Correlations on the Structure and Properties of Collagen Hydrogels Produced by E-Beam Crosslinking
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Synthesis of Hydrogels
2.3. Evaluation of Hydrogel Properties
2.3.1. Determination of Gel Fraction
2.3.2. Swelling Degree
2.3.3. Crosslinking Density
2.4. Instrumental Analysis
2.4.1. Rheological Analysis
2.4.2. FTIR Analysis
2.4.3. SEM Analysis
2.4.4. Thermal Analysis
3. Results and Discussion
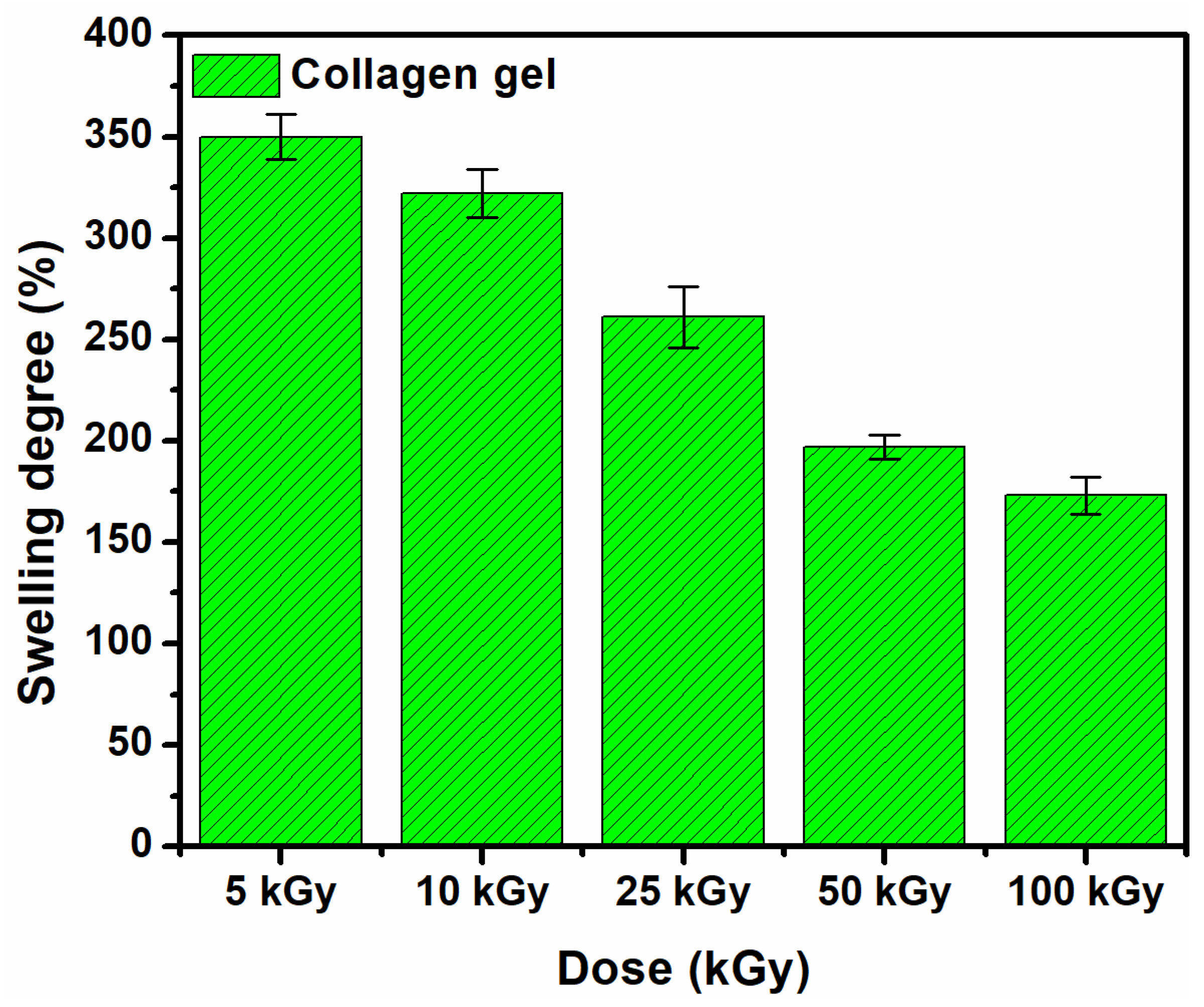
3.1. Gel Fraction and Swelling Degree
3.2. Crosslinking Density
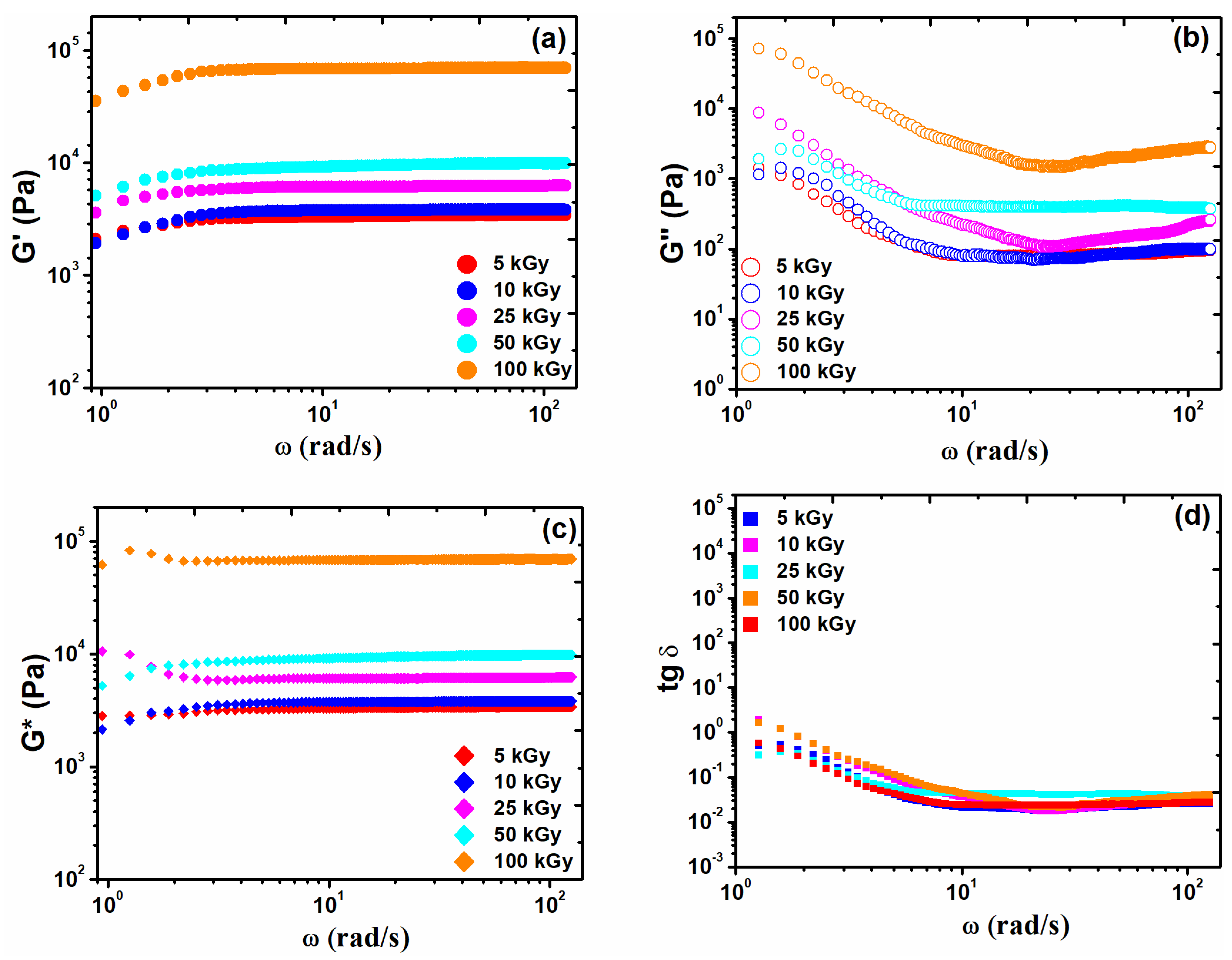
3.3. Rheological Analysis
3.4. FTIR Analysis
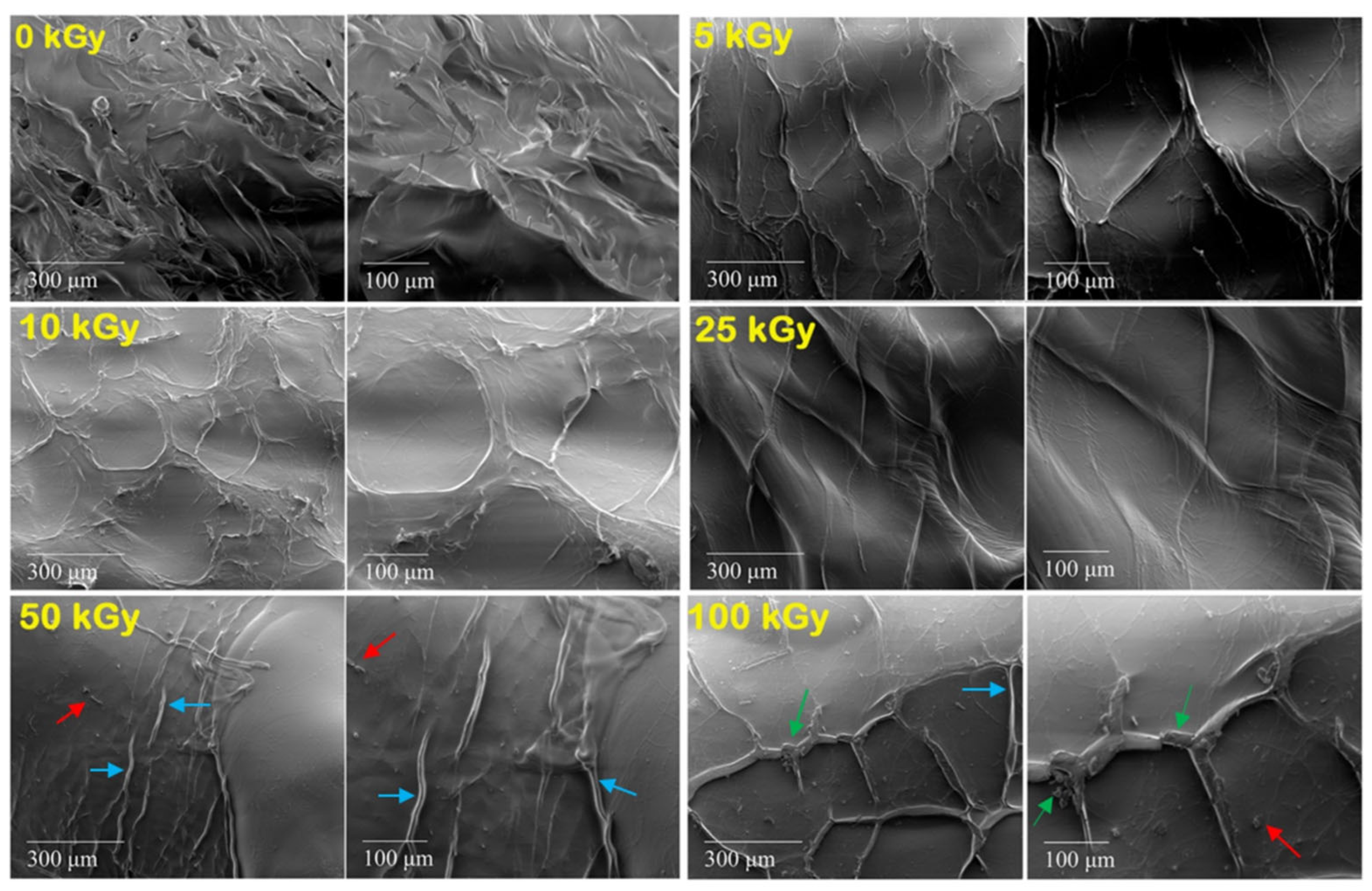
3.5. Surface Characterization—SEM Images
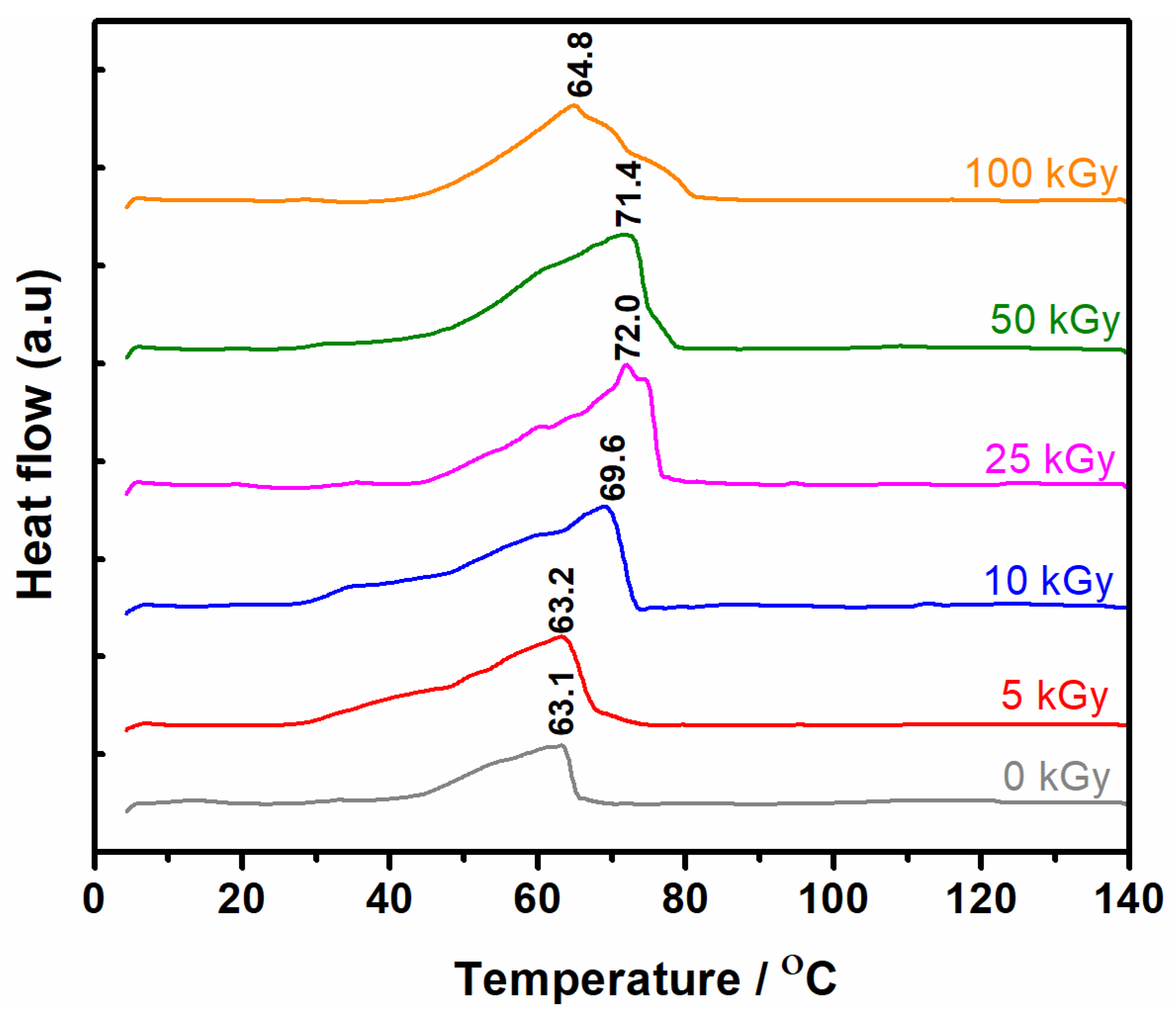
3.6. Thermal Analysis
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Albu, M.G.; Titorencu, I.; Violeta, M. Collagen-Based Drug Delivery Systems for Tissue Engineering. In Biomaterials Applications for Nanomedicine; Pignatello, R., Ed.; InTech: London, UK, 2011. [Google Scholar]
- Perron, R.R.; Wright, B.A. Alteration of Collagen Structure by Irradiation with Electrons. Nature 1950, 166, 863–864. [Google Scholar] [CrossRef] [PubMed]
- Bowes, J.H.; Moss, J.A. The Effect of Gamma Radiation on Collagen. Radiat. Res. 1962, 16, 211–223. [Google Scholar] [CrossRef] [PubMed]
- Inoue, N.; Bessho, M.; Furuta, M.; Kojima, T.; Okuda, S.; Hara, M. A Novel Collagen Hydrogel Cross-Linked by Gamma-Ray Irradiation in Acidic PH Conditions. J. Biomater. Sci. Polym. Ed. 2006, 17, 837–858. [Google Scholar] [CrossRef] [PubMed]
- Bailey, A.J. Irradiation-Induced Changes in the Denaturation Temperature and Intermolecular Cross-Linking of Tropocollagen. Radiat. Res. 1967, 31, 206–214. [Google Scholar] [CrossRef] [PubMed]
- Bailey, A.J.; Bendall, J.R.; Rhodes, D.N. The Effect of Irradiation on the Shrinkage Temperature of Collagen. Int. J. Appl. Radiat. Isot 1962, 13, 131–136. [Google Scholar] [CrossRef]
- Bailey, A.J.; Tromans, W.J. Effects of Ionizing Radiation on the Ultrastructure of Collagen Fibrils. Radiat. Res. 1964, 23, 145–155. [Google Scholar] [CrossRef]
- Koide, M.; Osaki, K.; Konishi, J.; Oyamada, K.; Katakura, T.; Takahashi, A.; Yoshizato, K. A New Type of Biomaterial for Artificial Skin: Dehydrothermally Cross-Linked Composites of Fibrillar and Denatured Collagens. J. Biomed. Mater. Res. 1993, 27, 79–87. [Google Scholar] [CrossRef]
- Popa, C.L.; Albu, M.; Bartha, C.; Costescu, A.; Luculescu, C.; Trusca, R.; Antohe, S. Structural Characterization and Optical Properties of Hydroxyapatite/Collagen Matrix. Rom. Rep. Phys. 2016, 68, 1149–1158. [Google Scholar]
- Ghica, M.V.; Albu, M.G.; Popa, L.; Leca, M.; Brăzdaru, L.; Cotruț, C.; Trandafir, V. Drug Delivery Systems Based on Collagen-Tannic Acid Matrices. Rev. Roum. Chim. 2009, 54, 1103–1110. [Google Scholar]
- Moise, V.; Vasilca, S.; Baltac, A.; Pintilie, C.; Virgolici, M.; Cutrubinis, M.; Kamerzan, C.; Dragan, D.; Ene, M.; Albota, F.; et al. Physicochemical Study for Characterization of Lyophilized Collagens Irradiated with Gamma Radiation and for Optimization of Medical Device Manufacturing Process. Radiat. Phys. Chem. 2020, 170, 108658. [Google Scholar] [CrossRef]
- Visser, C.E.; Voute, A.B.E.; Oosting, J.; Boon, M.E.; Kok, L.P. Microwave Irradiation and Cross-Linking of Collagen. Biomaterials 1992, 13, 34–37. [Google Scholar] [CrossRef]
- Ahmed, M.R.; Vairamuthu, S.; Shafiuzama, M.; Basha, S.H.; Jayakumar, R. Microwave Irradiated Collagen Tubes as a Better Matrix for Peripheral Nerve Regeneration. Brain Res. 2005, 1046, 55–67. [Google Scholar] [CrossRef] [PubMed]
- Xiangmei, W.; Jing, Z.; Hao, C.; Qingrui, W. Preparation and Characterization of Collagen-Based Composite Conduit for Peripheral Nerve Regeneration. J. Appl. Polym. Sci. 2009, 112, 3652–3662. [Google Scholar] [CrossRef]
- Jiang, B.; Wu, Z.; Zhao, H.; Tang, F.; Lu, J.; Wei, Q.; Zhang, X. Electron Beam Irradiation Modification of Collagen Membrane. Biomaterials 2006, 27, 15–23. [Google Scholar] [CrossRef]
- Ražem, D.; Katušin-Ražem, B. The Effects of Irradiation on Controlled Drug Delivery/Controlled Drug Release Systems. Radiat. Phys. Chem. 2008, 77, 288–344. [Google Scholar] [CrossRef]
- International Atomic Energy Agency. Trends in Radiation Sterilization of Health Care Products, Non-Serial Publications; IAEA: Vienna, Austria, 2008. [Google Scholar]
- Koshimizu, N.; Bessho, M.; Suzuki, S.; Yuguchi, Y.; Kitamura, S.; Hara, M. Gamma-Crosslinked Collagen Gel without Fibrils: Analysis of Structure and Heat Stability. Biosci. Biotechnol. Biochem. 2009, 73, 1915–1921. [Google Scholar] [CrossRef]
- Riedel, S.; Hietschold, P.; Krömmelbein, C.; Kunschmann, T.; Konieczny, R.; Knolle, W.; Mierke, C.T.; Zink, M.; Mayr, S.G. Design of Biomimetic Collagen Matrices by Reagent-Free Electron Beam Induced Crosslinking: Structure-Property Relationships and Cellular Response. Mater. Des. 2019, 168, 107606. [Google Scholar] [CrossRef]
- Albu, M.G. Collagen Gels and Matrices for Biomedical Applications: The Obtaining and Characterization of Collagen-Based Biomaterials as Support for Local Release; Lap Lambert Academic Publishing: Chisinau, Moldova, 2011. [Google Scholar]
- Nagasawa, N.; Yagi, T.; Kume, T.; Yoshii, F. Radiation Crosslinking of Carboxymethyl Starch. Carbohydr. Polym. 2004, 58, 109–113. [Google Scholar] [CrossRef]
- Ben Ammar, N.E.; Barbouche, M.; Hamzaoui, A.H. Chapter 15-Historical View of Hydrogel Characterization. In Hydrogels Based on Natural Polymers; Chen, Y., Ed.; Elsevier: Amsterdam, The Netherlands, 2020; pp. 459–479. ISBN 978-0-12-816421-1. [Google Scholar]
- Mark, J.E.; Erman, B. Rubberlike Elasticity: A Molecular Primer; Wiley Interscience: New York, NY, USA, 1988. [Google Scholar]
- Treloar, L.R.G. The Physics of Rubber Elasticity; Oxford University Press Inc.: New York, NY, USA, 1975. [Google Scholar]
- Cao, A.; Tang, Y.; Liu, Y.; Yuan, H.; Liu, L. A Strategy for Antimicrobial Regulation Based on Fluorescent Conjugated Oligomer–DNA Hybrid Hydrogels. Chem. Comm. 2013, 49, 5574–5576. [Google Scholar] [CrossRef]
- Holback, H.; Yeo, Y.; Park, K. Hydrogel Swelling Behavior and Its Biomedical Applications. In Biomedical Hydrogels: Biochemistry, Manufacture and Medical Applications; Rimmer, S., Ed.; Woodhead Publishing Ltd.: Sawston, UK, 2011; pp. 3–24. [Google Scholar]
- Inamura, P.Y.; Kraide, F.H.; Drumond, W.S.; de Lima, N.B.; Moura, E.A.B.; del Mastro, N.L. Ionizing Radiation Influence on the Morphological and Thermal Characteristics of a Biocomposite Prepared with Gelatin and Brazil Nut Wastes as Fiber Source. Radiat. Phys. Chem. 2013, 84, 66–69. [Google Scholar] [CrossRef]
- Tytgat, L.; Markovic, M.; Qazi, T.H.; Vagenende, M.; Bray, F.; Martins, J.C.; Rolando, C.; Thienpont, H.; Ottevaere, H.; Ovsianikov, A.; et al. Photo-Crosslinkable Recombinant Collagen Mimics for Tissue Engineering Applications. J. Mater. Chem. B 2019, 7, 3100–3108. [Google Scholar] [CrossRef]
- Gao, Y.; Duan, L.; Guan, S.; Gao, G.; Cheng, Y.; Ren, X.; Wang, Y. The Effect of Hydrophobic Alkyl Chain Length on the Mechanical Properties of Latex Particle Hydrogels. RSC Adv. 2017, 7, 44673–44679. [Google Scholar] [CrossRef]
- Mezger, T. The Rheology Handbook; Vincentz Network: Hannover, Germany, 2020; ISBN 9783748603702. [Google Scholar]
- Mori, H.; Shimizu, K.; Hara, M. Dynamic Viscoelastic Properties of Collagen Gels in the Presence and Absence of Collagen Fibrils. Mater. Sci. Eng. C 2012, 32, 2007–2016. [Google Scholar] [CrossRef]
- Ficai, A.; Andronescu, E.; Voicu, G.; Ghitulica, C.; Vasile, B.S.; Ficai, D.; Trandafir, V. Self-Assembled Collagen/Hydroxyapatite Composite Materials. Chem. Eng. J. 2010, 160, 794–800. [Google Scholar] [CrossRef]
- Fassett, J.; Tobolt, D.; Hansen, L.K. Type I Collagen Structure Regulates Cell Morphology and EGF Signaling in Primary Rat Hepatocytes through CAMP-Dependent Protein Kinase A. Mol. Biol. Cell 2006, 17, 345–356. [Google Scholar] [CrossRef]
- Muyonga, J.H.; Cole, C.G.B.; Duodu, K.G. Characterisation of Acid Soluble Collagen from Skins of Young and Adult Nile Perch (Lates Niloticus). Food Chem. 2004, 85, 81–89. [Google Scholar] [CrossRef]
- Lv, Q.; Hu, K.; Feng, Q.; Cui, F. Fibroin/Collagen Hybrid Hydrogels with Crosslinking Method: Preparation, Properties, and Cytocompatibility. J. Biomed. Mater. Res. A 2008, 84, 198–207. [Google Scholar] [CrossRef]
- Șulea, D.; Ghica, M.V.; Micutz, M.; Albu, M.G.; Brazdaru, L.; Staicu, T.; Leca, M.; Popa, L. Characterization and in Vitro Release of Chlorhexidine Digluconate Comprised in Type I Collagen Hydrogels. Rev. Roum. Chim. 2010, 55, 543–551. [Google Scholar]
- Garcia, Y.; Hemantkumar, N.; Collighan, R.; Griffin, M.; Rodriguez-Cabello, J.C.; Pandit, A. In Vitro Characterization of a Collagen Scaffold Enzymatically Cross-Linked with a Tailored Elastin-like Polymer. Tissue Eng. Part A 2008, 15, 887–899. [Google Scholar] [CrossRef]
- Popescu, C.; Budrugeac, P.; Wortmann, F.J.; Miu, L.; Demco, D.E.; Baias, M. Assessment of Collagen-Based Materials Which Are Supports of Cultural and Historical Objects. Polym. Degrad. Stab. 2008, 93, 976–982. [Google Scholar] [CrossRef]
- Park, J.B.; Bronzino, J.D. Biomaterials: Principles and Applications, 1st ed.; CRC Press: Boca Raton, FL, USA, 2002. [Google Scholar]
- Trandafir, V.; Popescu, G.; Albu, M.G.; Iovu, H.; Georgescu, M. Bioproduse Pe Baza de Colagen; Ars Docendi: Bucharest, Romania, 2007. [Google Scholar]
- Maslennikova, A.; Kochueva, M.; Ignatieva, N.; Vitkin, A.; Zakharkina, O.; Kamensky, V.; Sergeeva, E.; Kiseleva, E.; Bagratashvili, V. Effects of Gamma Irradiation on Collagen Damage and Remodeling. Int. J. Radiat. Biol. 2015, 91, 240–247. [Google Scholar] [CrossRef] [PubMed]
- Budrugeac, P.; Trandafir, V.; Albu, M.G. The Effect of the Hydration Degree on the Hydrothermal and Thermo-Oxidative Stability of Some Collageneous Matrices. J. Therm. Anal. Calorim. 2003, 72, 581–585. [Google Scholar] [CrossRef]
- Carşote, C.; Badea, E.; Miu, L.; Gatta, G. Della. Study of the Effect of Tannins and Animal Species on the Thermal Stability of Vegetable Leather by Differential Scanning Calorimetry. J. Therm. Anal. Calorim. 2016, 124, 1255–1266. [Google Scholar] [CrossRef]
- Carsote, C.; Badea, E. Micro Differential Scanning Calorimetry and Micro Hot Table Method for Quantifying Deterioration of Historical Leather. Herit. Sci. 2019, 7, 48. [Google Scholar] [CrossRef]
- Sendrea, C.; Badea, E.; Stanculescu, I.; Miu, L.; Iovu, H. Dose-Dependent Effects of Gamma Irradiation on Collagen in Vegetable Tanned Leather by Mobile NMR Spectroscopy. Leather Footwear J. 2015, 15, 139. [Google Scholar] [CrossRef]
- Miles, C.A.; Ghelashvili, M. Polymer-in-a-Box Mechanism for the Thermal Stabilization of Collagen Molecules in Fibers. Biophys. J. 1999, 76, 3243–3252. [Google Scholar] [CrossRef]
- Budrugeac, P.; Cucos, A. Application of Kissinger, Isoconversional and Multivariate Non-Linear Regression Methods for Evaluation of the Mechanism and Kinetic Parameters of Phase Transitions of Type I Collagen. Thermochim. Acta 2013, 565, 241–252. [Google Scholar] [CrossRef]



| Dose | ρ | G′ | G″ | Mc | Ve × 10−5 | ξ |
|---|---|---|---|---|---|---|
| (kGy) | (g/cm3) | (Pa) | (Pa) | (g/mol) | (mol/cm3) | (nm) |
| 5 | 1.0011 | 3386 | 86 | 107,538 | 0.93 | 23.06 |
| 10 | 1.0105 | 3807 | 89 | 145,522 | 0.69 | 23.67 |
| 25 | 1.0087 | 6174 | 174 | 99,356 | 1.02 | 18.62 |
| 50 | 1.0010 | 9713 | 395 | 95,903 | 1.04 | 16.93 |
| 100 | 1.0211 | 69,315 | 2267 | 16,693 | 6.11 | 6.61 |
| Dose, (kGy) | Amide I/Amide A | Amide III/A1450 | Δυ = υI − υII (cm−1) |
|---|---|---|---|
| 5 | 2.70 | 1.29 | 84.38 |
| 10 | 3.57 | 1.18 | 87.86 |
| 25 | 2.50 | 1.04 | 94.98 |
| 50 | 2.83 | 0.98 | 96.23 |
| 100 | 1.92 | 0.82 | 101.07 |
| Dose | Td | ΔHd | T1 | ΔH1 | T2 | ΔH2 | T3 | ΔH3 |
|---|---|---|---|---|---|---|---|---|
| (kGy) | (°C) | (J·g−1) | (°C) | (J·g−1) | (°C) | (J·g−1) | (°C) | (J·g−1) |
| 0 | 63.1 | 90.9 | - | - | 55.0 | 26.6 | 62.1 | 9.51 |
| 5 | 63.2 | 92.2 | 46.0 | 31.5 | 59.1 | 35.8 | 63.7 | 8.15 |
| 10 | 69.6 | 152.7 | 46.7 | 36.0 | 60.4 | 38.6 | 68.5 | 19.6 |
| 25 | 72.0 | 155.3 | 54.1 | 13.9 | 61.3 | 16.8 | 71.9 | 35.9 |
| 50 | 71.4 | 146. 6 | 56.1 | 31.1 | 65.2 | 41.7 | 71.7 | 19.8 |
| 100 | 64.8 | 92.1 | 52.7 | 12.36 | 65.3 | 68.2 | 76.8 | 4.18 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Demeter, M.; Călina, I.; Scărișoreanu, A.; Micutz, M.; Kaya, M.A. Correlations on the Structure and Properties of Collagen Hydrogels Produced by E-Beam Crosslinking. Materials 2022, 15, 7663. https://doi.org/10.3390/ma15217663
Demeter M, Călina I, Scărișoreanu A, Micutz M, Kaya MA. Correlations on the Structure and Properties of Collagen Hydrogels Produced by E-Beam Crosslinking. Materials. 2022; 15(21):7663. https://doi.org/10.3390/ma15217663
Chicago/Turabian StyleDemeter, Maria, Ion Călina, Anca Scărișoreanu, Marin Micutz, and Mădălina Albu Kaya. 2022. "Correlations on the Structure and Properties of Collagen Hydrogels Produced by E-Beam Crosslinking" Materials 15, no. 21: 7663. https://doi.org/10.3390/ma15217663
APA StyleDemeter, M., Călina, I., Scărișoreanu, A., Micutz, M., & Kaya, M. A. (2022). Correlations on the Structure and Properties of Collagen Hydrogels Produced by E-Beam Crosslinking. Materials, 15(21), 7663. https://doi.org/10.3390/ma15217663











