Mixed Ionic and Electronic Conduction in TeO2-ZnO-V2O5 Glasses towards Good Dielectric Features
Abstract
:1. Introduction
2. Experimental
3. Results and Analysis
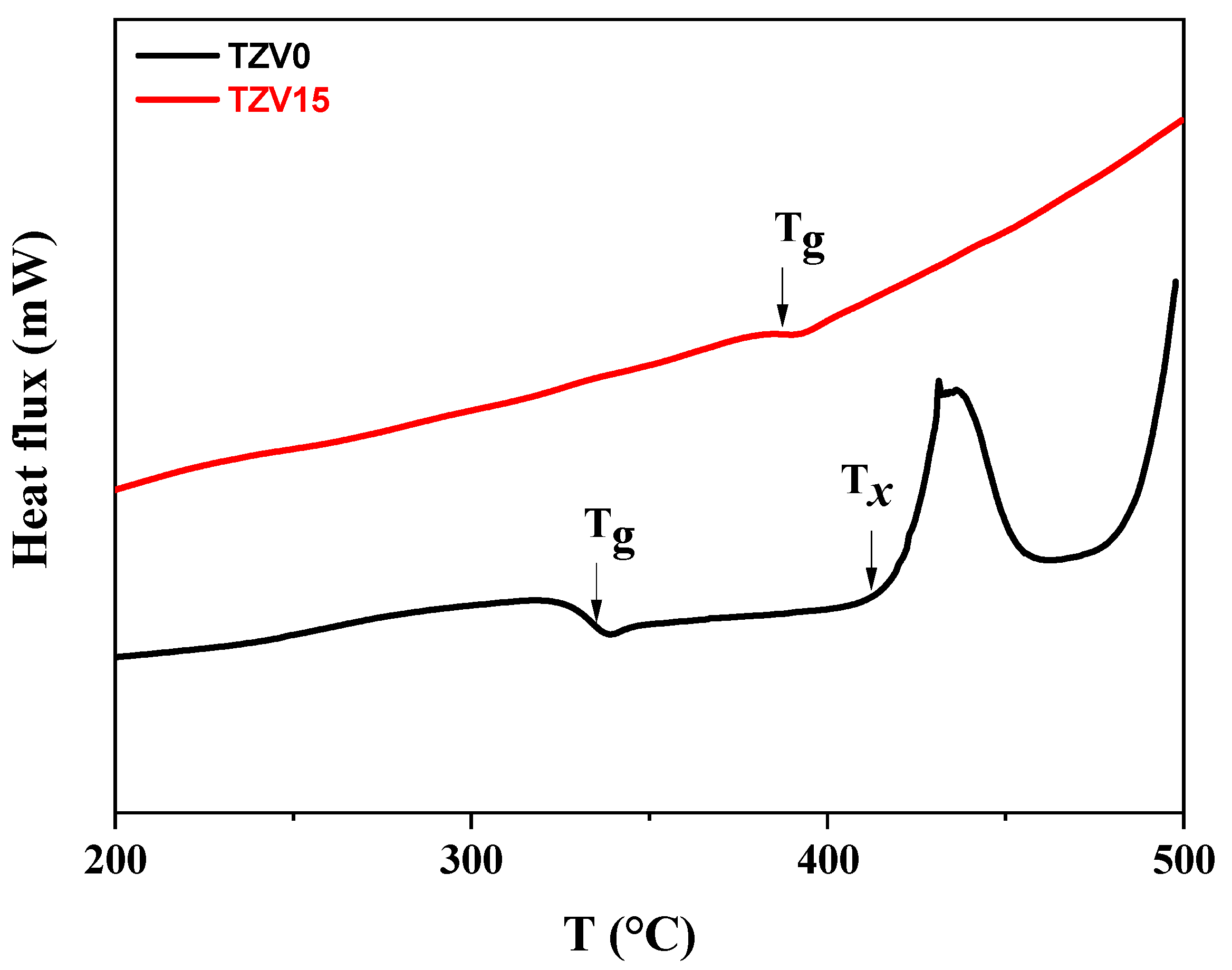
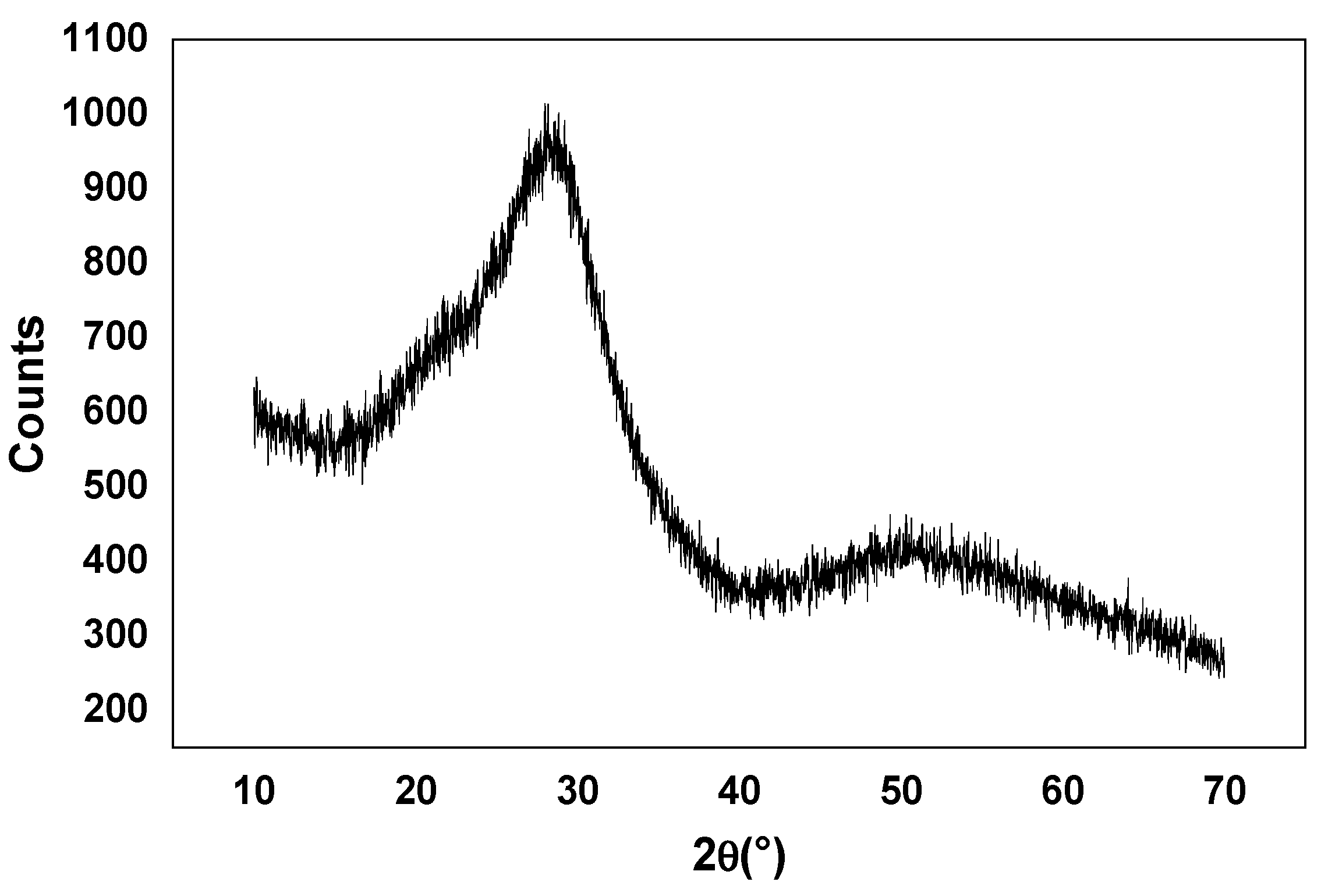
3.1. DSC, XRD, Raman, and FTIR Analysis
3.2. Impedance Spectroscopy
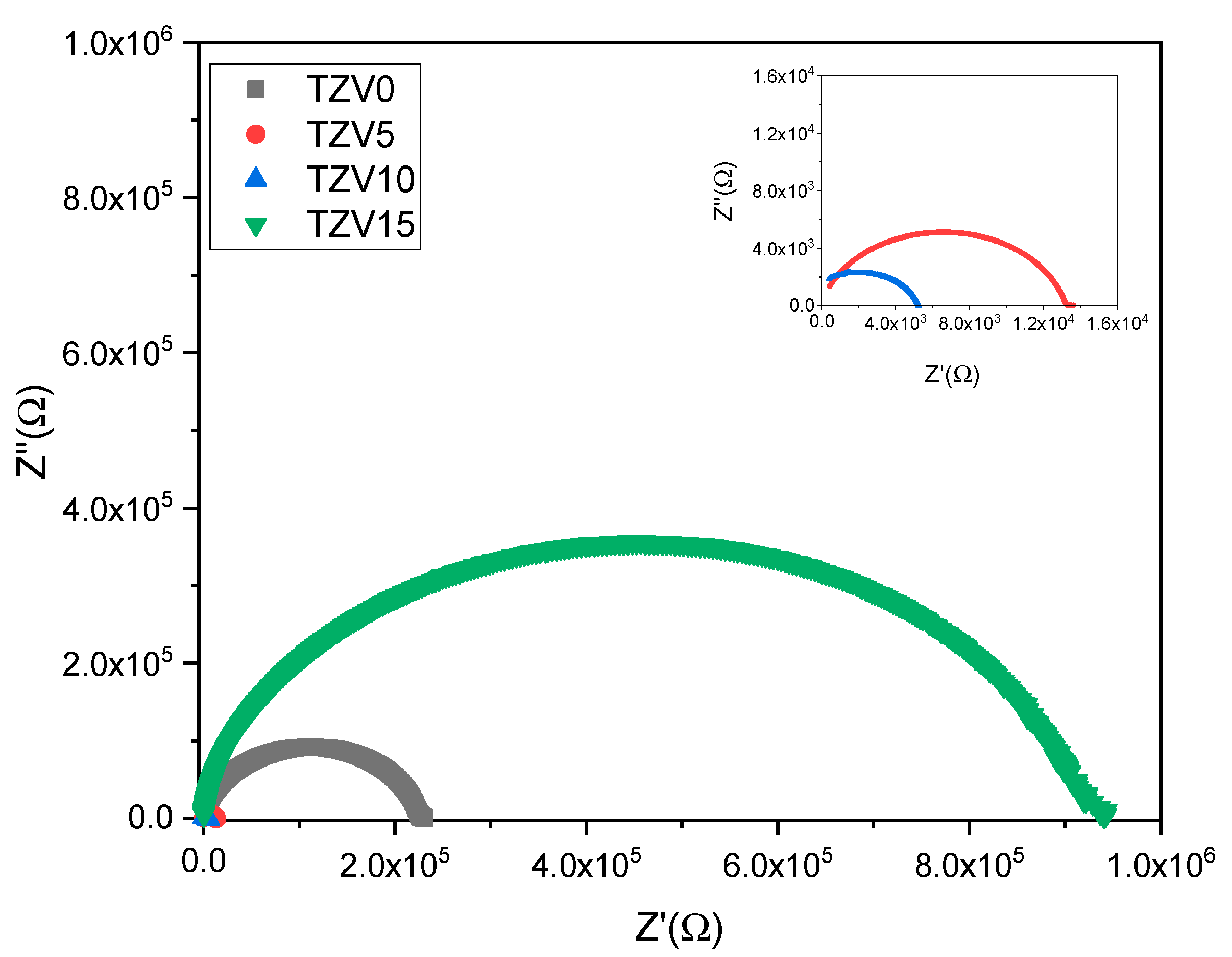
3.2.1. Nyquist Spectra
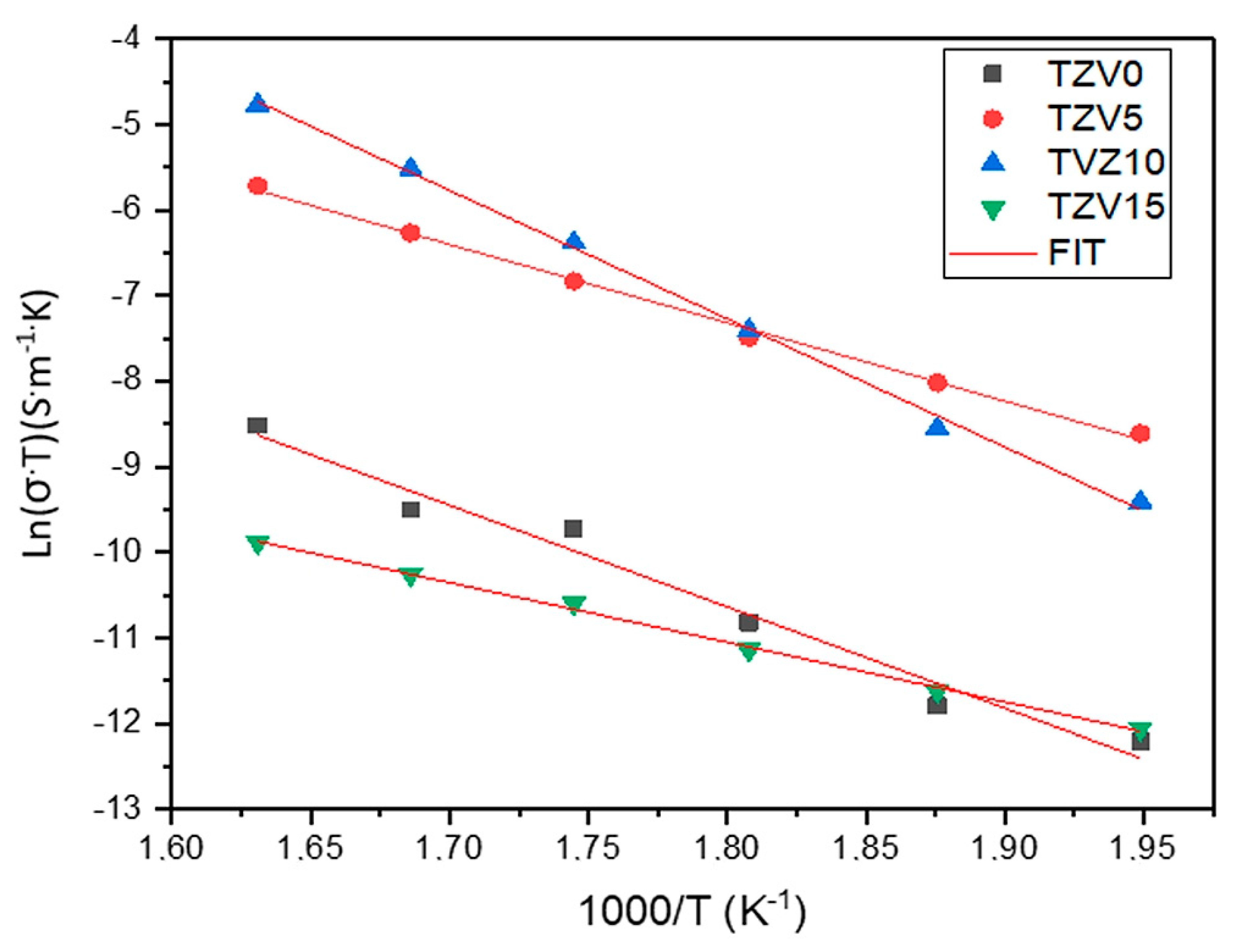
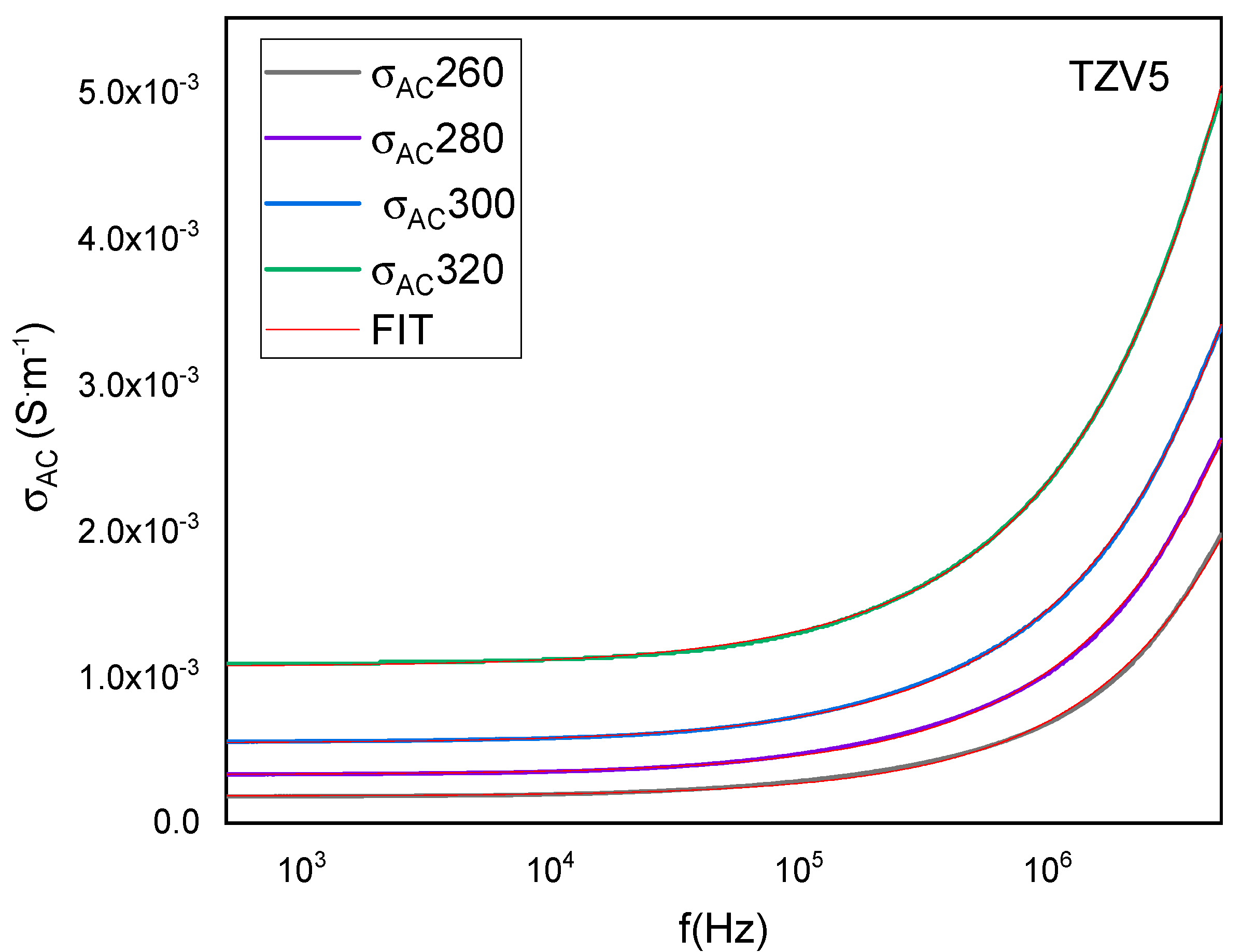
3.2.2. Electrical Conductivity
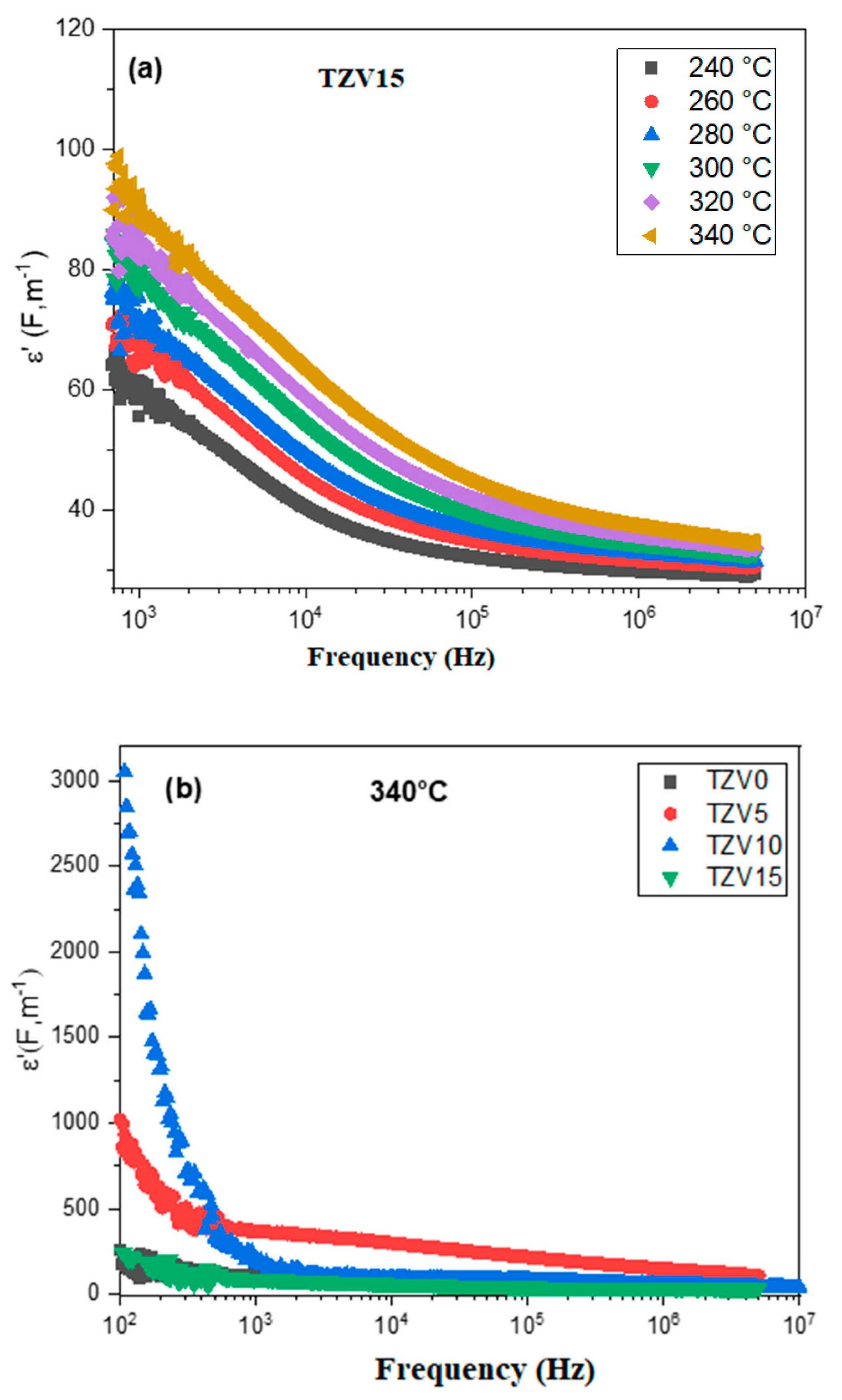
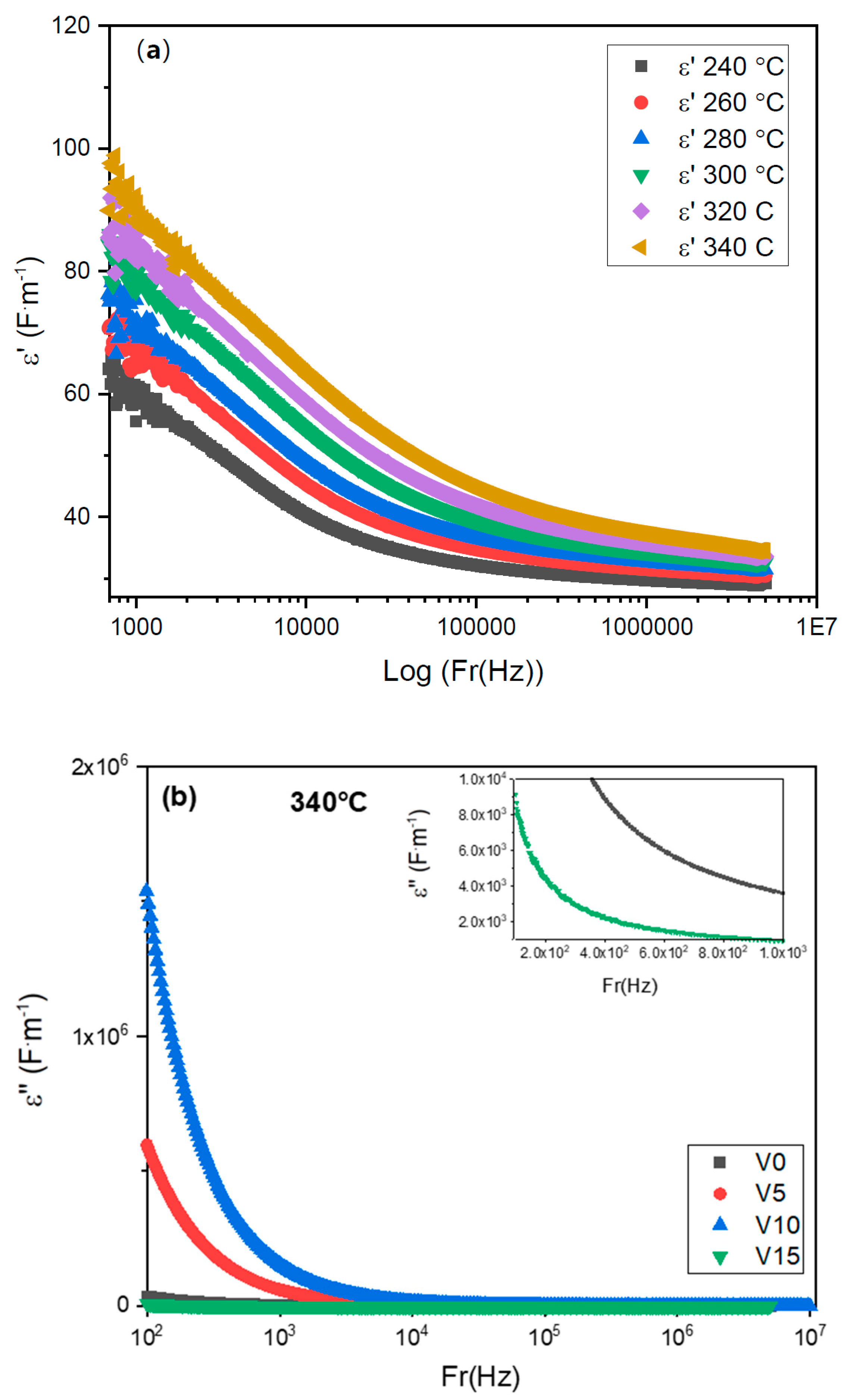
3.2.3. Analysis of Dielectric Constant
4. Conclusions
- -
- The glass transition temperature Tg was found to increase with the V2O5 content in the glass.
- -
- Both IR and Raman studies revealed a depolymerization of the glass network with rising the V2O5 concentration. A progressive change of the (VO4) groups to (VO5) units was shown when V2O5 composition was more than 10% by a change of the vanadium coordination due to the higher amount of NBO.
- -
- The conductivity of the glass is assured by a mixed ionic-polaronic process with a dominance of the ionic contribution up to 10% of V2O5, whereas the polaronic component becomes the more significant above this concentration due to the exchange of polarons between V4+ and V5+.
- -
- Variations of electric modulus and the dielectric loss with frequency and temperature exhibited dipolar relaxation effects mainly caused by the vanadate phases. In addition, the electric modulus variation shows a non-Debye dielectric dispersion.
- -
- The decrease of the exponent “s” with temperature is consistent with the CBH process.
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Kiliç, G.; İşsever, U.G.; İlik, E. The synthesis and characterization of zinc-tellurite semiconducting oxide glasses containing Ta2O5. Mater. Res. Express 2019, 6, 6. [Google Scholar] [CrossRef]
- Mallawany, R. Tellurite Glass Smart Materials: Application in Optics and Beyong, 1st ed.; Springer International Publishing AG: Cham, Switzerland, 2018; p. 297. [Google Scholar]
- Tagiara, N.; Palles, D.; Simandiras, E.; Psycharis, V.; Kyritsis, A.; Kamitsos, E. Synthesis, thermal and structural properties of pure TeO2 glass and zinc-tellurite glasses. J. Non-Crystalline Solids 2017, 457, 116–125. [Google Scholar] [CrossRef]
- McDonald, L.; Siligardi, C.; Vacchi, M.; Zieser, A.; Affatigato, M. Tellurium Vanadate Glasses: V4+colorimetric Measure and Its Effect on Conductivity. Front. Mater. 2020, 7, 103. [Google Scholar] [CrossRef]
- Fares, H.; Jlassi, I.; Elhouichet, H.; Férid, M. Investigations of thermal, structural and optical properties of tellurite glass with WO3 adding. J. Non-Crystalline Solids 2014, 396–397, 1–7. [Google Scholar] [CrossRef]
- Kaky, K.M.; Lakshminarayana, G.; Baki, S.; Kityk, I.; Taufiq-Yap, Y.; Mahdi, M. Structural, thermal and optical absorption features of heavy metal oxides doped tellurite rich glasses. Results Phys. 2017, 7, 166–174. [Google Scholar] [CrossRef]
- Aromäki, I.; Shestopalova, I.; Ponte, R.; Annurakshita, S.; Bautista, G.; Othmani, A.; Elhouichet, H.; Petit, L. (INVITED)Transparent Er3+ doped Ag2O containing tellurite glass-ceramics. Opt. Mater. X 2022, 15, 100164. [Google Scholar] [CrossRef]
- Saad, M.; Stambouli, W.; Sdiri, N.; Elhouichet, H. Effect of mixed sodium and vanadium on the electric and dielectric properties of zinc phosphate glass. Mater. Res. Bull. 2017, 89, 224–231. [Google Scholar] [CrossRef]
- Jlassi, I.; Sdiri, N.; Elhouichet, H. Electrical conductivity and dielectric properties of MgO doped lithium phosphate glasses. J. Non-Crystalline Solids 2017, 466–467, 45–51. [Google Scholar] [CrossRef]
- Arya, S.K.; Danewalia, S.S.; Arora, M.; Singh, K. Effect of Variable Oxidation States of Vanadium on the Structural, Optical, and Dielectric Properties of B2O3–Li2O–ZnO–V2O5 Glasses. J. Phys. Chem. B 2017, 120, 12168–12176. [Google Scholar] [CrossRef]
- Pavani, P.G.; Suresh, S.; Mouli, V.C. Studies on boro cadmium tellurite glasses. Opt. Mater. 2011, 34, 215–220. [Google Scholar] [CrossRef]
- Işsever, U.G.; Kilic, G.; Peker, M.; Ünaldi, T.; Aybek, A.Ş. Effect of low ratio V5+ doping on structural and optical properties of borotellurite semiconducting oxide glasses. J. Mater. Sci. Mater. Electron 2019, 30, 15156–15167. [Google Scholar] [CrossRef]
- Brovelli, S.; Galli, A.; Lorenzi, R.; Meinardi, F.; Spinolo, G.; Tavazzi, S.; Sigaev, V.; Sukhov, S.; Pernice, P.; Aronne, A.; et al. Efficient 1.53 μm erbium light emission in heavily Er-doped titania-modified aluminium tellurite glasses. J. Non. Cryst. Solids 2007, 353, 2150–2156. [Google Scholar] [CrossRef]
- Stambouli, W.; Elhouichet, H.; Ferid, M. Study of thermal, structural and optical properties of tellurite glass with different TiO2 composition. J. Mol. Struct. 2012, 1028, 39–43. [Google Scholar] [CrossRef]
- Hirdesh; Khanna, A.; Fábián, M.; Dippel, A.-C.; Gotowski, O. Structure of lithium tellurite and vanadium lithium tellurite glasses by high-energy X-ray and neutron diffraction. Acta Crystallogr. Sect. B Struct. Sci. Cryst. Eng. Mater. 2021, 77, 275–286. [Google Scholar] [CrossRef]
- Yahia, I.S.; Saddeek, Y.B.; Sakr, G.B.; Knoff, W.; Story, T.; Romcevic, N.; Dobrowolski, W. Spectroscopic analysis and magnetic susceptibility of CuO–TeO2–V2O5 glasses. J. Magn. Magn. Mat. 2009, 321, 4039–4044. [Google Scholar] [CrossRef]
- Abed, C.; Trabelsi, A.B.G.; Alkallas, F.H.; Fernandez, S.; Elhouichet, H. Transport Mechanisms and Dielectric Features of Mg-Doped ZnO Nanocrystals for Device Applications. Materials 2022, 15, 2265. [Google Scholar] [CrossRef] [PubMed]
- Ben Ali, M.; Elhouichet, H. Electrical and dielectric properties of Ni doped Zn2SnO4 nanoparticles. Ceram. Int. 2000, 46, 28686–28692. [Google Scholar] [CrossRef]
- Sdiri, N.; Elhouichet, H.; Elakermi, E.; Dhifallah, A.; Ferid, M. Structural investigation of amorphous Na2O–P2O5–B2O3 correlated with its ionic conductivity. J. Non Crystalline Solids 2015, 409, 34–42. [Google Scholar] [CrossRef]
- Nasser, R.; Othmen, W.B.H.; Elhouichet, H. Effect of Sb doping on the electrical and dielectric properties of ZnO nanocrystals. Ceram. Int. 2019, 45, 8000–8007. [Google Scholar] [CrossRef]
- Langar, A.; Sdiri, N.; Elhouichet, H.; Ferid, M. Conductivity and dielectric behavior of NaPO3–ZnO–V2O5 glasses. J. Alloy. Compds 2014, 590, 380–387. [Google Scholar] [CrossRef]
- Amara, S.F.C.B.; Hammami, H. Effect of iron oxide on the electrical conductivity of soda-lime silicate glasses by dielectric spectroscopy. J. Mater. Sci. Mater. Electron. 2019, 30, 13543–13555. [Google Scholar] [CrossRef]
- Saeed, M.A.M.; Abdullah, O.G. Effect of Structural Features on Ionic Conductivity and Dielectric Response of PVA Proton Conductor-Based Solid Polymer Electrolytes. J. Electron. Mater. 2020, 50, 432–442. [Google Scholar] [CrossRef]
- Sujatha, B.; Viswanatha, R.; Chethana, B.K.; Bhushana, N.; Reddy, C.N. Electrical conductivity and dielectric relaxation studies on microwave synthesized Na2SO4·NaPO3·MoO3 glasses. Ionics 2015, 22, 563–571. [Google Scholar] [CrossRef]
- Sankarappa, T.; Kumar, M.P.; Devidas, G.B.; Nagaraja, N.; Ramakrishnareddy, R. AC conductivity and dielectric studies in V2O5–TeO2 and V2O5–CoO–TeO2 glasses. J. Molecular Struct. 2008, 889, 308–315. [Google Scholar] [CrossRef]
- Souri, D.; Ghasemi, R.; Shiravand, M. The study of high-dc electric field effect on the conduction of V2O5–Sb–TeO2 glasses and the applicability of an electrothermal model. J. Mater. Sci. 2015, 50, 2554–2560. [Google Scholar] [CrossRef]
- Calahoo, C.; Wondraczek, L. Ionic glasses: Structure, properties and classification. J. Non-Cryst. Solids. X 2020, 8, 100054. [Google Scholar] [CrossRef]
- Desoky, M.M.E.L. Small polaron transport in V2O5–NiO–TeO2 glasses. J. Mater. Sci. Mater. Electron. 2003, 14, 215. [Google Scholar] [CrossRef]
- El-Desoky, M.M. Characterization and transport properties of V2O5-Fe2O3-TeO2 glasses. J. Non. Cryst. Solids 2005, 351, 3139. [Google Scholar] [CrossRef]
- Moawad, H.M.M.; Jain, H.; Ramadan, T.; El-Mallawany, R.; El-Sharbiny, M. Electrical Conductivity of Silver Vanadium Tellurite Glasses. J. Am. Ceram. Soc. 2002, 85, 2655–2659. [Google Scholar] [CrossRef]
- Watthaisong, P.; Jungthawan, S.; Hirunsit, P.; Suthirakun, S. Transport properties of electron small polarons in a V2O5 cathode of Li-ion batteries: A computational study. RSC Adv. 2019, 9, 19483–19494. [Google Scholar] [CrossRef]
- HSidek, A.A.; Rosmawati, S.; Talib, Z.A.; Halimah, M.K.; Halim, S.A. Effect of Zinc on the Elastic Behaviour of (TeO2) 90 (AlF3) 10-x (ZnO) x Glass System. Int. J. Basic Appl. Sci. 2009, 90, 41–44. [Google Scholar]
- Abdel-Khalek, E.; Ali, I.O. Structural, AC conductivity and dielectric properties of vanado-tellurite glasses containing BaTiO3. J. Non-Crystalline Solids 2014, 390, 31–36. [Google Scholar] [CrossRef]
- Mohamed, S.N.; Halimah, M.K.; Subban, R.H.Y.; Yahya, A.K. AC conductivity and dielectric properties in mixed ionic–electronic 20Na2O–20CaO–(60–x)B2O3–xV2O5 glasses. Phys. B Condens. Matter 2020, 602, 412480. [Google Scholar] [CrossRef]
- Kao, K.C. Electric Polarization and Relaxation in Dielectric Phenomena in Solids; Elsevier: Amsterdam, The Netherlands, 2004; pp. 41–114. [Google Scholar]
- Bruce, P. High and low frequency Jonscher behaviour of an ionically conducting glass. Solid State Ionics 1985, 15, 247–251. [Google Scholar] [CrossRef]
- Murugaraj, R. Ac conductivity and its scaling behavior in borate and bismuthate glasses. J. Mater. Sci. 2007, 42, 10065–10073. [Google Scholar] [CrossRef]
- Prezas, P.R.; Soares, M.J.; Freire, F.N.A.; Graça, M.P.F. Structural, electrical and dielectric characterization of TeO2-WO3-Y2O3-Er2O3-Yb2O3 glasses. Mater. Res. Bull. 2015, 68, 314–319. [Google Scholar] [CrossRef]
- Othmen, W.B.H.; Sdiri, N.; Elhouichet, H.; Férid, M. Study of charge transport in Fe-doped SnO2 nanoparticles prepared by hydrothermal method. Mat. Sci. Semicon. Proc. 2016, 52, 46–54. [Google Scholar] [CrossRef]
- Elliott, S.R. A theory of a.c. conduction in chalcogenide glasses. Philos. Mag. A J. Theor. Exp. Appl. Phys. 2017, 36, 8. [Google Scholar] [CrossRef]
- Cole, K.S.; Cole, R.H. Dispersion and absorption in dielectrics: II. Direct current characteristics. Chem. Phys. 1942, 10, 98–105. [Google Scholar] [CrossRef]
- Bouslama, W.; Ben Ali, M.; Sdiri, N.; Elhouichet, H. Conduction mechanisms and dielectric constant features of Fe doped ZnO nanocrystals. Ceram. Int. 2021, 47, 19106–19114. [Google Scholar] [CrossRef]
- Moynihan, C.T. Analysis of electrical relaxation in glasses and melts with large concentrations of mobile ions. J. Non-Crystalline Solids 1994, 172, 1395–1407. [Google Scholar] [CrossRef]
- Rayssi, C.; el Kossi, S.; Dhahri, J.; Khirouni, K. Frequency and temperature-dependence of dielectric permittivity and electric modulus studies of the solid solution Ca0.85Er0.1Ti1-xCo4x/3O3 (0 ≤ x ≤ 0.1). RSC Adv. 2018, 8, 17139–17150. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Macedo, P.B.; Moynihan, C.T.; Bose, R. Role of ionic diffusion in polarization in vitreous ionic conductors. Phys. Chem. Glas. 1973, 13, 171–179. [Google Scholar]
- Tabib, A.; Sdiri, N.; Elhouichet, H.; Férid, M. Investigations on electrical conductivity and dielectric properties of Na doped ZnO synthesized from sol gel method. J. Alloy. Compd. 2015, 622, 687–694. [Google Scholar] [CrossRef]



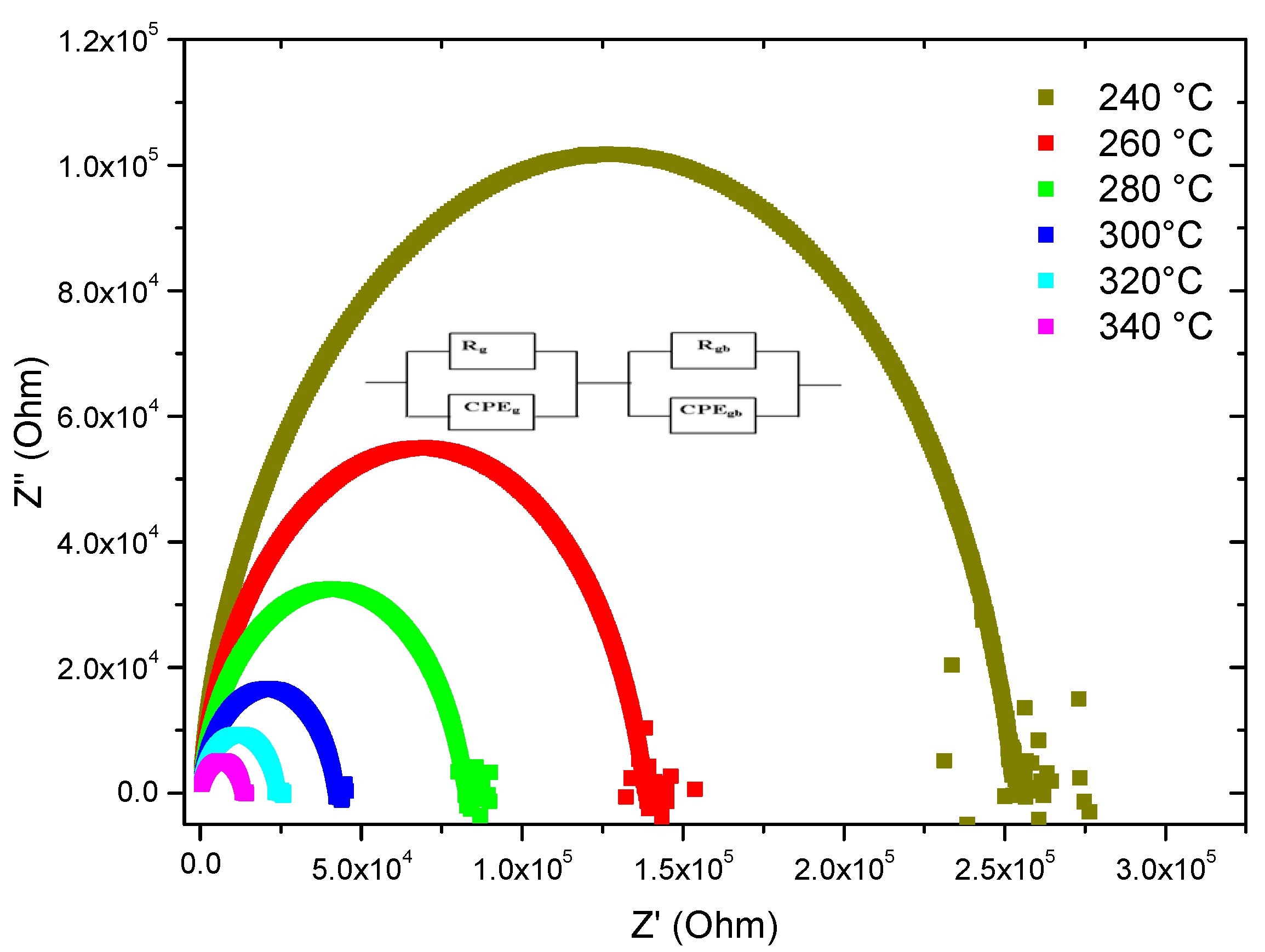

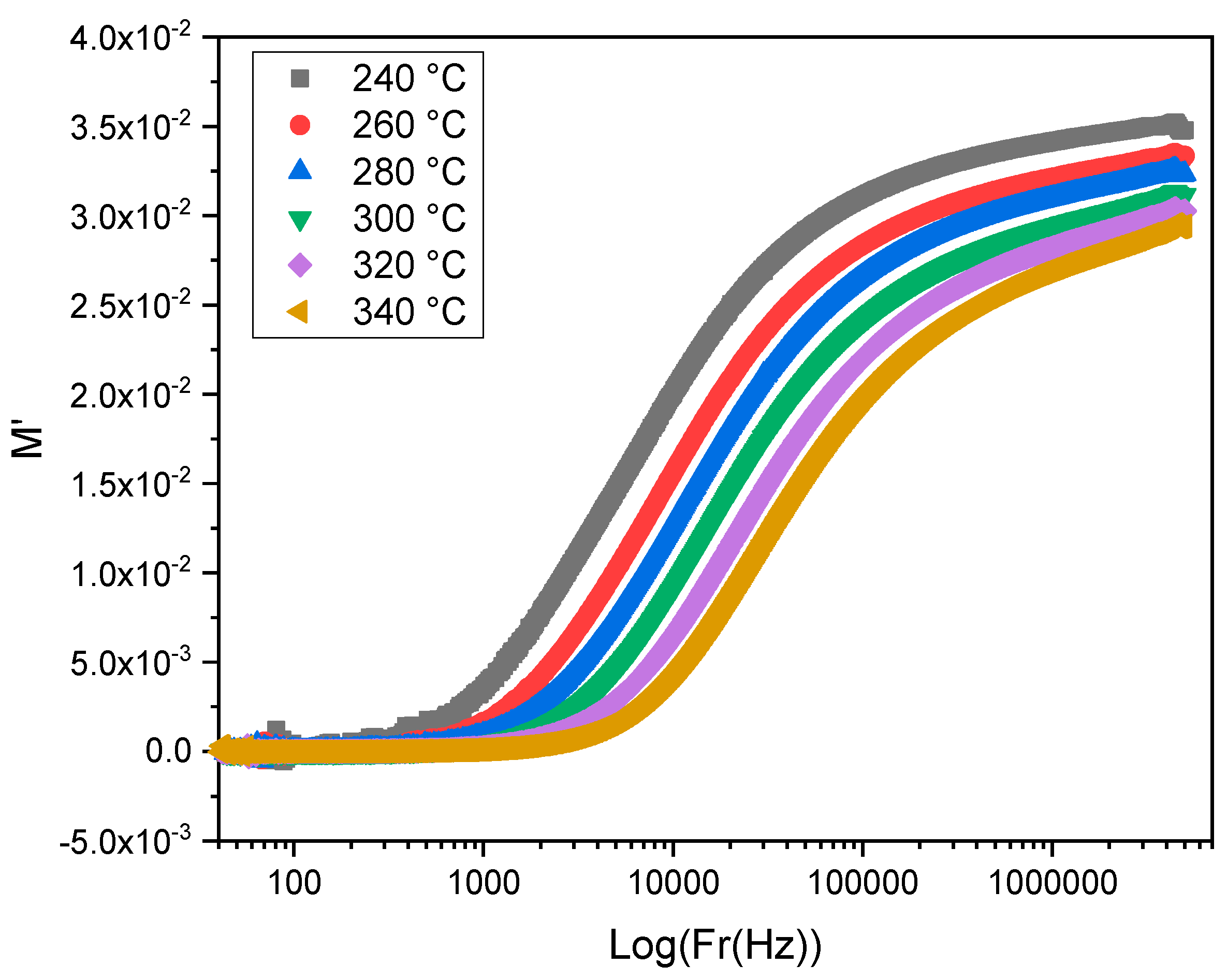
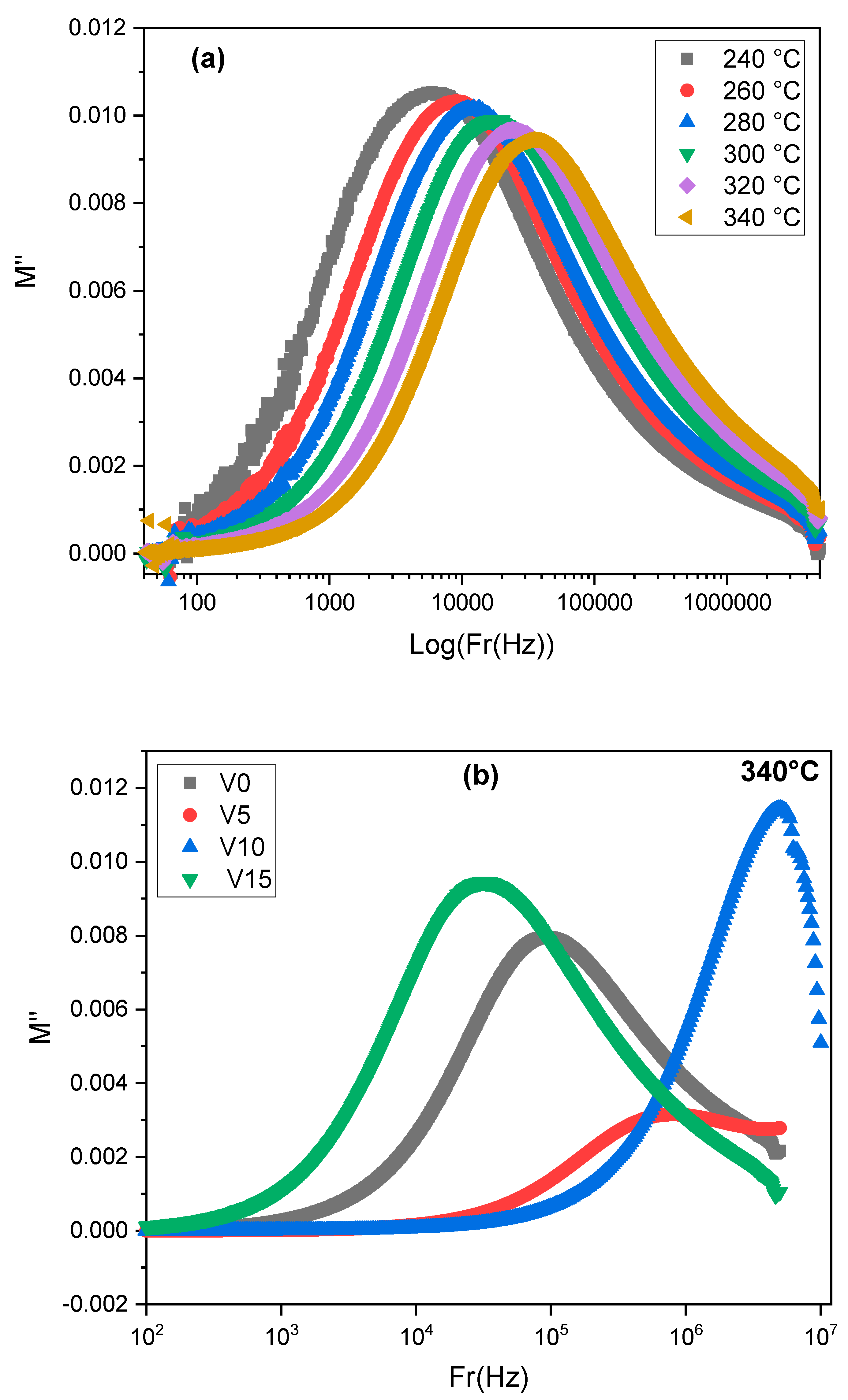
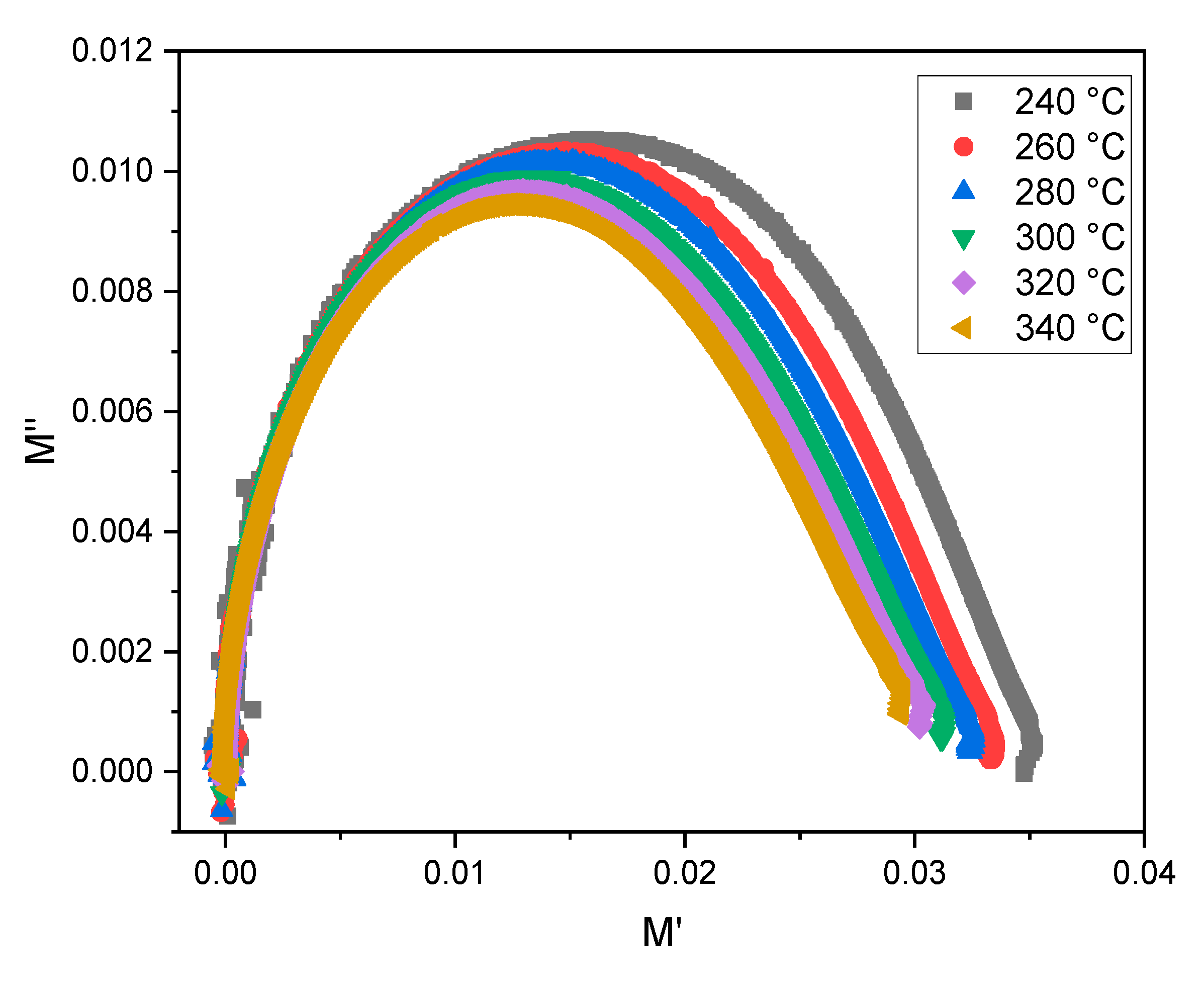

| T° | Bulk Region | Interfacial Impedance | ||||
|---|---|---|---|---|---|---|
| n1 | Ab (F cm2 sn−1) | Rb (Ω) | n2 | Aint (F cm2 sn−1) | Rint (Ω) | |
| 240 | 0.90 | 6.03 × 10−10 | 2.14 × 106 | 0.67 | 3.4 × 10−6 | 6.83 × 106 |
| 260 | 0.85 | 9.03 × 10−10 | 1.38 × 106 | 0.68 | 1 × 10−5 | 6.4 × 106 |
| 280 | 0.85 | 1.91 × 10−9 | 0.83 × 106 | 0.79 | 3.2 × 10−6 | 6.0 × 106 |
| 300 | 0.85 | 1.03 × 10−9 | 0.42 × 106 | 0.81 | 6.5 × 10−6 | 5.63 × 106 |
| 320 | 0.84 | 1.34 × 10−9 | 0.24 × 106 | 0.79 | 31 × 10−5 | 4.82 × 106 |
| 340 | 0.83 | 1.53 × 10−9 | 0.24 × 106 | 0.82 | 11 × 10−5 | 2.74 × 106 |
| LPMg [15] | 0.84 | 1.35 × 10−10 | 0.13 × 106 | |||
| NPZV10 [21] | 0.86 | 1.774 × 10−12 | 0.136 × 105 | 0.87 | 4.84 × 10−11 | 0.54 × 106 |
| PVB2.25 [18] | 0.89 | 4.15 × 10−11 | 4.5 × 105 | 0.87 | 2.7 × 10−12 | 2.3 × 105 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mechrgui, I.; Ben Gouider Trabelsi, A.; Alkallas, F.H.; Nasri, S.; Elhouichet, H. Mixed Ionic and Electronic Conduction in TeO2-ZnO-V2O5 Glasses towards Good Dielectric Features. Materials 2022, 15, 7659. https://doi.org/10.3390/ma15217659
Mechrgui I, Ben Gouider Trabelsi A, Alkallas FH, Nasri S, Elhouichet H. Mixed Ionic and Electronic Conduction in TeO2-ZnO-V2O5 Glasses towards Good Dielectric Features. Materials. 2022; 15(21):7659. https://doi.org/10.3390/ma15217659
Chicago/Turabian StyleMechrgui, Imen, Amira Ben Gouider Trabelsi, Fatemah. H. Alkallas, Saber Nasri, and Habib Elhouichet. 2022. "Mixed Ionic and Electronic Conduction in TeO2-ZnO-V2O5 Glasses towards Good Dielectric Features" Materials 15, no. 21: 7659. https://doi.org/10.3390/ma15217659





