Microstructural Characteristics of Al-Ti-B Inoculation Wires and Their Addition to the AlSi7Mg0.3 Alloy
Abstract
:1. Introduction
2. Materials and Methods
3. Results
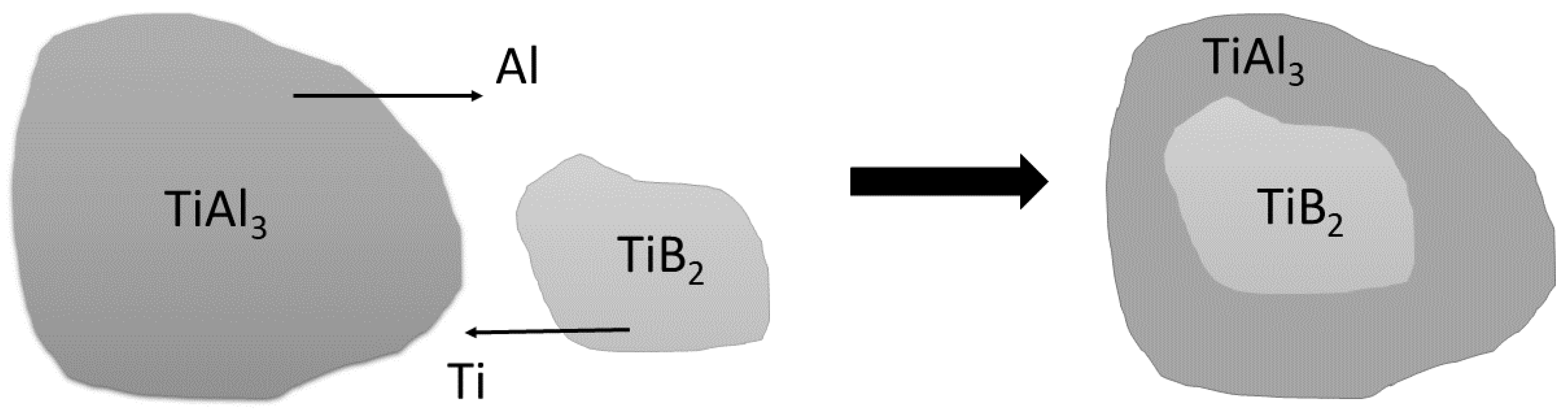
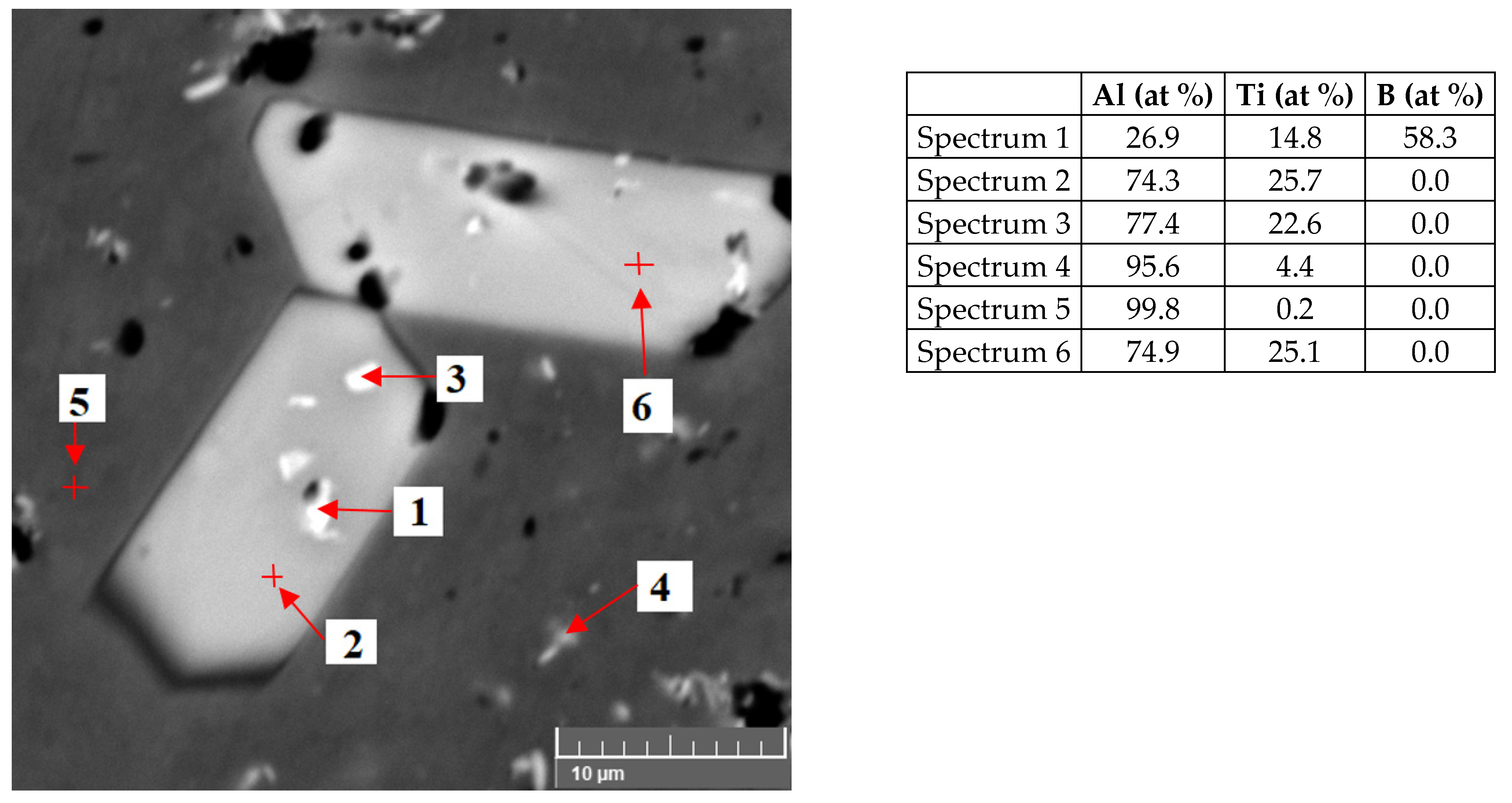
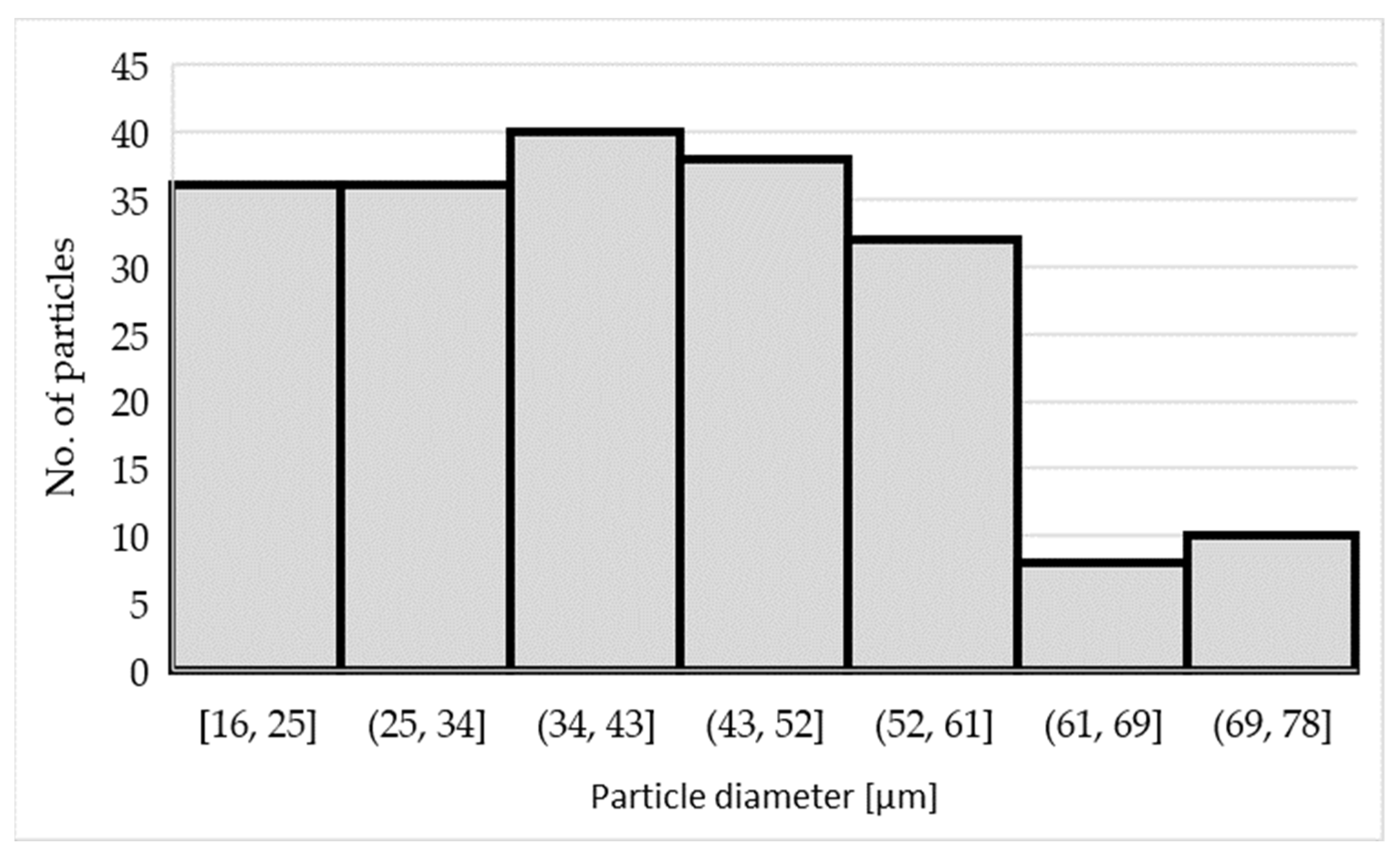
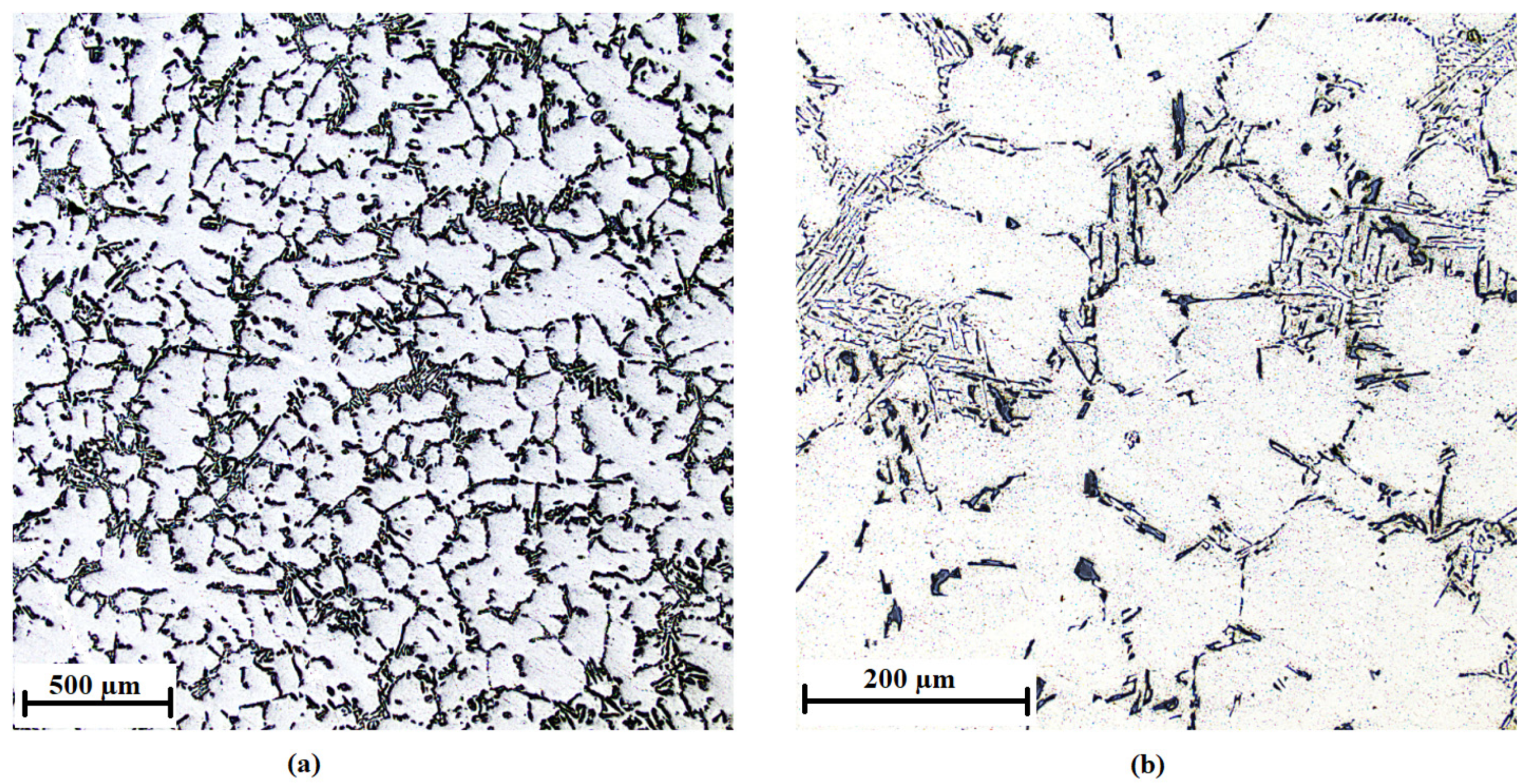
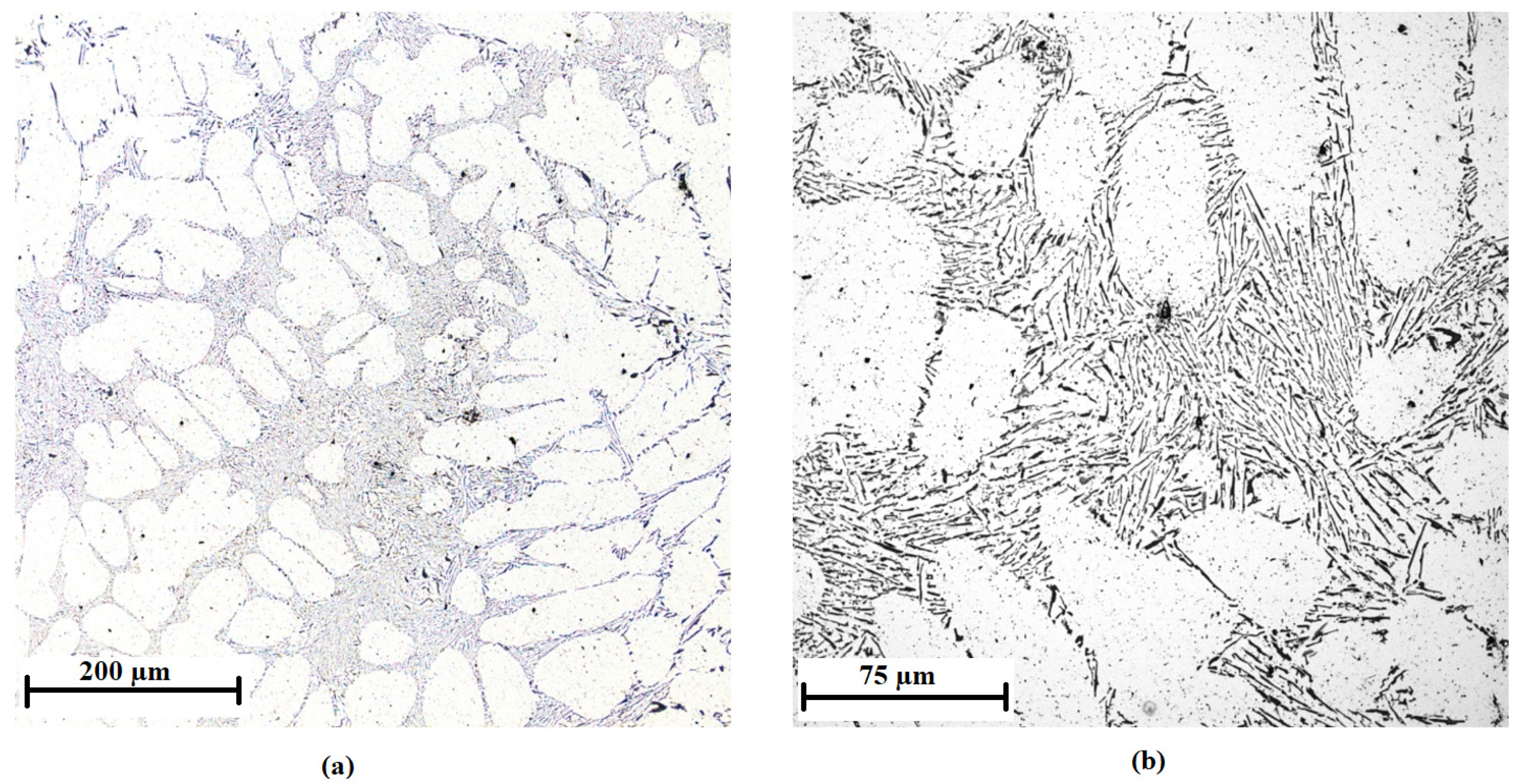
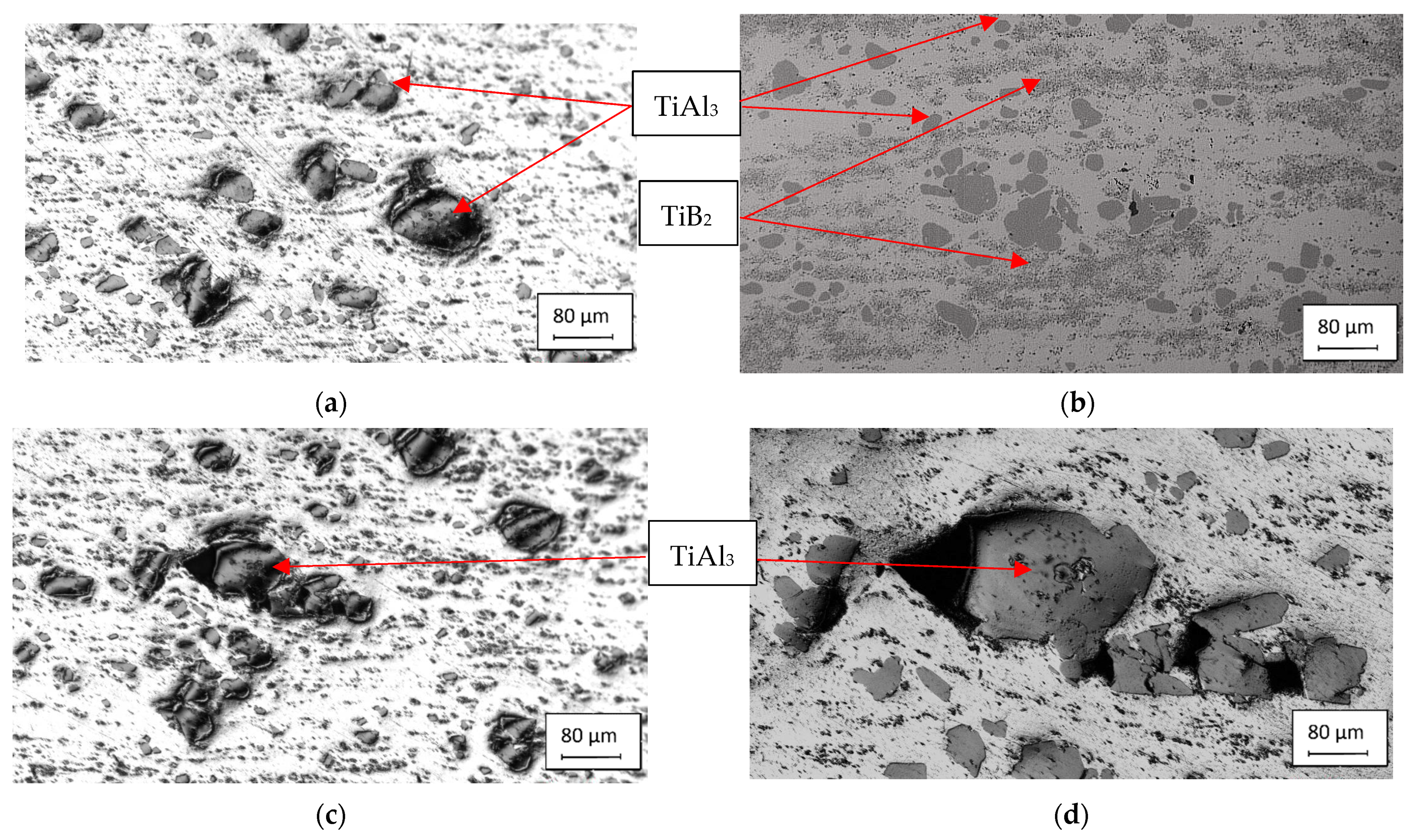
3.1. Characteristics of the Inoculants
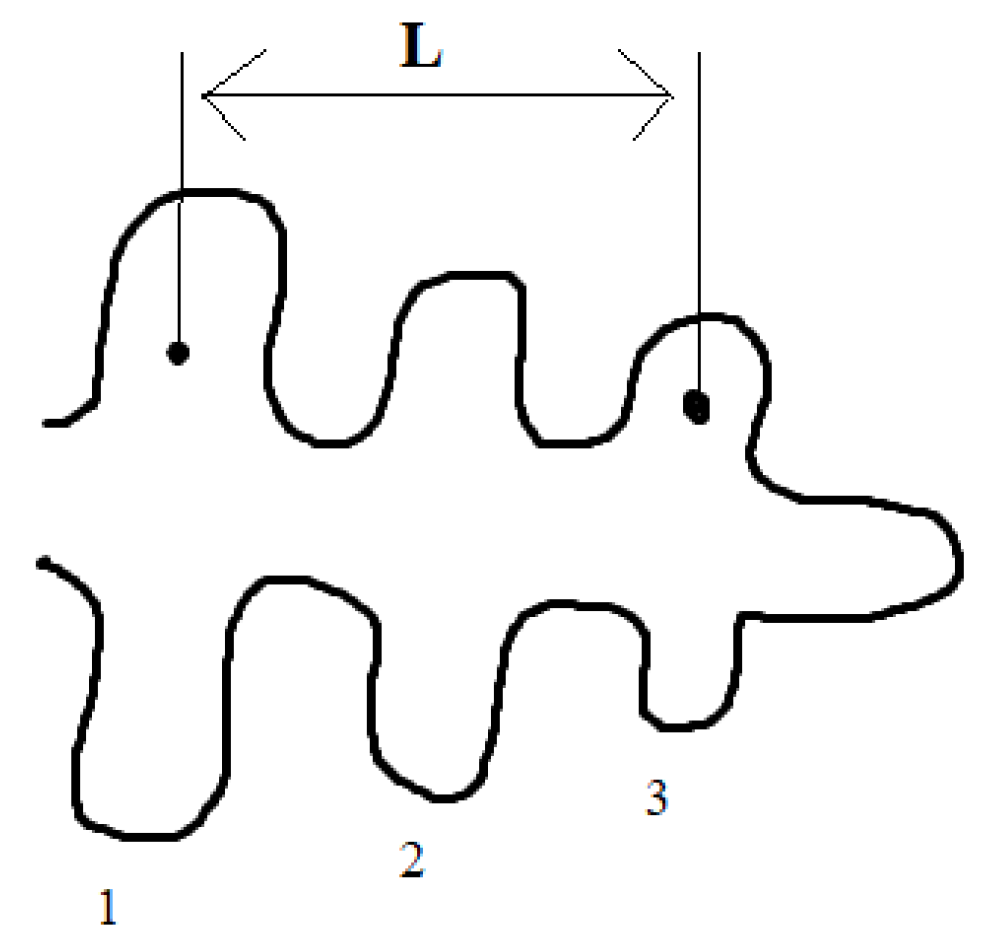
3.2. Optimizing the Inoculant in the AlSi7Mg0.3 Alloy
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Greer, A.L.; Cooper, P.S.; Meredith, M.W.; Schneider, W.; Schumacher, P.; Spittle, J.A.; Tronche, A. Grain Refinement of Aluminium Alloys by Inoculation. Adv. Eng. Mater. 2003, 5, 81–91. [Google Scholar] [CrossRef]
- Sunitha, K.; Gurusami, K. Study of Al-Si alloys grain refinement by inoculation. Mater. Today Proc. 2021, 43, 1825–1829. [Google Scholar] [CrossRef]
- Huang, B.; Liu, Y.; Zhou, Z.; Cheng, W.; Liu, X. Selective laser melting of 7075 aluminum alloy inoculated by Al–Ti–B: Grain refinement and superior mechanical properties. Vacuum 2022, 200, 111030. [Google Scholar] [CrossRef]
- Wannasin, J.; Canyook, R.; Wisutmethangoon, S.; Flemings, M.C. Grain refinement behavior of an aluminum alloy by inoculation and dynamic nucleation. Acta Mater. 2013, 61, 3897–3903. [Google Scholar] [CrossRef]
- Nowak, M.; Bolzoni, L.; Hari Babu, N. Grain refinement of Al–Si alloys by Nb–B inoculation. Part I: Concept development and effect on binary alloys. Mater. Des. (1980-2015) 2015, 66, 366–375. [Google Scholar] [CrossRef]
- Bolzoni, L.; Nowak, M.; Hari Babu, N. Grain refinement of Al–Si alloys by Nb–B inoculation. Part II: Application to commercial alloys. Mater. Des. (1980–2015) 2015, 66, 376–383. [Google Scholar] [CrossRef]
- Zhang, Y.; Zheng, H.; Liu, Y.; Shi, L.; Xu, R.; Tian, X. Cluster-assisted nucleation of silicon phase in hypoeutectic Al–Si alloy with further inoculation. Acta Mater. 2014, 70, 162–173. [Google Scholar] [CrossRef]
- Birol, Y. Effect of silicon content in grain refining hypoeutectic Al–Si foundry alloys with boron and titanium additions. Mater. Sci. Technol. 2012, 28, 385–389. [Google Scholar] [CrossRef]
- Sritharan, T.; Li, H. Influence of titanium to boron ratio on the ability to grain refine aluminium-silicon alloys. J. Mater. Process. Technol. 1997, 63, 585–589. [Google Scholar] [CrossRef]
- Abdelhamied, S.M.S. Effect of AlTi6 grain refiner on morphology, corrosion and mechanical properties of the commercial purity AA1050 aluminum alloy. Metall. Res. Technol. 2017, 114, 309. [Google Scholar] [CrossRef]
- Chen, Z.; Wang, T.; Gao, L.; Fu, H.; Li, T. Grain refinement and tensile properties improvement of aluminum foundry alloys by inoculation with Al–B master alloy. Mater. Sci. Eng. A 2012, 553, 32–36. [Google Scholar] [CrossRef]
- Chen, Z.; Kang, H.; Fan, G.; Li, J.; Lu, Y.; Jie, J.; Zhang, Y.; Li, T.; Jian, X.; Wang, T. Grain refinement of hypoeutectic Al-Si alloys with B. Acta Mater. 2016, 120, 168–178. [Google Scholar] [CrossRef]
- Birol, Y. A novel Al–Ti–B alloy for grain refining Al–Si foundry alloys. J. Alloy. Compd. 2009, 486, 219–222. [Google Scholar] [CrossRef]
- Wang, T.; Fu, H.; Chen, Z.; Xu, J.; Zhu, J.; Cao, F.; Li, T. A novel fading-resistant Al–3Ti–3B grain refiner for Al–Si alloys. J. Alloy. Compd. 2012, 511, 45–49. [Google Scholar] [CrossRef]
- Mohammadnejad, A.; Bahrami, A.; Tafaghodi Khajavi, L. Microstructure and Mechanical Properties of Spark Plasma Sintered Nanocrystalline TiAl-xB Composites (0.0 < x < 1.5 at.%) Containing Carbon Nanotubes. J. Mater. Eng. Perform. 2021, 30, 4380–4392. [Google Scholar] [CrossRef]
- Moustafa, E.B.; Mosleh, A.O. Effect of (Ti–B) modifier elements and FSP on 5052 aluminum alloy. J. Alloy. Compd. 2020, 823, 153745. [Google Scholar] [CrossRef]
- Yang, J.; Bao, S.; Akhtar, S.; Li, Y. The Interactions Between Oxide Film Inclusions and Inoculation Particles TiB2 in Aluminum Melt. Metall. Mater. Trans. B 2021, 52, 2497–2508. [Google Scholar] [CrossRef]
- Karabay, S.; Uzman, I. Inoculation of transition elements by addition of AlB2 and AlB12 to decrease detrimental effect on the conductivity of 99.6% aluminium in CCL for manufacturing of conductor. J. Mater. Process. Technol. 2005, 160, 174–182. [Google Scholar] [CrossRef]
- Dong, X.; Zhang, Y.; Amirkhanlou, S.; Ji, S. High performance gravity cast Al9Si0.45Mg0.4Cu alloy inoculated with AlB2 and TiB2. J. Mater. Process. Technol. 2018, 252, 604–611. [Google Scholar] [CrossRef] [Green Version]
- Karabay, S. Modification of AA-6201 alloy for manufacturing of high conductivity and extra high conductivity wires with property of high tensile stress after artificial aging heat treatment for all-aluminium alloy conductors. Mater. Des. 2006, 27, 821–832. [Google Scholar] [CrossRef]
- Prasada Rao, A.K. Influence of Vanadium on the Microstructure of A319 Alloy. Trans. Indian Inst. Met. 2011, 64, 447–451. [Google Scholar] [CrossRef]
- Uludağ, M. Influence of Al-B grain refiner on porosity formation of directionally solidified Al-Si alloys. China Foundry 2020, 17, 372–377. [Google Scholar] [CrossRef]
- Schuster, J.C.; Palm, M. Reassessment of the binary Aluminum-Titanium phase diagram. J. Phase Equilibria Diffus. 2006, 27, 255–277. [Google Scholar] [CrossRef]
- Bolibruchova, D.; Tillova, E. Zlievarenské Zliatiny Al-Si; University of Zilina: Zilina, Slovakia, 2005. [Google Scholar]
- McCartney, D.G. Grain refining of aluminium and its alloys using inoculants. Int. Mater. Rev. 1989, 34, 247–260. [Google Scholar] [CrossRef]
- Spittle, J.A.; Sadli, S. Effect of alloy variables on grain refinement of binary aluminium alloys with Al–Ti–B. Mater. Sci. Technol. 1995, 11, 533–537. [Google Scholar] [CrossRef]
- Mohanty, P.S.; Gruzleski, J.E. Mechanism of grain refinement in aluminium. Acta Metall. Et Mater. 1995, 43, 2001–2012. [Google Scholar] [CrossRef]
- Kumar, G.S.V.; Murty, B.S.; Chakraborty, M. Grain refinement response of LM25 alloy towards Al–Ti–C and Al–Ti–B grain refiners. J. Alloy. Compd. 2009, 472, 112–120. [Google Scholar] [CrossRef]
- Li, P.; Liu, S.; Zhang, L.; Liu, X. Grain refinement of A356 alloy by Al–Ti–B–C master alloy and its effect on mechanical properties. Mater. Des. 2013, 47, 522–528. [Google Scholar] [CrossRef]
- Prema, S.; Chandrashekharaiah, T.M.; Begum, P.F. Effect of Grain Refiners and/or Modifiers on the Microstructure and Mechanical Properties of Al-Si Alloy (LM6). Mater. Sci. Forum 2019, 969, 794–799. [Google Scholar] [CrossRef]
- Wang, Y.; Que, Z.; Hashimoto, T.; Zhou, X.; Fan, Z. Mechanism for Si Poisoning of Al-Ti-B Grain Refiners in Al Alloys. Metall. Mater. Trans. A 2020, 51, 5743–5757. [Google Scholar] [CrossRef]
- Michna, S.T. Aluminium Materials and Technologies from A to Z; Adin: Prešov, Slovakia, 2007. [Google Scholar]


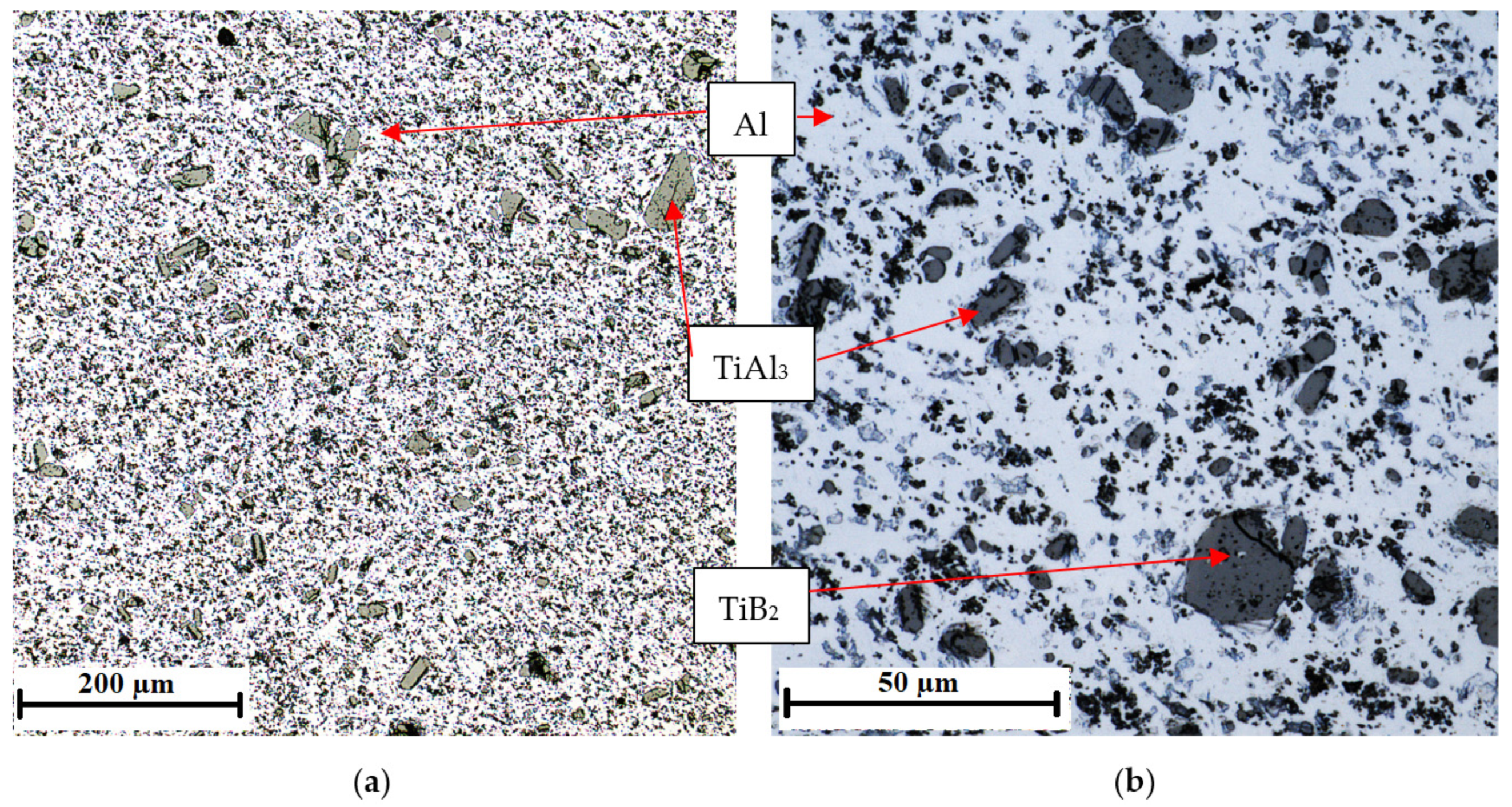
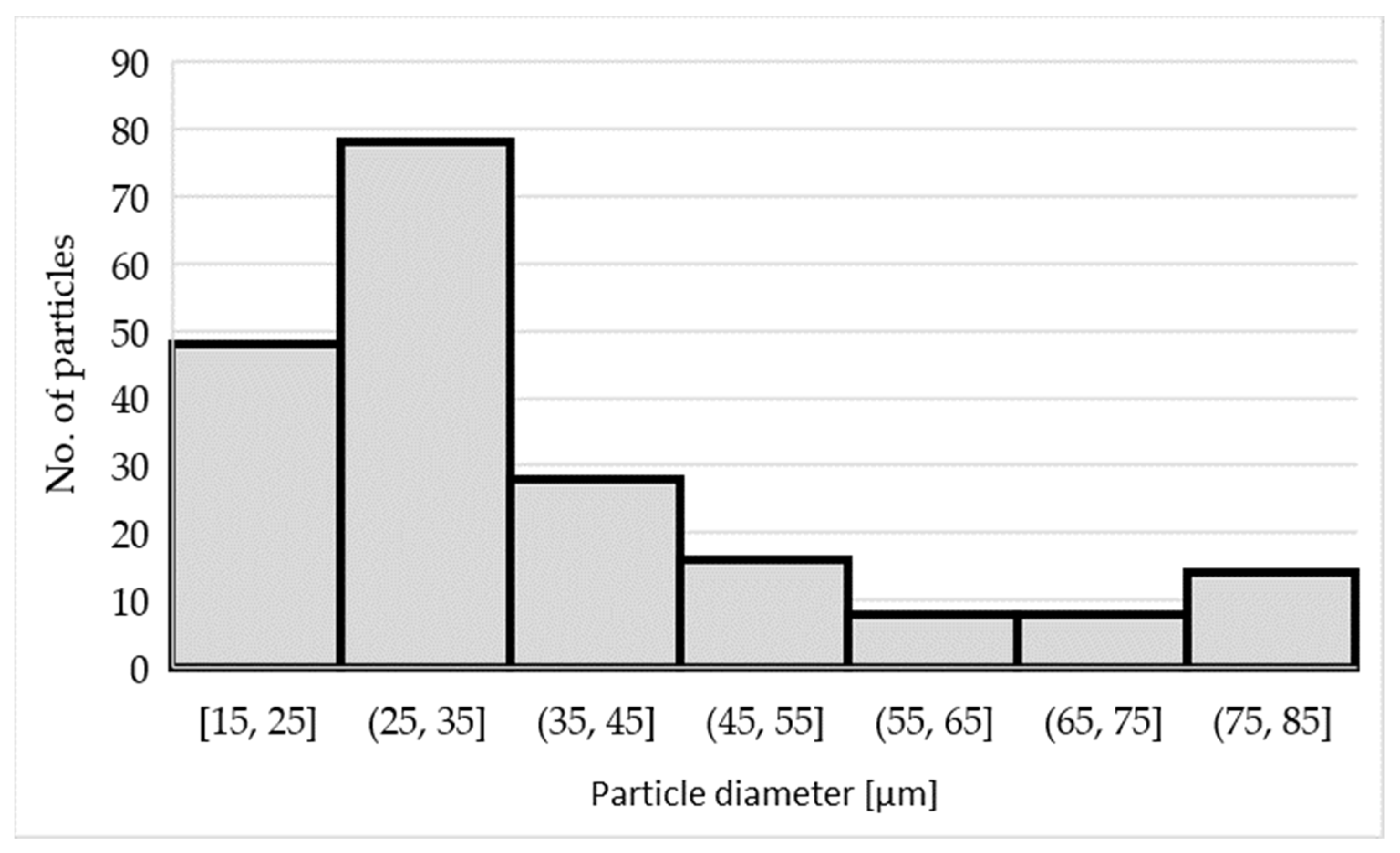
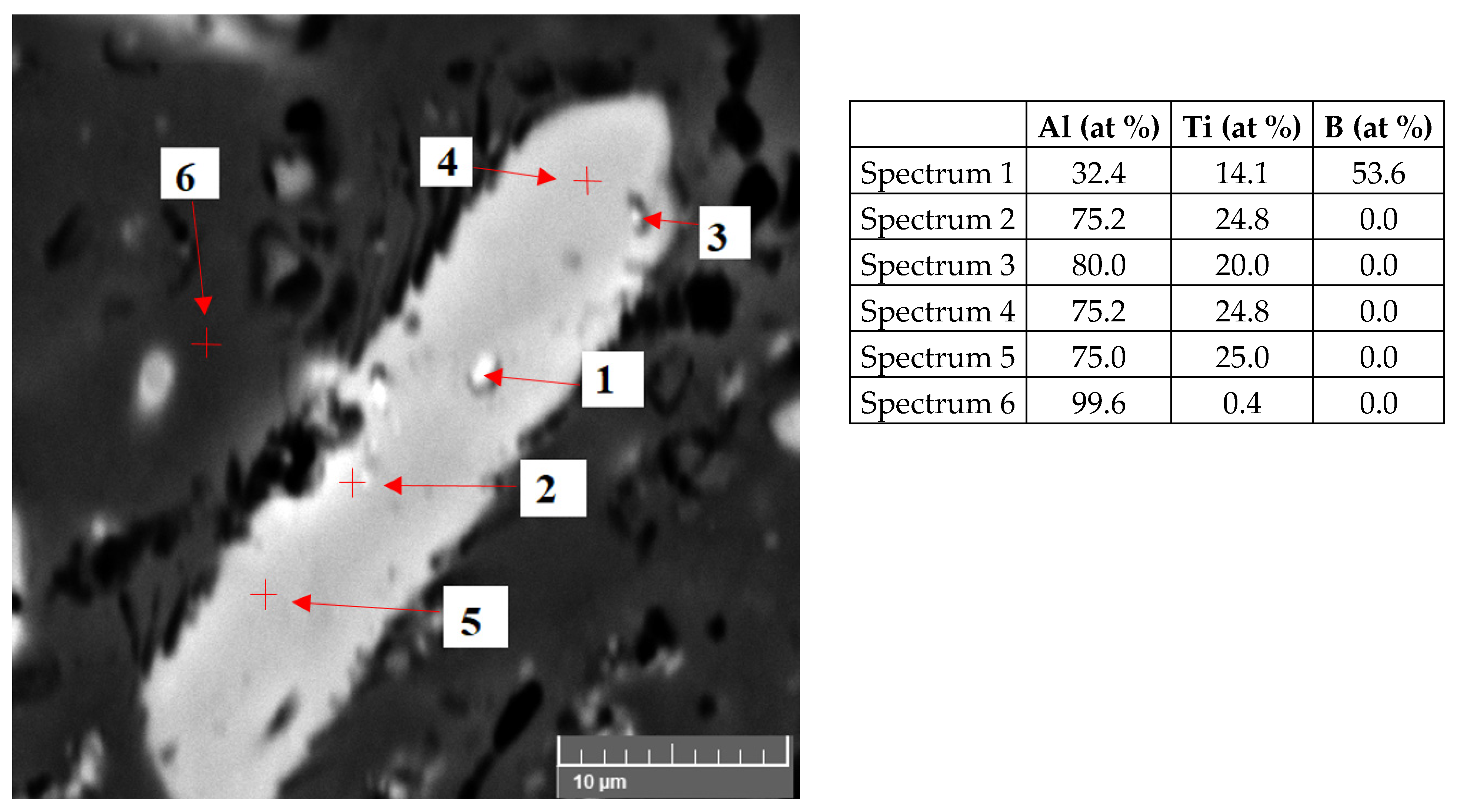
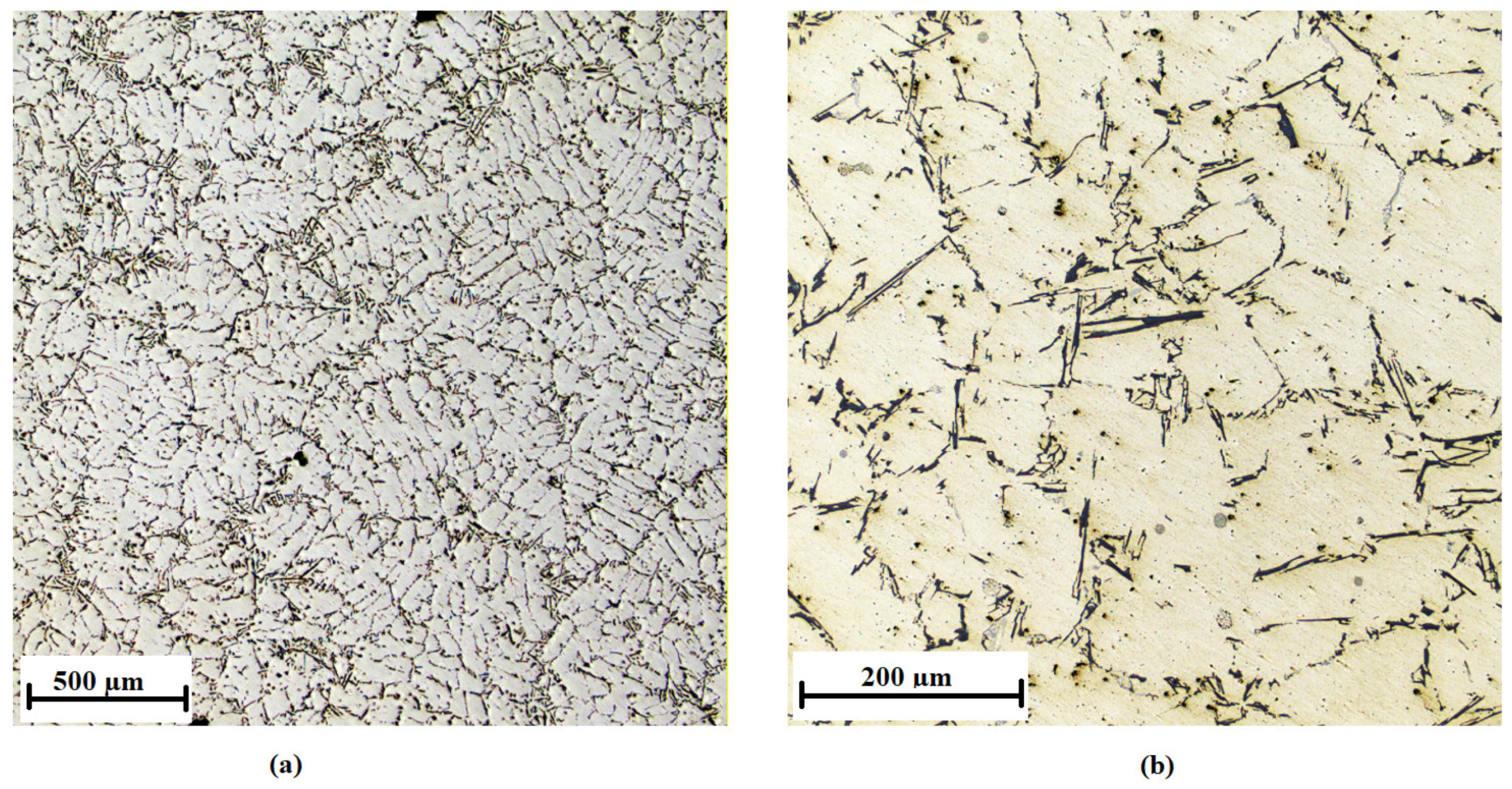



| Number of Sample | Alloy | Amount of the Inoculant Wire AlTi5B1 (wt %) | Amount of the Inoculant Wire AlTi3B1 (wt %) |
|---|---|---|---|
| 1 | AlSi7Mg0.3 | 0 | 0 |
| 2 | AlSi7Mg0.3 | 0.01 | 0 |
| 3 | AlSi7Mg0.3 | 0.05 | 0 |
| 4 | AlSi7Mg0.3 | 0.1 | 0 |
| 5 | AlSi7Mg0.3 | 0.2 | 0 |
| 6 | AlSi7Mg0.3 | 0 | 0.01 |
| AlTi5B1 | Average SDAS (µm) | Standard Deviation (µm) |
|---|---|---|
| 0 wt % Ti | 61 | 2.08 |
| 0.01 wt % Ti | 41 | 0.38 |
| 0.05 wt % Ti | 45 | 0.60 |
| 0.1 wt % Ti | 55 | 2.42 |
| 0.2 wt % Ti | 58 | 3.60 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Knaislová, A.; Michna, Š.; Hren, I.; Vlach, T.; Michalcová, A.; Novák, P.; Stančeková, D. Microstructural Characteristics of Al-Ti-B Inoculation Wires and Their Addition to the AlSi7Mg0.3 Alloy. Materials 2022, 15, 7626. https://doi.org/10.3390/ma15217626
Knaislová A, Michna Š, Hren I, Vlach T, Michalcová A, Novák P, Stančeková D. Microstructural Characteristics of Al-Ti-B Inoculation Wires and Their Addition to the AlSi7Mg0.3 Alloy. Materials. 2022; 15(21):7626. https://doi.org/10.3390/ma15217626
Chicago/Turabian StyleKnaislová, Anna, Štefan Michna, Iryna Hren, Tomáš Vlach, Alena Michalcová, Pavel Novák, and Dana Stančeková. 2022. "Microstructural Characteristics of Al-Ti-B Inoculation Wires and Their Addition to the AlSi7Mg0.3 Alloy" Materials 15, no. 21: 7626. https://doi.org/10.3390/ma15217626






