Shear Viscosity Overshoots in Polymer Modified Asphalts
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Asphalt Modification
2.3. Experimental
2.3.1. Shear Viscosity
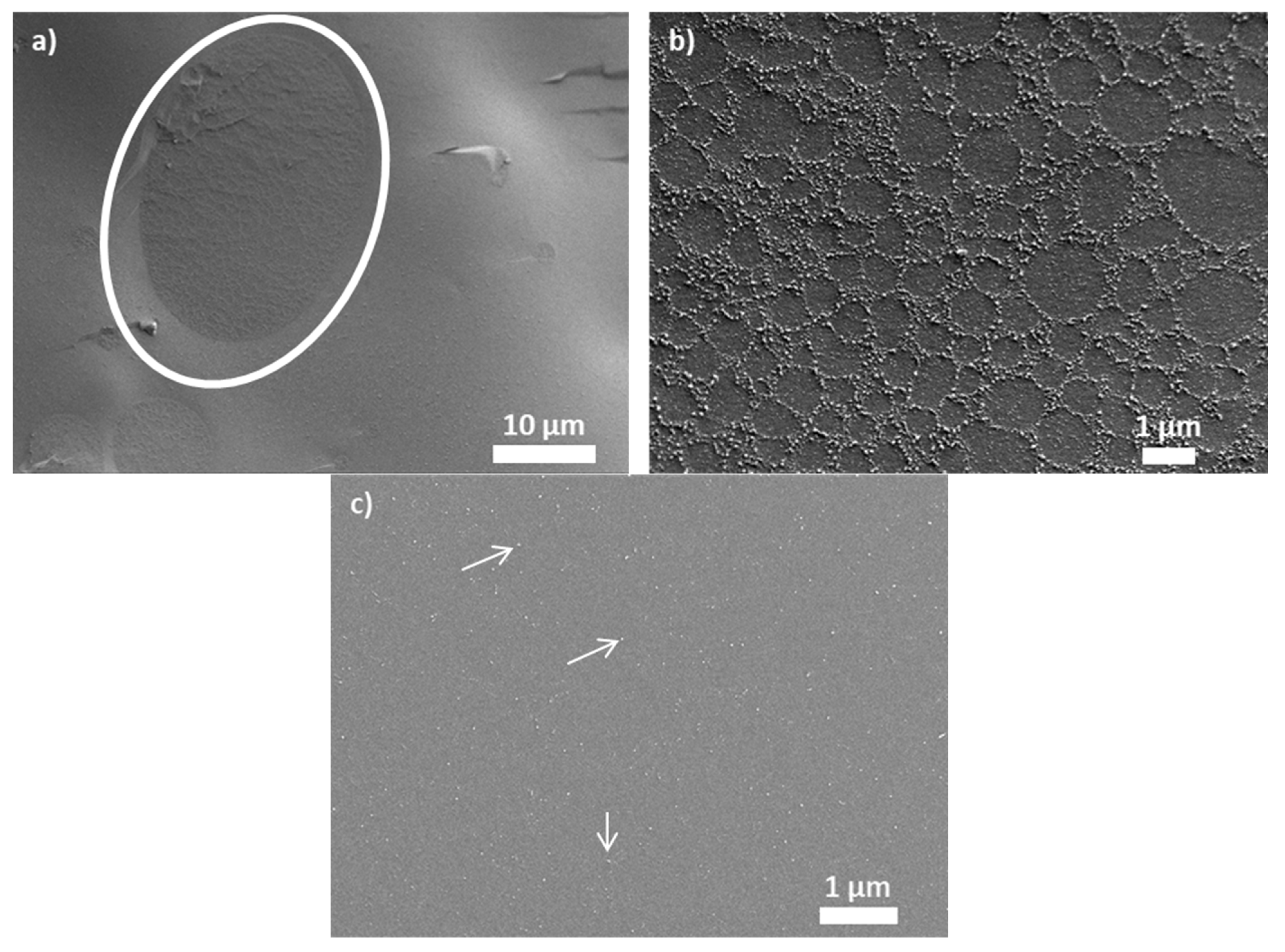
2.3.2. Cryo-SEM
3. Results and Discussion
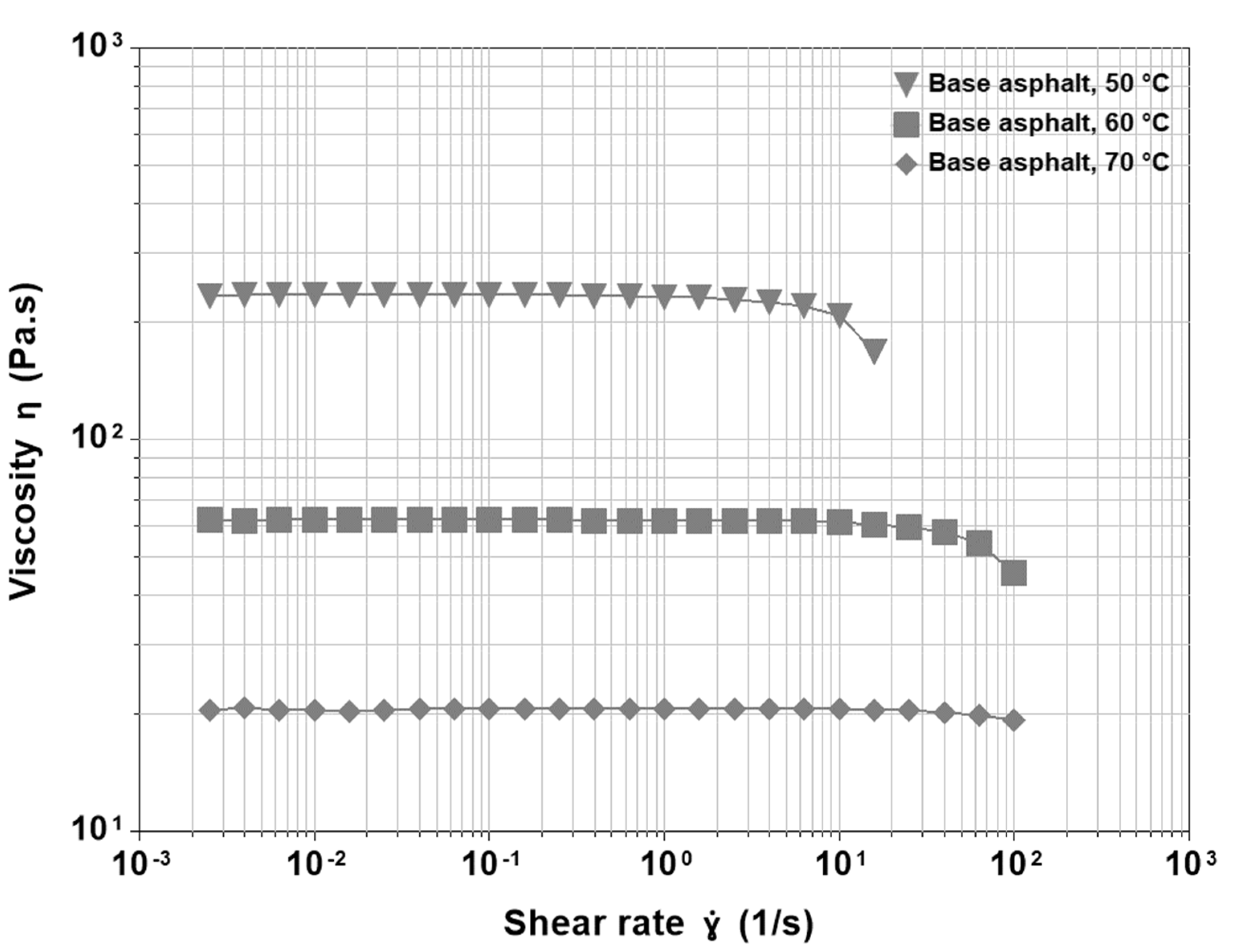
3.1. Base Asphalt
3.2. Base Asphalt and PPA
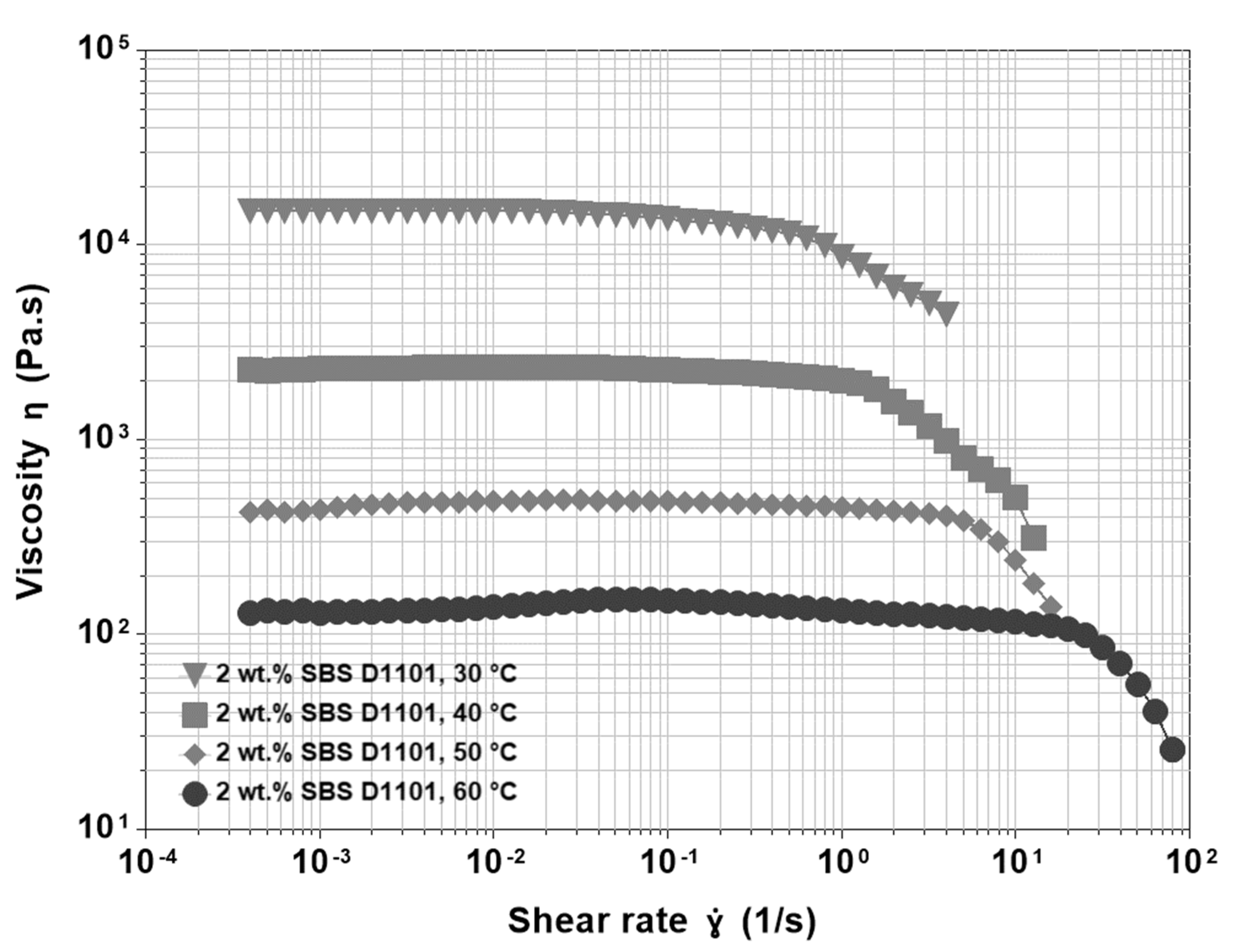
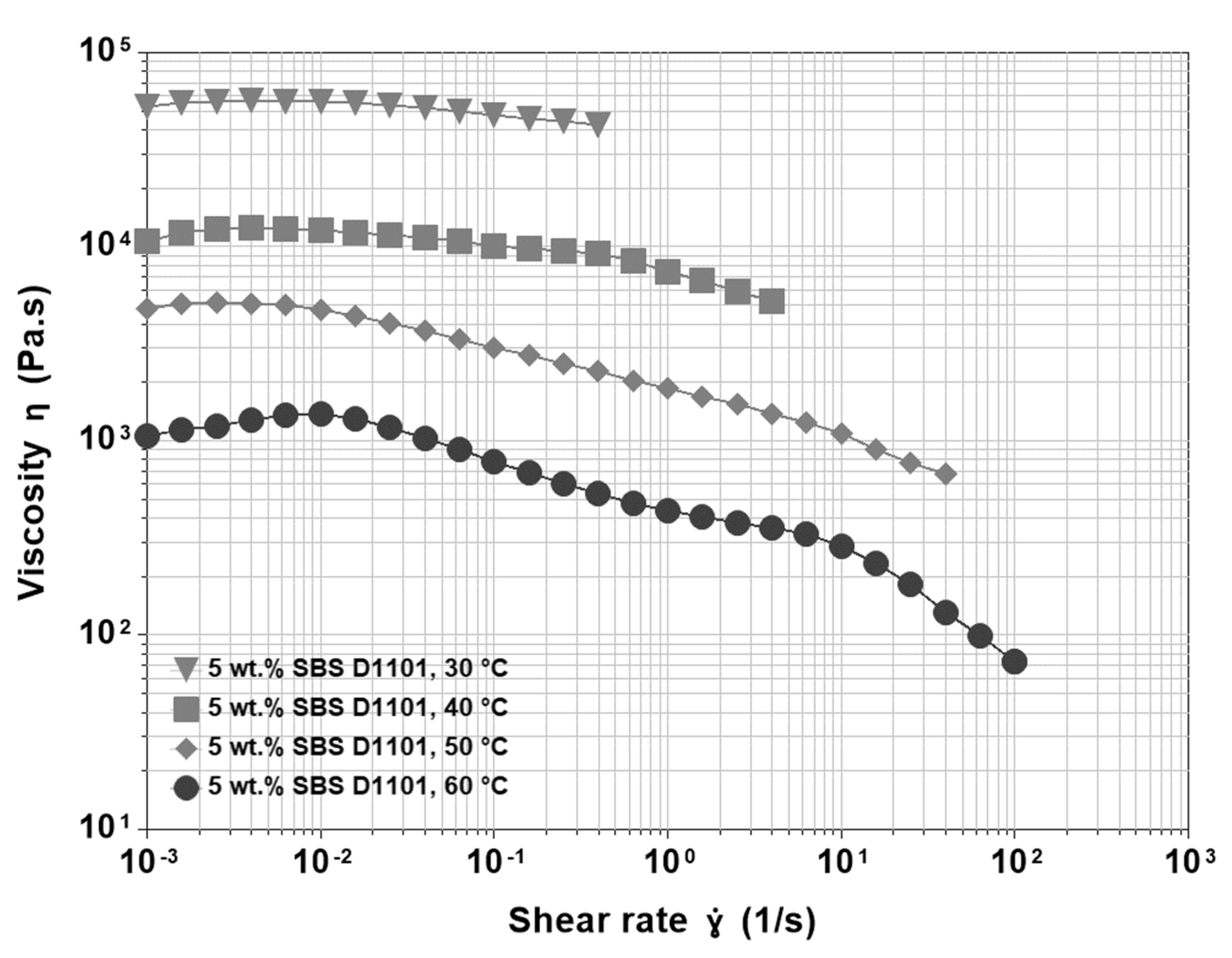
3.3. Base Asphalt and SBS
- The appearance of a shear thickening region that, at higher temperatures, is more pronounced and shifts to the left in the shear rate axis.
- The presence of a gradual shear thinning preceding the sharp final one.
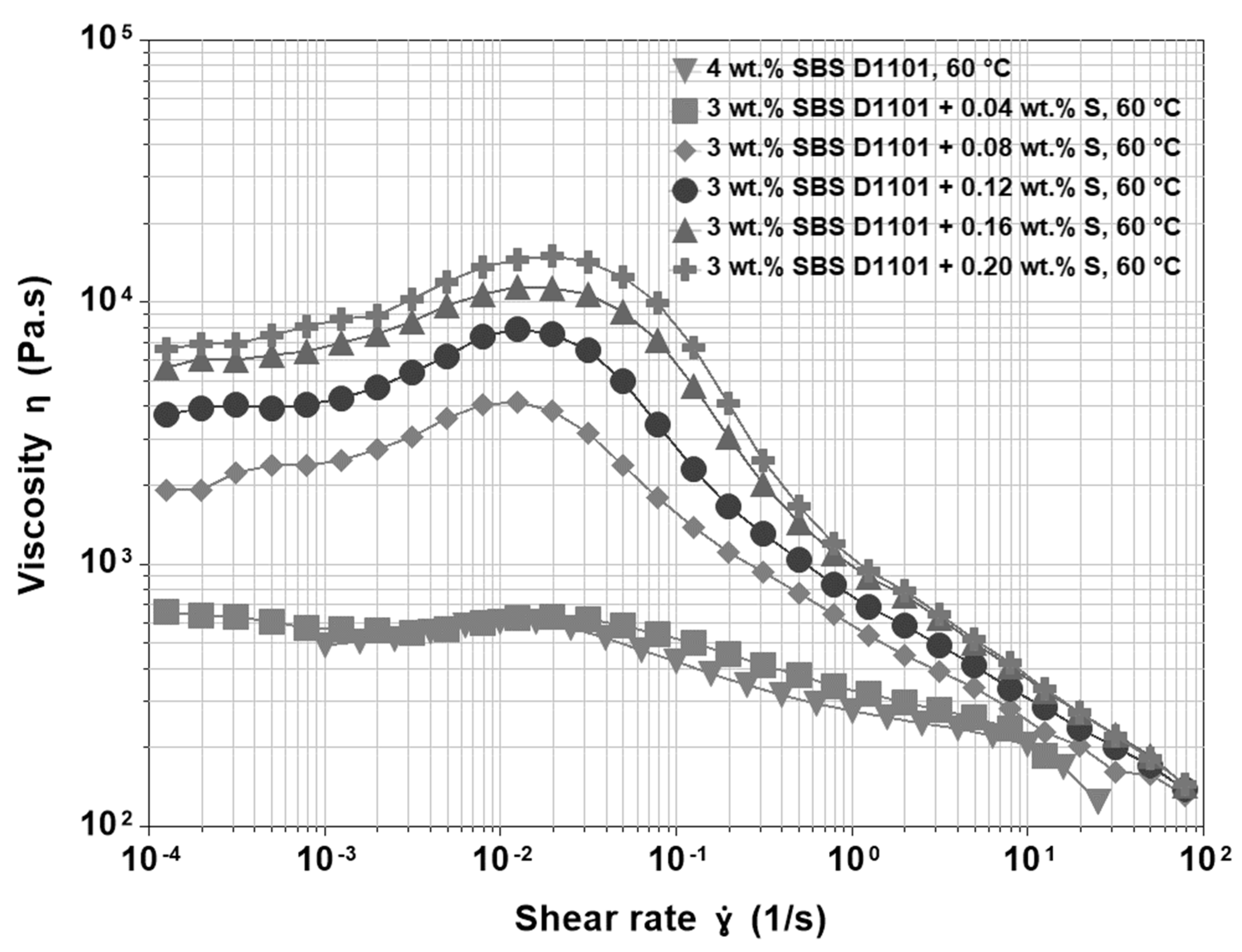
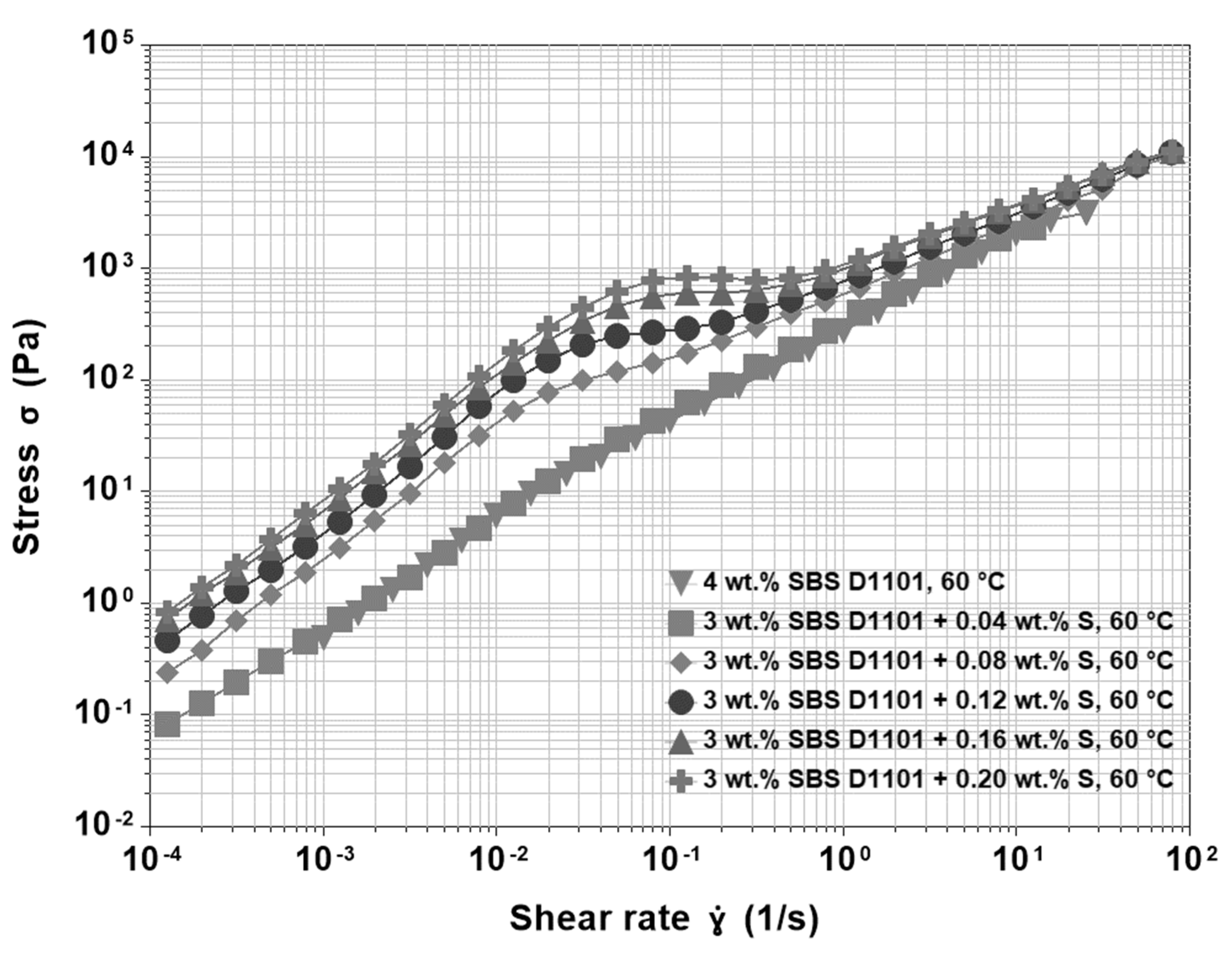
3.4. Base Asphalt, SBS and Sulfur
3.5. Base Asphalt, SBS, Sulfur and PPA
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Sebastian, J.M.; Lai, C.; Graessley, W.W.; Register, R.A.; Marchand, G.R. Steady-shear rheology of block copolymer melts: Zero-shear viscosity and shear disordering in body-centered-cubic systems. Macromolecules 2002, 35, 2700–2706. [Google Scholar] [CrossRef]
- Sebastian, J.M.; Lai, C.; Graessley, W.W.; Register, R.A. Steady-shear rheology of block copolymer melts and concentrated solutions: Disordering stress in body-centered-cubic systems. Macromolecules 2002, 35, 2707–2713. [Google Scholar] [CrossRef]
- Polacco, G. A review of the fundamentals of polymer-modified asphalts: Asphalt/polymer interactions and principles of compatibility. Adv. Colloid Interface Sci. 2015, 41, 77–112. [Google Scholar] [CrossRef] [PubMed]
- Adedeji, A.; Grünfelder, T.; Bates, F.S.; Macosko, C.W.; Stroup-Gardiner, M.; Newcomb, D.E. Asphalt modified by SBS triblock copolymer: Structures and properties: Asphalt modified by SBS triblock copolymer. Polym. Eng. Sci. 1996, 36, 1707–1723. [Google Scholar] [CrossRef]
- Ho, R.-M.; Adedeji, A.; Giles, D.W.; Hajduk, D.A.; Macosko, C.W.; Bates, F.S. Microstructure of Triblock Copolymers in Asphalt Oligomers, 2nd ed.; John Wiley & Sons: Hoboken, NJ, USA, 1997; pp. 2857–2877. [Google Scholar]
- Fawcett, A.; McNally, T. Polystyrene and asphaltene micelles within blends with a bitumen of an SBS block copolymer and styrene and butadiene homopolymers. Colloid Polym. Sci. 2003, 281, 203–213. [Google Scholar] [CrossRef]
- Liang, M.; Liang, P.; Fan, W.; Qian, C.; Xin, X.; Shi, J.; Nan, G. Thermo-rheological behavior and compatibility of modified asphalt with various styrene-butadiene structures in SBS copolymers. Mater. Des. 2015, 88, 177–185. [Google Scholar] [CrossRef]
- Koyun, A.N.; Büchner, J.; Wistuba, M.P.; Grothe, H. Laboratory and field ageing of SBS modified bitumen: Chemical properties and microstructural characterization. Colloids Surf. A Physicochem. Eng. Asp. 2021, 624, 126856. [Google Scholar] [CrossRef]
- Chen, J.S.; Huang, C.C. Fundamental characterization of SBS-modified asphalt mixed with sulfur. J. Appl. Polym. Sci. 2007, 103, 2817–2825. [Google Scholar] [CrossRef]
- Kaya Ozdemir, D.; Topal, A.; McNally, T. Relationship between microstructure and phase morphology of SBS modified bitumen with processing parameters studied using atomic force microscopy. Constr. Build. Mater. 2021, 268, 121061. [Google Scholar] [CrossRef]
- Mukhtar, N.; Mohd Hasan, M.R.; Mohd Ghazali, M.F.H.; Mohd Zin, Z.; Shariff, K.A.; Sani, A. Influence of concentration and packing of filler particles on the stiffening effect and shearing behaviour of asphalt mastic. Constr. Build. Mater. 2021, 295, 123660. [Google Scholar] [CrossRef]
- Gulzar, S.; Underwood, B.S. Nonlinear viscoelastic response of crumb rubber modified asphalt binder under large strains. Transp. Res. Record. 2020, 2674, 139–149. [Google Scholar] [CrossRef]
- Rathee, V.; Monti, A.; Rosti, M.E.; Shen, A.Q. Shear thickening behavior in dense repulsive and attractive suspensions of hard spheres. Soft Matter 2021, 17, 8047–8058. [Google Scholar] [CrossRef] [PubMed]
- Bonnaud, P.A.; Ushiyama, H.; Tejima, S.; Fujita, J.-I. Neat and aqueous polyelectrolytes under a steady-shear flow. J. Phys. Chem. B 2021, 125, 6930–6944. [Google Scholar] [CrossRef] [PubMed]
- Huamaní-Meléndez, V.J.; Mauro, M.A.; Darros-Barbosa, R. Physicochemical and rheological properties of aqueous Tara gum solutions. Food Hydrocoll. 2021, 111, 106195. [Google Scholar] [CrossRef]
- O’Brie, V.T.; Mackay, M.E. Stress components and shear thickening of concentrated hard sphere suspensions. Langmuir 2000, 16, 7931–7938. [Google Scholar] [CrossRef]
- Salehin, R.; Xu, R.-G.; Papanikolaou, S. Colloidal shear-thickening fluids using variable functional star-shaped particles: A molecular dynamics study. Materials 2021, 14, 6867. [Google Scholar] [CrossRef]
- Chissonde, S.; Azad, M.S.; Trivedi, J. Flow of hydrophobically associating polymers through unconsolidated sand pack: Role of extensional rheology and degree of association. J. Mol. Liq. 2021, 344, 117643. [Google Scholar] [CrossRef]
- Lalitha Sridhar, S.; Vernerey, F. The chain distribution tensor: Linking nonlinear rheology and chain anisotropy in transient polymers. Polymers 2018, 10, 848. [Google Scholar] [CrossRef]
- Khan, U.A.; Oelschlaeger, C.; Ali, F.I.; Roether, J.; Willenbacher, N.; Hashmi, I.A. Structural, macro- and micro-mechanical properties of supramolecular bi-component l-Lysine-sodium tetraphenyl borate based hydrogels. Colloids Surf. A Physicochem. Eng. Asp. 2018, 546, 366–377. [Google Scholar] [CrossRef]
- Andersson Trojer, M.; Andersson, M.; Bergenholtz, J.; Gatenholm, P. Elastic strain-hardening and shear-thickening exhibited by thermoreversible physical hydrogels based on poly(alkylene oxide)-grafted hyaluronic acid or carboxymethylcellulose. Phys. Chem. Chem. Phys. 2020, 22, 14579–14590. [Google Scholar] [CrossRef]
- Chassenieux, C.; Nicolai, T.; Benyahia, L. Rheology of associative polymer solutions. Curr. Opin. Colloid Interface Sci. 2011, 16, 18–26. [Google Scholar] [CrossRef]
- Koga, T.; Tanaka, F. Molecular origin of shear thickening in transient polymer networks: A molecular dynamics study. Eur. Phys. J. E 2005, 17, 115–118. [Google Scholar] [CrossRef] [PubMed]
- Castillo-Tejas, J.; Castrejón-González, O.; Carro, S.; González-Coronel, V.; Alvarado, J.F.J.; Manero, O. Associative polymers. Part III: Shear rheology from molecular dynamics. Colloids Surf. A Physicochem. Eng. Asp. 2016, 491, 37–49. [Google Scholar] [CrossRef]
- Royer, J.R.; Blair, D.L.; Hudson, S.D. Rheological signature of frictional interactions in shear thickening suspensions. Phys. Rev. Lett. 2016, 116, 188301. [Google Scholar] [CrossRef]
- Suzuki, S.; Uneyama, T.; Inoue, T.; Watanabe, H. Nonlinear Rheology of telechelic associative polymer networks: Shear thickening and thinning behavior of hydrophobically modified ethoxylated urethane (HEUR) in aqueous solution. Macromolecules 2012, 45, 888–898. [Google Scholar] [CrossRef]
- Berret, J.-F.; Séréro, Y. Evidence of shear-induced fluid fracture in telechelic polymer networks. Phys. Rev. Lett. 2001, 87, 048303. [Google Scholar] [CrossRef]
- Kaffashi, B.; Barmar, M.; Eyvani, J. The steady state and dynamic rheological properties of telechelic associative polymer solutions. Colloids Surf. A Physicochem. Eng. Asp. 2005, 254, 125–130. [Google Scholar] [CrossRef]
- Keane, D.P.; Mellor, M.D.; Poling-Skutvik, R. Responsive telechelic block copolymers for enhancing the elasticity of nanoemulsions. ACS Appl. Nano Mater. 2022, 5, 5934–5943. [Google Scholar] [CrossRef]
- Bourrianne, P.; Niggel, V.; Polly, G.; Divoux, T.; McKinley, G.H. Tuning the shear thickening of suspensions through surface roughness and physico-chemical interactions. Phys. Rev. Res. 2022, 4, 033062. [Google Scholar] [CrossRef]
- Cardiel, J.J.; Dohnalkova, A.C.; Dubash, N.; Zhao, Y.; Cheung, P.; Shen, A.Q. Microstructure and rheology of a flow-induced structured phase in wormlike micellar solutions. Proc. Natl. Acad. Sci. USA 2013, 110, E1653–E1660. [Google Scholar] [CrossRef]
- Lee, Y.-F.; Luo, Y.; Bai, T.; Velez, C.; Brown, S.C.; Wagner, N.J. Microstructure and rheology of shear-thickening colloidal suspensions with varying interparticle friction: Comparison of experiment with theory and simulation models. Phys. Fluids 2021, 33, 033316. [Google Scholar] [CrossRef]
- Jiang, W.; Sun, Y.; Xu, Y.; Peng, C.; Gong, X.; Zhang, Z. Shear-thickening behavior of polymethylmethacrylate particles suspensions in glycerine-water mixtures. Rheol. Acta 2010, 49, 1157–1163. [Google Scholar] [CrossRef]
- Guy, B.M.; Hermes, M.; Poon, W.C.K. Towards a unified description of the rheology of hard-particle suspensions. Phys. Rev. Lett. 2015, 115, 088304. [Google Scholar] [CrossRef]
- Malkin, A.Y.; Kulichikhin, V.G. Shear thickening and dynamic glass transition of concentrated suspensions. State of the problem. Colloid J. 2016, 78, 1–8. [Google Scholar] [CrossRef]
- Hoffman, R.L. Discontinuous and dilatant viscosity behavior in concentrated suspensions. II. Theory and experimental tests. J. Colloid Interface Sci. 1974, 46, 491–506. [Google Scholar] [CrossRef]
- Kim, J.; Helgeson, M.E. Shear-induced clustering of Brownian colloids in associative polymer networks at moderate Péclet number. Phys. Rev. Fluids 2016, 1, 043302. [Google Scholar] [CrossRef]
- Kamibayashi, M.; Ogura, H.; Otsubo, Y. Shear-thickening flow of nanoparticle suspensions flocculated by polymer bridging. J. Colloid Interface Sci. 2008, 321, 294–301. [Google Scholar] [CrossRef]
- Swarna; Pattanayek, S.K.; Ghosh, A.K. Dynamic shear rheology of colloidal suspensions of surface-modified silica nanoparticles in PEG. J. Nanopart Res. 2018, 20, 53. [Google Scholar] [CrossRef]
- Lesueur, D. The colloidal structure of bitumen: Consequences on the rheology and on the mechanisms of bitumen modification. Adv. Colloid Interface Sci. 2009, 145, 42–82. [Google Scholar] [CrossRef]
- Saboo, N.; Singh, B.; Kumar, P.; Vikram, D. Study on viscosity of conventional and polymer modified asphalt binders in steady and dynamic shear domain. Mech. Time-Depend. Mater. 2018, 22, 67–78. [Google Scholar] [CrossRef]
- Polacco, G.; Stastna, J.; Vlachovicova, Z.; Biondi, D.; Zanzotto, L. Temporary networks in polymer-modified asphalts. Polym. Eng. Sci. 2004, 44, 9. [Google Scholar] [CrossRef]
- Syroezhko, A.M.; Begak, O.Y.; Fedorov, V.V.; Gusarova, E.N. Modification of paving asphalts with sulfur. Russ. J. Appl. Chem. 2003, 76, 6. [Google Scholar]
- Baldino, N.; Gabriele, D.; Lupi, F.R.; Oliviero, C.; Rossi, P.; Caputo, T. Falvo, Rheological effects on bitumen of polyphosphoric acid (PPA) addition. Constr. Build. Mater. 2013, 40, 397–404. [Google Scholar] [CrossRef]
- Wagner, C.E.; Barbati, A.C.; Engmann, J.; Burbidge, A.S.; McKinley, G.H. Apparent shear thickening at low shear rates in polymer solutions can be an artifact of non-equilibration. Appl. Rheol. 2016, 26. [Google Scholar] [CrossRef]
- Chen, H.-X.; Zhang, E.-S.; Hong, M.; Liu, W.; Dai, X.-M.; Chen, Q.; Qiu, X.-P.; Ji, X.-L. Molecular weight dependence of associative behavior in polyimide/dmf solutions. Chin. J. Polym. Sci. 2020, 38, 629–637. [Google Scholar] [CrossRef]


Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Jasso, M.; Polacco, G.; Zanzotto, L. Shear Viscosity Overshoots in Polymer Modified Asphalts. Materials 2022, 15, 7551. https://doi.org/10.3390/ma15217551
Jasso M, Polacco G, Zanzotto L. Shear Viscosity Overshoots in Polymer Modified Asphalts. Materials. 2022; 15(21):7551. https://doi.org/10.3390/ma15217551
Chicago/Turabian StyleJasso, Martin, Giovanni Polacco, and Ludovit Zanzotto. 2022. "Shear Viscosity Overshoots in Polymer Modified Asphalts" Materials 15, no. 21: 7551. https://doi.org/10.3390/ma15217551
APA StyleJasso, M., Polacco, G., & Zanzotto, L. (2022). Shear Viscosity Overshoots in Polymer Modified Asphalts. Materials, 15(21), 7551. https://doi.org/10.3390/ma15217551






