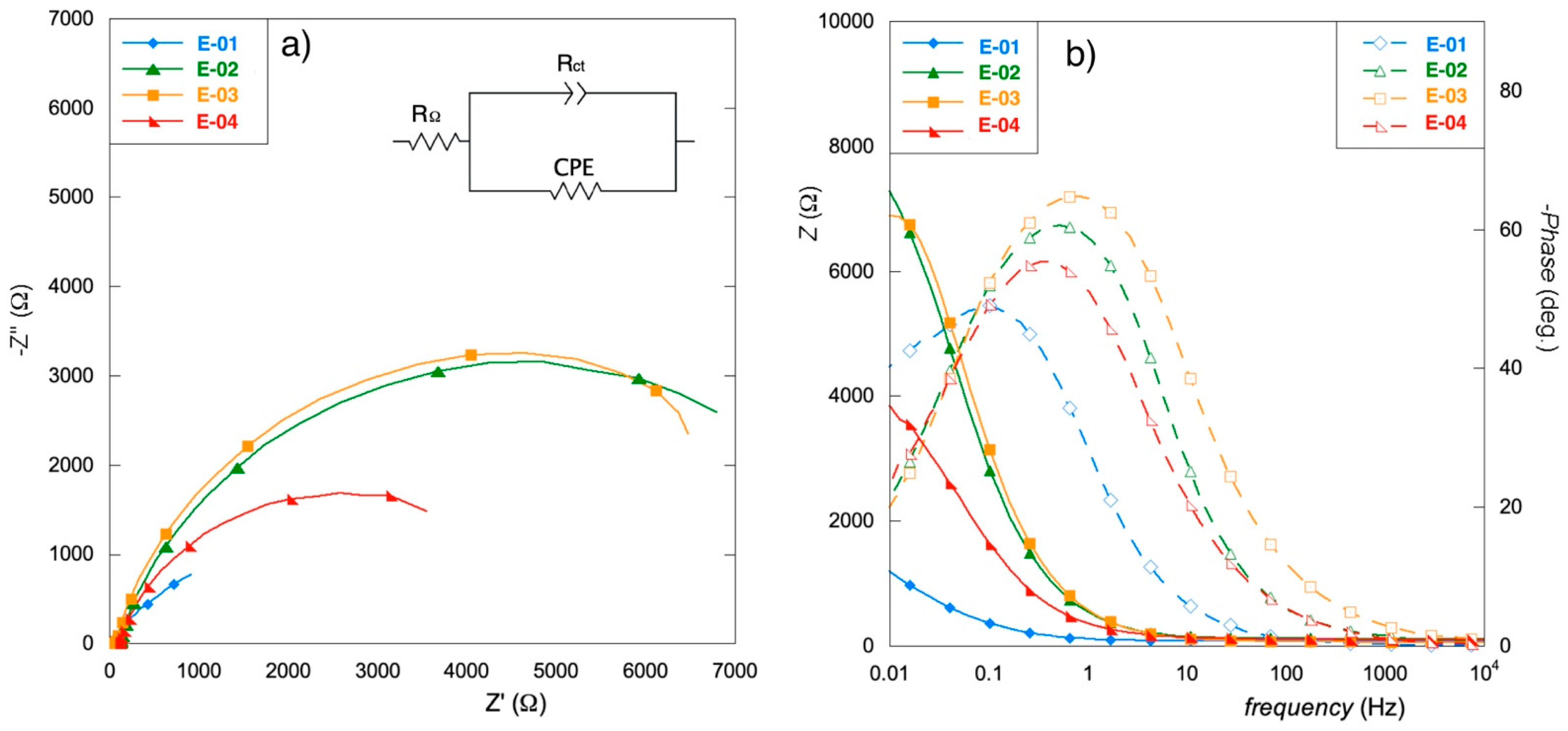
3.4. EIS Analysis
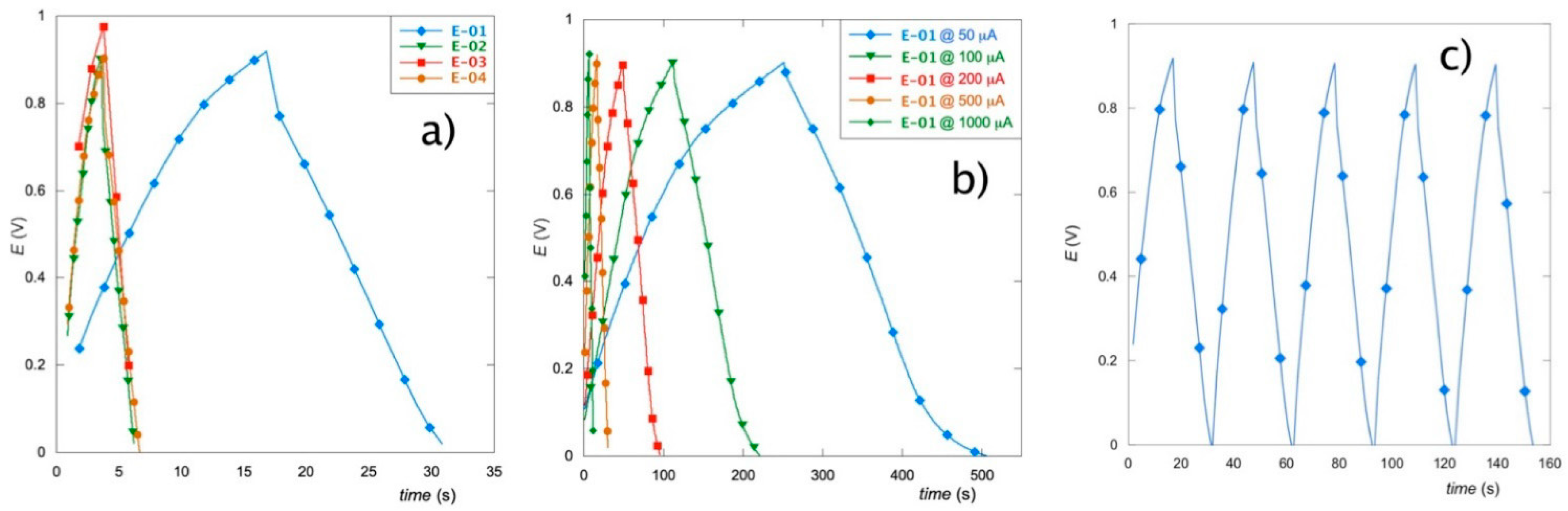
3.5. Galvanostatic Charge–Discharge Tests
4. Conclusions
- -
- The Ru inorganic precursor (E-01) promotes the growth of a thinner and less compact film with a larger specific surface area. This provides a higher amount of electrochemically active sites for OER and an enhancement of the capacitive behavior with a more remarkable tendency for charge storage. The replacement of Mn(NO3)2 with Mn(acac)3 does not affect the viability of the process as the price of the two salts are quite comparable
- -
- The replacement of RuCl3 with organic precursors improves the corrosion resistance and shifts the oxidation potential of the aqueous electrolyte to a more positive potential, thus promoting the processes based on chlorine evolution.
- -
- The electrodes obtained from only organic precursors (E-02 and E-03) exhibit thicker coatings that suffer from slow OCP stabilization but present the best charge distribution.
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Lokhande, C.D.; Dubal, D.P.; Joo, O.-S. Metal oxide thin film based supercapacitors. Curr. Appl. Phys. 2011, 11, 255–270. [Google Scholar] [CrossRef]
- Yu, M.; Feng, X. Thin-Film Electrode-Based Supercapacitors. Joule 2019, 3, 338–360. [Google Scholar] [CrossRef]
- Moumen, A.; Kumarage, G.C.W.; Comini, E. P-Type Metal Oxide Semiconductor Thin Films: Synthesis and Chemical Sensor Applications. Sensors 2022, 22, 1359. [Google Scholar] [CrossRef]
- Krishna, K.G.; Parne, S.; Pothukanuri, N.; Kathirvelu, V.; Gandi, S.; Joshi, D. Nanostructured metal oxide semiconductor-based gas sensors: A comprehensive review. Sens. Actuators A Phys. 2022, 341, 113578. [Google Scholar] [CrossRef]
- De Santis, S.; Sotgiu, G.; Porcelli, F.; Marsotto, M.; Iucci, G.; Orsini, M. A Simple Cerium Coating Strategy for Titanium Oxide Nano-tubes’ Bioactivity Enhancement. Nanomaterials 2021, 11, 445. [Google Scholar] [CrossRef]
- Battocchio, C.; Concolato, S.; De Santis, S.; Fahlman, M.; Iucci, G.; Santi, M.; Sotgiu, G.; Orsini, M. Chitosan functionalization of titanium and Ti6Al4V alloy with chloroacetic acid as linker agent. Mater. Sci. Eng. C 2019, 99, 1133–1140. [Google Scholar] [CrossRef]
- Ros, C.; Andreu, T.; Morante, J.R. Photoelectrochemical water splitting: A road from stable metal oxides to protected thin film solar cells. J. Mater. Chem. A 2020, 8, 10625–10669. [Google Scholar] [CrossRef]
- Kalinina, E.; Pikalova, E. Opportunities, challenges and prospects for electrodeposition of thin-film functional layers in solid oxide fuel cell technology. Materials 2021, 14, 5584. [Google Scholar] [CrossRef]
- Li, W.; Zhang, H.; Wang, J.; Qiao, W.; Ling, L.; Long, D. Flexible Ru/Graphene Aerogel with Switchable Surface Chemistry: Highly Efficient Catalyst for Room-Temperature CO Oxidation. Adv. Mater. Interfaces 2016, 3, 1500711. [Google Scholar] [CrossRef]
- Zan, G.; Wu, T.; Zhu, F.; He, P.; Cheng, Y.; Chai, S.; Wang, Y.; Huang, X.; Zhang, W.; Wan, Y.; et al. A biomimetic conductive super-foldable material. Matter 2021, 4, 3232–3247. [Google Scholar] [CrossRef]
- Zan, G.; Wu, T.; Dong, W.; Zhou, J.; Tu, T.; Xu, R.; Chen, Y.; Wang, Y.; Wu, Q. Two-Level Biomimetic Designs Enable Intelligent Stress Dispersion for Super-Foldable C/NiS Nanofiber Free-Standing Electrode. Adv. Fiber Mater. 2022, 4, 1177–1190. [Google Scholar] [CrossRef]
- Eda, G.; Fanchini, G.; Chhowalla, M. Large-area ultrathin films of reduced graphene oxide as a transparent and flexible electronic material. Nat. Nanotechnol. 2008, 3, 270–274. [Google Scholar] [CrossRef]
- Zhang, C.; Ji, C.; Park, Y.-B.; Guo, L.J. Thin-Metal-Film-Based Transparent Conductors: Material Preparation, Optical Design, and Device Applications. Adv. Opt. Mater. 2021, 9, 2001298. [Google Scholar] [CrossRef]
- Kumari, N.; Haider, M.A.; Anjum, U.; Basu, S. Identifying operating mechanism in the electrochemical reduction of CO2 on thin-film praseodymium-doped ceria electrodes. Ionics 2020, 26, 5673–5684. [Google Scholar] [CrossRef]
- Linge, J.M.; Erikson, H.; Kasikov, A.; Rähn, M.; Sammelselg, V.; Tammeveski, K. Oxygen reduction reaction on thin-film Ag electrodes in alkaline solution. Electrochim. Acta 2019, 325, 134922. [Google Scholar] [CrossRef]
- El-Kacemi, S.; Zazou, H.; Oturan, N.; Dietze, M.; Hamdani, M.; Es-Souni, M.; Oturan, M.A. Nanostructured ZnO-TiO2 thin film oxide as anode material in electrooxidation of organic pollutants. Application to the removal of dye Amido black 10B from water. Environ. Sci. Pollut. Res. 2017, 24, 1442–1449. [Google Scholar] [CrossRef]
- Tsin, F.; Pensel, A.; Vigneron, J.; Gonçalvès, A.-M.; Peulon, S. Innovative wastewater treatment based on electrodeposited thin film: Systematic studies of interfacial processes between birnessite and Mn(II) for a better efficiency. Appl. Surf. Sci. 2020, 525, 146555. [Google Scholar] [CrossRef]
- Ponchio, C.; Srevarit, W. Photoelectrocatalytic improvement of copper oxide thin film fabricated using anodization strategy application in nitrite degradation and promoting oxygen evolution. Chem. Pap. 2021, 75, 1123–1132. [Google Scholar] [CrossRef]
- Habibi, B.; Pashazadeh, A.; Pashazadeh, S.; Ali Saghatforoush, L. Electrocatalytic oxidation and determination of hydrazine in alkaline medium through in situ conversion thin film nanostructured modified carbon ceramic electrode. J. Electroanal. Chem. 2022, 907, 116038. [Google Scholar] [CrossRef]
- Amaterz, E.; Bouddouch, A.; Tara, A.; Taoufyq, A.; Bakiz, B.; Benlhachemi, A.; Jbara, O. Electrochemical degradation of Bisphenol A using electrodeposited SrHPO4 thin films. Nanotechnol. Environ. Eng. 2021, 6, 18. [Google Scholar] [CrossRef]
- Brüger, A.; Fafilek, G.; Neumann-Spallart, M. Treatment of cyanide: Photoelectrocatalytic degradation using TiO2 thin film electrodes and influence of volatilization. Sol. Energy 2020, 205, 74–78. [Google Scholar] [CrossRef]
- Petrucci, E.; Porcelli, F.; Orsini, M.; De Santis, S.; Sotgiu, G. Mixed Oxide Electrodes Based on Ruthenium and Copper: Electrochemical Properties as a Function of the Composition and Method of Manufacture. Metals 2022, 12, 316. [Google Scholar] [CrossRef]
- Sotgiu, G.; Orsini, M.; de Santis, S.; Porcelli, F.; Petrucci, E. Effect on durability and electrochemical response of the addition of a non-noble transition metal in mixed ruthenium and manganese oxide thin-film electrodes. Chem. Eng. Trans. 2021, 86, 1393–1398. [Google Scholar] [CrossRef]
- Petrucci, E.; Orsini, M.; Porcelli, F.; De Santis, S.; Sotgiu, G. Effect of spin coating parameters on the electrochemical properties of Ruthenium Oxide thin films. Electrochem 2022, 2, 83–94. [Google Scholar] [CrossRef]
- Sotgiu, G.; Tortora, L.; Petrucci, E. Influence of surface roughening of Titanium substrate in the electrochemical activity of Manganese oxide thin film electrode in anodic oxidation of dye-containing solutions. J. Appl. Electrochem. 2015, 45, 787–797. [Google Scholar] [CrossRef]
- Sotgiu, G.; Orsini, M.; Porcelli, F.; de Santis, S.; Petrucci, E. Wettability of micro and nanostructured surface of titanium based electrodes: Influence of chemical and electrochemical etching. Chem. Eng. Trans. 2021, 86, 1417–1422. [Google Scholar] [CrossRef]
- Petrucci, E.; Montanaro, D.; Orsini, M.; Sotgiu, G. Micro- and nanostructured TiO2 substrate: Influence on the electrocatalytic properties of manganese oxide based electrodes. J. Electroanal. Chem. 2018, 808, 380–386. [Google Scholar] [CrossRef]
- Trasatti, S.; Buzzanca, G. Ruthenium dioxide: A new interesting electrode material. Solid state structure and electrochemical behaviour. J. Electroanal. Chem. 1971, 29, A1–A5. [Google Scholar] [CrossRef]
- Kore, R.M.; Ghadge, T.S.; Ambare, R.C.; Lokhande, B.J. Effect of different nickel precursors on capacitive behavior of electrodeposited NiO thin films. AIP Conf. Proc. 2016, 1724, 20086. [Google Scholar] [CrossRef]
- Andrade, L.S.; Rocha-Filho, R.C.; Bocchi, N.; Biaggio, S.R. Study of the effect of precursor salts on the electrocatalytic properties of Ti-SnO2/Sb electrodes prepared by thermal decomposition [Estudo de efeito dos sais precursores sobre as propriedades eletrocatalíticas de eletrodos de Ti-SnO2/Sb preparados por decomposição térmica]. Quim. Nova 2004, 27, 866–872. [Google Scholar] [CrossRef]
- Ardizzone, S.; Falciola, M.; Trasatti, S. Effect of the Nature of the Precursor on the Electrocatalytic Properties of Thermally Prepared Ruthenium Oxide. J. Electrochem. Soc. 1989, 136, 1545–1550. [Google Scholar] [CrossRef]
- Fachinotti, E.; Guerrini, E.; Tavares, A.C.; Trasatti, S. Electrocatalysis of H2 evolution by thermally prepared ruthenium oxide. Effect of precursors: Nitrate vs. chloride. J. Electroanal. Chem. 2007, 600, 103–112. [Google Scholar] [CrossRef]
- Malmgren, C.; Eriksson, A.K.; Cornell, A.; Bäckström, J.; Eriksson, S.; Olin, H. Nanocrystallinity in RuO2 coatings-Influence of precursor and preparation temperature. Thin Solid Film. 2010, 518, 3615–3618. [Google Scholar] [CrossRef]
- Wen, J.; Ruan, X.; Zhou, Z. Preparation and electrochemical performance of novel ruthenium-manganese oxide electrode materials for electrochemical capacitors. J. Phys. Chem. Solids 2009, 70, 816–820. [Google Scholar] [CrossRef]
- Lei, J.; Chen, X. RuO2/MnO2 composite materials for high-performance supercapacitor electrodes. J. Semicond. 2015, 36, 083006. [Google Scholar] [CrossRef]
- Liu, X.; Pickup, P.G. Carbon fabric supported manganese and ruthenium oxide thin films for supercapacitors. J. Electrochem. Soc. 2011, 158, A241–A249. [Google Scholar] [CrossRef]
- Annamalai, K.P.; Zheng, X.; Gao, J.; Chen, T.; Tao, Y. Nanoporous ruthenium and manganese oxide nanoparticles/reduced graphene oxide for high-energy symmetric supercapacitors. Carbon 2019, 144, 185–192. [Google Scholar] [CrossRef]
- Cao, K.; Jin, T.; Yang, L.; Jiao, L. Recent progress in conversion reaction metal oxide anodes for Li-ion batteries. Mater. Chem. Front. 2017, 1, 2213–2242. [Google Scholar] [CrossRef]
- Sotgiu, G.; Montanaro, D.; Orsini, M.; Petrucci, E. Manganese-containing mixed oxide electrodes as anode materials for degradation of model organic pollutants. Chem. Eng. Trans. 2017, 57, 1639–1644. [Google Scholar] [CrossRef]
- Browne, M.P.; Nolan, H.; Duesberg, G.S.; Colavita, P.E.; Lyons, M.E.G. Low-Overpotential High-Activity Mixed Manganese and Ruthenium Oxide Electrocatalysts for Oxygen Evolution Reaction in Alkaline Media. ACS Catal. 2016, 6, 2408–2415. [Google Scholar] [CrossRef]
- Fernández, J.L.; De Chialvo, M.R.G.; Chialvo, A.C. Preparation and electrochemical characterization of Ti/RuxMn1-xO2 electrodes. J. Appl. Electrochem. 2002, 32, 513–520. [Google Scholar] [CrossRef]
- Aziz, M.A.; El-Madkhoum, A.; Hakeem, A.S.; Shaikh, M.N.; Rehman, A.U.; Yamani, Z.H. Effect of Mn precursors on the morphology and electrocatalytic activity toward water oxidation of micro-nanostructured MnOx films prepared by voltammetric deposition. J. Mater. Sci. Mater. Electron. 2017, 28, 18463–18473. [Google Scholar] [CrossRef]
- Park, H.H.; Park, I.S.; Kim, K.S.; Jeon, W.Y.; Park, B.K.; Kim, H.S.; Bae, T.S.; Lee, M.H. Bioactive and electrochemical characterization of TiO2 nanotubes on titanium via anodic oxidation. Electrochim. Acta 2010, 55, 6109–6114. [Google Scholar] [CrossRef]
- El-Sayed, A.R.; Mohran, H.S.; Shilkamy, H.A.S. Role of Indium Alloying with Lead as a Means to Reduce the Passivation Phenomena in Lead/Acid Batteries. Int. J. Electrochem. 2014, 932654. [Google Scholar] [CrossRef]
- Iqbal, M.N.; Abdel-Magied, A.F.; Abdelhamid, H.N.; Olsén, P.; Shatskiy, A.; Zou, X.; Åkermark, B.; Kärkäs, M.D.; Johnston, E.V. Mesoporous Ruthenium Oxide: A Heterogeneous Catalyst for Water Oxidation. ACS Sustain. Chem. Eng. 2017, 5, 9651–9656. [Google Scholar] [CrossRef]
- Duan, Y.; Gao, F.; Teplyakov, A.V. Role of the deposition precursor molecules in defining oxidation state of deposited copper in surface reduction reactions on H-terminated Si(111) Surface. J. Phys. Chem. C 2015, 119, 27018–27027. [Google Scholar] [CrossRef]
- Gao, L.; Cui, X.; Sewell, C.D.; Li, J.; Lin, Z. Recent advances in activating surface reconstruction for the high-efficiency oxygen evolution reaction. Chem. Soc. Rev. 2021, 50, 8428–8569. [Google Scholar] [CrossRef]
- Thulasi, K.M.; Manikkoth, S.T.; Paravannoor, A.; Palantavida, S.; Bhagiyalakshmi, M.; Vijayan, B.K. Ceria deposited titania nanotubes for high performance supercapacitors. J. Phys. Chem. Solids 2019, 135, 109111. [Google Scholar] [CrossRef]
- Dawoud, H.D.; Al Tahtamouni, T.; Bensalah, N. Sputtered manganese oxide thin film on carbon nanotubes sheet as a flexible and binder-free electrode for supercapacitors. Int. J. Energy Res. 2019, 43, 1245–1254. [Google Scholar] [CrossRef]
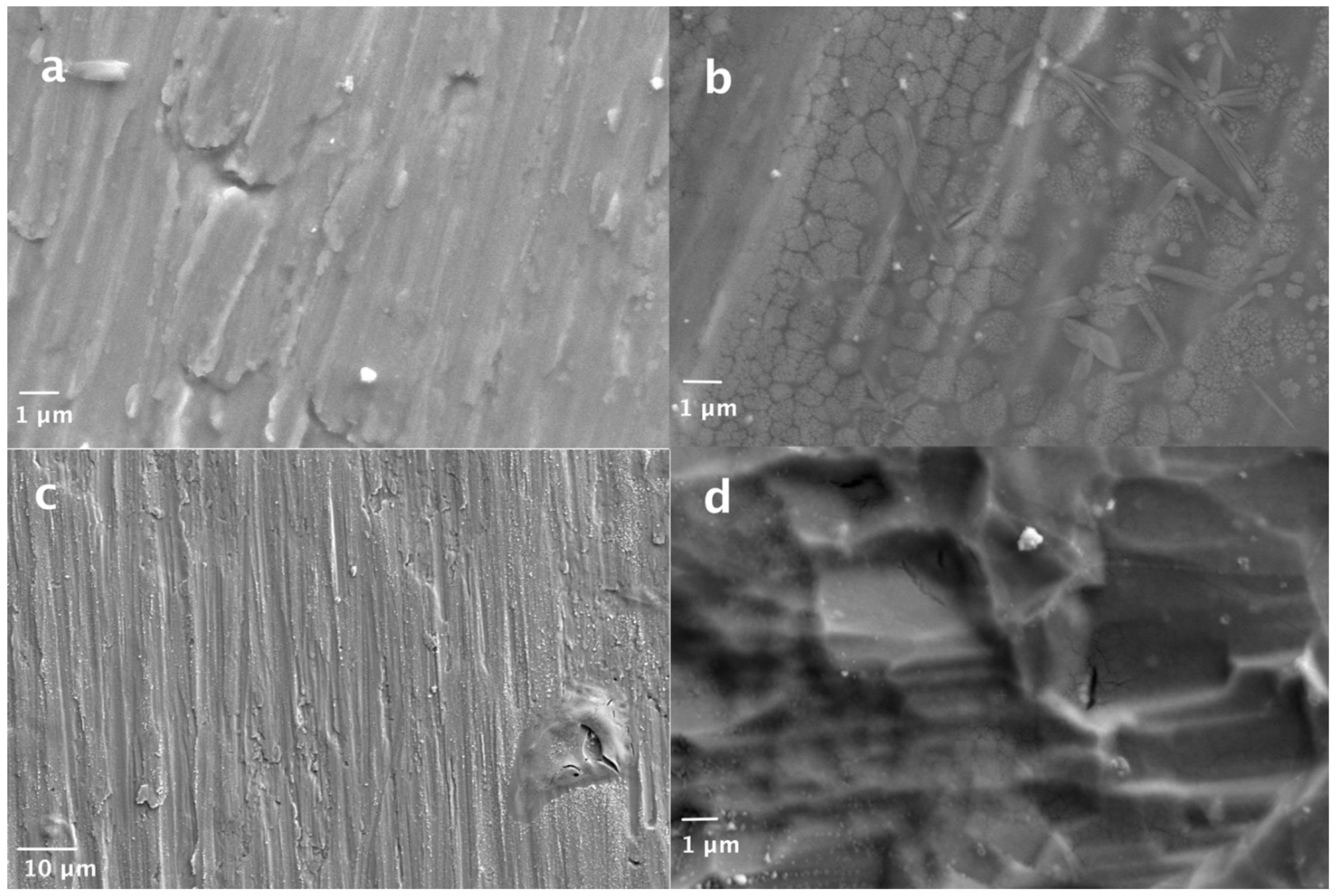

| Item | Surface Finish | RuCl3 | Ru(acac)3 | Mn(NO3)2 | Mn(acac)3 | OCP (V) | Ecorr (V) | J0 (mA cm−2) |
|---|---|---|---|---|---|---|---|---|
| E-01 | smooth | X | X | 0.428 | −0.096 | 6.55 | ||
| E-02 | smooth | X | X | 0.453 | −0.013 | 2.02 | ||
| E-03 | etched | X | X | 0.474 | 0.024 | 1.47 | ||
| E-04 | smooth | X | X | 0.528 | −0.036 | 3.06 |
| E-01 | E-02 | E-03 | E-04 | |
|---|---|---|---|---|
| RΩ (Ω) | 81.1 | 117.0 | 95.0 | 107.0 |
| RCT (kΩ) | 2.3 | 8.4 | 8.6 | 4.9 |
| Q0 (mMho sn) | 4.30 | 0.45 | 0.40 | 0.78 |
| n | 0.74 | 0.81 | 0.81 | 0.76 |
| C (mF) | 9.43 | 0.61 | 0.53 | 1.18 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Petrucci, E.; Porcelli, F.; Orsini, M.; De Santis, S.; Sotgiu, G. Effect of Precursors on the Electrochemical Properties of Mixed RuOx/MnOx Electrodes Prepared by Thermal Decomposition. Materials 2022, 15, 7489. https://doi.org/10.3390/ma15217489
Petrucci E, Porcelli F, Orsini M, De Santis S, Sotgiu G. Effect of Precursors on the Electrochemical Properties of Mixed RuOx/MnOx Electrodes Prepared by Thermal Decomposition. Materials. 2022; 15(21):7489. https://doi.org/10.3390/ma15217489
Chicago/Turabian StylePetrucci, Elisabetta, Francesco Porcelli, Monica Orsini, Serena De Santis, and Giovanni Sotgiu. 2022. "Effect of Precursors on the Electrochemical Properties of Mixed RuOx/MnOx Electrodes Prepared by Thermal Decomposition" Materials 15, no. 21: 7489. https://doi.org/10.3390/ma15217489
APA StylePetrucci, E., Porcelli, F., Orsini, M., De Santis, S., & Sotgiu, G. (2022). Effect of Precursors on the Electrochemical Properties of Mixed RuOx/MnOx Electrodes Prepared by Thermal Decomposition. Materials, 15(21), 7489. https://doi.org/10.3390/ma15217489










