Excessive Dental Bleaching with 22% Carbamide Peroxide Combined with Erosive and Abrasive Challenges: New Insights into the Morphology and Surface Properties of Enamel
Abstract
1. Introduction
2. Materials and Methods
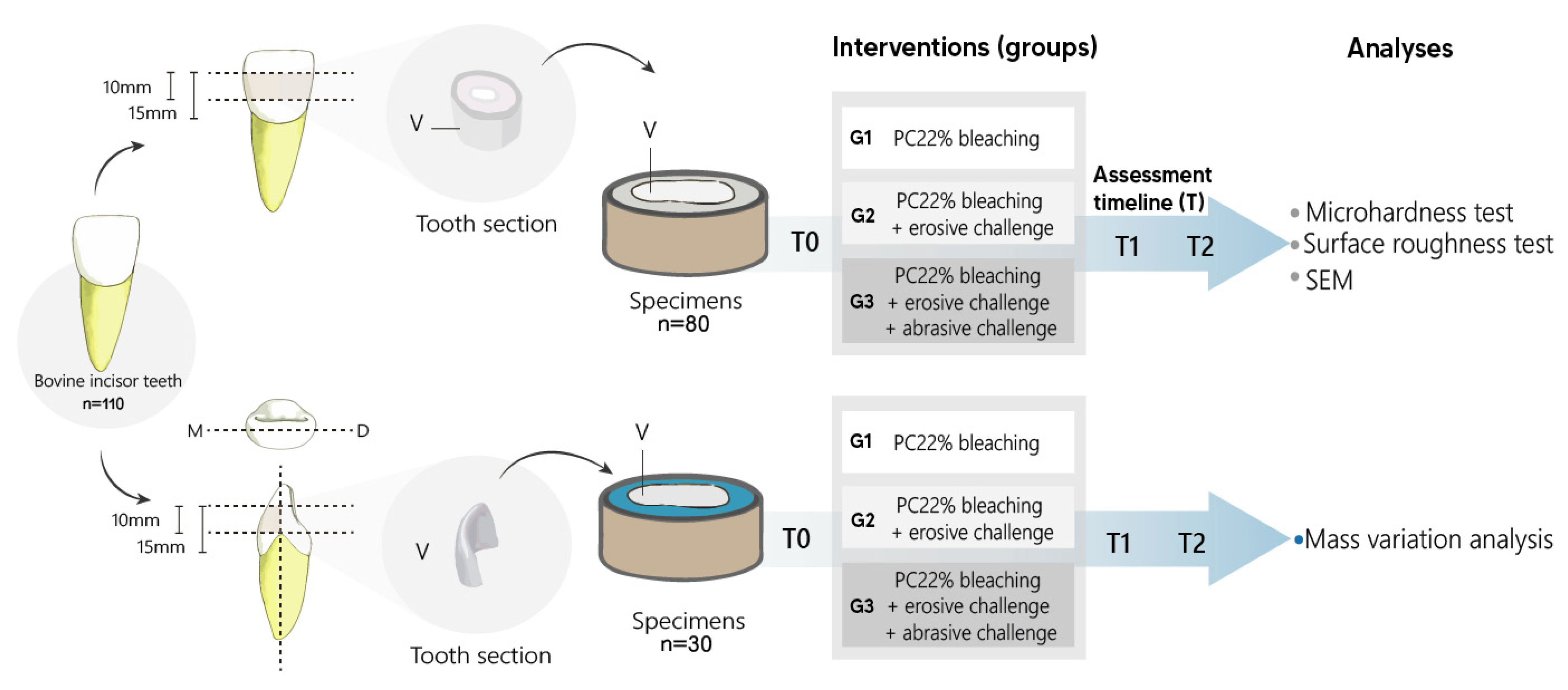
2.1. Ethical Aspects and Sample Definition
2.2. Teeth Specimens
2.3. Tooth Bleaching
2.4. Erosive Challenge
2.5. Abrasive Challenge
2.6. Microhardness and Roughness Tests
2.7. Mass Variation Evaluation
2.8. Ultrastructure Analysis
2.9. Statistical Analysis
3. Results
3.1. The Roughness in the Enamel Surface Was Modified Only in the Erosive and Abrasive Challenge
3.2. The Microhardness Decreased at All Times in the Erosive and Abrasive Challenges, with Lower Values at 28 Days
3.3. Bleaching Alone Decreased the Mass in an Excessive Regimen, While Erosive and Abrasive Challenges Affected the Enamel after 14 Days of Treatment
3.4. Ultrastructural Damage Was More Evident in the Excessive Bleaching Combined with Erosive and Abrasive Challenges Groups
4. Discussion
5. Conclusions
- 1.
- Supervised at-home tooth bleaching with a high-concentration (22% carbamide peroxide) gel did not affect the SR, even when performed excessively. Tooth bleaching with a high-concentration (22% carbamide peroxide) gel negatively affected the MH and mass of the dental enamel only when performed excessively (28 days).
- 2.
- Tooth bleaching combined with exposure to an acidic beverage significantly increased the SR, with a loss of enamel mass and MH.
- 3.
- Excessive tooth bleaching combined with exposure to acidic drinks and tooth brushing exacerbated the deleterious effects on the enamel, qualitatively evidenced by the visual inspection of the enamel ultrastructure.
Author Contributions
Funding
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Pinto, M.M.; De Godoy, C.H.L.; Bortoletto, C.C.; Olivan, S.R.G.; Motta, L.J.; Altavista, O.M.; Bussadori, S.K. Tooth whitening with hydrogen peroxide in adolescents: Study protocol for a randomized controlled trial. Trials 2014, 15, 395–401. [Google Scholar] [CrossRef] [PubMed]
- Alqahtani, M.Q. Tooth-bleaching procedures and their controversial effects: A literature review. Saudi Dent. J. 2014, 26, 33–46. [Google Scholar] [CrossRef] [PubMed]
- Meireles, S.S.; Fontes, S.T.; Coimbra, L.A.; Della Bona, Á.; Demarco, F.F. Effectiveness of different carbamide peroxide concentrations used for tooth bleaching: An in vitro study. J. Appl. Oral Sci. 2012, 20, 186–191. [Google Scholar] [CrossRef] [PubMed]
- Da Rosa, G.; Maran, B.M.; Schmitt, V.L.; Loguercio, A.D.; Reis, A.; Naufel, F.S. Effectiveness of Whitening Strips Use Compared with Supervised Dental Bleaching: A Systematic Review and Meta-analysis. Oper. Dent. 2020, 45, 289–307. [Google Scholar] [CrossRef] [PubMed]
- Carey, C.M. Tooth whitening: What we now know. J. Evid.-Based Dent. Pract. 2014, 14, 70–76. [Google Scholar] [CrossRef] [PubMed]
- Ribeiro, M.S.E.; Santos, H.S.B.; Baia, J.C.P.; Oliveira, R.P.; Souza Júnior, M.H.S.; Loretto, S.C. Influence of prolonged tooth bleaching on enamel mass variation. Int. J. Odontostomat. 2019, 13, 305–309. [Google Scholar] [CrossRef]
- Peskersoy, C.; Tetik, A.; Ozturk, V.O.; Gokay, N. Spectrophotometric and computerized evaluation of tooth bleaching employing 10 different home-bleaching procedures: In-vitro study. Eur. J. Dent. 2014, 8, 538–545. [Google Scholar] [CrossRef]
- Abouassi, T.; Wolkewitz, M.; Hahn, P. Effect of carbamide peroxide and hydrogen peroxide on enamel surface: An in vitro study. Clin. Oral Investig. 2011, 15, 673–680. [Google Scholar] [CrossRef]
- Vilhena, K.F.B.; Nogueira, B.C.L.; Fagundes, N.C.F.; Loretto, S.C.; Angelica, R.S.; Lima, R.R.; Silva e Souza, M.H. Dental enamel bleached for a prolonged and excessive time: Morphological changes. PLoS ONE 2019, 14, e0214948. [Google Scholar] [CrossRef]
- Bodanezi, A.; De Bittencourt, M.E.; Bodanezi, R.V.; Zottis, T.; Munhoz, E.A.; Carlini-Júnior, B. Surface modifications on aesthetically restored teeth following home bleaching with 16% peroxide carbamide. Eur. J. Dent. 2011, 5, 157–162. [Google Scholar] [CrossRef]
- Meireles, S.S.; Santos, I.S.; Bona, A.D.; Demarco, F.F. A double-blind randomized clinical trial of two carbamide peroxide tooth bleaching agents: 2-year follow-up. J. Dent. 2010, 38, 956–963. [Google Scholar] [CrossRef] [PubMed]
- Soares, D.G.; Ribeiro, A.P.; Sacono, N.T.; Coldebella, C.R.; Hebling, J.; De Souza Costa, C.A. Transenamel and transdentinal cytotoxicity of carbamide peroxide bleaching gels on odontoblast-like MDPC-23 cells. Int. Endod. J. 2011, 44, 116–125. [Google Scholar] [CrossRef] [PubMed]
- Santos, L.F.; Torres, C.R.; Caneppele, T.M.; Magalhães, A.C.; Borges, A.B. Effect of home-bleaching gels modified by calcium and/or fluoride and the application of nano-hydroxyapatite paste on in vitro enamel erosion susceptibility. Acta Odontol. Scand. 2016, 74, 121–126. [Google Scholar] [CrossRef] [PubMed]
- Liporoni, P.C.S.; Wan Bakar, W.Z.; Zanatta, R.F.; Ambrosano, G.M.; Aguiar, F.H.B.; Amaechi, B.T. Influence of Erosion/Abrasion and the Dentifrice Abrasiveness Concomitant with Bleaching Procedures. Clin. Cosmet. Investig. Dent. 2020, 12, 101–109. [Google Scholar] [CrossRef]
- Ribeiro, M.E.S.; Lopes, R.M.; Aranha, A.C.C.; Medeiros, I.S.; Lima, R.R.; Souza Júnior, M.H.S.E.; Loretto, S.C. Is prolonged bleaching more harmful to dental enamel than daily dietary and hygienic oral habits? Braz. Oral Res. 2021, 19, 107–113. [Google Scholar] [CrossRef]
- Cavalli, V.; Reis, A.F.; Giannini, M.; Ambrosano, G.M. The effect of time following bleaching on enamel bond strength of resin composite. Oper. Dent. 2001, 26, 597–602. [Google Scholar]
- Soares, L.E.; De Carvalho Filho, A.C. Protective effect of fluoride varnish and fluoride gel on enamel erosion: Roughness, SEM-EDS, and µ-EDXRF studies. Microsc. Res. Tech. 2015, 78, 240–248. [Google Scholar] [CrossRef]
- Jassé, F.F.; de Campos, E.; Lefever, D.; Di Bella, E.; Salomon, J.P.; Krejci, I.; Ardu, S. Influence of filler charge on gloss of composite materials before and after in vitro toothbrushing. J. Dent. 2013, 14, 41–44. [Google Scholar] [CrossRef]
- Ostrowska, A.; Szymański, W.; Kołodziejczyk, L.; Bołtacz, E.R. Evaluation of the Erosive Potential of Selected Isotonic Drinks: In Vitro Studies. Adv. Clin. Exp. Med. 2016, 25, 1313–1319. [Google Scholar] [CrossRef]
- Olivan, S.R.G.; Sfalcin, R.A.; Fernandes, K.P.S.; Ferrari, R.A.M.; Horliana, A.C.R.T.; Motta, L.J.; Bussadori, S.K. Preventive effect of the remineralizing materials on dental erosion lesions by speckle technique: An in vitro analysis. Photodiagn. Photodyn. Ther. 2020, 7, 101655. [Google Scholar] [CrossRef]
- Lussi, A.; Carvalho, T.S. Erosive tooth wear: A multifactorial condition of growing concern and increasing knowledge. Monogr. Oral Sci. 2014, 25, 1–15. [Google Scholar] [PubMed]
- Wang, C.; Zhao, Y.; Zheng, S.; Xue, J.; Zhou, J.; Tang, Y.; Jiang, L.; Li, W. Effect of enamel morphology on nanoscale adhesion forces of streptococcal bacteria: An AFM study. Scanning 2015, 37, 313–321. [Google Scholar] [CrossRef] [PubMed]
- Wiegand, A.; Begic, M.; Attin, T. In vitro evaluation of abrasion of eroded enamel by different manual, power, and sonic toothbrushes. Caries Res. 2005, 40, 60–65. [Google Scholar] [CrossRef] [PubMed]




| Material (Manufacturer) | Composition |
|---|---|
| Whiteness Perfect bleaching gel (FGM Produtos Odontológicos Ltd.a, Joinville, SC, Brazil) | 22% carbamide peroxide, carbopol, potassium hydroxide, sodium fluoride, glycerol, deionized water, and pH around 7. |
| Artificial saliva (A fórmula—Farmácia de Manipulação, Belém, PA, Brazil) | Sodium bicarbonate 2190 mg, potassium phosphate 1270 mg, magnesium chloride 125 mg, calcium chloride 441 mg, potassium chloride 820 mg, sodium fluoride 4.5 mg, nipazol 100 mg, carboxymethylcellulose 8 mg, distilled water 3000 mL. |
| Colgate toothpaste Total 12 (Colgate-Palmolive, São Bernardo do Campo, SP, Brazil) | Sodium fluoride (1450 F ppm), water, sorbitol, hydrated silica, sodium laurylsulfate, PVM/MA copolymer, flavor, carragenine, Sodium hydroxide, triclosan, titanium dioxide (Cl 77,891), dipenteno and RDA 70. |
| Gatorade® isotonic of citrus fruits (PepsiCo Inc., Purchase, NY, USA) | Water, sucrose, glucose, sodium chloride, sodium citrate, monobasic potassium phosphate, acidulant (citric acid), natural orange and grapefruit aroma, artificial colors, and pH = 3. |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Barros Júnior, E.d.S.; Ribeiro, M.E.S.; Lima, R.R.; Souza Júnior, M.H.d.S.e.; Loretto, S.C. Excessive Dental Bleaching with 22% Carbamide Peroxide Combined with Erosive and Abrasive Challenges: New Insights into the Morphology and Surface Properties of Enamel. Materials 2022, 15, 7496. https://doi.org/10.3390/ma15217496
Barros Júnior EdS, Ribeiro MES, Lima RR, Souza Júnior MHdSe, Loretto SC. Excessive Dental Bleaching with 22% Carbamide Peroxide Combined with Erosive and Abrasive Challenges: New Insights into the Morphology and Surface Properties of Enamel. Materials. 2022; 15(21):7496. https://doi.org/10.3390/ma15217496
Chicago/Turabian StyleBarros Júnior, Edson de Sousa, Mara Eliane Soares Ribeiro, Rafael Rodrigues Lima, Mário Honorato da Silva e Souza Júnior, and Sandro Cordeiro Loretto. 2022. "Excessive Dental Bleaching with 22% Carbamide Peroxide Combined with Erosive and Abrasive Challenges: New Insights into the Morphology and Surface Properties of Enamel" Materials 15, no. 21: 7496. https://doi.org/10.3390/ma15217496
APA StyleBarros Júnior, E. d. S., Ribeiro, M. E. S., Lima, R. R., Souza Júnior, M. H. d. S. e., & Loretto, S. C. (2022). Excessive Dental Bleaching with 22% Carbamide Peroxide Combined with Erosive and Abrasive Challenges: New Insights into the Morphology and Surface Properties of Enamel. Materials, 15(21), 7496. https://doi.org/10.3390/ma15217496







