Influences of Ag Addition on the Microstructure and Mechanical Properties of Al-4Mg Alloy
Abstract
:1. Introduction
2. Experimental
3. Results and Discussions
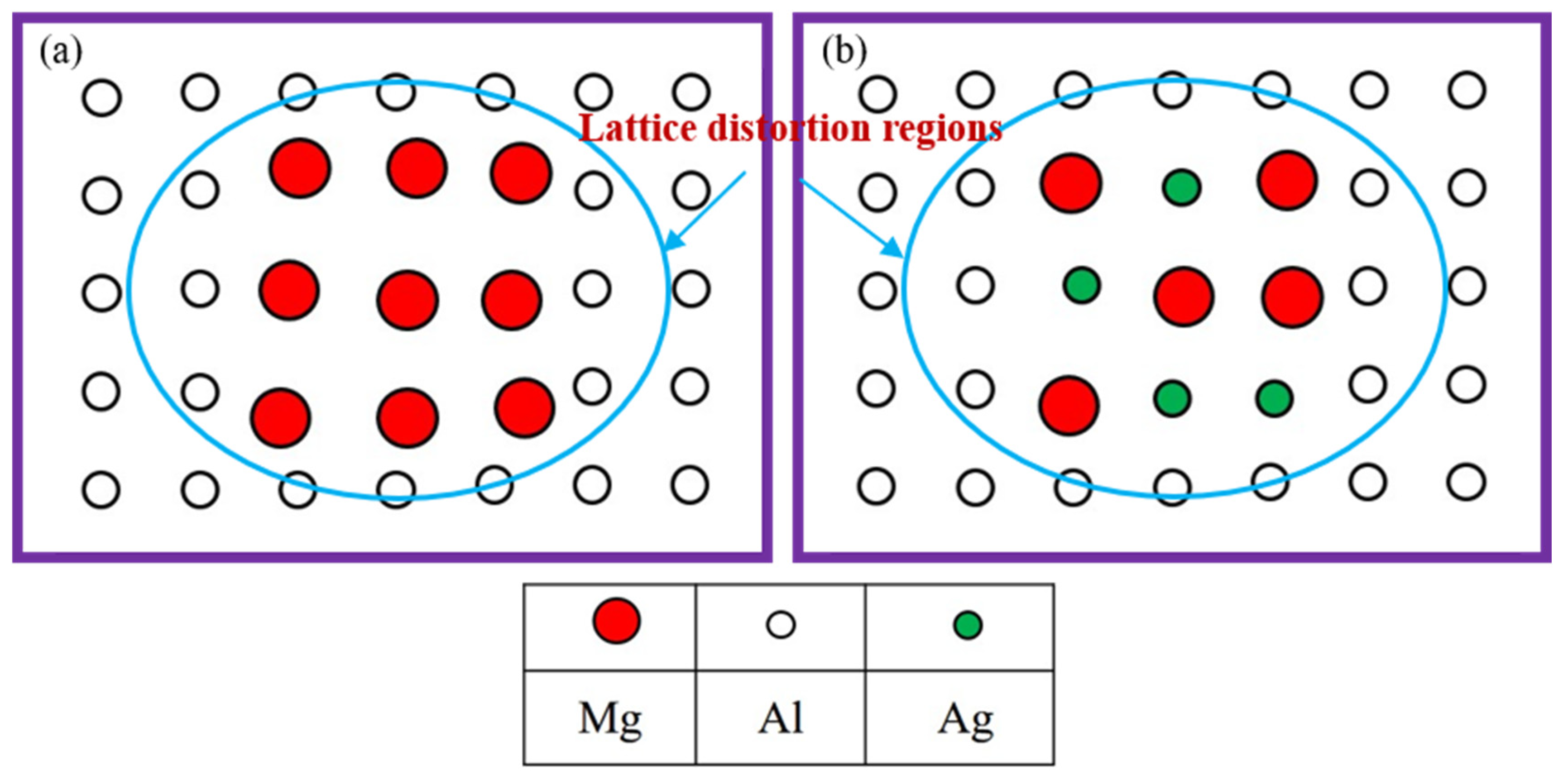
3.1. Microstructural Observations
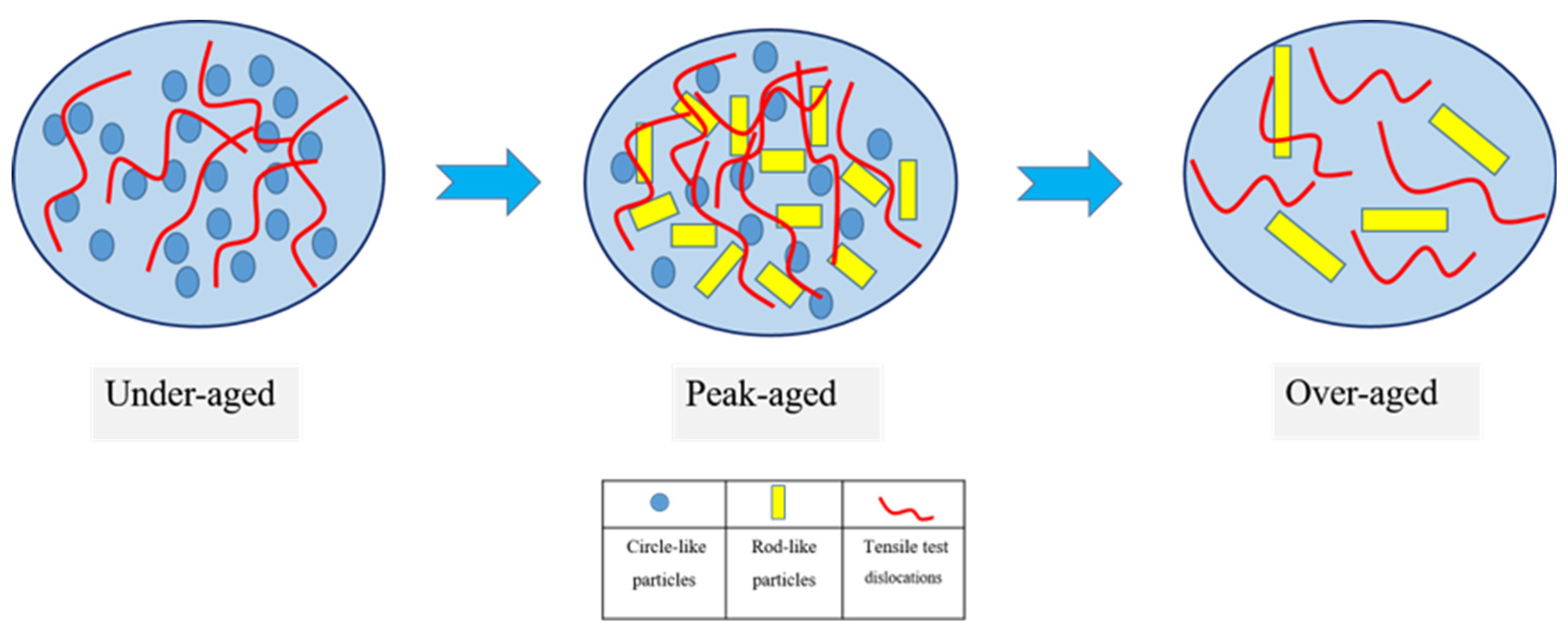
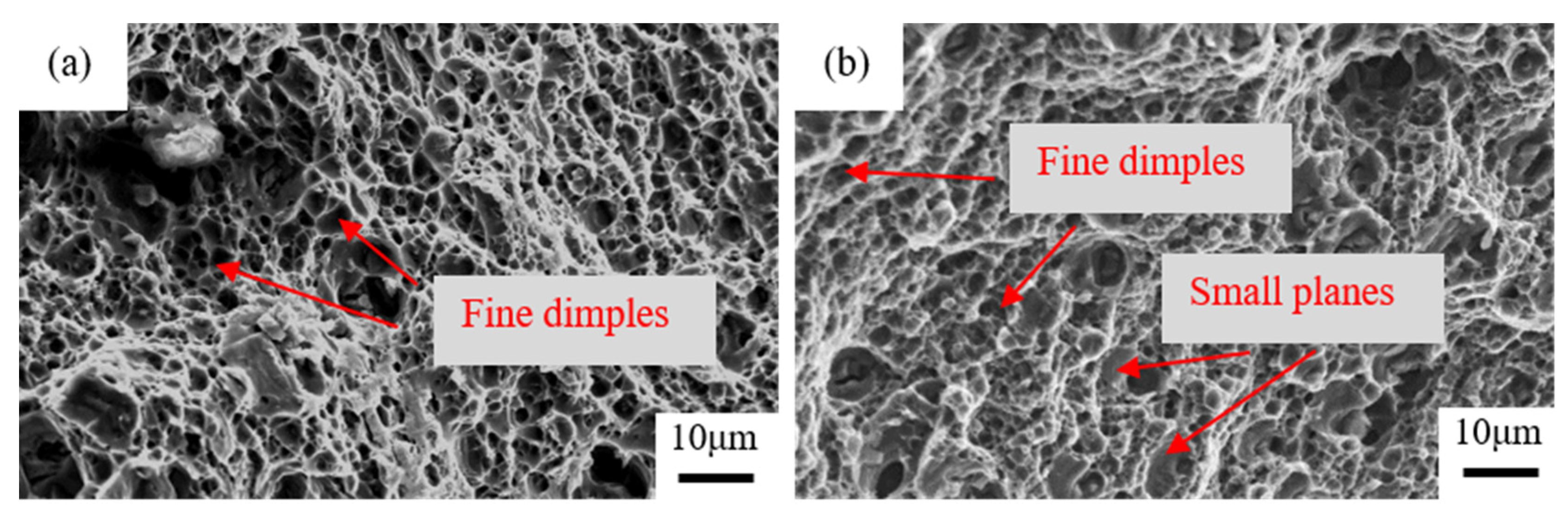
3.2. Mechanical Properties and Precipitation Process
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- He, L.; Li, X.; Wang, X.; Zhang, H.; Cui, J. Effects of homogenization on microstructures and properties of a new type Al-Mg-Mn-Zr-Ti-Er alloy. Mater. Sci. Eng. A 2010, 527, 7510–7518. [Google Scholar] [CrossRef]
- Król, M.; Tański, T.; Snopiński, P.; Tomiczek, B. Structure and properties of aluminium-magnesium casting alloys after heat treatment. J. Therm. Anal. Calorim. 2017, 127, 299–308. [Google Scholar] [CrossRef]
- Lee, B.H.; Kim, S.H.; Park, J.H.; Kim, H.W.; Lee, J.C. Role of Mg in simultaneously improving the strength and ductility of Al-Mg alloys. Mater. Sci. Eng. A 2016, 657, 115–122. [Google Scholar] [CrossRef]
- Kubota, M. Observation of beta phase particles in an isothermally aged Al-10 mass%Mg alloy with and without 0.5 mass%Ag. Mater. Trans. 2008, 49, 235–241. [Google Scholar] [CrossRef] [Green Version]
- Hamana, D.; Azizi, A. Low temperature post-precipitation after precipitation of β′ and β phases in Al-12 wt.% Mg alloy. Mater. Sci. Eng. A 2008, 476, 357–365. [Google Scholar] [CrossRef]
- Engler, O.; Miller-Jupp, S. Control of second-phase particles in the Al-Mg-Mn alloy AA 5083. J. Alloy. Compd. 2016, 689, 998–1010. [Google Scholar] [CrossRef]
- Fang, H.; Liu, H.; Yan, Y.; Luo, X.; Xu, X.; Chu, X.; Lu, Y.; Kun, Y.; Wang, D. Evolution of texture, microstructure, tensile strength and corrosion properties of annealed Al-Mg-Sc-Zr alloys. Mater. Sci. Eng. A 2021, 804, 140682. [Google Scholar] [CrossRef]
- Chen, H.; Chen, Z.; Ji, G.; Zhong, S.; Wang, H.; Borbély, A.; Ke, Y.; Bréchet, Y. Experimental and modelling assessment of ductility in a precipitation hardening AlMgScZr alloy. Int. J. Plast. 2021, 139, 102971. [Google Scholar] [CrossRef]
- Wheeler, M.J.; Blankenburgs, G.; Staddon, R.W. Evidence for a Ternary Phase in the Aluminium–Magnesium–Silver System. Nature 1965, 207, 746–747. [Google Scholar] [CrossRef]
- Stemper, L.; Tunes, M.A.; Oberhauser, P.; Uggowitzer, P.J.; Pogatscher, S. Age-hardening response of AlMgZn alloys with Cu and Ag additions. Acta Mater. 2020, 195, 541–554. [Google Scholar] [CrossRef]
- Weng, Y.; Ding, L.; Zhang, Z.; Jia, Z.; Wen, B.; Liu, Y.; Muraishi, S.; Li, Y.; Liu, Q. Effect of Ag addition on the precipitation evolution and interfacial segregation for Al-Mg-Si alloy. Acta Mater. 2019, 180, 301–316. [Google Scholar] [CrossRef] [Green Version]
- Kubota, M. Characterisation of Precipitate Microstructures of natural and artificial ageing in Al-Mg(Ag) alloys. Mater. Trans. 2005, 46, 241–250. [Google Scholar] [CrossRef] [Green Version]
- Kubota, M.; Muddle, B.C. Effect of trace additions of Ag on precipitation in Al-Mg alloys. Mater. Trans. 2005, 46, 2968–2974. [Google Scholar] [CrossRef] [Green Version]
- Zhu, X.; Blake, P.; Ji, S. The formation of Al6 (Fe, Mn) phase in die-cast Al-Mg alloys. Cryst. Eng. Comm. 2018, 20, 3839–3848. [Google Scholar] [CrossRef] [Green Version]
- Kim, J.H.; Kobayashi, E.; Sato, T. Effects of Cu addition on behavior of nanoclusters during multi-step aging in Al-Mg-Si alloys. Mater. Trans. 2011, 52, 906–913. [Google Scholar] [CrossRef] [Green Version]
- Liu, X.T.; Dong, J.; Cui, J.Z.; Zhao, G. Homogenizing treatment of high strength aluminium alloy cast under electric magnetic field. Chin. J. Nonferrous Met. 2003, 13, 909–913. [Google Scholar]
- Hono, K.; Murayama, M.; Reich, L. Clustering and segregation of Mg and Ag atoms during the precipitation processes in Al(-Li)-Cu-Mg-Ag alloys. Rev. Met. 1999, 12, 97–104. [Google Scholar]
- Howe, J. Analytical transmission electron microscopy analysis of Ag and Mg segregation to {111} θ precipitate plates in an Al-Cu-Mg-Ag alloy, Philos. Mag. Lett. 1994, 70, 111–120. [Google Scholar] [CrossRef]
- Kubota, M.; Nie, J.F.; Muddle, B.C. Identification of metastable rod-like particles in an isothermally aged Al–10Mg–0.5Ag (mass%) alloy. Mater. Trans. 2005, 46, 1288–1294. [Google Scholar] [CrossRef] [Green Version]
- Sato, T.; Kojima, Y.; Takahashi, T. Modulated structures and GP zones in Al-Mg alloys. Metall. Trans. A 1982, 13, 1373–1378. [Google Scholar] [CrossRef]
- Bouchear, M.; Hamana, D.; Laoui, T. GP zones and precipitate morphology in aged Al-Mg alloys. Philos. Mag. A 1996, 73, 1733–1740. [Google Scholar] [CrossRef]
- Nakamura, J.; Matsuda, K.; Sato, T.; Marioara, C.D.; Andersen, S.J.; Holmestad, R.; Ikeno, S. The Crystal Structure of the β’-Phase Including Ag in Al-Mg-Si-Ag Alloy. Adv. Mater. Res. Trans. Tech. Publ. 2012, 409, 67–70. [Google Scholar]
- Murayama, M.; Hono, K. Three dimensional atom probe analysis of pre-precipitate clustering in an Al-Cu-Mg-Ag alloy. Scr. Mater. 1998, 38, 1315–1319. [Google Scholar] [CrossRef]
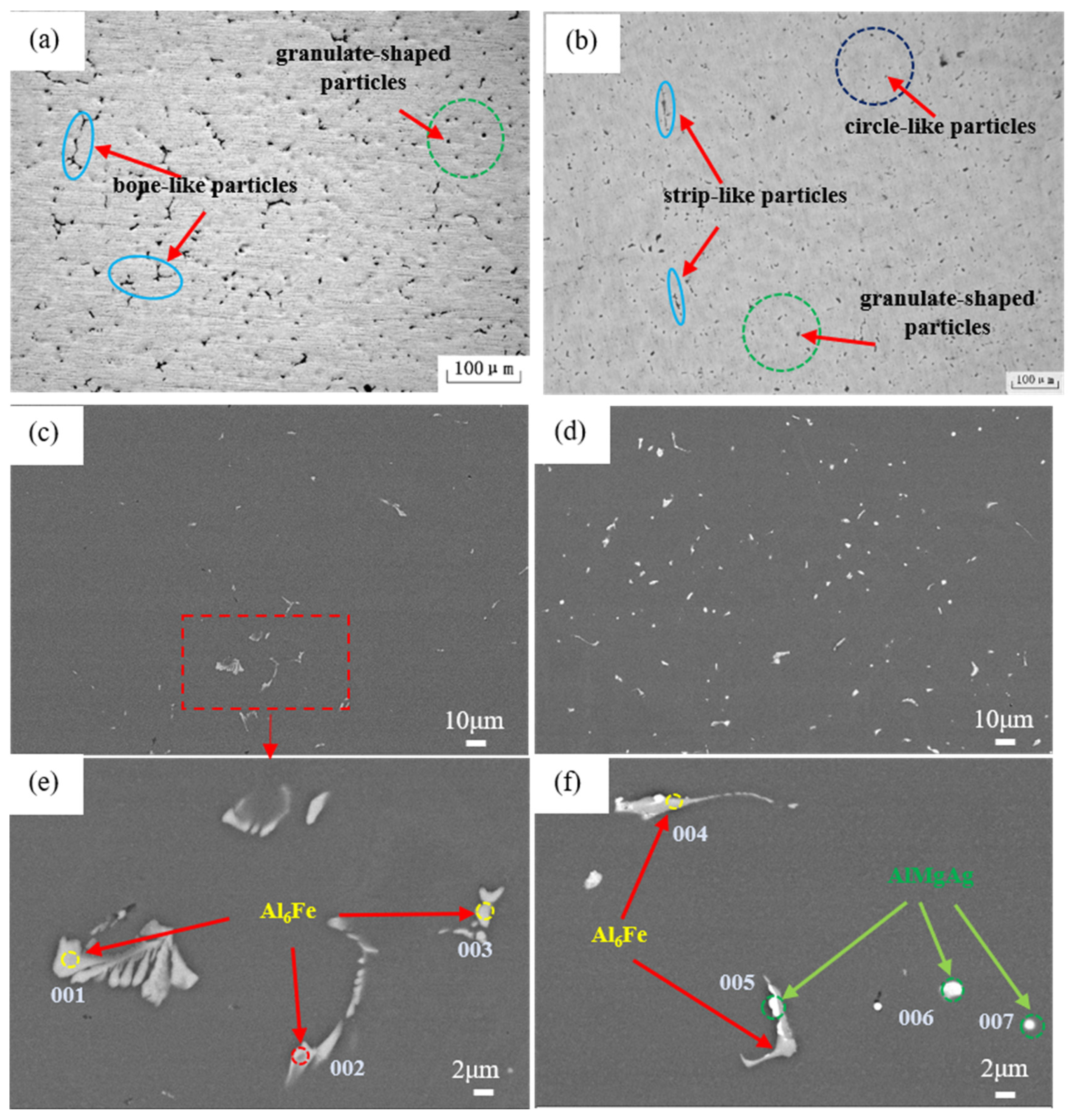
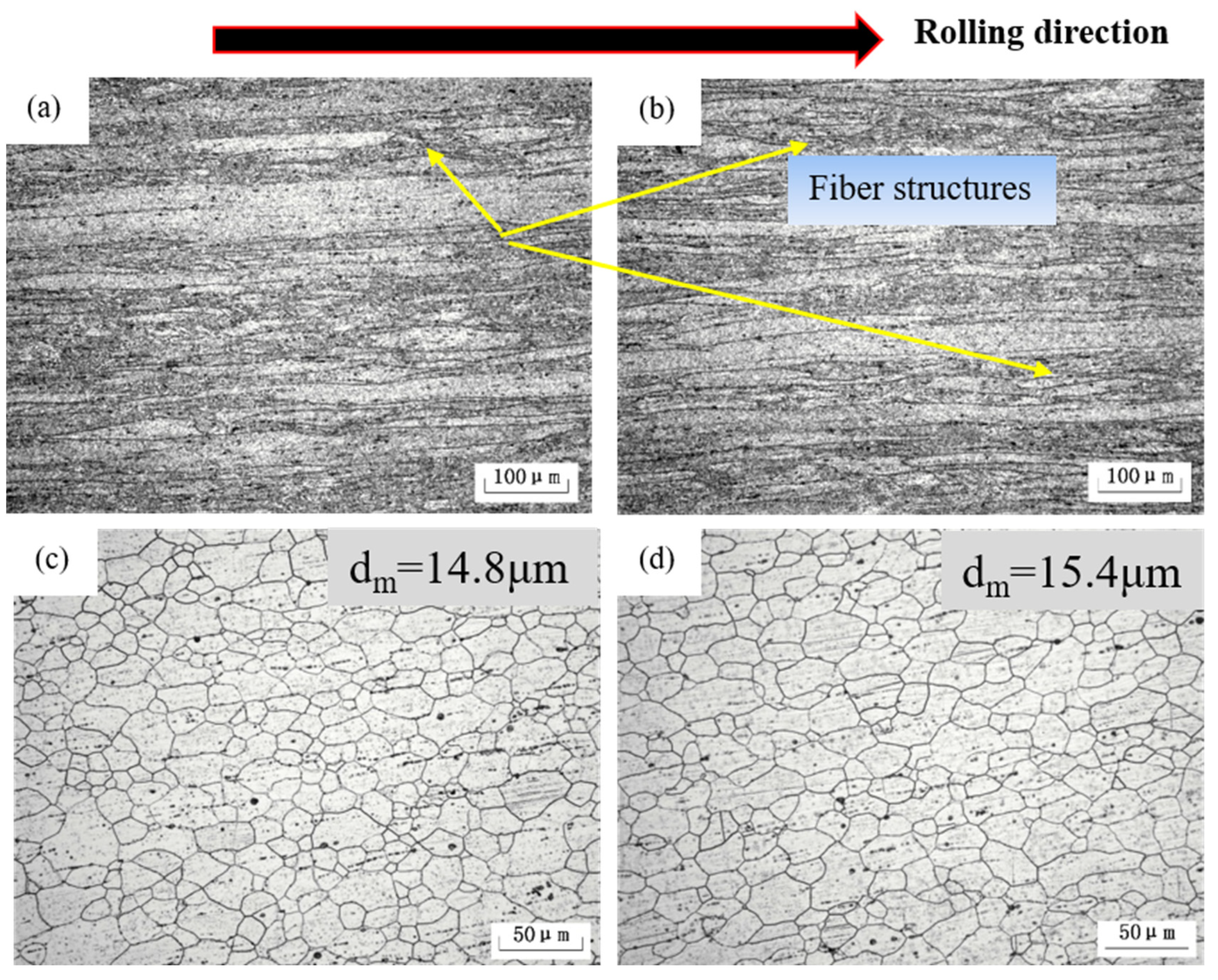

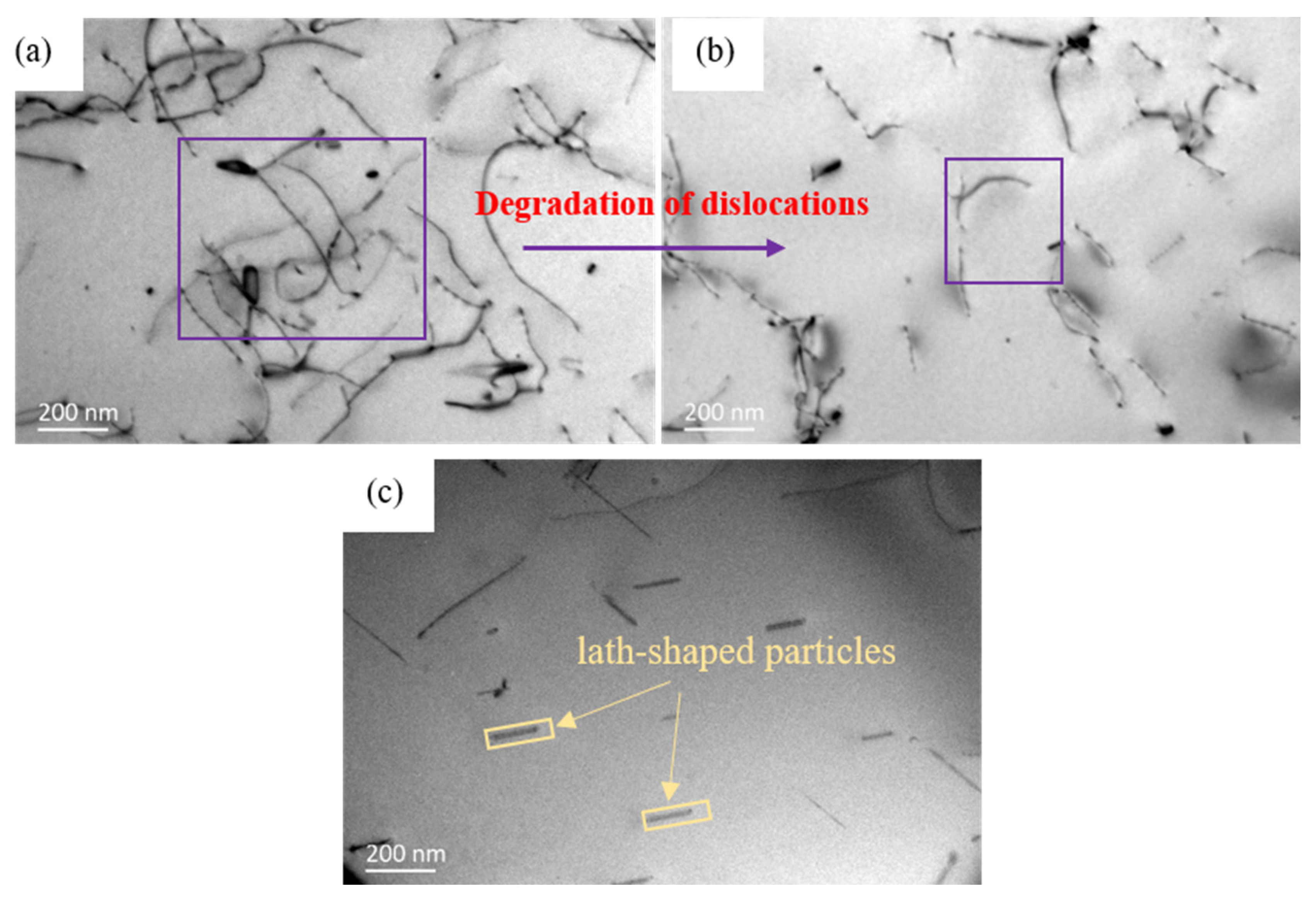
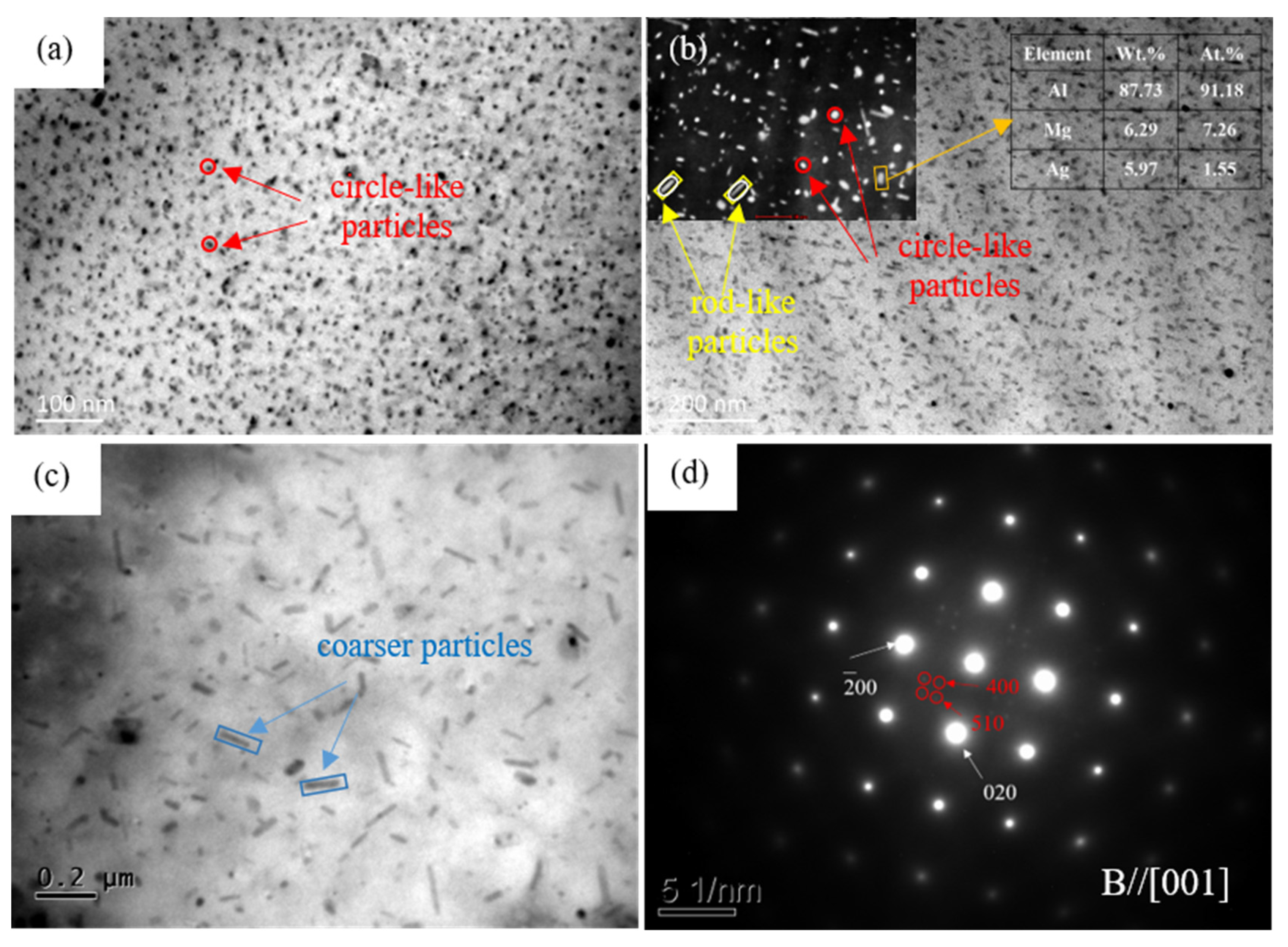

| Position | Mg | Ag | Fe | Si | Al |
|---|---|---|---|---|---|
| 001 | 0.95 | 31.96 | 67.09 | ||
| 002 | 4.39 | 15.98 | 79.63 | ||
| 003 | 4.16 | 21.05 | 74.79 | ||
| 004 | 1.12 | 0.82 | 32.72 | 65.33 | |
| 005 | 2.26 | 53.51 | 3.23 | 1.83 | 39.17 |
| 006 | 6.97 | 23.31 | 0.91 | 82.65 | |
| 007 | 17.64 | 28.92 | 1.37 | 52.07 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Guo, C.; Chen, Y.; Zhang, H. Influences of Ag Addition on the Microstructure and Mechanical Properties of Al-4Mg Alloy. Materials 2022, 15, 7705. https://doi.org/10.3390/ma15217705
Guo C, Chen Y, Zhang H. Influences of Ag Addition on the Microstructure and Mechanical Properties of Al-4Mg Alloy. Materials. 2022; 15(21):7705. https://doi.org/10.3390/ma15217705
Chicago/Turabian StyleGuo, Cheng, Yifei Chen, and Haitao Zhang. 2022. "Influences of Ag Addition on the Microstructure and Mechanical Properties of Al-4Mg Alloy" Materials 15, no. 21: 7705. https://doi.org/10.3390/ma15217705





