Investigations on the Phase Transformations, Equilibria and Athermal ω in Ni-Ga-Cr Ternary System
Abstract
:1. Introduction
2. Experimental Details
3. Results and Discussions
3.1. Microstructure Analyses and Phase Identifications
3.2. Observations of the ω Phase
3.3. Determination of the Phase Boundary
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Conflicts of Interest
References
- Yeh, J.W.; Chen, S.K.; Lin, S.J.; Gan, J.Y.; Chin, T.S.; Shun, T.T.; Tsau, C.H.; Chang, S.Y. Nanostructured high-entropy alloys with multiple principal elements: Novel alloy design concepts and outcomes. Adv. Eng. Mater. 2004, 6, 299–303. [Google Scholar] [CrossRef]
- Cantor, B.; Chang, I.T.H.; Knight, P.; Vincent, A.J.B. Microstructural development in equiatomic multicomponent alloys. Mat. Sci. Eng. A Struct. 2004, 375, 213–218. [Google Scholar] [CrossRef]
- Yang, T.; Zhao, Y.L.; Tong, Y.; Jiao, Z.B.; Wei, J.; Cai, J.X.; Han, X.D.; Chen, D.; Hu, A.; Kai, J.J.; et al. Multicomponent intermetallic nanoparticles and superb mechanical behaviors of complex alloys. Science 2018, 362, 933–937. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Miracle, D.B.; Senkov, O.N. A critical review of high entropy alloys and related concepts. Acta Mater. 2017, 122, 448–511. [Google Scholar] [CrossRef] [Green Version]
- Ye, Y.F.; Wang, Q.; Lu, J.; Liu, C.T.; Yang, Y. High-entropy alloy: Challenges and prospects. Mater. Today 2016, 19, 349–362. [Google Scholar] [CrossRef]
- Chen, J.; Zhou, X.Y.; Wang, W.L.; Liu, B.; Lv, Y.K.; Yang, W.; Xu, D.P.; Liu, Y. A review on fundamental of high entropy alloys with promising high–temperature properties. J. Alloy. Compd. 2018, 760, 15–30. [Google Scholar] [CrossRef]
- Oikawa, K.; Ota, T.; Gejima, F.; Ohmori, T.; Kainuma, R.; Ishida, K. Phase equilibria and phase transformations in new B2-type ferromagnetic shape memory alloys of Co-Ni-Ga and Co-Ni-Al systems. Mater. Trans. 2001, 42, 2472–2475. [Google Scholar] [CrossRef] [Green Version]
- Oikawa, K.; Ota, T.; Ohmori, T.; Tanaka, Y.; Morito, H.; Fujita, A.; Kainuma, R.; Fukamichi, K.; Ishida, K. Magnetic and martensitic phase transitions in ferromagnetic Ni-Ga-Fe shape memory alloys. Appl. Phys. Lett. 2002, 81, 5201–5203. [Google Scholar] [CrossRef]
- Gerstein, G.; Firstov, G.S.; Kosorukova, T.A.; Koval, Y.N.; Maier, H.J. Development of B2 shape memory intermetallics beyond NiAl, CoNiAl and CoNiGa. Shape Mem. Superelast. 2018, 4, 360–368. [Google Scholar] [CrossRef]
- Decker, P.; Fortmann, J.; Salomon, S.; Krooß, P.; Niendorf, T.; Ludwig, A. Influence of Cr alloying (1.5 to 5 at.%) on martensitic phase transformation temperatures in Co-Ni-Ga-Cr thin films. Shape Mem. Superelast. 2019, 5, 106–112. [Google Scholar] [CrossRef]
- Andersson, J.O.; Helander, T.; Höglund, L.; Shi, P.; Sundman, B. Thermo-Calc & DICTRA, Computational Tools For Materials Science. Calphad 2002, 26, 273–312. [Google Scholar]
- Nash, P. Phase Diagrams of Binary Nickel Alloys; ASM International: Materials Park, OH, USA, 1991; pp. 75–84. [Google Scholar]
- Okamoto, H. Ga-Ni (Gallium-Nickel). J. Phase Equilib. Diff. 2010, 31, 575–576. [Google Scholar] [CrossRef]
- Belgacem-Bouzida, A.; Djaballah, Y.; Notin, M. Calorimetric measurement of the intermetallic compounds Cr3Ga and CrGa4 and thermodynamic assessment of the (Cr-Ga) system. J. Alloy. Compd. 2005, 397, 155–160. [Google Scholar] [CrossRef]
- Ochial, S.; Oya, Y.; Suzuki, T. Alloying behaviour of Ni3Al, Ni3Ga, Ni3Si and Ni3Ge. Acta Metall. 1984, 32, 289–298. [Google Scholar] [CrossRef]
- Mishima, Y.; Ochiai, S.; Suzuki, T. Lattice parameters of Ni (γ), Ni3Al (γ’) and Ni3Ga (γ’) solid solutions with additions of transition and B-subgroup elements. Acta Metall. 1985, 33, 1161–1169. [Google Scholar] [CrossRef]
- Shao, G.; Miodownik, A.P.; Tsakiropoulos, P. ω-phase formation in V-Al and Ti-Al-V alloys. Philos. Mag. A 1995, 71, 1389–1408. [Google Scholar] [CrossRef]
- Sikka, S.K.; Vohra, Y.K.; Chidambaram, R. Omega phase in materials. Prog. Mater. Sci. 1982, 27, 245–310. [Google Scholar] [CrossRef]
- Okunishi, E.; Kawai, T.; Mitsuhara, M.; Farjami, S.; Itakura, M.; Hara, T.; Nishida, M. HAADF-STEM studies of athermal and isothermal ω-phases in β-Zr alloy. J. Alloy. Compd. 2013, 577, S713–S716. [Google Scholar] [CrossRef]
- Sass, S.L. The structure and decomposition of Zr and Ti bcc solid solutions. J. Less-Common Met. 1972, 28, 157–173. [Google Scholar] [CrossRef]
- Strychor, R.; Williams, J.C.; Soffa, W.A. Phase transformations and modulated microstructures in Ti-Al-Nb alloys. Metall. Trans. A 1988, 19, 225–234. [Google Scholar] [CrossRef]
- Shao, G.; Tsakiropoulos, P. On the ω phase formation in Cr-Al and Ti-Al-Cr alloys. Acta Mater. 2000, 48, 3671–3685. [Google Scholar] [CrossRef]
- Bendersky, L.A.; Boettinger, W.J.; Burton, B.P.; Biancaniello, F.S.; Shoemaker, C.B. The formation of ordered ω-related phases in alloys of composition Ti4Al3Nb. Acta Metall. Mater. 1990, 38, 931–943. [Google Scholar] [CrossRef]
- Dawson, C.W.; Sass, S.L. The as-quenched form of the omega phase in Zr-Nb alloys. Metall. Trans. 1970, 1, 2225–2233. [Google Scholar] [CrossRef]
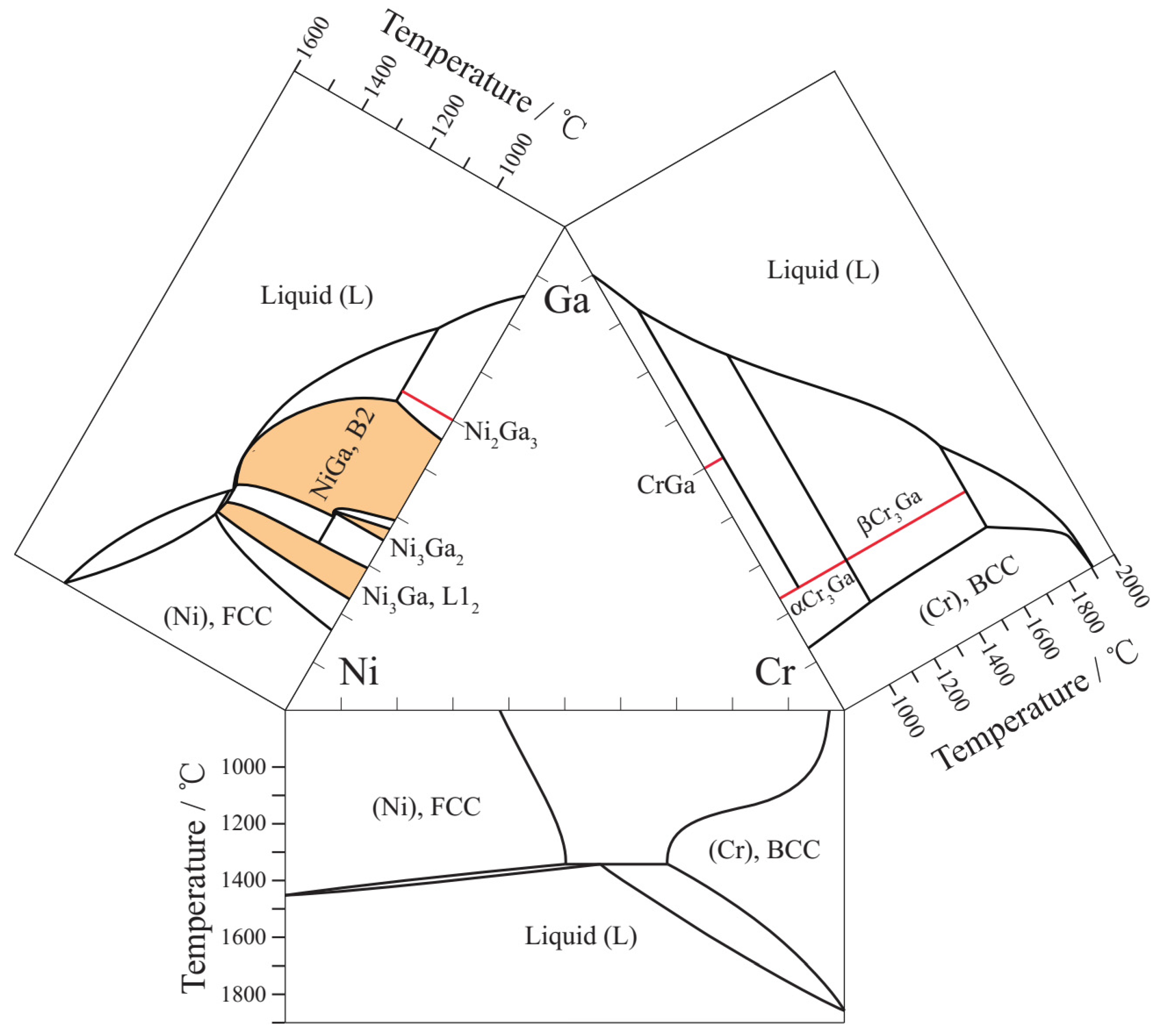

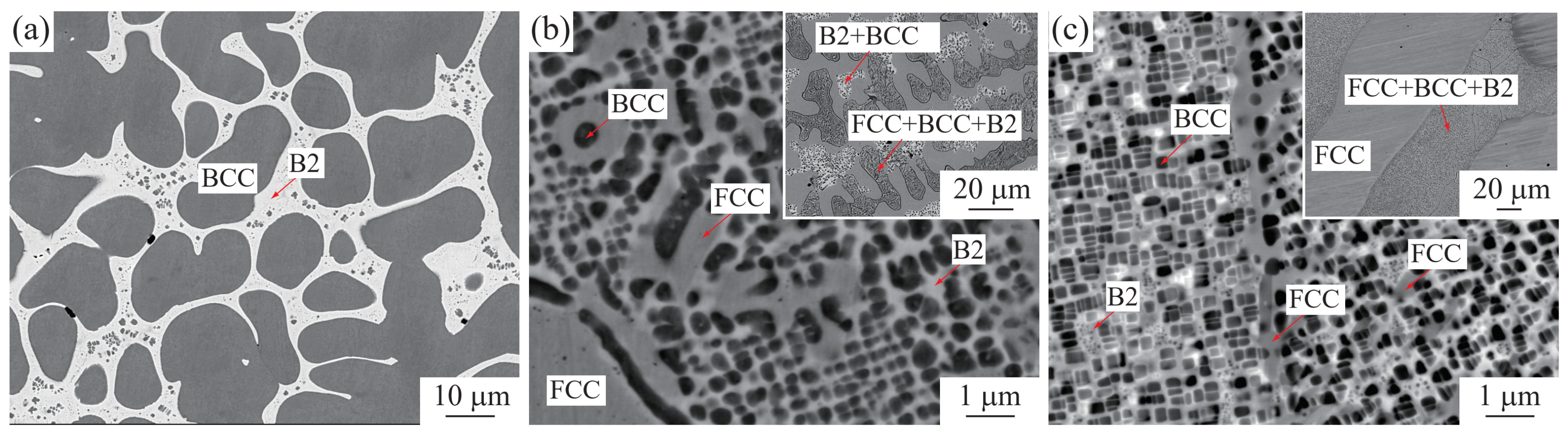


| T/°C | Alloys/at. % (EDS) Cr Balance | Equilibrium Phase (WDS) | Composition/at. % | |||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Phase 1 | Phase 2 | Phase 3 | Phase 1 | Phase 2 | Phase 3 | |||||
| Ni | Ga | Ni | Ga | Ni | Ga | |||||
| 850 | Ni1.34-Ga25.24 | B2 | α-Cr3Ga | 42.66 | 52.1 | 0.67 | 22.46 | |||
| Ni15.19-Ga14.09 | B2 | BCC | 50.27 | 39.89 | 1.12 | 2.27 | ||||
| Ni50.48-Ga15.50 | B2 | L12 | BCC | 60.8 | 28.71 | 67.44 | 20.12 | 1.54 | 0.19 | |
| Ni67.61-Ga27.87 | L12 | B2 | 71.49 | 23.64 | 63.98 | 30.78 | ||||
| Ni14.47-Ga48.11 | L | α-Cr3Ga | Ni2Ga3 | 15.13 | 70.53 | 0.05 | 23.37 | 29.81 | 55.44 | |
| Ni39.91-Ga4.0 | BCC | FCC | 1.81 | 0.03 | 59.66 | 6.6 | ||||
| Ni9.24-Ga20.81 | BCC | α-Cr3Ga | B2 | 0.68 | 7.98 | 0.59 | 22.05 | 44.88 | 45.95 | |
| Ni40.02-Ga29.62 | BCC | B2 | 1.57 | 0.93 | 54.32 | 36.6 | ||||
| Ni66.27-Ga13.64 | L12 | FCC | 69.86 | 16.97 | 65.5 | 12.75 | ||||
| Ni78.94-Ga18.02 | FCC | L12 | 82.13 | 13.63 | 77.84 | 18.44 | ||||
| Ni60.30-Ga13.21 | FCC | BCC | 61.87 | 12.63 | 2.65 | 0.1 | ||||
| Ni73.75-Ga15.99 | FCC | L12 | 73.89 | 10.04 | 75.13 | 15.21 | ||||
| Ni33.23-Ga62.74 | Ni2Ga3 | L | 41.38 | 57.49 | 19.46 | 72.56 | ||||
| Ni6.52-Ga60.09 | α-Cr3Ga | L | 0 | 22.95 | 7.89 | 74.9 | ||||
| Ni46.31-Ga25.36 | B2 | BCC | 58.03 | 31.44 | 1.94 | 0.45 | ||||
| 1000 | Ni18.11-Ga37.27 | α-Cr3Ga | B2 | 0.51 | 22.21 | 36.78 | 47.61 | |||
| Ni16.16-Ga14.37 | BCC | B2 | 1.38 | 3.23 | 47.46 | 36.44 | ||||
| Ni50.20-Ga21.02 | BCC | FCC | B2 | 2.38 | 0.65 | 55.11 | 15.09 | 55.07 | 26.91 | |
| Ni66.65-Ga28.55 | L12 | B2 | 72.22 | 22.84 | 65.91 | 29.34 | ||||
| Ni10.70-Ga49.40 | L | α-Cr3Ga | 17.76 | 65.28 | 0.08 | 22.88 | ||||
| Ni39.61-Ga4.82 | BCC | FCC | 3.43 | 0.12 | 54.74 | 5.98 | ||||
| Ni7.46-Ga17.28 | BCC | B2 | 1.81 | 11.76 | 40.22 | 42.6 | ||||
| Ni42.63-Ga28.48 | BCC | B2 | 1.65 | 1.94 | 51.15 | 34 | ||||
| Ni4.82-Ga47.26 | L | α-Cr3Ga | 10.67 | 68.6 | 0.11 | 22.8 | ||||
| Ni66.37-Ga13.04 | FCC | 66.37 | 13.04 | |||||||
| Ni74.57-Ga22.70 | L12 | 74.57 | 22.7 | |||||||
| Ni77.85-Ga18.47 | FCC | L12 | 81.24 | 14.27 | 78.71 | 17.89 | ||||
| Ni58.65-Ga14.28 | FCC | 58.65 | 14.28 | |||||||
| Ni44.19-Ga26.80 | BCC | B2 | 2.2 | 0.89 | 53.89 | 29.13 | ||||
| Ni32.70-Ga58.53 | L | B2 | 27.41 | 60.59 | 40.54 | 53.51 | ||||
| Ni75.75-Ga18.44 | L12 | 75.75 | 18.44 | |||||||
| Ni72.52-Ga19.79 | L12 | 72.52 | 19.79 | |||||||
| Ni65.0-Ga20.0 | L12 | FCC | 68.45 | 21.02 | 66.52 | 18.08 | ||||
| Ni65.0-Ga21.60 | FCC | B2 | 64.64 | 17.74 | 62.04 | 29.27 | ||||
| Ni26.55-Ga49.10 | L | α-Cr3Ga | B2 | 24.66 | 58.9 | 0.25 | 22.55 | 35.33 | 49.51 | |
| 1150 | Ni19.16-Ga37.33 | β-Cr3Ga | L | 3.21 | 16.37 | 28.48 | 45.74 | |||
| Ni16.54-Ga13.63 | BCC | B2 including tiny BCC (EDS) | 4.31 | 6.14 | 41.03 | 30.96 | ||||
| Ni49.14-Ga21.94 | B2 including tiny BCC (EDS) | FCC | 50.02 | 25.3 | 50.62 | 15.65 | ||||
| Ni70.30-Ga24.02 | B2 | FCC | 67.93 | 27.82 | 72.57 | 19.42 | ||||
| Ni12.87-Ga53.65 | L | 12.87 | 53.65 | 0 | 0 | |||||
| Ni38.75-Ga4.69 | BCC | FCC | 10.68 | 1.8 | 50.34 | 5.54 | ||||
| Ni12.17-Ga20.77 | β-Cr3Ga | L | 3.83 | 12.43 | 34.6 | 43.26 | ||||
| Ni4.63-Ga43.05 | L | β-Cr3Ga | 8.3 | 55.05 | 0.51 | 22.91 | ||||
| Ni74.92-Ga22.46 | L12 | 74.92 | 22.46 | |||||||
| Ni42.23-Ga15.70 | FCC | B2 | BCC | 48.66 | 14.8 | Not determined | Not determined | Not determined | Not determined | |
| Ni17.69-Ga18.96 | BCC | L | 4.23 | 8.33 | 40.04 | 38.98 | ||||
| Ni74.93-Ga14.21 | FCC | 74.93 | 14.21 | |||||||
| Ni67.03-Ga16.88 | FCC | 67.03 | 16.88 | |||||||
| Ni45.98-Ga25.50 | B2 including tiny BCC (EDS) | 45.98 | 25.5 | |||||||
| Ni57.25-Ga27.13 | B2 including tiny BCC | 57.25 | 27.13 | |||||||
| Ni51.96-Ga32.25 | B2 including tiny BCC | 51.96 | 32.25 | |||||||
| Ni63.03-Ga22.96 | FCC | B2 | 64.94 | 17.58 | 62.6 | 27.18 | ||||
| Ni57.26-Ga19.50 | FCC | B2 | 58.59 | 16.68 | 57.72 | 26.2 | ||||
| Ni7.40-Ga2.68 | FCC | B2 including tiny BCC (EDS) | BCC | 48.34 | 15.06 | 45.42 | 22.14 | 4.5 | 1.71 | |
| Ni12.60-Ga2.81 | FCC | BCC | 48.93 | 12.36 | 5.21 | 1.26 | ||||
| Ni49.74-Ga37.35 | B2 | 49.74 | 37.35 | |||||||
| Ni49.89-Ga42.38 | B2 | 49.89 | 42.38 | |||||||
| Ni49.97-Ga39.84 | B2 | 49.97 | 39.84 | |||||||
| Ni45.52-Ga34.92 | B2 including tiny BCC (EDS) | 45.52 | 34.92 | |||||||
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ruan, J.; Chen, Y.; Kobayashi, K.; Ueshima, N.; Oikawa, K. Investigations on the Phase Transformations, Equilibria and Athermal ω in Ni-Ga-Cr Ternary System. Materials 2022, 15, 7617. https://doi.org/10.3390/ma15217617
Ruan J, Chen Y, Kobayashi K, Ueshima N, Oikawa K. Investigations on the Phase Transformations, Equilibria and Athermal ω in Ni-Ga-Cr Ternary System. Materials. 2022; 15(21):7617. https://doi.org/10.3390/ma15217617
Chicago/Turabian StyleRuan, Jingjing, Yuyuan Chen, Kosei Kobayashi, Nobufumi Ueshima, and Katsunari Oikawa. 2022. "Investigations on the Phase Transformations, Equilibria and Athermal ω in Ni-Ga-Cr Ternary System" Materials 15, no. 21: 7617. https://doi.org/10.3390/ma15217617





