Sol-Gel Synthesis and Characterization of Yttrium-Doped MgFe2O4 Spinel
Abstract
1. Introduction
2. Materials and Methods
3. Results
4. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Amiri, M.; Salavati-Niasari, M.; Akbari, A. Magnetic nanocarriers: Evolution of spinel ferrites for medical applications. Adv. Colloid Interface Sci. 2019, 265, 29–44. [Google Scholar] [CrossRef] [PubMed]
- Vinnik, D.A.; Sherstyuk, D.P.; Zhivulin, V.E.; Zhivulin, D.E.; Starikov, A.Y.; Gudkova, S.A.; Zherebtsov, D.A.; Pankratov, D.A.; Alekhina, Y.A.; Perov, N.S. Impact of the Zn–Co content on structural and magnetic characteristics of the Ni spinel ferrites. Ceram. Int. 2022, 48, 18124–18133. [Google Scholar] [CrossRef]
- Kefeni, K.K.; Msagati, T.A.M.; Nkambule, T.T.I.; Mamba, B.B. Spinel ferrite nanoparticles and nanocomposites for biomedical applications and their toxicity. Mater. Sci. Eng. C 2020, 107, 110314. [Google Scholar] [CrossRef] [PubMed]
- Ivanets, A.; Prozorovich, V.; Roshchina, M.; Grigoraviciute-Puroniene, I.; Zarkov, A.; Kareiva, A.; Wang, Z.; Srivastava, V.; Sillanpää, M. Heterogeneous Fenton oxidation using magnesium ferrite nanoparticles for ibuprofen removal from wastewater: Optimization and kinetics studies. J. Nanomater. 2020, 2020, 8159628. [Google Scholar] [CrossRef]
- Hu, W.; Qin, N.; Wu, G.; Lin, Y.; Li, S.; Bao, D. Opportunity of Spinel Ferrite Materials in Nonvolatile Memory Device Applications Based on Their Resistive Switching Performances. J. Am. Chem. Soc. 2012, 134, 14658–14661. [Google Scholar] [CrossRef]
- Ivanets, A.; Prozorovich, V.; Sarkisov, V.; Roshchina, M.; Grigoraviciute-Puroniene, I.; Zarkov, A.; Kareiva, A.; Masindi, V.; Wang, C.; Srivastava, V. Effect of magnesium ferrite doping with lanthanide ions on dark-, visible-and UV-driven methylene blue degradation on heterogeneous Fenton-like catalysts. Ceram. Int. 2021, 47, 29786–29794. [Google Scholar] [CrossRef]
- Šutka, A.; Gross, K.A. Spinel ferrite oxide semiconductor gas sensors. Sens. Actuators B Chem. 2016, 222, 95–105. [Google Scholar] [CrossRef]
- Xie, X.; Wang, B.; Wang, Y.; Ni, C.; Sun, X.; Du, W. Spinel structured MFe2O4 (M = Fe, Co, Ni, Mn, Zn) and their composites for microwave absorption: A review. Chem. Eng. J. 2022, 428, 131160. [Google Scholar] [CrossRef]
- Jiang, Z.; Chen, K.; Zhang, Y.; Wang, Y.; Wang, F.; Zhang, G.; Dionysiou, D.D. Magnetically recoverable MgFe2O4/conjugated polyvinyl chloride derivative nanocomposite with higher visible-light photocatalytic activity for treating Cr (VI)-polluted water. Sep. Purif. Technol. 2020, 236, 116272. [Google Scholar] [CrossRef]
- Sharma, L.; Kakkar, R. Magnetically retrievable one-pot fabrication of mesoporous magnesium ferrite (MgFe2O4) for the re-mediation of chlorpyrifos and real pesticide wastewater. J. Environ. Chem. Eng. 2018, 6, 6891–6903. [Google Scholar] [CrossRef]
- Shahjuee, T.; Masoudpanah, S.M.; Mirkazemi, S.M. Thermal Decomposition Synthesis of MgFe2O4 Nanoparticles for Magnetic Hyperthermia. J. Supercond. Nov. Magn. 2019, 32, 1347–1352. [Google Scholar] [CrossRef]
- Reza Barati, M.; Selomulya, C.; Suzuki, K. Particle size dependence of heating power in MgFe2O4 nanoparticles for hyper-thermia therapy application. J. Appl. Phys. 2014, 115, 17B522. [Google Scholar] [CrossRef]
- Ajeesha, T.; Ashwini, A.; George, M.; Manikandan, A.; Mary, J.A.; Slimani, Y.; Almessiere, M.A.; Baykal, A. Nickel substituted MgFe2O4 nanoparticles via co-precipitation method for photocatalytic applications. Phys. B: Condens. Matter 2021, 606, 412660. [Google Scholar] [CrossRef]
- Shen, Y.; Wu, Y.; Li, X.; Zhao, Q.; Hou, Y. One-pot synthesis of MgFe2O4 nanospheres by solvothermal method. Mater. Lett. 2013, 96, 85–88. [Google Scholar] [CrossRef]
- Heidari, P.; Masoudpanah, S.M. A facial synthesis of MgFe2O4/RGO nanocomposite powders as a high performance micro-wave absorber. J. Alloys. Compd. 2020, 834, 155166. [Google Scholar] [CrossRef]
- Ali, N.A.; Idris, N.H.; Din, M.F.M.; Mustafa, N.S.; Sazelee, N.A.; Yap, F.A.H.; Sulaiman, N.N.; Yahya, M.S.; Ismail, M. Nanolayer-like-shaped MgFe2O4 synthesised via a simple hydrothermal method and its catalytic effect on the hydrogen storage properties of MgH2. RSC Adv. 2018, 8, 15667–15674. [Google Scholar] [CrossRef]
- Chen, D.; Zhang, Y.; Tu, C. Preparation of high saturation magnetic MgFe2O4 nanoparticles by microwave-assisted ball milling. Mater. Lett. 2012, 82, 10–12. [Google Scholar] [CrossRef]
- Feng, Y.; Li, S.; Zheng, Y.; Yi, Z.; He, Y.; Xu, Y. Preparation and characterization of MgFe2O4 nanocrystallites via PVA sol-gel route. J. Alloys. Compd. 2017, 699, 521–525. [Google Scholar] [CrossRef]
- Araújo, J.C.R.; Araujo-Barbosa, S.; Souza, A.L.R.; Iglesias, C.A.M.; Xavier, J.; Souza, P.B.; Pla Cid, C.C.; Azevedo, S.; da Silva, R.B.; Correa, M.A.; et al. Tuning structural, magnetic, electrical, and dielectric properties of MgFe2O4 synthesized by sol-gel followed by heat treatment. J. Phys. Chem. Solids 2021, 154, 110051. [Google Scholar] [CrossRef]
- Uke, S.J.; Mardikar, S.P.; Bambole, D.R.; Kumar, Y.; Chaudhari, G.N. Sol-gel citrate synthesized Zn doped MgFe2O4 nanocrystals: A promising supercapacitor electrode material. Mater. Sci. Energy Technol. 2020, 3, 446–455. [Google Scholar] [CrossRef]
- Phor, L.; Chahal, S.; Kumar, V. Zn2+ substituted superparamagnetic MgFe2O4 spinel-ferrites: Investigations on structural and spin-interactions. J. Adv. Ceram. 2020, 9, 576–587. [Google Scholar] [CrossRef]
- Wang, X.; Kan, X.; Liu, X.; Feng, S.; Zheng, G.; Cheng, Z.; Wang, W.; Chen, Z.; Liu, C. Characterization of microstructure and magnetic properties for Co2+ ions doped MgFe2O4 spinel ferrites. Mater. Today Commun. 2020, 25, 101414. [Google Scholar] [CrossRef]
- Ahmad, Y.; Raina, B.; Thakur, S.; Bamzai, K.K. Magnesium and yttrium doped superparamagnetic manganese ferrite nano-particles for magnetic and microwave applications. J. Magn. Magn. Mater. 2022, 552, 169178. [Google Scholar] [CrossRef]
- Almessiere, M.A.; Güner, S.; Slimani, Y.; Baykal, A.; Shirsath, S.E.; Korkmaz, A.D.; Badar, R.; Manikandan, A. Investigation on the structural, optical, and magnetic features of D3+ and Y3+ co-doped Mn0.5Zn0.5Fe2O4 spinel ferrite nanoparticles. J. Mol. Struct. 2022, 1248, 131412. [Google Scholar] [CrossRef]
- Sharma, R.; Kumar, V.; Bansal, S.; Singhal, S. Boosting the catalytic performance of pristine CoFe2O4 with yttrium (Y3+) in-clusion in the spinel structure. Mater. Res. Bull. 2017, 90, 94–103. [Google Scholar] [CrossRef]
- Amin, N.; Hasan, M.S.U.; Majeed, Z.; Latif, Z.; un Nabi, M.A.; Mahmood, K.; Ali, A.; Mehmood, K.; Fatima, M.; Akhtar, M. Structural, electrical, optical and dielectric properties of yttrium substituted cadmium ferrites prepared by Co-Precipitation method. Ceram. Int. 2020, 46, 20798–20809. [Google Scholar] [CrossRef]
- Cvejić, Ž.; Rakić, S.; Jankov, S.; Skuban, S.; Kapor, A. Dielectric properties and conductivity of zinc ferrite and zinc ferrite doped with yttrium. J. Alloys. Compd. 2009, 480, 241–245. [Google Scholar] [CrossRef]
- Ishaque, M.; Khan, M.A.; Ali, I.; Khan, H.M.; Iqbal, M.A.; Islam, M.U.; Warsi, M.F. Investigations on structural, electrical and dielectric properties of yttrium substituted Mg-ferrites. Ceram. Int. 2015, 41, 4028–4034. [Google Scholar] [CrossRef]
- Shannon, R.D. Revised effective ionic radii and systematic studies of interatomic distances in halides and chalcogenides. Acta Crystallogr. Sect. A 1976, 32, 751–767. [Google Scholar] [CrossRef]
- Zhang, Y.; Yang, J.; Xu, J.; Gao, Q.; Hong, Z. Controllable synthesis of hexagonal and orthorhombic YFeO3 and their visi-ble-light photocatalytic activities. Mater. Lett. 2012, 81, 1–4. [Google Scholar] [CrossRef]
- Zhang, R.-L.; Chen, C.-L.; Jin, K.-X.; Niu, L.-W.; Xing, H.; Luo, B. Dielectric behavior of hexagonal and orthorhombic YFeO3 prepared by modified sol-gel method. J. Electroceramics 2014, 32, 187–191. [Google Scholar] [CrossRef]
- Waldron, R.D. Infrared Spectra of Ferrites. Phys. Rev. 1955, 99, 1727–1735. [Google Scholar] [CrossRef]
- Pradeep, A.; Priyadharsini, P.; Chandrasekaran, G. Sol–gel route of synthesis of nanoparticles of MgFe2O4 and XRD, FTIR and VSM study. J. Magn. Magn. Mater. 2008, 320, 2774–2779. [Google Scholar] [CrossRef]
- Seeman, V.; Feldbach, E.; Kärner, T.; Maaroos, A.; Mironova-Ulmane, N.; Popov, A.I.; Shablonin, E.; Vasil’chenko, E.; Lush-chik, A. Fast-neutron-induced and as-grown structural defects in magnesium aluminate spinel crystal with different morphology. Opt. Mater. 2019, 91, 42–49. [Google Scholar] [CrossRef]
- Šepelák, V.; Baabe, D.; Mienert, D.; Litterst, F.J.; Becker, K.D. Enhanced magnetisation in nanocrystalline high-energy milled MgFe2O4. Scr. Mater. 2003, 48, 961–966. [Google Scholar] [CrossRef]
- De Grave, E.; Govaert, A.; Chambaere, D.; Robbrecht, G. A Mössbauer effect study of MgFe2O4. Phys. B C 1979, 96, 103–110. [Google Scholar] [CrossRef]
- Franco Jr, A.; Silva, M.S. High temperature magnetic properties of magnesium ferrite nanoparticles. J. Appl. Phys. 2011, 109, 07B505. [Google Scholar] [CrossRef]
- Majetich, S.A.; Sachan, M. Magnetostatic interactions in magnetic nanoparticle assemblies: Energy, time and length scales. J. Phys. D Appl. Phys. 2006, 39, R407–R422. [Google Scholar] [CrossRef]
- Karoblis, D.; Zarkov, A.; Garskaite, E.; Mazeika, K.; Baltrunas, D.; Niaura, G.; Beganskiene, A.; Kareiva, A. Study of gadolinium substitution effects in hexagonal yttrium manganite YMnO3. Sci. Rep. 2021, 11, 2875. [Google Scholar] [CrossRef]
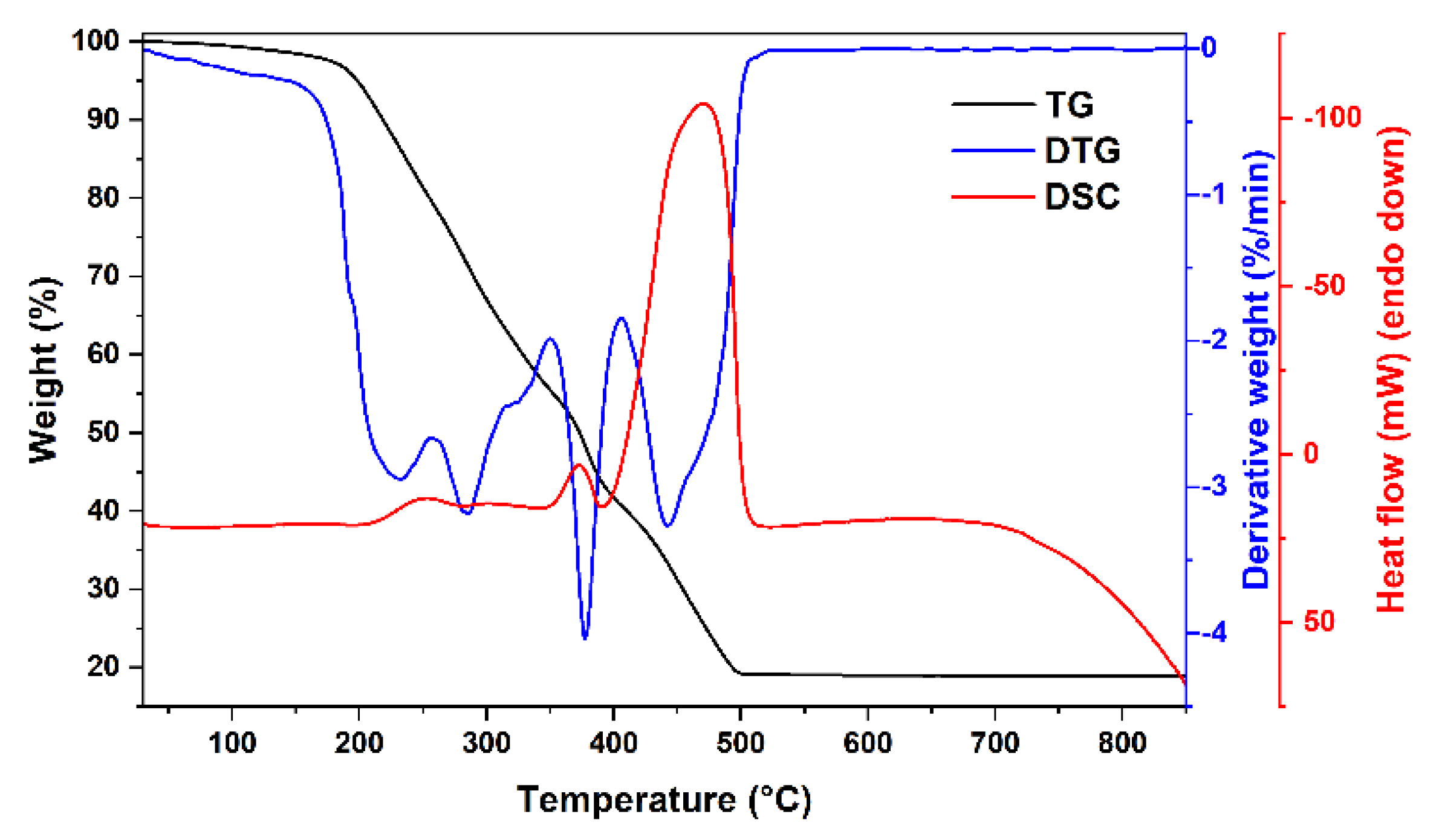
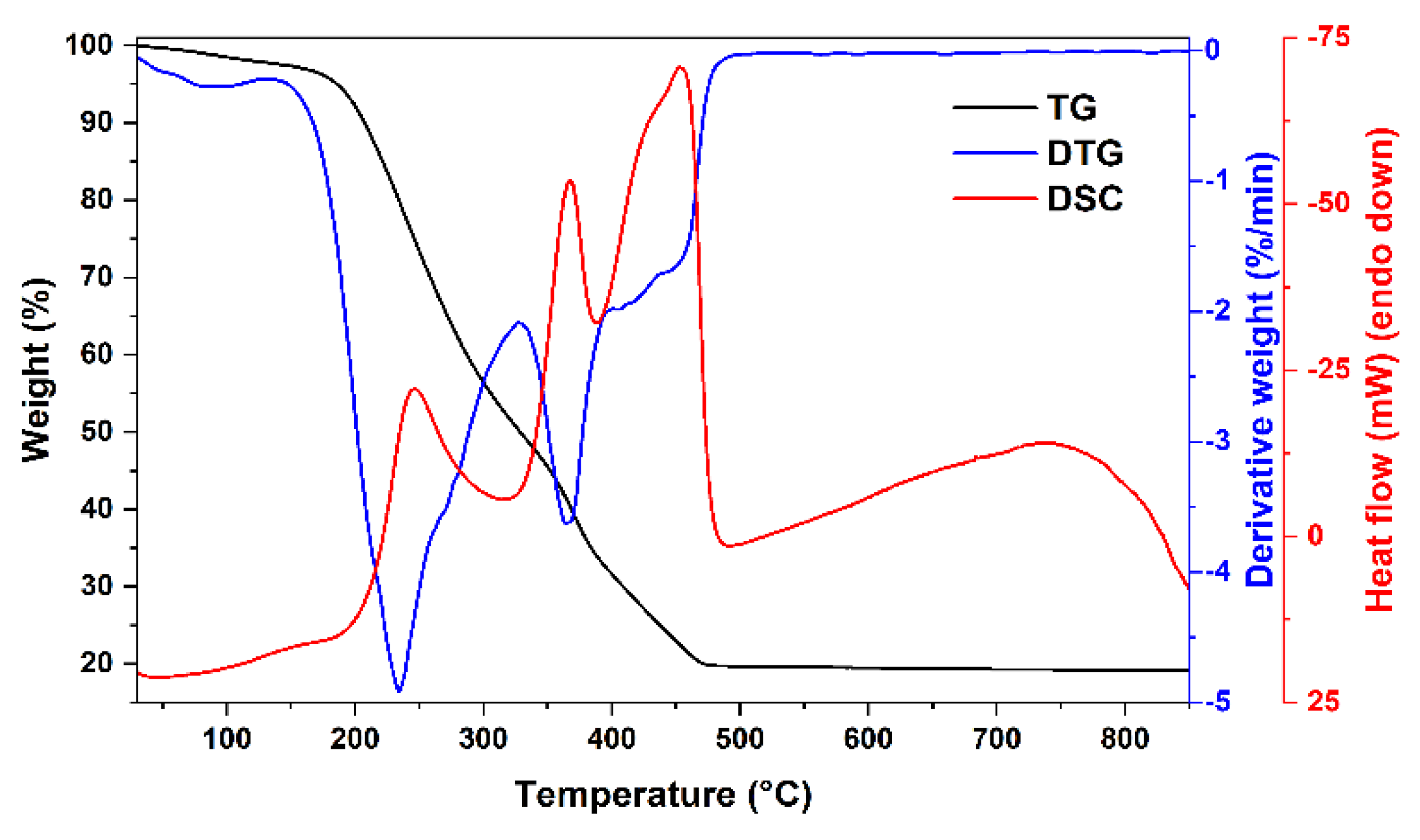

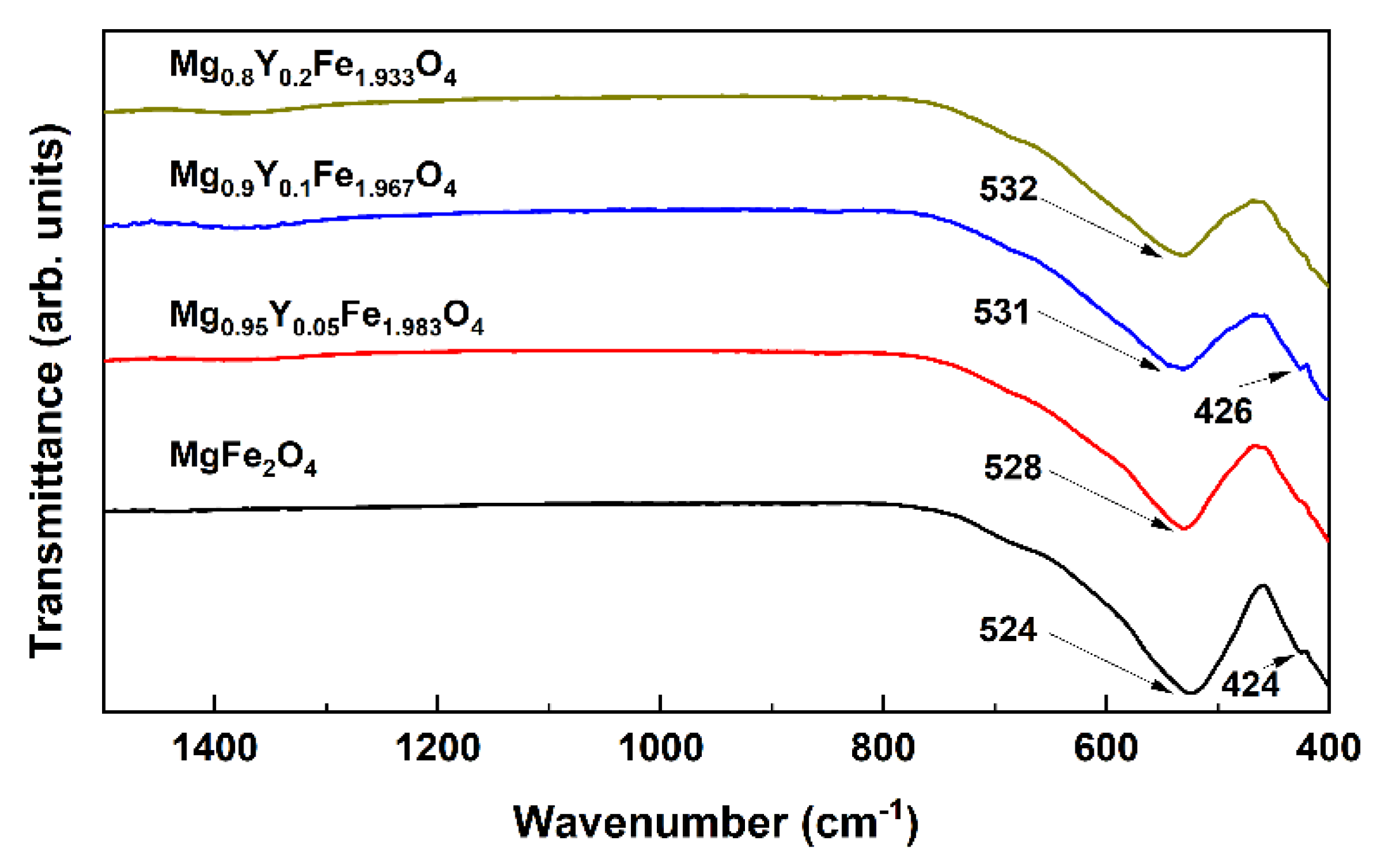


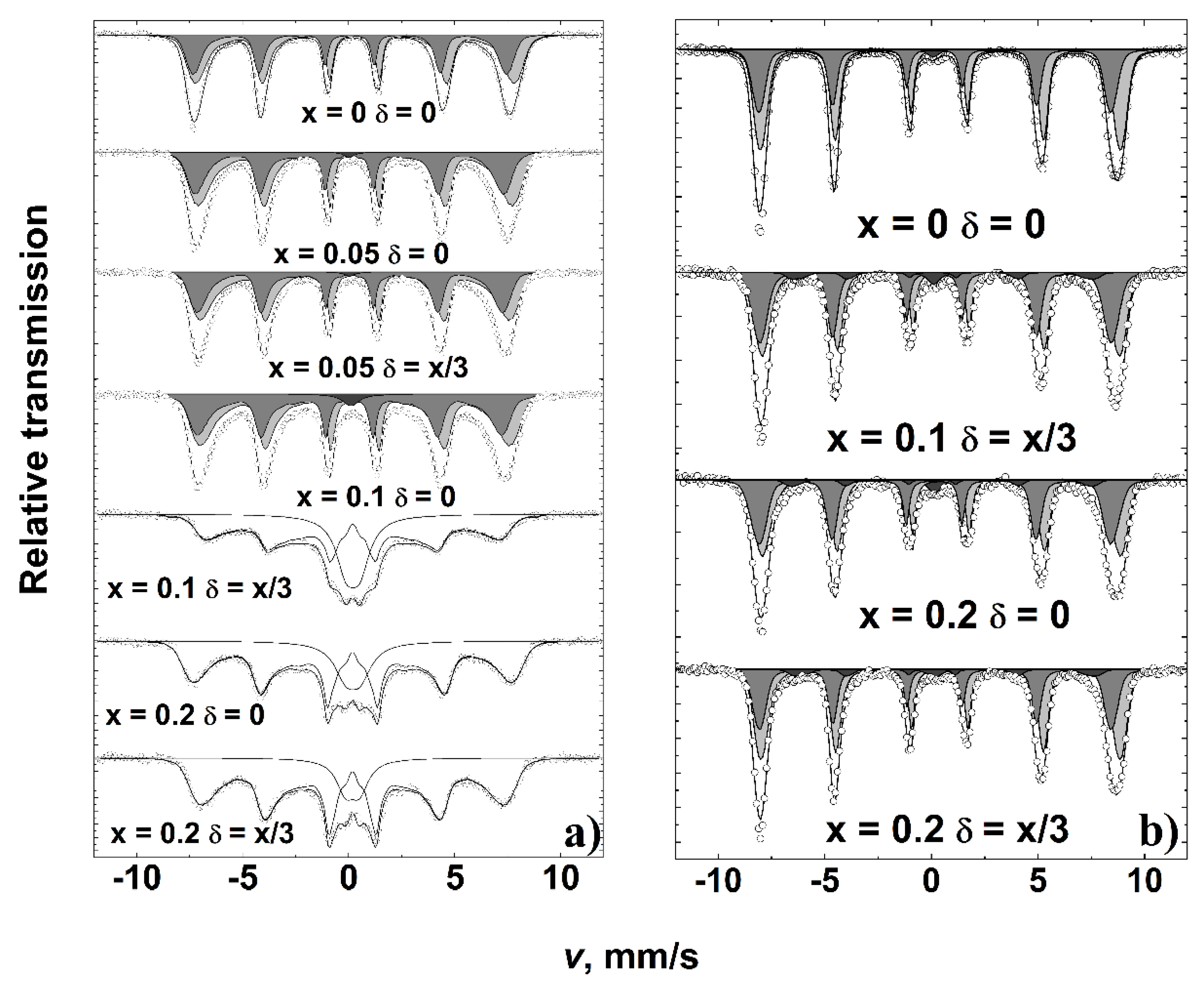
| x | δ | <BA>, T | <BB>, T | IA/IB | Is, % | δA, mm/s | δB, mm/s |
|---|---|---|---|---|---|---|---|
| 0 | 0 | 45.0 | 45.0 | 0.62 | 0 | 0.16 ± 0.01 | 0.39 ± 0.01 |
| 0.05 | 0 | 41.6 | 43.4 | 0.7 | 1 | 0.14 ± 0.01 | 0.40 ± 0.01 |
| 0.05 | 0.017 | 41.2 | 42.3 | 0.7 | 2 | 0.16 ± 0.01 | 0.40 ± 0.01 |
| 0.1 | 0 | 39.6 | 40.8 | 0.74 | 1 | 0.15 ± 0.01 | 0.40 ± 0.01 |
| <BAB>, T | <Ball>, T | δAB, mm/s | δs, mm/s | ||||
| 0.1 | 0.034 | 31.4 | 21.8 | - | 31 | 0.30 ± 0.01 | 0.31 ± 0.01 |
| 0.2 | 0 | 35.3 | 28.4 | - | 20 | 0.29 ± 0.01 | 0.31 ± 0.01 |
| 0.2 | 0.067 | 34.1 | 29.4 | - | 14 | 0.29 ± 0.01 | 0.29 ± 0.01 |
| x | δ | I, % | δ, mm/s | 2ε, mm/s | <B>, T | |
|---|---|---|---|---|---|---|
| 0 | 0 | 37 | 0.27 ± 0.01 | 0.01 ± 0.01 | 51.3 | Tetrahedral A |
| 63 | 0.51 ± 0.01 | 0.05 ± 0.01 | 52.3 | Octahedral B | ||
| 0.1 | 0.034 | 34 | 0.27 * | 0.10 ± 0.01 | 51.0 | Tetrahedral A |
| 61 | 0.51 * | −0.01 ± 0.01 | 51.7 | Octahedral B | ||
| 5 | 0.39 ± 0.02 | 0.54 ± 0.05 | 42.7 | Disordered/h-YFeO3 | ||
| 0.2 | 0.067 | 36 | 0.27 ± 0.01 | 0.03 ± 0.01 | 51.2 | Tetrahedral A |
| 59 | 0.51 ± 0.01 | 0.03 ± 0.01 | 51.9 | Octahedral B | ||
| 5 | 0.45 ± 0.02 | 0.63 ± 0.05 | 42.6 | Disordered/YFeO3 | ||
| 0.2 | 0 | 35 | 0.27 * | 0.12 ± 0.01 | 51.2 | Tetrahedral A |
| 60 | 0.51 * | 0.01 ± 0.01 | 51.7 | Octahedral B | ||
| 5 | 0.33 ± 0.03 | 0.55 ± 0.07 | 43.4 | Disordered/YFeO3 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Karoblis, D.; Mazeika, K.; Raudonis, R.; Zarkov, A.; Kareiva, A. Sol-Gel Synthesis and Characterization of Yttrium-Doped MgFe2O4 Spinel. Materials 2022, 15, 7547. https://doi.org/10.3390/ma15217547
Karoblis D, Mazeika K, Raudonis R, Zarkov A, Kareiva A. Sol-Gel Synthesis and Characterization of Yttrium-Doped MgFe2O4 Spinel. Materials. 2022; 15(21):7547. https://doi.org/10.3390/ma15217547
Chicago/Turabian StyleKaroblis, Dovydas, Kestutis Mazeika, Rimantas Raudonis, Aleksej Zarkov, and Aivaras Kareiva. 2022. "Sol-Gel Synthesis and Characterization of Yttrium-Doped MgFe2O4 Spinel" Materials 15, no. 21: 7547. https://doi.org/10.3390/ma15217547
APA StyleKaroblis, D., Mazeika, K., Raudonis, R., Zarkov, A., & Kareiva, A. (2022). Sol-Gel Synthesis and Characterization of Yttrium-Doped MgFe2O4 Spinel. Materials, 15(21), 7547. https://doi.org/10.3390/ma15217547








