Development of Shrimp Freshness Indicating Films by Embedding Anthocyanins-Rich Rhododendron simsii Flower Extract in Locust Bean Gum/Polyvinyl Alcohol Matrix
Abstract
:1. Introduction
2. Materials and Methods
2.1. Material and Reagents
2.2. Extraction and Purification of Anthocyanins
2.3. Preparation of Films
2.4. Structural Characterization of Films
2.5. Determination of Physical and Functional Properties of Films
2.5.1. Thickness
2.5.2. Color
2.5.3. Light Transmittance
2.5.4. Water Vapor Permeability (WVP)
2.5.5. Oxygen Permeability (OP)
2.5.6. Water Contact Angle (WCA)
2.5.7. Mechanical Properties
2.5.8. Thermogravimetric Analysis (TGA)
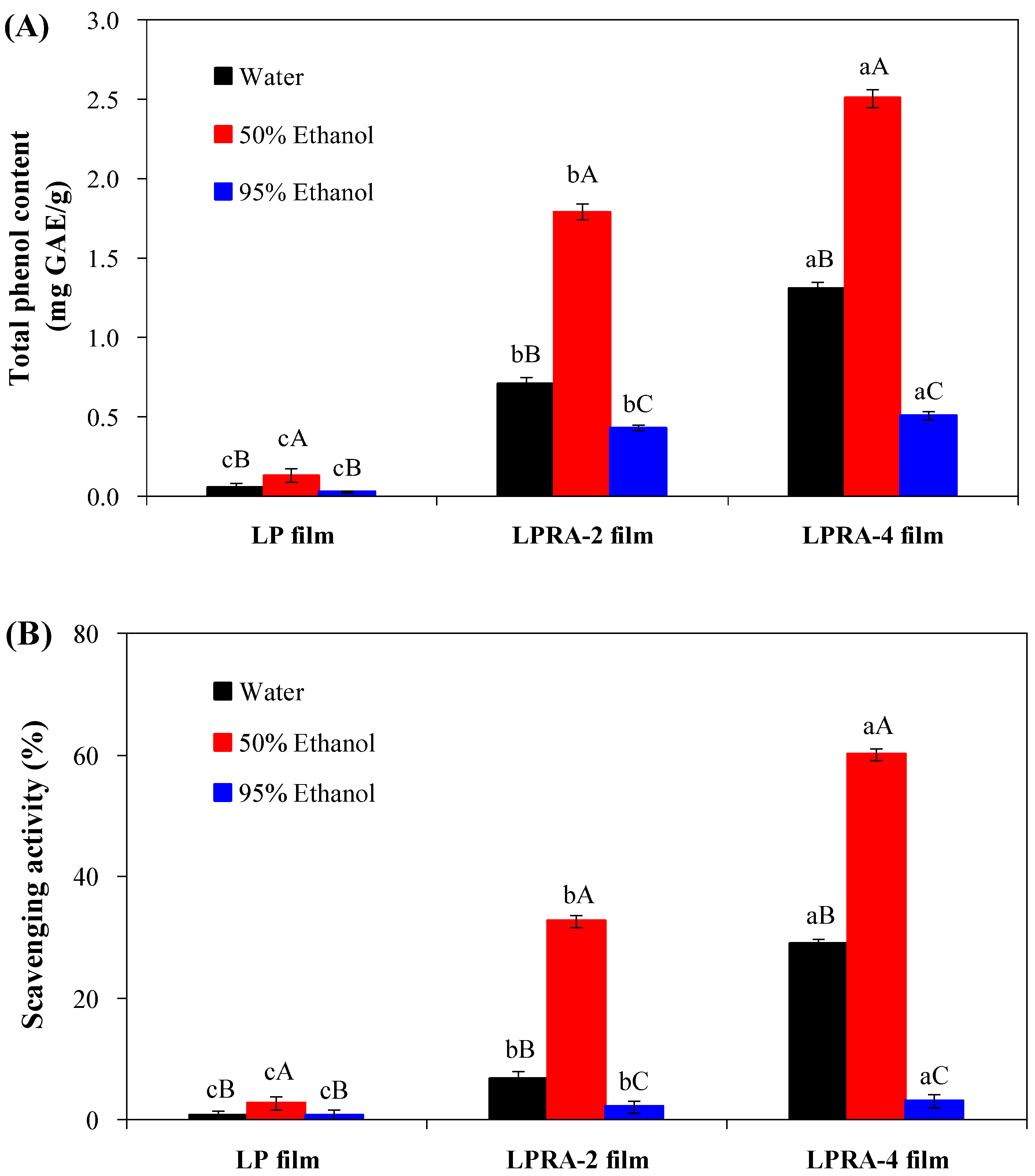
2.5.9. Antioxidant Activity
2.5.10. pH-Sensitivity and Ammonia-Sensitivity
2.6. Application of Films
2.7. Statistical Analysis
3. Results and Discussion
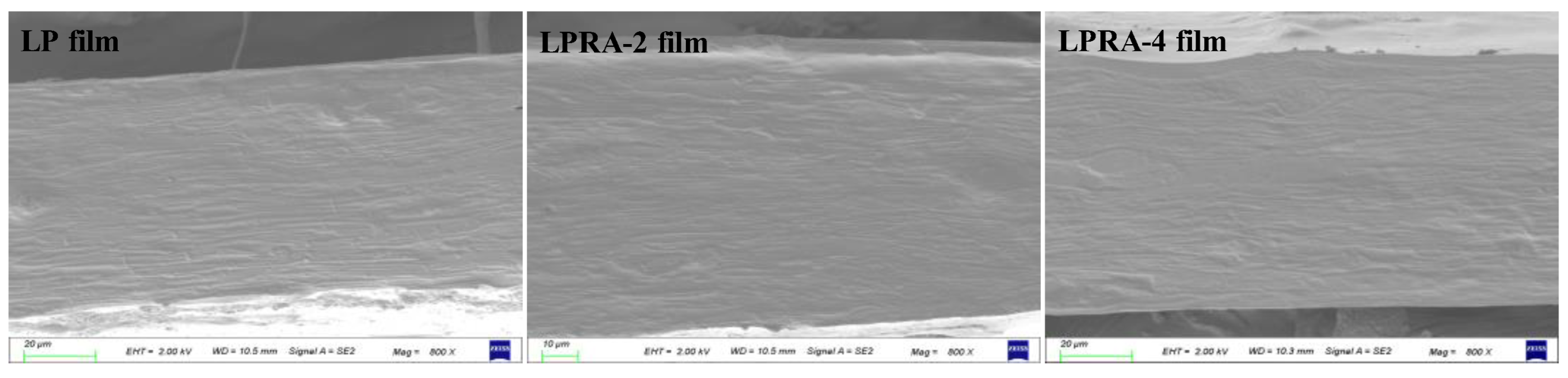
3.1. Cross-Sectional Microstructure
3.2. FT-IR Spectra
3.3. Thickness
3.4. Color
3.5. Light Transmittance
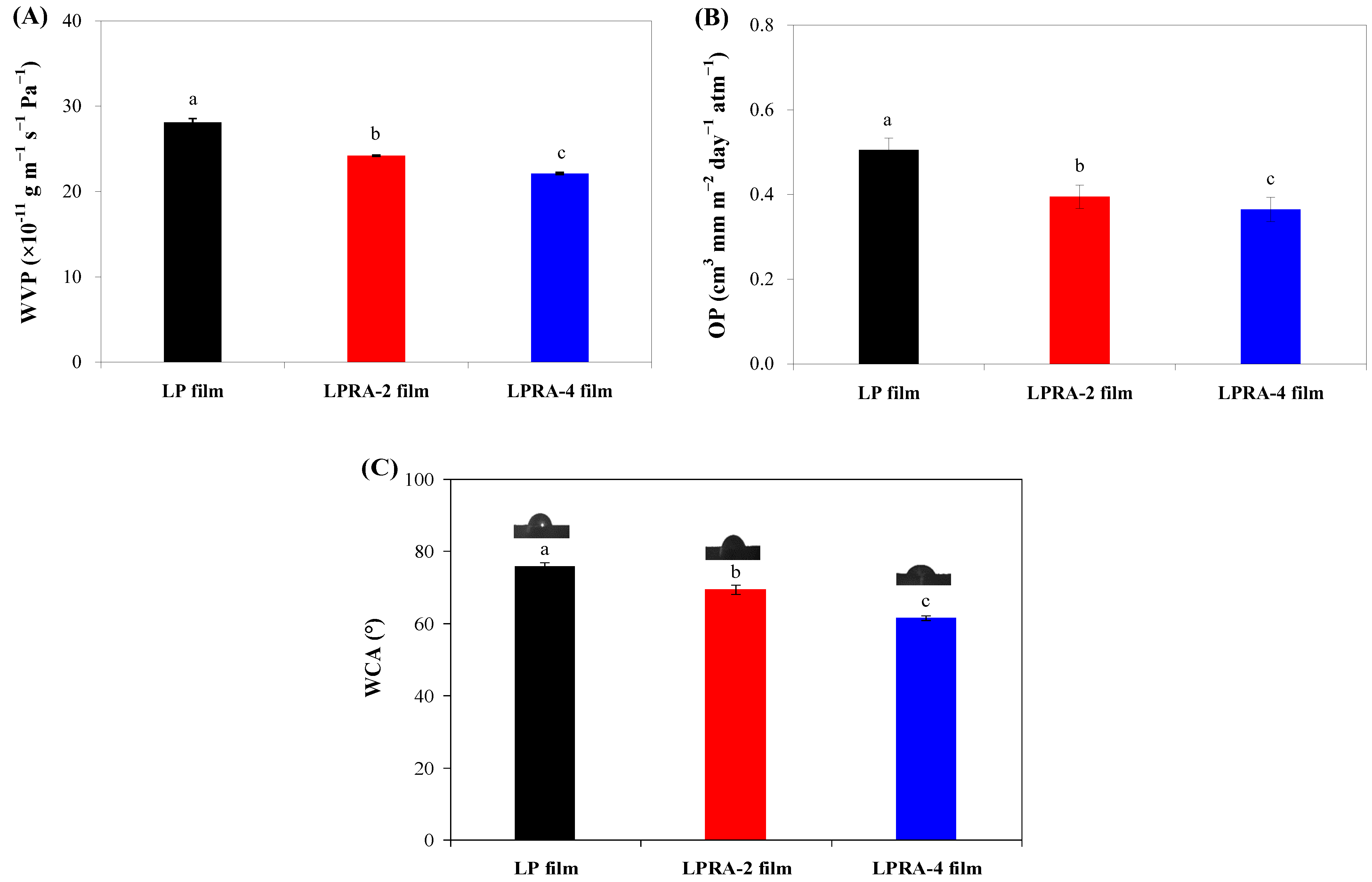
3.6. WVP
3.7. OP
3.8. WCA
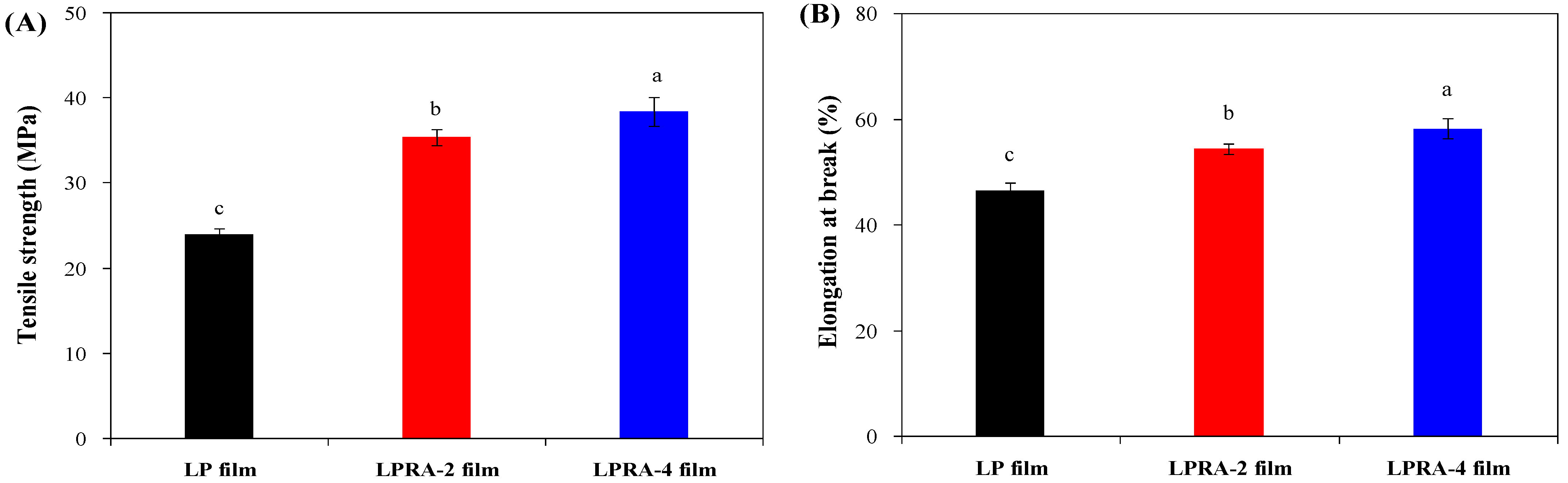
3.9. Mechanical Properties
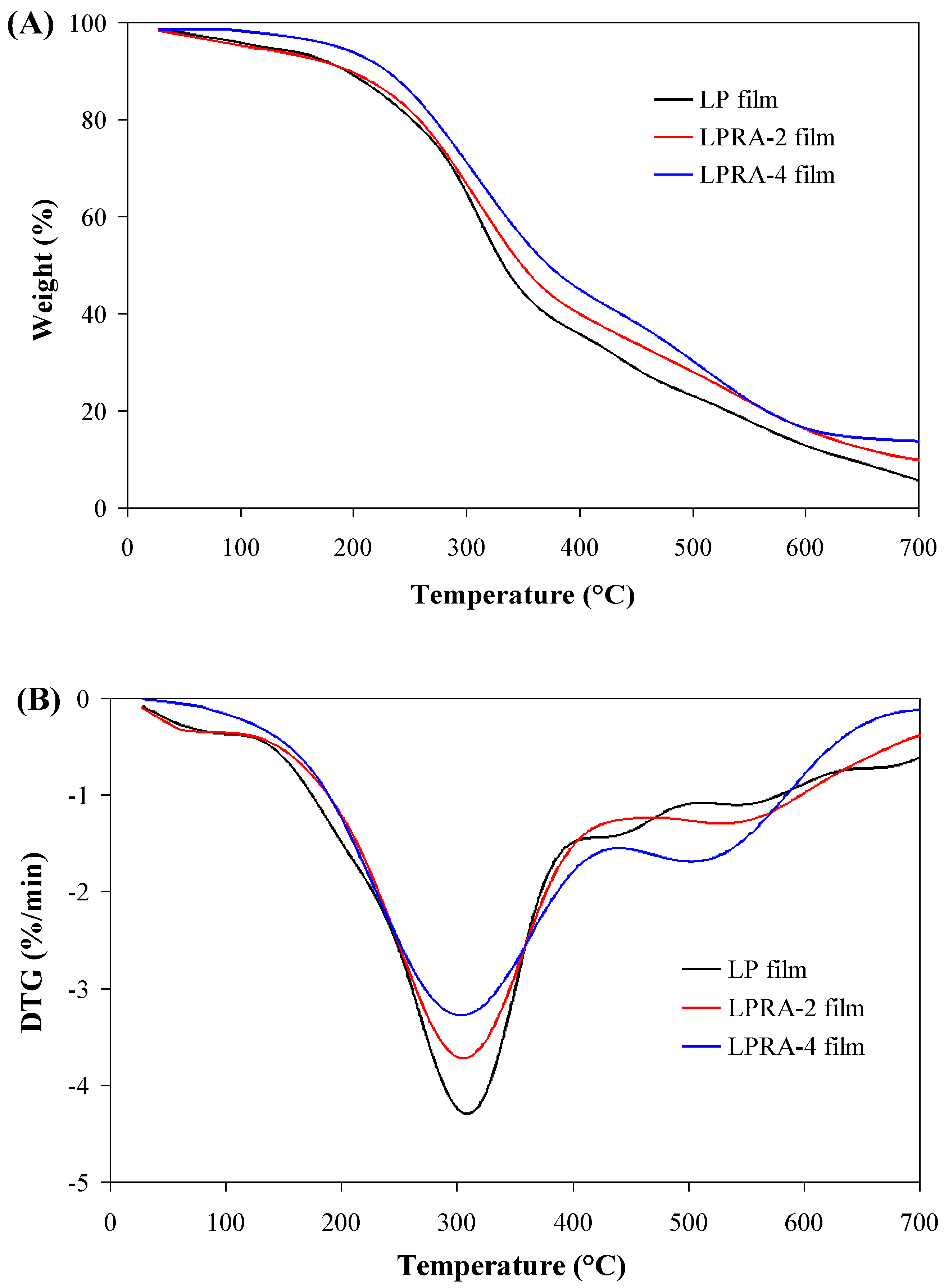
3.10. Thermal Stability
3.11. Antioxidant Activity
3.12. pH Sensitivity and Ammonia Sensitivity
3.13. Application of Films
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Bhargava, N.; Sharanagat, V.S.; Mor, R.S.; Kumar, K. Active and intelligent biodegradable packaging films using food and food waste–derived bioactive compounds: A review. Trends Food Sci. Technol. 2020, 105, 385–401. [Google Scholar] [CrossRef]
- Realini, C.E.; Marcos, B. Active and intelligent packaging systems for a modern society. Meat Sci. 2014, 98, 404–419. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Peralta, J.; Bitencourt-Cervi, C.M.; Maciel, V.B.; Yoshida, C.M.; Carvalho, R.A. Aqueous hibiscus extract as a potential natural pH indicator incorporated in natural polymeric films. Food Packag. Shelf Life 2019, 19, 47–55. [Google Scholar] [CrossRef]
- Yong, H.; Liu, J. Recent advances in the preparation, physical and functional properties, and applications of anthocyanins-based active and intelligent packaging films. Food Packag. Shelf Life 2020, 26, 100550. [Google Scholar] [CrossRef]
- Cheng, M.; Yan, X.; Cui, Y.; Han, M.; Wang, X.; Wang, J.; Zhang, R. An eco-friendly film of pH-responsive indicators for smart packaging. J. Food Eng. 2022, 321, 110943. [Google Scholar] [CrossRef]
- Gupta, V.; Biswas, D.; Roy, S. A Comprehensive Review of biodegradable polymer-based films and coatings and their food packaging applications. Materials 2022, 15, 5899. [Google Scholar] [CrossRef]
- Zheng, L.; Liu, L.; Yu, J.; Shao, P. Novel trends and applications of natural pH-responsive indicator film in food packaging for improved quality monitoring. Food Control 2022, 134, 108769. [Google Scholar] [CrossRef]
- Boccalon, E.; Viscusi, G.; Lamberti, E.; Fancello, F.; Zara, S.; Sassi, P.; Marinozzi, M.; Nocchetti, M.; Gorrasi, G. Composite films containing red onion skin extract as intelligent pH indicators for food packaging. Appl. Surf. Sci. 2022, 593, 153319. [Google Scholar] [CrossRef]
- Capello, C.; Leandro, G.C.; Gagliardi, T.R.; Valencia, G.A. Intelligent Films from chitosan and biohybrids based on anthocyanins and Laponite®: Physicochemical properties and food packaging applications. J. Polym. Environ. 2021, 29, 3988–3999. [Google Scholar] [CrossRef]
- Lozano-Navarro, J.I.; Díaz-Zavala, N.P.; Velasco-Santos, C.; Melo-Banda, J.A.; Páramo-García, U.; Paraguay-Delgado, F.; García-Alamilla, R.; Martínez-Hernández, A.L.; Zapién-Castillo, S. Chitosan-starch films with natural extracts: Physical, chemical, morphological and thermal properties. Materials 2018, 11, 120. [Google Scholar] [CrossRef]
- Zhao, L.; Liu, Y.; Zhao, L.; Wang, Y. Anthocyanin-based pH-sensitive smart packaging films for monitoring food freshness. J. Agric. Food Res. 2022, 9, 100340. [Google Scholar] [CrossRef]
- Kan, J.; Liu, J.; Xu, F.; Yun, D.; Yong, H.; Liu, J. Development of pork and shrimp freshness monitoring labels based on starch/polyvinyl alcohol matrices and anthocyanins from 14 plants: A comparative study. Food Hydrocoll. 2022, 124, 107293. [Google Scholar] [CrossRef]
- Yong, H.; Liu, J.; Kan, J.; Liu, J. Active/intelligent packaging films developed by immobilizing anthocyanins from purple sweetpotato and purple cabbage in locust bean gum, chitosan and κ-carrageenan-based matrices. Int. J. Biol. Macromol. 2022, 211, 238–248. [Google Scholar] [CrossRef] [PubMed]
- Qin, Y.; Yun, D.; Xu, F.; Chen, D.; Kan, J.; Liu, J. Smart packaging films based on starch/polyvinyl alcohol and Lycium ruthenicum anthocyanins-loaded nano-complexes: Functionality, stability and application. Food Hydrocoll. 2021, 119, 106850. [Google Scholar] [CrossRef]
- Boonsiriwit, A.; Lee, M.; Kim, M.; Inthamat, P.; Siripatrawan, U.; Lee, Y.S. Hydroxypropyl methylcellulose/microcrystalline cellulose biocomposite film incorporated with butterfly pea anthocyanin as a sustainable pH-responsive indicator for intelligent food-packaging applications. Food Biosci. 2021, 44, 101392. [Google Scholar] [CrossRef]
- Kang, S.; Wang, H.; Xia, L.; Chen, M.; Li, L.; Cheng, J.; Li, X.; Jiang, S. Colorimetric film based on polyvinyl alcohol/okra mucilage polysaccharide incorporated with rose anthocyanins for shrimp freshness monitoring. Carbohydr. Polym. 2020, 229, 115402. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.; Zou, X.; Zhai, X.; Huang, X.; Jiang, C.; Holmes, M. Preparation of an intelligent pH film based on biodegradable polymers and roselle anthocyanins for monitoring pork freshness. Food Chem. 2019, 272, 306–312. [Google Scholar] [CrossRef]
- Gautam, V.; Sharma, A.; Arora, S.; Bhardwaj, R.; Ahmad, A.; Ahamad, B.; Ahmad, P. In-vitro antioxidant, antimutagenic and cancer cell growth inhibition activities of Rhododendron arboreum leaves and flowers. Saudi J. Biol. Sci. 2020, 27, 1788–1796. [Google Scholar] [CrossRef]
- Heursel, J. Diversity of flower colours in Rhododendron simsii Planch. and prospects for breeding. Euphytica 1981, 30, 9–14. [Google Scholar] [CrossRef]
- Cheng, X.; Zhang, J.; Chen, Z. Effects of total flavone from Rhododendron simsii Planch. flower on postischemic cardiac dysfunction and cardiac remodeling in rats. Evidence-Based Integr. Med. 2017, 2017, 5389272. [Google Scholar]
- Jiao, Y.; Fan, Y.F.; Wang, Y.L.; Zhang, J.Y.; Chen, S.; Chen, Z.W. Protective effect and mechanism of total flavones from Rhododendron simsii planch flower on cultured rat cardiomyocytes with anoxia and reoxygenation. Evidence-Based Integr. Med. 2015, 2015, 863531. [Google Scholar]
- Hang, N.T.T.; Miyajima, I.; Ureshino, K.; Kobayashi, N.; Kurashige, Y.; Matsui, T.; Okubo, H. Anthocyanins of wild Rhododendron simsii Planch. flowers in Vietnam and Japan. J. Jpn. Soc. Hortic. Sci. 2011, 80, 206–213. [Google Scholar] [CrossRef]
- Huyen, D.T.T.; Ureshino, K.; Van, D.T.; Miyajima, I. Co-pigmentation of anthocyanin-flavonol in the blotch area of Rhododendron simsii Planch. flowers. Hort. J. 2016, 85, 232–237. [Google Scholar] [CrossRef] [Green Version]
- Yun, D.; He, Y.; Zhu, H.; Hui, Y.; Li, C.; Chen, D.; Liu, J. Smart packaging films based on locust bean gum, polyvinyl alcohol, the crude extract of Loropetalum chinense var. rubrum petals and its purified fractions. Int. J. Biol. Macromol. 2022, 205, 141–153. [Google Scholar] [CrossRef] [PubMed]
- Halász, K.; Csóka, L. Black chokeberry (Aronia melanocarpa) pomace extract immobilized in chitosan for colorimetric pH indicator film application. Food Packag. Shelf Life 2018, 16, 185–193. [Google Scholar] [CrossRef]
- Wu, Y.; Tang, P.; Quan, S.; Zhang, H.; Wang, K.; Liu, J. Preparation, characterization and application of smart packaging films based on locust bean gum/polyvinyl alcohol blend and betacyanins from cockscomb (Celosia cristata L.) flower. Int. J. Biol. Macromol. 2021, 191, 679–688. [Google Scholar] [CrossRef] [PubMed]
- Luo, Y.; Liu, H.; Yang, S.; Zeng, J.; Wu, Z. Sodium alginate-based green packaging films functionalized by guava leaf extracts and their bioactivities. Materials 2019, 12, 2923. [Google Scholar] [CrossRef] [Green Version]
- Qin, Y.; Liu, Y.; Yuan, L.; Yong, H.; Liu, J. Preparation and characterization of antioxidant, antimicrobial and pH-sensitive films based on chitosan, silver nanoparticles and purple corn extract. Food Hydrocoll. 2019, 96, 102–111. [Google Scholar] [CrossRef]
- Bi, F.; Zhang, X.; Liu, J.; Yong, H.; Gao, L.; Liu, J. Development of antioxidant and antimicrobial packaging films based on chitosan, D-α-tocopheryl polyethylene glycol 1000 succinate and silicon dioxide nanoparticles. Food Packag. Shelf Life 2020, 24, 100503. [Google Scholar] [CrossRef]
- Żołek-Tryznowska, Z.; Kałuża, A. The influence of starch origin on the properties of starch films: Packaging performance. Materials 2021, 14, 1146. [Google Scholar] [CrossRef]
- Yao, X.; Liu, J.; Hu, H.; Yun, D.; Liu, J. Development and comparison of different polysaccharide/PVA-based active/intelligent packaging films containing red pitaya betacyanins. Food Hydrocoll. 2022, 124, 107305. [Google Scholar] [CrossRef]
- Sani, M.A.; Tavassoli, M.; Salim, S.A.; Azizi-lalabadi, M.; McClements, D.J. Development of green halochromic smart and active packaging materials: TiO2 nanoparticle-and anthocyanin-loaded gelatin/κ-carrageenan films. Food Hydrocoll. 2022, 124, 107324. [Google Scholar] [CrossRef]
- Jiang, G.; Hou, X.; Zeng, X.; Zhang, C.; Wu, H.; Shen, G.; Li, S.; Luo, Q.; Li, M.; Liu, X.; et al. Preparation and characterization of indicator films from carboxymethyl-cellulose/starch and purple sweet potato (Ipomoea batatas (L.) lam) anthocyanins for monitoring fish freshness. Int. J. Biol. Macromol. 2020, 143, 359–372. [Google Scholar] [CrossRef] [PubMed]
- Yun, D.; Cai, H.; Liu, Y.; Xiao, L.; Song, J.; Liu, J. Development of active and intelligent films based on cassava starch and Chinese bayberry (Myrica rubra Sieb. et Zucc.) anthocyanins. RSC Adv. 2019, 9, 30905–30916. [Google Scholar] [CrossRef] [Green Version]
- Qin, Y.; Liu, Y.; Yong, H.; Liu, J.; Zhang, X.; Liu, J. Preparation and characterization of active and intelligent packaging films based on cassava starch and anthocyanins from Lycium ruthenicum Murr. Int. J. Biol. Macromol. 2019, 134, 80–90. [Google Scholar] [CrossRef]
- Yuan, L.; Wu, Y.; Qin, Y.; Yong, H.; Liu, J. Recent advances in the preparation, characterization and applications of locust bean gum-based films. J. Renew. Mater. 2020, 8, 1565–1579. [Google Scholar] [CrossRef]
- Liu, J.; Meng, C.G.; Liu, S.; Kan, J.; Jin, C.H. Preparation and characterization of protocatechuic acid grafted chitosan films with antioxidant activity. Food Hydrocoll. 2017, 63, 457–466. [Google Scholar] [CrossRef]
- Zhang, X.; Liu, J.; Qian, C.; Kan, J.; Jin, C. Effect of grafting method on the physical property and antioxidant potential of chitosan film functionalized with gallic acid. Food Hydrocoll. 2019, 89, 1–10. [Google Scholar] [CrossRef]
- Zhai, X.; Shi, J.; Zou, X.; Wang, S.; Jiang, C.; Zhang, J.; Huang, X.; Zhang, W.; Holmes, M. Novel colorimetric films based on starch/polyvinyl alcohol incorporated with roselle anthocyanins for fish freshness monitoring. Food Hydrocoll. 2017, 69, 308–317. [Google Scholar] [CrossRef] [Green Version]
- Chen, S.; Wu, M.; Lu, P.; Gao, L.; Yan, S.; Wang, S. Development of pH indicator and antimicrobial cellulose nanofibre packaging film based on purple sweet potato anthocyanin and oregano essential oil. Int. J. Biol. Macromol. 2020, 149, 271–280. [Google Scholar] [CrossRef]
- Gasti, T.; Dixit, S.; D’souza, O.J.; Hiremani, V.D.; Vootla, S.K.; Masti, S.P.; Chougale, R.B.; Malabadi, R.B. Smart biodegradable films based on chitosan/methylcellulose containing Phyllanthus reticulatus anthocyanin for monitoring the freshness of fish fillet. Int. J. Biol. Macromol. 2020, 187, 451–461. [Google Scholar] [CrossRef] [PubMed]
- Gutiérrez, T.J.; Alvarez, V.A. Bionanocomposite films developed from corn starch and natural and modified nano-clays with or without added blueberry extract. Food Hydrocoll. 2018, 77, 407–420. [Google Scholar] [CrossRef]
- Amaregouda, Y.; Kamanna, K.; Gasti, T. Fabrication of intelligent/active films based on chitosan/polyvinyl alcohol matrices containing Jacaranda cuspidifolia anthocyanin for real-time monitoring of fish freshness. Int. J. Biol. Macromol. 2022, 218, 799–815. [Google Scholar] [CrossRef] [PubMed]
- Sohany, M.; Tawakkal, I.S.M.A.; Ariffin, S.H.; Shah, N.N.A.K.; Yusof, Y.A. Characterization of anthocyanin associated purple sweet potato starch and peel-based pH indicator films. Foods 2021, 10, 2005. [Google Scholar] [CrossRef] [PubMed]
- Shahid, M.; Mohammad, F. Recent advancements in natural dye applications: A review. J. Clean. Prod. 2013, 53, 310–331. [Google Scholar] [CrossRef]
- Yong, H.; Wang, X.; Zhang, X.; Liu, Y.; Qin, Y.; Liu, J. Effects of anthocyanin-rich purple and black eggplant extracts on the physical, antioxidant and pH-sensitive properties of chitosan film. Food Hydrocoll. 2019, 94, 93–104. [Google Scholar] [CrossRef]
- Yong, H.; Liu, J. Active packaging films and edible coatings based on polyphenol-rich propolis extract: A review. Compr. Rev. Food Sci. Food Saf. 2021, 20, 2106–2145. [Google Scholar] [CrossRef]




| Film | Appearance | Thickness (mm) | L* | a* | b* | ΔE |
|---|---|---|---|---|---|---|
| LP film |  | 0.090 ± 0.003 a | 88.95 ± 0.06 a | −0.71 ± 0.01 c | 0.54 ± 0.01 b | 1.65 ± 0.06 c |
| LPRA-2 film |  | 0.089 ± 0.004 a | 68.91 ± 0.87 b | 7.66 ± 0.53 b | 16.20 ± 1.07 a | 28.41 ± 1.44 b |
| LPRA-4 film |  | 0.087 ± 0.002 b | 59.60 ± 1.08 c | 10.61 ± 0.57 a | 18.05 ± 0.34 a | 37.62 ± 1.22 a |
| Time (day) | TVB-N Level (mg/100 g) | LP film | LPRA-2 film | LPRA-4 film |
|---|---|---|---|---|
| 0 | 5.10 ± 0.31 g |  |  |  |
| 1 | 11.83 ± 0.25 f |  |  |  |
| 2 | 17.38 ± 0.12 e |  |  |  |
| 3 | 25.93 ± 0.57 d |  |  |  |
| 4 | 33.59 ± 0.13 c |  |  |  |
| 5 | 41.68 ± 0.23 b |  |  |  |
| 6 | 56.35 ± 0.19 a |  |  |  |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Li, C.; Yun, D.; Wang, Z.; Xu, F.; Tang, C.; Liu, J. Development of Shrimp Freshness Indicating Films by Embedding Anthocyanins-Rich Rhododendron simsii Flower Extract in Locust Bean Gum/Polyvinyl Alcohol Matrix. Materials 2022, 15, 7557. https://doi.org/10.3390/ma15217557
Li C, Yun D, Wang Z, Xu F, Tang C, Liu J. Development of Shrimp Freshness Indicating Films by Embedding Anthocyanins-Rich Rhododendron simsii Flower Extract in Locust Bean Gum/Polyvinyl Alcohol Matrix. Materials. 2022; 15(21):7557. https://doi.org/10.3390/ma15217557
Chicago/Turabian StyleLi, Chenchen, Dawei Yun, Zeyu Wang, Fengfeng Xu, Chao Tang, and Jun Liu. 2022. "Development of Shrimp Freshness Indicating Films by Embedding Anthocyanins-Rich Rhododendron simsii Flower Extract in Locust Bean Gum/Polyvinyl Alcohol Matrix" Materials 15, no. 21: 7557. https://doi.org/10.3390/ma15217557
APA StyleLi, C., Yun, D., Wang, Z., Xu, F., Tang, C., & Liu, J. (2022). Development of Shrimp Freshness Indicating Films by Embedding Anthocyanins-Rich Rhododendron simsii Flower Extract in Locust Bean Gum/Polyvinyl Alcohol Matrix. Materials, 15(21), 7557. https://doi.org/10.3390/ma15217557







